Genetic Basis of Brugada Syndrome
Abstract
1. Introduction
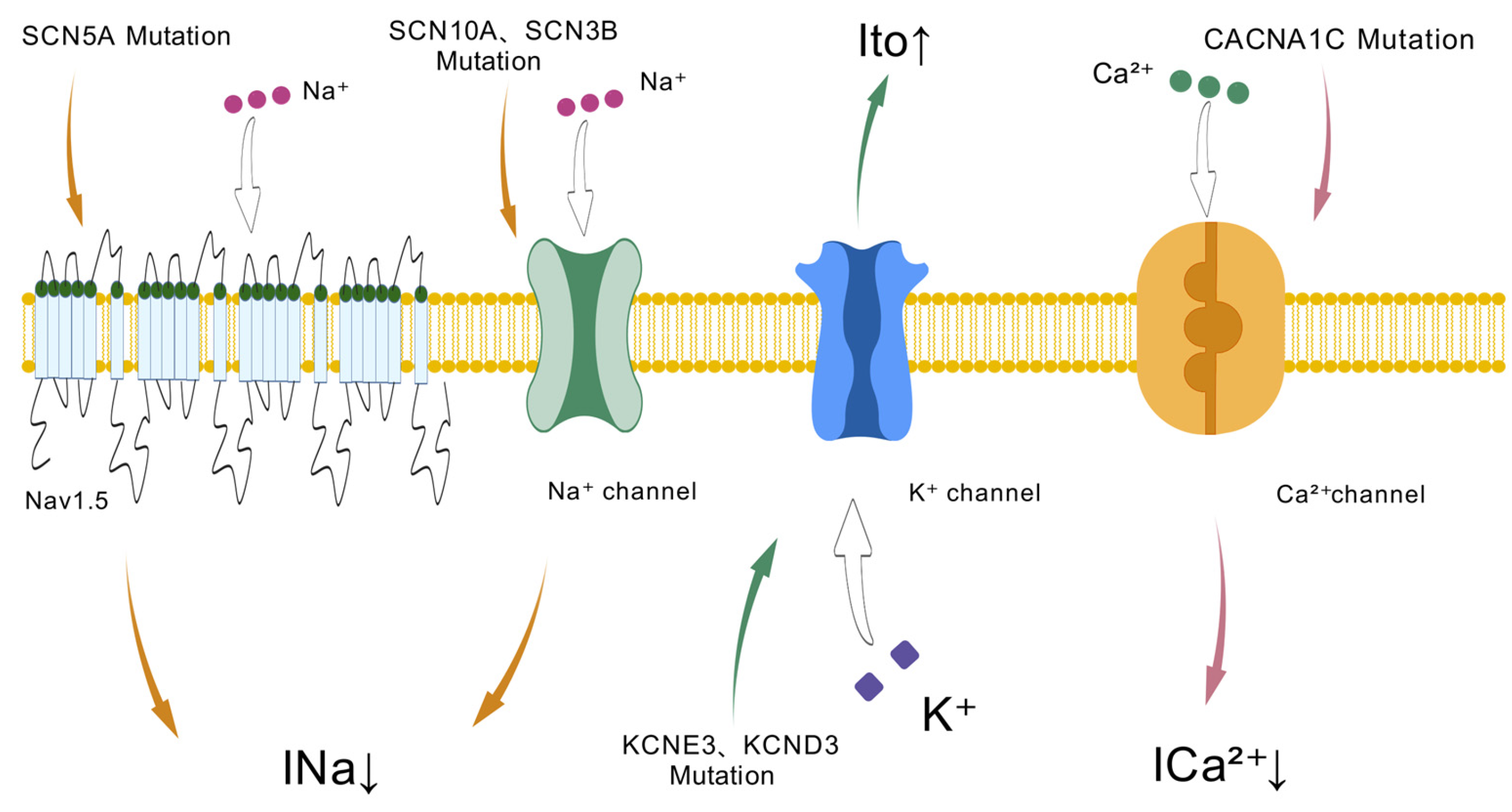
2. Genetic Basis
3. Mutations Causing a Loss-of-Function of Sodium Channel Current
3.1. SCN5A
3.2. SCN10A
3.3. SCN1-4B
3.4. GPD1L
3.5. PKP2
3.6. HEY2
3.7. Other Genes Related to Sodium Channel Current
4. Mutations Causing a Gain-of-Function of Potassium Channel Currents
4.1. KCNE
4.2. KCND
4.3. KCNJ8
4.4. KCNH2
4.5. HCN4
4.6. Other Genes Related to Potassium Channel Currents
5. Mutations Causing a Loss-of-Function of Calcium Channel Current
5.1. CACNA1C
5.2. CASQ2
5.3. RYR2
5.4. CALM
6. Other Candidate Genes
6.1. TRPM4
6.2. DLG1
6.3. Mitochondrial DNA (mtDNA)
6.4. RRAD
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Brugada, P. Brugada Syndrome: 30 Years of Scientific Adventure. Turk Kardiyol. Dern. Ars.-Arch. Turk. Soc. Cardiol. 2022, 50, 452–458. [Google Scholar] [CrossRef] [PubMed]
- Brugada, P.; Brugada, J. Right bundle branch block, persistent ST segment elevation and sudden cardiac death: A distinct clinical and electrocardiographic syndrome: A multicenter report. J. Am. Coll. Cardiol. 1992, 20, 1391–1396. [Google Scholar] [CrossRef] [PubMed]
- Mariani, M.V.; Pierucci, N.; Fanisio, F.; Laviola, D.; Silvetti, G.; Piro, A.; La Fazia, V.M.; Chimenti, C.; Rebecchi, M.; Drago, F.; et al. Inherited Arrhythmias in the Pediatric Population: An Updated Overview. Medicina 2024, 60, 94. [Google Scholar] [CrossRef] [PubMed]
- Amin, A.S.; de Groot, E.A.; Ruijter, J.M.; Wilde, A.A.; Tan, H.L. Exercise-induced ECG changes in Brugada syndrome. Circ. Arrhythm. Electrophysiol. 2009, 2, 531–539. [Google Scholar] [CrossRef] [PubMed]
- Bébarová, M. Brugada syndrome. Vnitr. Lek. 2011, 57, 551–560. [Google Scholar] [PubMed]
- Besli, G.E.; Yıldırım, S.; Akalın, İ.; Ayhan, Y.I.; Kısıoğlu, M.; Berdeli, A. Fever-induced Brugada syndrome in a 9-year-old boy presenting with acute chest pain. Turk. J. Pediatr. 2018, 60, 571–575. [Google Scholar] [CrossRef] [PubMed]
- Antzelevitch, C.; Patocskai, B. Brugada Syndrome: Clinical, Genetic, Molecular, Cellular, and Ionic Aspects. Curr. Probl. Cardiol. 2016, 41, 7–57. [Google Scholar] [CrossRef] [PubMed]
- Popa, I.P.; Șerban, D.N.; Mărănducă, M.A.; Șerban, I.L.; Tamba, B.I.; Tudorancea, I. Brugada Syndrome: From Molecular Mechanisms and Genetics to Risk Stratification. Int. J. Mol. Sci. 2023, 24, 3328. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Minamino, T. Role of mutations in L-type calcium channel genes in Brugada syndrome, early repolarization syndrome, and idiopathic ventricular fibrillation associated with right bundle branch block. Circ. J. 2013, 77, 1689–1690. [Google Scholar] [CrossRef] [PubMed]
