Abstract
Periodontitis is a prevalent chronic inflammatory disease that has been increasingly recognized for its systemic impacts, including its connection to respiratory diseases such as pneumonia, chronic obstructive pulmonary disease (COPD), Obstructive Sleep Apnea (OSA), asthma, lung cancer, and COVID-19. This review explores the potential role of periodontal pathobionts, particularly Porphyromonas gingivalis (Pg), Treponema denticola (Td), Fusobacterium nucleatum (Fn), Aggregatibacter actinomycetemcomitans (Aa), and Tannerella forsythia (Tf), in respiratory health. These pathobionts contribute to respiratory diseases by facilitating pathogen adhesion, inducing epithelial cell apoptosis, and promoting inflammation. The review also highlights the beneficial effects of periodontal treatment in reducing pathobiont burden and systemic inflammation, thereby mitigating the risk of respiratory complications. This interdisciplinary approach underscores the need to consider oral health as a critical component in managing and preventing respiratory diseases, with future research needed to further clarify these associations and develop targeted interventions.
1. Introduction
1.1. Background of Periodontal Microorganisms and Periodontitis
Periodontitis is a chronic inflammatory and destructive disease of the periodontal tissues, caused by a combination of bacteria, host immunological responses, and other contributing factors [1]. It is prevalent globally and remains the primary etiological driver of adult tooth loss, affecting roughly 12% of the global adult population with severe periodontal disease [1,2]. The bacterial composition in periodontal pockets plays a crucial role in disease progression. Bacterial components like lipopolysaccharides initiate local and systemic inflammation, contributing to tissue destruction [3]. Recent advances in periodontal microbiology have demonstrated a complex interaction between oral microbiota and systemic health, with specific associations between periodontitis and a variety of systemic conditions [3,4,5].
1.2. Close Relationship Between Periodontitis and Systemic Diseases
A growing body of evidence has identified a close relationship between periodontitis and systemic diseases, such as cardiovascular diseases, diabetes mellitus, rheumatoid arthritis, and neurodegenerative disorders [6,7,8]. For example, Porphyromonas gingivalis and other periodontal pathogens have been detected in atherosclerotic plaques, implicating them in cardiovascular disease [7,8]. Additionally, bacteria such as Actinobacillus actinomycetemcomitans have been detected in half of the analyzed carotid endarterectomy specimens from clinically confirmed atherosclerotic patients, supporting the hypothesis that periodontal pathogens contribute to systemic bacteremia [9]. Accumulating evidence has demonstrated a periodontal–systemic axis manifesting as neurocognitive decline (including Alzheimer’s disease), gestational complications (preterm birth/low birth weight), and autoimmune arthritis pathogenesis [10,11]. Mechanistic studies further reveal that chronic periodontitis exhibits pathophysiological synergism with cardiovascular disease determinants mediated by circulating mediators including interleukin-6 (IL-6), tumor necrosis factor-alpha (TNF-α), and high-sensitivity C-reactive protein (hsCRP) [9]. Furthermore, it has been recognized by the European Federation of Periodontology and the International Diabetes Federation that periodontitis and diabetes are linked through pathways involving IL-1β, TNF-α, and IL-6, with professional periodontal therapy contributing to a reduction in glycated hemoglobin (HbA1c) levels in diabetic patients [12]. Non-surgical periodontal treatment has also been associated with improvements in systemic inflammation markers and joint disease activity in rheumatoid arthritis patients [13].
1.3. Close Relationship Between Periodontitis and Pneumonia
There is ample evidence linking respiratory illnesses, especially pneumonia, to periodontitis [14]. Respiratory pathogens such as Klebsiella pneumoniae and Pseudomonas aeruginosa can colonize the oral cavity and be aspirated into the lungs, leading to pneumonia [15,16]. Patients with lung diseases exhibit a significantly worse periodontal condition, with the majority of them having a high periodontal index indicating a heightened risk of infection [17]. Studies have demonstrated that patients with moderate to severe periodontitis are four times more likely to experience community-acquired pneumonia (CAP) compared to those without a disease of the periodontal tissues [18]. Moreover, a 7-year longitudinal study revealed that patients with periodontitis undergoing hemodialysis had significantly higher mortality rates from pneumonia than their periodontally healthy counterparts [19]. The oral cavity can serve as a reservoir for respiratory infections, as periodontal disease promotes the accumulation of dental plaque, which fosters the colonization of respiratory pathogens [20,21,22].
This review aims to explore the intricate relationship between periodontitis and systemic health, with a particular focus on its connection to respiratory diseases such as pneumonia, chronic obstructive pulmonary disease (COPD), obstructive sleep apnea (OSA), asthma, lung cancer, and COVID-19. By examining the current literature, we will investigate the role of specific periodontal microorganisms—namely Porphyromonas gingivalis (Pg), Treponema denticola (TD), Fusobacterium nucleatum (Fn), Aggregatibacter actinomycetemcomitans (Aa), and Tannerella forsythia (Tf)—in influencing respiratory health. These pathogens contribute to the development of respiratory diseases by promoting pathogen adhesion, inducing epithelial cell apoptosis, and triggering inflammatory responses. Furthermore, the review will highlight the potential benefits of periodontal treatment, such as reducing microbial load and systemic inflammation, which may help mitigate the risk of respiratory complications. The importance of incorporating oral health into respiratory disease prevention and therapy is highlighted by this multidisciplinary approach and calls for further research to clarify these associations and develop targeted interventions aimed at improving clinical outcomes.
2. Relationship Between Periodontal Microorganisms and Respiratory Health
2.1. The Relationship Between Periodontal Microorganisms and Respiratory Diseases
Periodontitis is closely linked to respiratory diseases, including pneumonia. The lungs can be infected by oral pathogens including Porphyromonas gingivalis (Pg) and Fusobacterium nucleatum (Fn), potentially leading to infections like community-acquired pneumonia (CAP) [23]. In hospital settings, molecular studies of mechanically ventilated patients demonstrate that respiratory pathogens such as Staphylococcus aureus, Pseudomonas aeruginosa, Acinetobacter species, and enteric species recovered from dental plaque are genetically indistinguishable (>95% similarity by pulsed-field gel electrophoresis) from those isolated from bronchoalveolar lavage fluid, confirming that dental plaque serves as an important reservoir for respiratory pathogens [24]. Molecular evidence further confirms that periodontal biofilms serve as reservoirs for respiratory pathogens. Supporting evidence from institutionalized elders demonstrates this relationship: it was reported that 57% of such patients (28/49) had dental plaques colonized by aerobic pathogens, predominantly Staphylococcus aureus (45%), Gram-negative bacilli (42%), and Pseudomonas aeruginosa (13%). Crucially, pulsed-field gel electrophoresis (PFGE) genetically matched respiratory pathogens from dental plaques to those causing hospital-acquired pneumonia (HAP) in 8 of 10 microbiologically confirmed cases, establishing the oral cavity as a direct source of respiratory infection in high-risk populations with compromised oral hygiene [16]. These organisms spread from the mouth into the trachea and subsequently into the lung to initiate infection [24].
Patients with periodontal disease, particularly those with weakened immune systems, face an increased risk of respiratory complications [23]. Early detection and management of periodontal disease—through the collaboration between dental and medical professionals—are essential to reducing the risk of pneumonia and improving respiratory health outcomes [23].
Oral pathogens such as Porphyromonas gingivalis (Pg), Fusobacterium nucleatum (Fn), and chronic obstructive pulmonary disease (COPD), which is linked to periodontal disease, have also been linked to Aggregatibacter actinomycetemcomitans (Aa), Tannerella forsythia (Tf), and Treponema denticola (TD) [25]. Aspiration of these microbes can lead to inflammation and infection in the lungs, and the inflammation caused by periodontal disease may exacerbate COPD symptoms [26]. Additionally, oral dysbiosis exacerbates COPD via the aspiration of pathogens (e.g., Pg and Fn activating lung inflammation), and conversely, systemic inflammation from COPD may disrupt oral microbiota homeostasis [27]. In addition, periodontal disease, dry mouth, and bruxism are frequently linked to obstructive sleep apnea (OSA) [28]. The systemic inflammation caused by OSA can worsen periodontal disease, while mouth breathing exacerbates xerostomia and increases the risk of dental cavities. Patients with OSA, especially those using therapeutic oral appliances for OSA management, require frequent dental check-ups due to the potential changes in dental occlusion [28]. The importance of interdisciplinary care is highlighted, as early dental evaluation and intervention are critical in managing oral health issues associated with OSA [28]. A potential link between periodontal disease and asthma has also been suggested. Asthma may contribute to periodontal disease through the use of inhalers, mouth breathing, and hypoxia [29]. Conversely, the onset and worsening of asthma symptoms may be influenced by periodontal bacteria and the inflammation they cause [29]. According to a number of studies, people with asthma are more likely to acquire periodontal disease, and the association between the two disorders is influenced by common risk factors like smoking and gastroesophageal reflux disease (GERD) [29]. Some research suggests that treating periodontal disease may help alleviate asthma symptoms [29]. Additionally, there might be a link between lung cancer and periodontal disease [25]. Research indicates that a systemic inflammatory response plays a central role in linking periodontal disease to lung cancer. Inflammation caused by periodontal bacteria stimulates the immune system, potentially increasing the risk of lung cancer development [25]. Evidence also suggests that the systemic effects of periodontal bacteria can account for the correlation between periodontal disease and tooth loss and an increased risk of lung cancer. To create a more robust link between periodontal disease and lung cancer, further prospective research is required [25].
Finally, periodontitis may cause systemic inflammation, which could accelerate the spread of COVID-19. Periodontal disease and COVID-19 are believed to be related through the cytokine storm mechanism [30]. Maintaining proper oral hygiene is crucial in reducing the risk of respiratory complications, though further research is required to fully elucidate this connection [30]. As summarized, emerging evidence suggests periodontal inflammation may exacerbate COVID-19 severity through amplified cytokine release (e.g., IL-6, TNF-α) [30]. Maintaining proper oral hygiene is crucial in reducing the respiratory complications associated with SARS-CoV-2 infection, as depicted in Figure 1’s pathway analysis, which further illustrates the proposed biological pathways linking periodontal dysbiosis to SARS-CoV-2 complications. However, causal mechanisms require validation in large-scale longitudinal studies. The synthesized mechanistic framework of these interactions is presented in Figure 1.
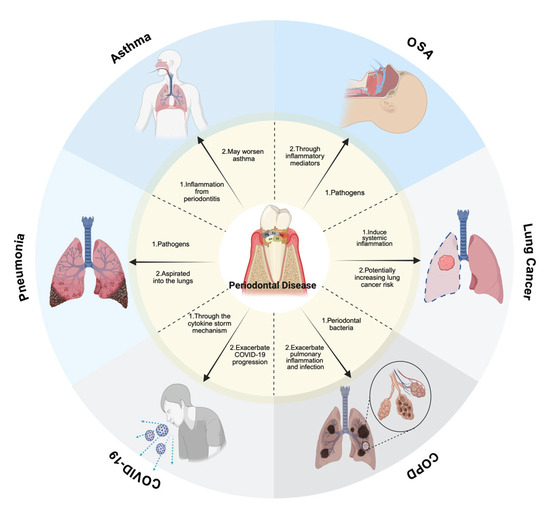
Figure 1.
Mechanistic pathways linking periodontal pathogens to respiratory diseases, with oral appliances for OSA treatment specified. The diagram is of the associations with respiratory diseases. Pneumonia: oral pathogens community-acquired pneumonia (CAP) can result by aspirating Porphyromonas gingivalis (Pg) and Fusobacterium nucleatum (Fn) into the lungs, with immunocompromised individuals at higher risk. COPD: Pg, Fn, Treponema denticola (Td), Aggregatibacter actinomycetemcomitans (Aa), and Tannerella forsythia (Tf) contribute to lung inflammation and infection, exacerbating COPD progression. OSA: periodontal disease, dry mouth, and bruxism are common in OSA patients. Mouth breathing worsens xerostomia and dental caries, while prolonged use of oral appliances may affect occlusion. Asthma: inhaler use, mouth breathing, and hypoxia may worsen periodontitis, while periodontal inflammation may, in turn, exacerbate asthma symptoms. Lung Cancer: periodontal bacteria trigger a systemic inflammatory response, potentially increasing lung cancer risk. An increased incidence of lung cancer is linked to both periodontitis and tooth loss. COVID-19: periodontitis may worsen COVID-19 progression via the cytokine storm mechanism, and maintaining oral health may help reduce respiratory complications. This figure was created with Created in BioRender. Kim, B. (2025) https://BioRender.com/p86m475.
2.2. Types and Functions of Periopathogens
Porphyromonas gingivalis (Pg), Treponema denticola (Td), Fusobacterium nucleatum (Fn), Aggregatibacter actinomycetemcomitans (Aa), and Tannerella forsythia (Tf) are among the many microorganisms that are found in the oral cavity, all of which are implicated in periodontitis [22]. An addition to compromising periodontal health, these microorganisms are implicated in systemic diseases such respiratory problems, rheumatoid arthritis, and cardiovascular disease [31,32]. Patients with chronic periodontitis frequently have saliva that contains Porphyromonas gingivalis (Pg), a highly pathogenic, Gram-negative anaerobic bacterium that lives in periodontal pockets. It contains a number of virulence factors, such as fimbriae, gingipains (gingipain protests, GP), and lipopolysaccharide (LPS), which are species-specific trypsin-like proteinases. Kgp targets lysine residues, while Rgp cleaves arginine residues [33]. These gingipains increase the expression of PAFR on human alveolar epithelial cells, which improves Streptococcus pneumoniae’s ability to adhere to these cells. This approach raises the possibility that Porphyromonas gingivalis (Pg) has a role in pneumonia development [33]. Additionally, Pg’s gingipains (Rgp and Kgp) promote PAFR expression on alveolar cells, enhancing Streptococcus pneumoniae adhesion and potentially contributing to pneumonia. Treponema denticola (Td) secretes Td-CTLP, a key virulence factor that plays a significant role in periodontitis development [34]. Moreover, Td-CTLP has been detected in various orodigestive cancers, such as esophageal, gastric, and pancreatic tumors, and in vitro studies suggest that it activates matrix metalloproteinases (MMP-8 and MMP-9), critical players in tumor progression [34]. Td-CTLP may contribute to carcinogenesis through immunomodulation. Fusobacterium nucleatum (Fn), a Gram-negative, spindle-shaped anaerobe, is one of the most prevalent bacteria in the human oral cavity [35]. It acts as a pathobiont, proliferating during dysbiosis that often precedes periodontal disease, and supports keystone species such as Porphyromonas gingivalis (Pg) in disrupting host–microbe homeostasis [35]. Fusobacterium nucleatum (Fn) is known for its strong adhesion mechanisms and can coaggregate with early colonizers like Streptococcus species and anaerobic secondary colonizers such as Porphyromonas gingivalis (Pg), Treponema denticola (Td), and Aggregatibacter actinomycetemcomitans (Aa). By linking these species together, Fn plays a critical role in biofilm formation, which resists host defenses like saliva and gingival crevicular fluid [35]. Furthermore, Fn promotes cancer progression by manipulating β-catenin signaling, immune evasion, and chemoresistance, with FadA and LPS playing key roles. Aggregatibacter actinomycetemcomitans (Aa) weakens the host’s mucosal defenses, promoting disease onset. A comprehensive review of clinical, molecular, and interventional research that has elucidated the genetic, phenotypic, and biogeographical strategies Aa uses to survive and contribute to disease pathogenesis by suppressing host mucosal defenses can be found at [36]. Aggregatibacter actinomycetemcomitans (Aa) can enhance the expression of complement resistance genes and leukotoxin production, thereby modulating the local host immune response to facilitate the overgrowth of a consortium of pathobionts [36]. The consortium collaboratively overcomes the host’s innate immune defenses, triggering the release of inflammatory cytokines. This process ultimately leads to the degradation of connective tissue and bone, resulting in the disruption of the periodontal attachment apparatus [36]. Tannerella forsythia (Tf) is an anaerobic bacterium within the “red-complex” of the oral microbiota, which is strongly linked to periodontitis. This bacterium produces sialidases, enzymes that cleave sialic acids from the glycans present on the surface of host cells. Among these sialidases, NanH plays a crucial role in T. forsythia’s virulence [37]. Tannerella forsythia (Tf)’s NanH sialidase targets both α2,3 and α2,6-linked sialic acids, preferring the α2,3 linkage, when cleaving sialic acid from host glycans. Beyond aiding in nutrient acquisition, this enzyme facilitates biofilm formation and enhances interactions with host cells by removing sialic acid [37]. Specifically, NanH aids nutrient acquisition, promotes biofilm formation, and enhances interactions with host cells by removing sialic acid. Collectively, the virulence mechanisms of these key periodontal pathogens include Porphyromonas gingivalis (Pg), Treponema denticola (Td), Fusobacterium nucleatum (Fn), Aggregatibacter actinomycetemcomitans (Aa), and Tannerella forsythia (Tf) (Figure 2).