- Gourraud, J.-B.; Barc, J.; Thollet, A.; Le Scouarnec, S.; Le Marec, H.; Schott, J.-J.; Redon, R.; Probst, V. The Brugada Syndrome: A Rare Arrhythmia Disorder with Complex Inheritance. Front. Cardiovasc. Med. 2016, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Kirsch, G.E.; Zhang, D.; Brugada, R.; Brugada, J.; Brugada, P.; Potenza, D.; Moya, A.; Borggrefe, M.; Breithardt, G.; et al. Genetic basis and molecular mechanism for idiopathic ventricular fibrillation. Nature 1998, 392, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, T.; Surber, R. SCN5A channelopathies—An update on mutations and mechanisms. Prog. Biophys. Mol. Biol. 2008, 98, 120–136. [Google Scholar] [CrossRef] [PubMed]
- Doundoulakis, I.; Pannone, L.; Chiotis, S.; Della Rocca, D.G.; Sorgente, A.; Tsioufis, P.; Del Monte, A.; Vetta, G.; Piperis, C.; Overeinder, I.; et al. SCN5A gene variants and arrhythmic risk in Brugada syndrome: An updated systematic review and meta-analysis. Heart Rhythm 2024, 21, 1987–1997. [Google Scholar] [CrossRef] [PubMed]
- Kapplinger, J.D.; Tester, D.J.; Alders, M.; Benito, B.; Berthet, M.; Brugada, J.; Brugada, P.; Fressart, V.; Guerchicoff, A.; Harris-Kerr, C.; et al. An international compendium of mutations in the SCN5A-encoded cardiac sodium channel in patients referred for Brugada syndrome genetic testing. Heart Rhythm 2010, 7, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Kapplinger, J.D.; Giudicessi, J.R.; Ye, D.; Tester, D.J.; Callis, T.E.; Valdivia, C.R.; Makielski, J.C.; Wilde, A.A.; Ackerman, M.J. Enhanced Classification of Brugada Syndrome-Associated and Long-QT Syndrome-Associated Genetic Variants in the SCN5A-Encoded Na(v)1.5 Cardiac Sodium Channel. Circ. Cardiovasc. Genet. 2015, 8, 582–595. [Google Scholar] [CrossRef] [PubMed]
- Kroncke, B.M.; Glazer, A.M.; Smith, D.K.; Blume, J.D.; Roden, D.M. SCN5A (Na(V)1.5) Variant Functional Perturbation and Clinical Presentation: Variants of a Certain Significance. Circ. Genom. Precis. Med. 2018, 11, e002095. [Google Scholar] [CrossRef] [PubMed]
- Akai, J.; Makita, N.; Sakurada, H.; Shirai, N.; Ueda, K.; Kitabatake, A.; Nakazawa, K.; Kimura, A.; Hiraoka, M. A novel SCN5A mutation associated with idiopathic ventricular fibrillation without typical ECG findings of Brugada syndrome. FEBS Lett. 2000, 479, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Andorin, A.; Behr, E.R.; Denjoy, I.; Crotti, L.; Dagradi, F.; Jesel, L.; Sacher, F.; Petit, B.; Mabo, P.; Maltret, A.; et al. Impact of clinical and genetic findings on the management of young patients with Brugada syndrome. Heart Rhythm 2016, 13, 1274–1282. [Google Scholar] [CrossRef] [PubMed]