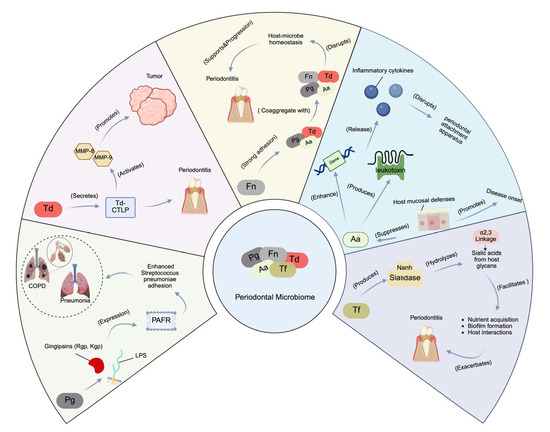
Figure 2.
Virulence mechanisms of key periodontal pathogens. Pg: a Gram-negative anaerobe that produces virulence factors like gingipains (Rgp, Kgp), which promote PAFR expression on alveolar epithelial cells, enhancing Streptococcus pneumoniae adhesion and potentially contributing to pneumonia. Td: secretes Td-CTLP, a virulence factor linked to periodontitis and orodigestive cancers. Td-CTLP activates MMP-8 and MMP-9, key enzymes in tumor progression. Fn: a pathobiont that coaggregates with Pg, Td, and Aa, facilitating biofilm formation and resisting host defenses. Aa: suppresses host mucosal defenses by enhancing complement resistance genes and leukotoxin production, contributing to immune evasion and periodontal tissue destruction. Tf: produces NanH sialidase, which cleaves sialic acids from host glycans, aiding biofilm formation and bacterial–host interactions. This figure was created with Created in BioRender. Kim, B. (2025) https://BioRender.com/7t3mdxt.
2.3. Interactions Between Periodontal Microorganisms and the Respiratory System: Mechanism Analysis
Periodontal pathogens play a critical role in modulating respiratory health by promoting the adhesion of respiratory pathogens to lung tissues. For example, Porphyromonas gingivalis (Pg) upregulates platelet-activating factor receptors (PAFR), which facilitate the attachment of Streptococcus pneumoniae to respiratory epithelial cells [32]. Additionally, certain periodontal microorganisms, such as Fusobacterium nucleatum (Fn), have been shown to increase ACE2 receptor expression, increasing SARS-CoV-2’s ability to enter host cells during COVID-19 [38,39]. The mechanisms by which periodontal microorganisms affect respiratory health include promoting mucus hypersecretion, inducing apoptosis in lung epithelial cells, and dysregulating immune responses [40,41]. Fusobacterium nucleatum (Fn) and Porphyromonas gingivalis (Pg) stimulate the production of MUC5AC, leading to mucus overproduction and airway obstruction in diseases such as COPD [40,41]. These pathogens also induce proinflammatory cytokine release, which contributes to the exacerbation of respiratory diseases like pneumonia and COVID-19 [42,43,44].
2.4. The Impact of Respiratory Diseases on Periodontal Microbiota
Chronic obstructive pulmonary disease (COPD) and community-acquired pneumonia (CAP) are respiratory illnesses directly associated with the periodontal microbiome. Anaerobic bacteria, commonly found in periodontal disease, have been identified as pathogenic factors in CAP [45]. COPD directly influences periodontal microbiota diversity and abundance. Studies show that patients with both COPD and periodontitis exhibit distinct salivary microbiome profiles compared to those with periodontitis alone or healthy controls [46], characterized by an increased Rothia, Veillonella, and Actinomyces abundance, higher bacterial richness/diversity, and unique Lachnospiraceae prevalence—shifts correlating with aggravated periodontal inflammation suggest that COPD exacerbates oral dysbiosis [46]. Furthermore, the presence of oral pathogens in COPD patients’ sputum and bronchoalveolar lavage fluid confirms the oral microbiota’s crucial role in disease pathogenesis [47]. Pathogens including Streptococcus pneumoniae, Klebsiella pneumoniae, and Staphylococcus aureus colonize dental plaque, periodontal pockets, and saliva, establishing the oral cavity as a significant reservoir for respiratory pathogens [45]. This creates a foundation for lower respiratory infections when host immunity is compromised [45].
Crucially, respiratory diseases facilitate the colonization of these pathogens by promoting oral microbiota dysbiosis and impairing immune function. Conditions such as periodontitis, denture stomatitis, and smoking enable colonization, while aging, radiotherapy, chemotherapy, and inhalation therapies (e.g., beta-2 agonists, anticholinergics, and corticosteroids) exacerbate this process [45]. Inhalation therapies alter oral ecology through a reduced salivary flow (diminishing IgA/lysozyme protection) [48], acidic pH shifts promoting cariogenic bacteria [48], and corticosteroid-induced immunosuppression increasing oral candidiasis/ulceration risk [48]. Periodontal pathogens (Porphyromonas gingivalis, Treponema denticola) prevalent in COVID-19 patients exacerbate respiratory inflammation and enhance SARS-CoV-2 infectivity by degrading its spike protein [49]. They may enter the bloodstream, causing bacteremia and endotoxemia that worsen COVID-19 outcomes [49]. Anatomically, the oral cavity serves as a reservoir for respiratory pathogens due to its connection with the upper respiratory tract [50]. When immunity is compromised (by COPD, smoking, or prolonged hospitalization), oral microbes enter the lungs contributing to respiratory disease development [50]. Collectively, these mechanisms demonstrate that respiratory diseases and their treatments initiate a vicious cycle: COPD/inhalation therapy → oral dysbiosis → pathogen colonization → worsened respiratory disease [46,48]. As illustrated in Figure 3, periodontal treatment provides systemic benefits across various interdisciplinary diseases, including diabetes, cardiovascular disease, rheumatoid arthritis, and Alzheimer’s disease.
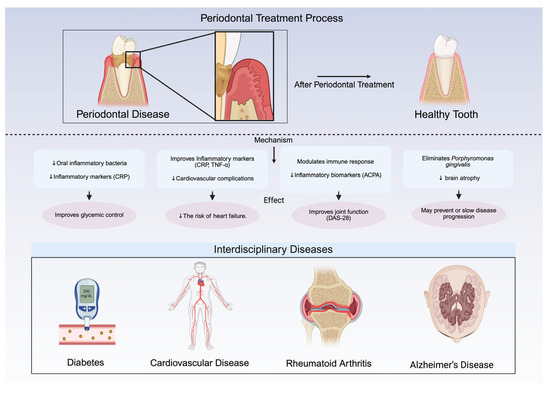
Figure 3.
Systemic benefits of periodontal treatment across interdisciplinary diseases. Diabetes: reduces oral inflammation, improves glycemic control, and lowers inflammatory markers like C-reactive protein (CRP); crucial for type 2 diabetes management. Cardiovascular disease (CVD): lowers the risk of myocardial infarction and heart failure by improving inflammatory markers (CRP, TNF-α); particularly beneficial for diabetic patients. Rheumatoid arthritis (RA): reduces inflammation, improves disease activity score 28 (DAS-28), and lowers anti-citrullinated protein antibody (ACPA) levels; interdisciplinary management recommended. Alzheimer’s disease (AD): eliminates Porphyromonas gingivalis, slows brain atrophy, and may delay cognitive decline; early intervention is key. This figure was created Created in BioRender. Kim, B. (2025) https://BioRender.com/6lo1gi5.
3. Effects of Periodontal Treatment on Respiratory Health
Periodontal treatment has been shown to reduce the risk of respiratory diseases, particularly in reducing hospital-acquired infections like pneumonia [51]. Professional oral care interventions significantly decrease the oral microbial burden, thereby mitigating the occurrence of ventilator-associated pneumonia among critically ill patients; furthermore, chlorhexidine-based oral rinses have demonstrated efficacy in curtailing respiratory pathogens in the oropharynx, which in turn minimizes the risk of aspiration pneumonia [51]. In individuals suffering from COPD (Chronic Obstructive Pulmonary Disease), periodontal therapy has been linked to improved respiratory function and a reduction in adverse respiratory events [52], and treatment in patients with periodontitis and COPD can slow down lung function deterioration, reduce exacerbation frequency, and improve respiratory function [53]. Patients receiving periodontal treatment exhibit a reduced incidence of respiratory complications—including acute exacerbations, pneumonia, and acute respiratory failure—while COPD patients undergoing periodontal therapy experience fewer visits to medical facilities, hospitalizations, and ICU admissions [52]. Furthermore, compared to the control group, the therapy group’s all-cause death rate is substantially lower [52]. Both COPD and periodontal diseases involve systemic inflammation, and periodontal treatment may help alleviate COPD symptoms by reducing inflammation through the reduction in dental plaque, oral mucosal colonization, and serum inflammatory markers, potentially preventing airway inflammation [52]. Although its influence on quality of life remains uncertain, these findings suggest that periodontal treatment may be beneficial for managing COPD, but additional studies are required to validate these outcomes [53]. Periodontal disease extends beyond the oral cavity and has been linked to a range of systemic conditions, including respiratory diseases [54]. While the exact nature of the causal relationship remains unclear, significant evidence suggests that periodontal treatment can influence the symptoms, events, and biomarkers of several systemic diseases, potentially reducing their severity or prevalence [54]. Recent interventional studies have concentrated on assessing how periodontal treatment influences systemic conditions, with growing recognition of its benefits in managing disorders such as cardiovascular disease, diabetes, and rheumatoid arthritis [54]. Extensive research demonstrates significant beneficial effects beyond the oral cavity: in diabetes, treatment enhances glycemic control and reduces systemic inflammation [55,56,57,58]. For cardiovascular disease (CVD) in periodontitis patients, therapy improves key biomarkers (CRP, TNF-α) and specifically reduces myocardial infarction and heart failure risk in diabetics [59,60,61]. Periodontal therapy also reduces systemic inflammation and improves disease activity scores (DAS-28) in rheumatoid arthritis patients [62,63,64]. Furthermore, evidence links periodontal treatment to potentially slowing Alzheimer’s disease progression, including reduced brain atrophy, suggesting a role in managing early AD risk [65,66]. These results underscore the prospective benefits of periodontal treatment in preventing and managing respiratory health issues, particularly within hospital settings [51].
Daily toothbrushing is a highly effective intervention for reducing hospital-acquired pneumonia (HAP), particularly in critically ill and mechanically ventilated patients, as incorporating it into routine oral care significantly lowers pneumonia incidence and improves critical clinical outcomes, with evidence that brushing reduces plaque, decreases oral pathogens (Pg, Fn), restores the oral microbiome balance, and lowers the frequency of acute COPD exacerbation [27]. Key advantages include its safety profile (no major adverse events reported), feasibility across hospital settings, and equivalence of twice-daily brushing to more frequent regimens, strongly supporting prioritizing it over antiseptic-only protocols in infection prevention guidelines [67]. This is critical because oral hygiene significantly impacts respiratory infection risk in hospitalized patients: Oral microbiota can migrate to the respiratory tract, where specific genera like Prevotella (promoting bronchitis) and Streptococcus pneumoniae (exacerbating pneumonia) worsen infections; dysbiosis, especially in mechanically ventilated patients, increases ventilator-associated pneumonia (VAP) risk, and enhanced oral care (e.g., antimicrobial rinses, debridement) plus targeted microbiome modulation (e.g., probiotics) effectively reduce respiratory infections in this population [68].
4. Impact of Oral Care Interventions on Respiratory Infections in Hospitalized Patients
The oral microbiome, as the body’s second most complex microbial ecosystem, is closely linked to respiratory health through anatomical proximity and immune modulation. A two-sample Mendelian randomization study in an East Asian population [68] confirmed causal associations between specific oral microbial genera and five respiratory infections: Prevotella promotes bronchitis but inhibits pneumonia and tonsillitis; Streptococcus pneumoniae exacerbates pneumonia, while Streptococcus pseudopneumoniae inhibits bronchitis; Fusobacterium significantly promotes chronic sinusitis, bronchiectasis, and bronchitis; Pauljensenia and Capnocytophaga are associated with a reduced risk of bronchitis/tonsillitis and inhibited pneumonia progression, respectively, indicating that translocation of oral microbes to the respiratory tract can influence respiratory diseases via the modulation of inflammatory cytokines (e.g., IL-6, IL-8) and enhancement of pathogen virulence. In hospitalized patients, oral hygiene interventions have shown significant preventive effects on respiratory infections: A systematic review and meta-analysis of 15 randomized controlled trials involving 10,742 patients demonstrated that twice-daily mechanical toothbrushing reduced the risk of hospital-acquired pneumonia (HAP) by 33% (RR = 0.67, 95% CI: 0.56–0.81) [67], with similar benefits in mechanically ventilated patients (RR = 0.68, 95% CI: 0.57–0.82) [67], and no major adverse events reported; the intervention also decreased ICU mortality by 19% (RR = 0.81, 95% CI: 0.69–0.95) [67], shortened the duration of mechanical ventilation by 1.24 days (95% CI: −2.42 to −0.06) [67], and reduced the ICU length of stay by 1.78 days (95% CI: −2.85 to −0.70) [67]. Because twice-daily brushing was as effective as more frequent regimens and far superior to antiseptic-only protocols, mechanical oral cleaning interventions should be prioritized in infection prevention guidelines [67]. Combined strategies—antiseptic rinses, mechanical debridement, and targeted microbiome modulation (e.g., probiotics)—effectively reduce respiratory infections in hospitalized, particularly mechanically ventilated, patients, improving clinical outcomes and survival [67,68].
5. Recent Progress in the Study of Periodontal Microorganisms and Respiratory Health
5.1. New Technologies and Methods for Periodontal Microbiome Research
Recent progress in high-throughput sequencing and metagenomic analysis has transformed the study of the periodontal microbiome, enabling deeper insights into microbial diversity and function [69,70]. Complementing these modern approaches, traditional molecular typing techniques like pulsed-field gel electrophoresis and multilocus sequence typing remain vital for determining the genetic relatedness of microbial strains in clinical settings [16]. These technologies provide researchers with the means to investigate the interactions between periodontal pathogens and respiratory health at an unprecedented level of detail.
5.2. New Discoveries and Theoretical Breakthroughs in the Field of Respiratory Health
Recent discoveries have underscored how periodontal pathogens contribute to the entry and replication of respiratory viruses, including SARS-CoV-2 [38,39]. These findings have significant implications for understanding how oral health influences respiratory health amid viral infections such as COVID-19. Beyond viral infections, an HIV study demonstrates that oral dysbiosis correlates with impaired pulmonary function and systemic inflammation, suggesting oral microbiota as a biomarker for respiratory outcomes in comorbid lung diseases [27].
5.3. Frontier Trends in Interdisciplinary Research
The integration of periodontal and respiratory health into interdisciplinary care models is an emerging trend in medical research. Studies are increasingly recognizing the importance of addressing the oral–pulmonary axis to improve overall patient outcomes. Interdisciplinary approaches that involve the collaboration between dental and medical professionals hold promise for enhancing the management of both periodontal and respiratory diseases [42,43].
6. Challenges and Prospects
6.1. Limitations of Periodontal Microorganisms and Respiratory Health Research
Even while there is mounting evidence linking periodontal microbes to poor respiratory health, several limitations continue to challenge the field. Many studies are constrained by small sample sizes, which limit their generalizability across larger populations [71]. Furthermore, the diagnostic criteria for both periodontitis and respiratory diseases are often inconsistent, complicating efforts to standardize findings and make cross-study comparisons [71].
Another significant limitation is the predominance of cross-sectional studies, which can only establish correlations rather than causality between periodontal disease and respiratory conditions. Equally important is the unresolved debate regarding directionality (oral → lung vs. lung → oral), particularly the lack of direct evidence on how COPD specifically alters periodontal microbiota, emphasizing the need for longitudinal studies on respiratory disease-induced oral dysbiosis [27]. Longitudinal and interventional studies are essential to gain a clearer understanding of the relationship between these conditions over time [72].