- Nishii, N.; Ogawa, M.; Morita, H.; Nakamura, K.; Banba, K.; Miura, D.; Kumagai, N.; Matsunaga, A.; Kawamura, H.; Urakawa, S.; et al. SCN5A mutation is associated with early and frequent recurrence of ventricular fibrillation in patients with Brugada syndrome. Circ. J. 2010, 74, 2572. [Google Scholar] [CrossRef] [PubMed]
- Russo, V.; Caturano, A.; Migliore, F.; Guerra, F.; Francia, P.; Nesti, M.; Conte, G.; Perini, A.P.; Mascia, G.; Albani, S.; et al. Long-term clinical outcomes of patients with drug-induced type 1 Brugada electrocardiographic pattern: A nationwide cohort registry study. Heart Rhythm 2024, 21, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Fukuyama, M.; Ohno, S.; Makiyama, T.; Horie, M. Novel SCN10A variants associated with Brugada syndrome. Europace 2016, 18, 905–911. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Barajas-Martínez, H.; Pfeiffer, R.; Dezi, F.; Pfeiffer, J.; Buch, T.; Betzenhauser, M.J.; Belardinelli, L.; Kahlig, K.M.; Rajamani, S.; et al. Mutations in SCN10A are responsible for a large fraction of cases of Brugada syndrome. J. Am. Coll. Cardiol. 2014, 64, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Cavalli, M.; Fossati, B.; Vitale, R.; Brigonzi, E.; Ricigliano, V.A.G.; Saraceno, L.; Cardani, R.; Pappone, C.; Meola, G. Flecainide-induced Brugada Syndrome in a Patient with Skeletal Muscle Sodium Channelopathy: A Case Report with Critical Therapeutical Implications and Review of the Literature. Front. Neurol. 2018, 9, 385. [Google Scholar] [CrossRef] [PubMed]
- Bissay, V.; Van Malderen, S.C.; Keymolen, K.; Lissens, W.; Peeters, U.; Daneels, D.; Jansen, A.C.; Pappaert, G.; Brugada, P.; De Keyser, J.; et al. SCN4A variants and Brugada syndrome: Phenotypic and genotypic overlap between cardiac and skeletal muscle sodium channelopathies. Eur. J. Hum. Genet. 2016, 24, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Banfi, P.; Coll, M.; Oliva, A.; Alcalde, M.; Striano, P.; Mauri, M.; Princiotta, L.; Campuzano, O.; Versino, M.; Brugada, R. Lamotrigine induced Brugada-pattern in a patient with genetic epilepsy associated with a novel variant in SCN9A. Gene 2020, 754, 144847. [Google Scholar] [CrossRef] [PubMed]
- Peeters, U.; Scornik, F.; Riuró, H.; Pérez, G.; Komurcu-Bayrak, E.; Van Malderen, S.; Pappaert, G.; Tarradas, A.; Pagans, S.; Daneels, D.; et al. Contribution of Cardiac Sodium Channel β-Subunit Variants to Brugada Syndrome. Circ. J. 2015, 79, 2118–2129. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Koopmann, T.T.; Le Scouarnec, S.; Yang, T.; Ingram, C.R.; Schott, J.