In addition, while most research focuses on bacterial pathogens, the potential role of viruses, fungi, and other microorganisms in the oral–respiratory axis remains underexplored. This gap leaves much to be discovered about the full spectrum of microbial involvement in respiratory health. Furthermore, confounding factors such as smoking, immunosuppressive therapy, and systemic conditions like diabetes are not always adequately accounted for, introducing potential bias [73].
The lack of randomized controlled studies (RCTs) examining how periodontal care affects respiratory outcomes is another drawback. Most of the current literature focuses on observational data, which does not allow for definitive conclusions regarding the impact of treating periodontitis on lung health [72].
6.2. Future Research Directions and Suggestions
To address these challenges, future research should prioritize the design and implementation of well-powered longitudinal cohort studies and interventional studies that utilize consistent diagnostic criteria. Such studies will help to establish stronger causal links between periodontitis and respiratory diseases. Moreover, it will be critical to incorporate standardized assessments for oral and respiratory health, alongside a comprehensive control for confounding variables like smoking and medication use [72].
There is also a pressing need to expand research beyond bacterial pathogens to explore the roles of viruses, fungi, and polymicrobial interactions in the oral–respiratory axis. Advanced microbiome analysis techniques, such as metagenomics and multi-omics approaches, should be employed to deepen our understanding of the interactions between oral pathogens and the host immune system [72,74,75]. This will enhance our ability to investigate how periodontal pathogens contribute to respiratory diseases like pneumonia, asthma, and COPD.
Future research should also investigate the use of probiotics, prebiotics, and other microbial interventions to restore balance within the oral microbiome and potentially improve respiratory health outcomes [76,77]. Additionally, identifying specific biomarkers that predict the development of respiratory disease based on the oral microbiome composition could open up new avenues for early diagnosis and preventive care [78,79,80]. Critically, research insights must translate into clinical practice, particularly in high-risk settings. Current oral care in institutional settings often remains inadequate, with healthcare providers in these facilities rarely assisting residents with tooth/denture cleaning—even when trained. Implementing evidence-based, structured oral care programs (e.g., professional weekly cleaning combined with post-meal brushing) are essential, as such interventions have been shown to significantly reduce pneumonia-related mortality [16].
Finally, interdisciplinary collaboration between dentists, pulmonologists, and infectious disease specialists will be critical for advancing this field. Such cooperation will foster the development of novel treatment strategies targeting both oral and respiratory health [52,77,80,81,82].
7. Conclusions
It is commonly known that periodontitis is a major risk factor for a number of respiratory conditions, such as pneumonia and Chronic Obstructive Pulmonary Disease (COPD), Obstructive Sleep Apnea (OSA), asthma, lung cancer, and COVID-19. Periodontal pathogens such as Porphyromonas gingivalis (Pg), Treponema denticola (Td), Fusobacterium nucleatum (Fn), Aggregatibacter actinomycetemcomitans (Aa), and Tannerella forsythia (Tf) play a crucial role in respiratory diseases by promoting pathogen adhesion, inducing epithelial cell apoptosis, and triggering inflammatory responses. Molecular studies definitively identify dental plaque colonizers as key reservoirs for hospital-acquired pneumonia (HAP) pathogens in hospitalized elders. Consequently, integrating routine oral hygiene into respiratory health protocols is imperative for mitigating infection risks in vulnerable populations.
In addition to respiratory diseases, periodontal disease is closely associated with systemic conditions, including diabetes, cardiovascular diseases, rheumatoid arthritis, and Alzheimer’s disease. By intensifying systemic inflammation, these infections may aid in the development of various illnesses.
Although significant evidence supports these associations, more large-scale, rigorous studies are needed to establish definitive causality. The current limitations primarily stem from the lack of high-quality Randomized Controlled Trials (RCTs) and longitudinal studies, which are essential for establishing causal links between periodontal disease and respiratory health outcomes. Future research should prioritize designing and implementing high-quality clinical trials with long-term follow-up to establish clearer causal relationships. Moreover, research should expand beyond bacterial pathogens to include other microorganisms, such as viruses and fungi, to provide a more comprehensive understanding of the oral–respiratory axis.
An interdisciplinary approach is crucial, as this review emphasizes, particularly in integrating oral health as a critical component in managing and preventing respiratory diseases. Periodontal treatment, by reducing the microbial burden and systemic inflammation, may effectively alleviate symptoms and reduce the occurrence of respiratory complications. This interdisciplinary approach not only promotes collaboration between dental and medical fields but also offers a new perspective on integrating oral health into overall health management. Integrating the gut–oral–lung axis framework further supports that oral microbiome modulation (e.g., via statins or hygiene) may improve COPD outcomes, while acknowledging bidirectional influences between respiratory and periodontal health [27].
Additionally, periodontal treatment shows potential benefits in managing systemic diseases, including diabetes, cardiovascular diseases, rheumatoid arthritis, and Alzheimer’s disease. Studies suggest that periodontal treatment can improve glycemic control in diabetic patients, reduce cardiovascular risk, lower disease activity in rheumatoid arthritis, and delay the progression of Alzheimer’s disease. Early screening and treatment of periodontal disease, particularly in high-risk groups such as COPD and diabetes patients, could serve as an effective strategy to reduce respiratory complications. Therefore, we advocate for incorporating oral health into comprehensive health management in clinical practice, particularly in the prevention and treatment of respiratory diseases, diabetes, cardiovascular diseases, rheumatoid arthritis, and Alzheimer’s disease, with future studies expected to provide more evidence to support this field.
Author Contributions
Conceptualization, B.K. and N.H.; methodology, B.K. and N.H.; writing—original draft preparation, B.K. and N.H.; writing—review and editing, B.K. and N.H.; supervision, N.H.; project administration, N.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the grants from the National Natural Science Foundation of China (NSFC No. 81500844).
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| Aa | Aggregatibacter actinomycetemcomitans |
| ACPA | Anti-Citrullinated Protein Antibodies |
| AD | Alzheimer’s Disease |
| CAP | Community-Acquired Pneumonia |
| COPD | Chronic Obstructive Pulmonary Disease |
| CRP | C-Reactive Protein |
| CVD | Cardiovascular Disease |
| DAS-28 | Disease Activity Score 28 |
| Fn | Fusobacterium nucleatum |
| GERD | Gastroesophageal Reflux Disease |
| HbA1c | Glycated Hemoglobin A1C |
| ICU | Intensive Care Unit |
| IL-1β | Interleukin-1 Beta |
| IL-6 | Interleukin-6 |
| Kgp | Lysine-specific Gingipain |
| LPS | Lipopolysaccharide |
| MMP-8 | Matrix Metalloproteinase-8 |
| MMP-9 | Matrix Metalloproteinase-9 |
| MUC5AC | Mucin 5AC |
| OSA | Obstructive Sleep Apnea |
| PAFR | Platelet-Activating Factor Receptor |
| Pg | Porphyromonas gingivalis |
| Ra | Rheumatoid Arthritis |
| RCTs | Randomized Controlled Trials |
| Rgp | Arginine-specific Gingipain |
| Td | Treponema denticola |
| Td-CTLP | Treponema denticola Chymotrypsin-Like Proteinase |
| Tf | Tannerella forsythia |
| TNF-α | Tumor Necrosis Factor-Alpha |
References
- Slots, J. Periodontitis: Facts, fallacies and the future. Periodontol. 2000 2017, 75, 7–23. [Google Scholar] [CrossRef]
- Genco, R.J.; Sanz, M. Clinical and public health implications of periodontal and systemic diseases: An overview. Periodontol. 2000 2020, 83, 7–13. [Google Scholar] [CrossRef]
- Sanz, M.; Beighton, D.; Curtis, M.A.; Cury, J.A.; Dige, I.; Dommisch, H.; Ellwood, R.; Giacaman, R.A.; Herrera, D.; Herzberg, M.C.; et al. Role of microbial biofilms in the maintenance of oral health and in the development of dental caries and periodontal diseases. Consensus report of group 1 of the joint EFP/ORCA workshop on the boundaries between caries and periodontal disease. J. Clin. Periodontol. 2017, 44 (Suppl. S18), S5–S11. [Google Scholar] [CrossRef]
- Cardoso, E.M.; Reis, C.; Manzanares-Cespedes, M.C. Chronic periodontitis, inflammatory cytokines, and interrelationship with other chronic diseases. Postgrad. Med. 2018, 130, 98–104. [Google Scholar] [CrossRef]
- Fiorillo, L.; Cervino, G.; Laino, L.; D’Amico, C.; Mauceri, R.; Tozum, T.F.; Gaeta, M.; Cicciu, M. Porphyromonas gingivalis, periodontal and systemic implications: A systematic review. Dent. J. 2019, 7, 114. [Google Scholar] [CrossRef] [PubMed]
- Mei, F.; Xie, M.; Huang, X.; Long, Y.; Lu, X.; Wang, X.; Chen, L. Porphyromonas gingivalis and its systemic impact: Current status. Pathogens 2020, 9, 944. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Shi, G. Smoking and microbiome in oral, airway, gut and some systemic diseases. J. Transl. Med. 2019, 17, 225. [Google Scholar] [CrossRef] [PubMed]
- Haraszthy, V.I.; Zambon, J.J.; Trevisan, M.; Zeid, M.; Genco, R.J. Identification of periodontal pathogens in atheromatous plaques. J. Periodontol. 2000, 71, 1554–1560. [Google Scholar] [CrossRef]
- Hajishengallis, G. Interconnection of periodontal disease and comorbidities: Evidence, mechanisms, and implications. Periodontol. 2000 2022, 89, 9–18. [Google Scholar] [CrossRef]
- Liccardo, D.; Marzano, F.; Carraturo, F.; Guida, M.; Femminella, G.D.; Bencivenga, L.; Agrimi, J.; Addonizio, A.; Melino, I.; Valletta, A.; et al. Potential bidirectional relationship between periodontitis and Alzheimer’s disease. Front. Physiol. 2020, 11, 683. [Google Scholar] [CrossRef]
- Singhrao, S.K.; Olsen, I. Assessing the role of Porphyromonas gingivalis in periodontitis to determine a causative relationship with Alzheimer’s disease. J. Oral Microbiol. 2019, 11, 1563405. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Ceriello, A.; Buysschaert, M.; Chapple, I.; Demmer, R.T.; Graziani, F.; Herrera, D.; Jepsen, S.; Lione, L.; Madianos, P.; et al. Scientific evidence on the links between periodontal diseases and diabetes: Consensus report and guidelines of the joint workshop on periodontal diseases and diabetes by the international diabetes federation and the European federation of periodontology. J. Clin. Periodontol. 2018, 45, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Febles, J.; Sanz, M. Periodontitis and rheumatoid arthritis: What have we learned about their connection and their treatment? Periodontol. 2000 2021, 87, 181–203. [Google Scholar] [CrossRef]
- Kelly, N.; El Karim, I. Periodontitis may be associated with respiratory diseases such as asthma, COPD, and pneumonia. J. Evid.-Based Dent. Pract. 2020, 20, 101498. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, K.; Matsumoto, K.; Furuta, M.; Fukuyama, S.; Takeshita, T.; Ogata, H.; Suma, S.; Shibata, Y.; Shimazaki, Y.; Hata, J.; et al. Periodontitis is associated with chronic obstructive pulmonary disease. J. Dent. Res. 2019, 98, 534–540. [Google Scholar] [CrossRef]
- El-Solh, A.A.; Pietrantoni, C.; Bhat, A.; Okada, M.; Zambon, J.; Aquilina, A.; Berbary, E. Colonization of dental plaques: A reservoir of respiratory pathogens for hospital-acquired pneumonia in institutionalized elders. Chest 2004, 126, 1575–1582. [Google Scholar] [CrossRef]
- Rastogi, T.; Chowdhary, Z.; Krishna, M.K.; Mehrotra, S.; Mohan, R. Prevalence of periodontitis in patients with pulmonary disease: A cross-sectional survey in the industrial district of India. J. Indian Soc. Periodontol. 2019, 23, 269–274. [Google Scholar] [CrossRef]
- Iwasaki, M.; Taylor, G.W.; Awano, S.; Yoshida, A.; Kataoka, S.; Ansai, T.; Nakamura, H. Periodontal disease and pneumonia mortality in haemodialysis patients: A 7-year cohort study. J. Clin. Periodontol. 2018, 45, 38–45. [Google Scholar] [CrossRef]
- Benedyk, M.; Mydel, P.M.; Delaleu, N.; Plaza, K.; Gawron, K.; Milewska, A.; Maresz, K.; Koziel, J.; Pyrc, K.; Potempa, J. Gingipains: Critical factors in the development of aspiration pneumonia caused by Porphyromonas gingivalis. J. Innate Immun. 2016, 8, 185–198. [Google Scholar] [CrossRef]
- Scannapieco, F.A.; Shay, K. Oral health disparities in older adults: Oral bacteria, inflammation, and aspiration pneumonia. Dent. Clin. N. Am. 2014, 58, 771–782. [Google Scholar] [CrossRef]
- Gomes-Filho, I.S.; Cruz, S.S.D.; Trindade, S.C.; Passos-Soares, J.D.S.; Carvalho-Filho, P.C.; Figueiredo, A.C.M.G.; Lyrio, A.O.; Hintz, A.M.; Pereira, M.G.; Scannapieco, F. Periodontitis and respiratory diseases: A systematic review with meta-analysis. Oral Dis. 2020, 26, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Hua, F.; Xie, H.; Worthington, H.V.; Furness, S.; Zhang, Q.; Li, C. Oral hygiene care for critically ill patients to prevent ventilator-associated pneumonia. Cochrane Database Syst. Rev. 2016, 2016, CD008367. [Google Scholar] [CrossRef] [PubMed]
- Herrera, D.; Sanz, M.; Shapira, L.; Brotons, C.; Chapple, I.; Frese, T.; Graziani, F.; Hobbs, F.D.R.; Huck, O.; Hummers, E.; et al. Association between periodontal diseases and cardiovascular diseases, diabetes and respiratory diseases: Consensus report of the joint workshop by the European federation of periodontology (EFP) and the European arm of the world organization of family doctors (WONCA Europe). J. Clin. Periodontol. 2023, 50, 819–841. [Google Scholar] [CrossRef] [PubMed]