-J.; Demolombe, S.; Probst, V.; Anselme, F.; Escande, D.; et al. Sodium channel β1 subunit mutations associated with Brugada syndrome and cardiac conduction disease in humans. J. Clin. Investig. 2008, 118, 2260–2268. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Barajas-Martínez, H.; Medeiros-Domingo, A.; Crotti, L.; Veltmann, C.; Schimpf, R.; Urrutia, J.; Alday, A.; Casis, O.; Pfeiffer, R. A novel rare variant in SCN1Bb linked to Brugada syndrome and SIDS by combined modulation of Na(v)1.5 and K(v)4.3 channel currents. Heart Rhythm 2012, 9, 760–769. [Google Scholar] [CrossRef] [PubMed]
- Riuró, H.; Beltran-Alvarez, P.; Tarradas, A.; Selga, E.; Campuzano, O.; Vergés, M.; Pagans, S.; Iglesias, A.; Brugada, J.; Brugada, P.; et al. A missense mutation in the sodium channel β2 subunit reveals SCN2B as a new candidate gene for brugada syndrome. Hum. Mutat. 2013, 34, 961–966. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Barajas-Martinez, H.; Burashnikov, E.; Springer, M.; Wu, Y.; Varro, A.; Pfeiffer, R.; Koopmann, T.T.; Cordeiro, J.M.; Guerchicoff, A.; et al. A mutation in the β3 subunit of the cardiac sodium channel associated with Brugada ECG phenotype. Circ. Cardiovasc. Genet. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- London, B.; Michalec, M.; Mehdi, H.; Zhu, X.; Kerchner, L.; Sanyal, S.; Viswanathan, P.C.; Pfahnl, A.E.; Shang, L.L.; Madhusudanan, M.; et al. Mutation in glycerol-3-phosphate dehydrogenase 1 like gene (GPD1-L) decreases cardiac Na+ current and causes inherited arrhythmias. Circulation 2007, 116, 2260–2268. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Chen, Y.; Fan, L.; Guo, S.; Li, J.; Jin, J.; Xiang, R. Whole-exome sequencing identifies a novel mutation of GPD1L (R189X) associated with familial conduction disease and sudden death. J. Cell. Mol. Med. 2018, 22, 1350–1354. [Google Scholar] [CrossRef] [PubMed]
- Cerrone, M.; Lin, X.; Zhang, M.; Agullo-Pascual, E.; Pfenniger, A.; Chkourko Gusky, H.; Novelli, V.; Kim, C.; Tirasawadichai, T.; Judge, D.P.; et al. Missense mutations in plakophilin-2 cause sodium current deficit and associate with a Brugada syndrome phenotype. Circulation 2014, 129, 1092–1103. [Google Scholar] [CrossRef] [PubMed]
- Campuzano, O.; Fernández-Falgueras, A.; Iglesias, A.; Brugada, R. Brugada Syndrome and PKP2: Evidences and uncertainties. Int. J. Cardiol. 2016, 214, 403–405. [Google Scholar] [CrossRef] [PubMed]
- Bezzina, C.R.; Barc, J.; Mizusawa, Y.; Remme, C.A.; Gourraud, J.-B.; Simonet, F.; Verkerk, A.O.; Schwartz, P.J.; Crotti, L.; Dagradi, F.; et al. Common variants at SCN5A-SCN10A and HEY2 are associated with Brugada syndrome, a rare disease with high risk of sudden cardiac death. Nat. Genet. 2013, 45, 1044–1049. [Google Scholar] [CrossRef] [PubMed]