- Heo, S.M.; Haase, E.M.; Lesse, A.J.; Gill, S.R.; Scannapieco, F.A. Genetic relationships between respiratory pathogens isolated from dental plaque and bronchoalveolar lavage fluid from patients in the intensive care unit undergoing mechanical ventilation. Clin. Infect. Dis. 2008, 47, 1562–1570. [Google Scholar] [CrossRef]
- Michaud, D.S.; Fu, Z.; Shi, J.; Chung, M. Periodontal disease, tooth loss, and cancer risk. Epidemiol. Rev. 2017, 39, 49–58. [Google Scholar] [CrossRef]
- Lin, P.; Liu, A.; Tsuchiya, Y.; Noritake, K.; Ohsugi, Y.; Toyoshima, K.; Tsukahara, Y.; Shiba, T.; Nitta, H.; Aoki, A.; et al. Association between periodontal disease and chronic obstructive pulmonary disease. Jpn. Dent. Sci. Rev. 2023, 59, 389–402. [Google Scholar] [CrossRef]
- Asai, N.; Ohkuni, Y.; Kato, H.; Hagihara, M.; Mikamo, H.; Kaneko, N. COPD Pathogenesis and Alterations in the Oral, Lung, and Gut Microbiomes. Microbiol. Res. 2024, 15, 1605–1615. [Google Scholar] [CrossRef]
- Maniaci, A.; Lavalle, S.; Anzalone, R.; Lo Giudice, A.; Cocuzza, S.; Parisi, F.M.; Torrisi, F.; Iannella, G.; Sireci, F.; Fadda, G.; et al. Oral health implications of obstructive sleep apnea: A literature review. Biomedicines 2024, 12, 1382. [Google Scholar] [CrossRef]
- Tamiya, H.; Abe, M.; Nagase, T.; Mitani, A. The link between periodontal disease and asthma: How do these two diseases affect each other? J. Clin. Med. 2023, 12, 6747. [Google Scholar] [CrossRef]
- Sukumar, K.; Tadepalli, A. Nexus between COVID-19 and periodontal disease. J. Int. Med. Res. 2021, 49, 675903673. [Google Scholar] [CrossRef]
- Liu, C.; Cao, Y.; Lin, J.; Ng, L.; Needleman, I.; Walsh, T.; Li, C. Oral care measures for preventing nursing home-acquired pneumonia. Cochrane Database Syst. Rev. 2018, 2018, CD012416. [Google Scholar] [CrossRef]
- Khadka, S.; Khan, S.; King, A.; Goldberg, L.R.; Crocombe, L.; Bettiol, S. Poor oral hygiene, oral microorganisms and aspiration pneumonia risk in older people in residential aged care: A systematic review. Age Ageing 2021, 50, 81–87. [Google Scholar] [CrossRef]
- Kamio, N.; Hayata, M.; Tamura, M.; Tanaka, H.; Imai, K. Porphyromonas gingivalis enhances pneumococcal adhesion to human alveolar epithelial cells by increasing expression of host platelet-activating factor receptor. FEBS Lett. 2021, 595, 1604–1612. [Google Scholar] [CrossRef]
- Nieminen, M.T.; Listyarifah, D.; Hagstrom, J.; Haglund, C.; Grenier, D.; Nordstrom, D.; Uitto, V.; Hernandez, M.; Yucel-Lindberg, T.; Tervahartiala, T.; et al. Treponema denticola chymotrypsin-like proteinase may contribute to orodigestive carcinogenesis through immunomodulation. Br. J. Cancer 2018, 118, 428–434. [Google Scholar] [CrossRef]
- Alon-Maimon, T.; Mandelboim, O.; Bachrach, G. Fusobacterium nucleatum and cancer. Periodontol. 2000 2022, 89, 166–180. [Google Scholar] [CrossRef]
- Fine, D.H.; Patil, A.G.; Velusamy, S.K. Aggregatibacter actinomycetemcomitans (Aa) under the radar: Myths and misunderstandings of Aa and its role in aggressive periodontitis. Front. Immunol. 2019, 10, 728. [Google Scholar] [CrossRef] [PubMed]
- Satur, M.J.; Urbanowicz, P.A.; Spencer, D.I.R.; Rafferty, J.; Stafford, G.P. Structural and functional characterisation of a stable, broad-specificity multimeric sialidase from the oral pathogen Tannerella forsythia. Biochem. J. 2022, 479, 1785–1806. [Google Scholar] [CrossRef]
- Takahashi, Y.; Watanabe, N.; Kamio, N.; Yokoe, S.; Suzuki, R.; Sato, S.; Iinuma, T.; Imai, K. Expression of the SARS-CoV-2 receptor ACE2 and proinflammatory cytokines induced by the periodontopathic bacterium Fusobacterium nucleatum in human respiratory epithelial cells. Int. J. Mol. Sci. 2021, 22, 1352. [Google Scholar] [CrossRef]
- Scialo, F.; Daniele, A.; Amato, F.; Pastore, L.; Matera, M.G.; Cazzola, M.; Castaldo, G.; Bianco, A. ACE2: The major cell entry receptor for SARS-CoV-2. Lung 2020, 198, 867–877. [Google Scholar] [CrossRef]
- Nagaoka, K.; Yanagihara, K.; Harada, Y.; Yamada, K.; Migiyama, Y.; Morinaga, Y.; Hasegawa, H.; Izumikawa, K.; Kakeya, H.; Nishimura, M.; et al. Macrolides inhibit Fusobacterium nucleatum-induced MUC5AC production in human airway epithelial cells. Antimicrob. Agents. Chemother. 2013, 57, 1844–1849. [Google Scholar] [CrossRef]
- Miya, C.; Cueno, M.E.; Suzuki, R.; Maruoka, S.; Gon, Y.; Kaneko, T.; Yonehara, Y.; Imai, K. Porphyromonas gingivalis gingipains potentially affect MUC5AC gene expression and protein levels in respiratory epithelial cells. FEBS Open Bio 2021, 11, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Bunte, K.; Beikler, T. Th17 cells and the IL-23/IL-17 axis in the pathogenesis of periodontitis and immune-mediated inflammatory diseases. Int. J. Mol. Sci. 2019, 20, 3394. [Google Scholar] [CrossRef]
- Thomas, R.; Qiao, S.; Yang, X. Th17/Treg imbalance: Implications in lung inflammatory diseases. Int. J. Mol. Sci. 2023, 24, 4865. [Google Scholar] [CrossRef]
- Wang, W.; Wang, X.; Lu, S.; Lv, H.; Zhao, T.; Xie, G.; Du, Y.; Fan, Y.; Xu, L. Metabolic disturbance and Th17/Treg imbalance are associated with progression of gingivitis. Front. Immunol. 2021, 12, 670178. [Google Scholar] [CrossRef]
- Dong, J.; Li, W.; Wang, Q.; Chen, J.; Zu, Y.; Zhou, X.; Guo, Q. Relationships between oral microecosystem and respiratory diseases. Front. Mol. Biosci. 2021, 8, 718222. [Google Scholar] [CrossRef]
- Lin, M.; Li, X.; Wang, J.; Cheng, C.; Zhang, T.; Han, X.; Song, Y.; Wang, Z.; Wang, S. Saliva Microbiome Changes in Patients with Periodontitis With and Without Chronic Obstructive Pulmonary Disease. Front. Cell. Infect. Microbiol. 2020, 15, 124. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, Z.; Wang, Z. The relationship between oral microbiota and chronic obstructive pulmonary disease. Sichuan Da Xue Xue Bao Yi Xue Ban 2023, 54, 54–60. [Google Scholar] [CrossRef]
- Godara, N.; Godara, R.; Khullar, M. Impact of inhalation therapy on oral health. Lung India 2011, 28, 272–275. [Google Scholar] [CrossRef]
- Carmona Loayza, D.A.; Lafebre, M.F. Periodontal disease and COVID-19: Prognosis and potential pathways of association in their pathogenesis. Can. J. Dent. Hyg. 2023, 57, 44–51. [Google Scholar] [PubMed]
- Pathak, J.L.; Yan, Y.; Zhang, Q.; Wang, L.; Ge, L. The role of oral microbiome in respiratory health and diseases. Respir. Med. 2021, 185, 106475. [Google Scholar] [CrossRef] [PubMed]
- Bellissimo-Rodrigues, W.T.; Menegueti, M.G.; Gaspar, G.G.; Nicolini, E.A.; Auxiliadora-Martins, M.; Basile-Filho, A.; Martinez, R.; Bellissimo-Rodrigues, F. Effectiveness of a dental care intervention in the prevention of lower respiratory tract nosocomial infections among intensive care patients: A randomized clinical trial. Infect. Control. Hosp. Epidemiol. 2014, 35, 1342–1348. [Google Scholar] [CrossRef]
- Shen, T.; Chang, P.; Lin, C.; Chen, C.; Tu, C.; Hsia, T.; Shih, C.; Hsu, W.; Sung, F.; Kao, C. Periodontal treatment reduces risk of adverse respiratory events in patients with chronic obstructive pulmonary disease: A propensity-matched cohort study. Medicine 2016, 95, e3735. [Google Scholar] [CrossRef]
- Apessos, I.; Voulgaris, A.; Agrafiotis, M.; Andreadis, D.; Steiropoulos, P. Effect of periodontal therapy on COPD outcomes: A systematic review. BMC Pulm. Med. 2021, 21, 92. [Google Scholar] [CrossRef]
- Katayama, Y.; Takanishi, H.; Sato, Y.; Fujita, S.; Enomoto, M. Effect of oral care in reducing the incidence of early-onset ventilator-associated pneumonia in preterm infants. Pediatr. Pulmonol. 2021, 56, 2570–2575. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xie, X.; Wu, Y.; Yan, F. Advances in research on the mechanism of association between periodontitis and diabetes mellitus. Sichuan Da Xue Xue Bao Yi Xue Ban 2023, 54, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Rapone, B.; Ferrara, E.; Corsalini, M.; Qorri, E.; Converti, I.; Lorusso, F.; Delvecchio, M.; Gnoni, A.; Scacco, S.; Scarano, A. Inflammatory status and glycemic control level of patients with type 2 diabetes and periodontitis: A randomized clinical trial. Int. J. Environ. Res. Public Health 2021, 18, 3018. [Google Scholar] [CrossRef] [PubMed]
- Simpson, T.C.; Clarkson, J.E.; Worthington, H.V.; Macdonald, L.; Weldon, J.C.; Needleman, I.; Iheozor-Ejiofor, Z.; Wild, S.H.; Qureshi, A.; Walker, A.; et al. Treatment of periodontitis for glycaemic control in people with diabetes mellitus. Cochrane Database Syst. Rev. 2022, 2022, CD004714. [Google Scholar] [CrossRef]
- Teeuw, W.J.; Gerdes, V.E.A.; Loos, B.G. Effect of periodontal treatment on glycemic control of diabetic patients: A systematic review and meta-analysis. Diabetes Care 2010, 33, 421–427. [Google Scholar] [CrossRef]
- Roca-Millan, E.; Gonzalez-Navarro, B.; Sabater-Recolons, M.; Mari-Roig, A.; Jane-Salas, E.; Lopez-Lopez, J. Periodontal treatment on patients with cardiovascular disease: Systematic review and meta-analysis. Med. Oral Patol. Oral Cir. Bucal 2018, 23, e681–e690. [Google Scholar] [CrossRef]
- Peng, C.; Yang, Y.; Chan, K.; Kornelius, E.; Chiou, J.; Huang, C. Periodontal treatment and the risks of cardiovascular disease in patients with type 2 diabetes: A retrospective cohort study. Intern. Med. 2017, 56, 1015–1021. [Google Scholar] [CrossRef]
- Li, C.; Lv, Z.; Shi, Z.; Zhu, Y.; Wu, Y.; Li, L.; Iheozor-Ejiofor, Z. Periodontal therapy for the management of cardiovascular disease in patients with chronic periodontitis. Cochrane Database Syst. Rev. 2014, 2014, CD009197. [Google Scholar] [CrossRef]
- Grigg, J. The platelet activating factor receptor: A new anti-infective target in respiratory disease? Thorax 2012, 67, 840–841. [Google Scholar] [CrossRef][Green Version]
- Petit, C.; Culshaw, S.; Weiger, R.; Huck, O.; Sahrmann, P. Impact of treatment of rheumatoid arthritis on periodontal disease: A review. Mol. Oral Microbiol. 2024, 39, 199–224. [Google Scholar] [CrossRef]
- Mustufvi, Z.; Twigg, J.; Kerry, J.; Chesterman, J.; Pavitt, S.; Tugnait, A.; Mankia, K. Does periodontal treatment improve rheumatoid arthritis disease activity? A systematic review. Rheumatol. Adv. Pract. 2022, 6, rkac061. [Google Scholar] [CrossRef] [PubMed]
- Borsa, L.; Dubois, M.; Sacco, G.; Lupi, L. Analysis the link between periodontal diseases and Alzheimer’s disease: A systematic review. Int. J. Environ. Res. Public Health 2021, 18, 9312. [Google Scholar] [CrossRef] [PubMed]
- Schwahn, C.; Frenzel, S.; Holtfreter, B.; Van der Auwera, S.; Pink, C.; Bulow, R.; Friedrich, N.; Volzke, H.; Biffar, R.; Kocher, T.; et al. Effect of periodontal treatment on preclinical Alzheimer’s disease-results of a trial emulation approach. Alzheimer’s Dement. 2022, 18, 127–141. [Google Scholar] [CrossRef] [PubMed]
- Ehrenzeller, S.; Klompas, M. Association Between Daily Toothbrushing and Hospital-Acquired Pneumonia: A Systematic Review and Meta-Analysis. JAMA Intern. Med. 2024, 184, 131–142. [Google Scholar] [CrossRef]
- He, J.; Mao, N.; Lyu, W.; Zhou, S.; Zhang, Y.; Liu, Z.; Xu, Z. Association between oral microbiome and five types of respiratory infections: A two-sample Mendelian randomization study in east Asian population. Front. Microbiol. 2024, 15, 1392473. [Google Scholar] [CrossRef]
- Pan, Y.; Teng, D.; Burke, A.C.; Haase, E.M.; Scannapieco, F.A. Oral bacteria modulate invasion and induction of apoptosis in HEp-2 cells by Pseudomonas aeruginosa. Microb. Pathog. 2009, 46, 73–79. [Google Scholar] [CrossRef]
- Li, Q.; Pan, C.; Teng, D.; Lin, L.; Kou, Y.; Haase, E.M.; Scannapieco, F.A.; Pan, Y. Porphyromonas gingivalis modulates Pseudomonas aeruginosa-induced apoptosis of respiratory epithelial cells through the STAT3 signaling pathway. Microbes Infect. 2014, 16, 17–27. [Google Scholar] [CrossRef]
- Sampson, V.; Kamona, N.; Sampson, A. Could there be a link between oral hygiene and the severity of SARS-CoV-2 infections? Br. Dent. J. 2020, 228, 971–975. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, R.; Yi, Z.; Li, Y.; Fu, Y.; Zhang, Y.; Li, P.; Li, X.; Pan, Y. Porphyromonas gingivalis induced inflammatory responses and promoted apoptosis in lung epithelial cells infected with H1N1 via the Bcl-2/Bax/Caspase-3 signaling pathway. Mol. Med. Rep. 2018, 18, 97–104. [Google Scholar] [CrossRef]
- Larvin, H.; Wilmott, S.; Wu, J.; Kang, J. The impact of periodontal disease on hospital admission and mortality during COVID-19 pandemic. Front. Med. 2020, 7, 604980. [Google Scholar] [CrossRef]
- Li, Q.; Wang, H.; Tan, L.; Zhang, S.; Lin, L.; Tang, X.; Pan, Y. Oral pathogen Fusobacterium nucleatum coaggregates with Pseudomonas aeruginosa to modulate the inflammatory cytotoxicity of pulmonary epithelial cells. Front. Cell. Infect. Microbiol. 2021, 11, 643913. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, C.; Liu, J.; Pan, Y.; Li, Y. In vitro effect of Porphyromonas gingivalis combined with influenza a virus on respiratory epithelial cells. Arch. Oral Biol. 2018, 95, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Prasanna, S.J. Causal relationship between periodontitis and chronic obstructive pulmonary disease. J. Indian Soc. Periodontol. 2011, 15, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Thomas, M.S.; Parolia, A.; Kundabala, M.; Vikram, M. Asthma and oral health: A review. Aust. Dent. J. 2010, 55, 128–133. [Google Scholar] [CrossRef]
- Gomes-Filho, I.S.; Soledade-Marques, K.R.; Seixas Da Cruz, S.; de Santana Passos-Soares, J.; Trindade, S.C.; Souza-Machado, A.; Fischer Rubira-Bullen, I.R.; de Moraes Marcilio Cerqueira, E.; Barreto, M.L.; Costa De Santana, T.; et al. Does periodontal infection have an effect on severe asthma in adults? J. Periodontol. 2014, 85, e179–e187. [Google Scholar] [CrossRef]
- Soledade-Marques, K.R.; Gomes-Filho, I.S.; Da Cruz, S.S.; Passos-Soares, J.D.S.; Trindade, S.C.; Cerqueira, E.D.M.M.; Coelho, J.M.F.; Barreto, M.L.; Costa, M.D.C.N.; Vianna, M.I.P.; et al. Association between periodontitis and severe asthma in adults: A case-control study. Oral Dis. 2018, 24, 442–448. [Google Scholar] [CrossRef]
- Zhang, Z.; Wen, S.; Liu, J.; Ouyang, Y.; Su, Z.; Chen, D.; Liang, Z.; Wang, Y.; Luo, T.; Jiang, Q.; et al. Advances in the relationship between periodontopathogens and respiratory diseases (review). Mol. Med. Rep. 2024, 29, 42. [Google Scholar] [CrossRef]
- Khassawneh, B.; Alhabashneh, R.; Ibrahim, F. The association between bronchial asthma and periodontitis: A case-control study in Jordan. J. Asthma 2019, 56, 404–410. [Google Scholar] [CrossRef]
- Han, E.; Choi, I.S.; Kim, H.; Kang, Y.; Park, J.; Lim, J.; Seo, J.; Choi, J. Inhaled corticosteroid-related tooth problems in asthmatics. J. Asthma 2009, 46, 160–164. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).