- Mohler, P.J. Ankyrins and human disease: What the electrophysiologist should know. J. Cardiovasc. Electrophysiol. 2006, 17, 1153–1159. [Google Scholar] [CrossRef] [PubMed]
- Park, D.S.; Shekhar, A.; Santucci, J.; Redel-Traub, G.; Solinas, S.; Mintz, S.; Lin, X.; Chang, E.W.; Narke, D.; Xia, Y.; et al. Ionic Mechanisms of Impulse Propagation Failure in the FHF2-Deficient Heart. Circ. Res. 2020, 127, 1536–1548. [Google Scholar] [CrossRef] [PubMed]
- Hennessey, J.A.; Wei, E.Q.; Pitt, G.S. Fibroblast growth factor homologous factors modulate cardiac calcium channels. Circ. Res. 2013, 113, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Hennessey, J.A.; Marcou, C.A.; Wang, C.; Wei, E.Q.; Wang, C.; Tester, D.J.; Torchio, M.; Dagradi, F.; Crotti, L.; Schwartz, P.J.; et al. FGF12 is a candidate Brugada syndrome locus. Heart Rhythm 2013, 10, 1886–1894. [Google Scholar] [CrossRef] [PubMed]
- Campuzano, O.; Berne, P.; Selga, E.; Allegue, C.; Iglesias, A.; Brugada, J.; Brugada, R. Brugada syndrome and p.E61X_RANGRF. Cardiol. J. 2014, 21, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Mlynarova, J.; Trentin-Sonoda, M.; da Silva, F.G.; Major, J.L.; Salih, M.; Carneiro-Ramos, M.S.; Tuana, B.S.; Backx, P.H. SLMAP3 isoform modulates cardiac gene expression and function. PLoS ONE 2019, 14, e0214669. [Google Scholar] [CrossRef] [PubMed]
- Guzzo, R.M.; Sevinc, S.; Salih, M.; Tuana, B.S. A novel isoform of sarcolemmal membrane-associated protein (SLMAP) is a component of the microtubule organizing centre. J. Cell Sci. 2004, 117, 2271–2281. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T.; Sato, A.; Marcou, C.A.; Tester, D.J.; Ackerman, M.J.; Crotti, L.; Schwartz, P.J.; On, Y.K.; Park, J.-E.; Nakamura, K.; et al. A novel disease gene for Brugada syndrome: Sarcolemmal membrane-associated protein gene mutations impair intracellular trafficking of hNav1. Circ. Arrhythm. Electrophysiol. 2012, 5, 1098–1107. [Google Scholar] [CrossRef] [PubMed]
- Delpón, E.; Cordeiro, J.M.; Núñez, L.; Thomsen, P.E.B.; Guerchicoff, A.; Pollevick, G.D.; Wu, Y.; Larsen, C.T.; Hofman-Bang, J.; Burashnikov, E.; et al. Functional effects of KCNE3 mutation and its role in the development of Brugada syndrome. Circ. Arrhythm. Electrophysiol. 2008, 1, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Hedley, P.L.; Haundrup, O.; Andersen, P.S.; Aidt, F.H.; Jensen, M.; Moolman-Smook, J.C.; Bundgaard, H.; Christiansen, M. The KCNE genes in hypertrophic cardiomyopathy: A candidate gene study. J. Negat. Results Biomed. 2011, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Liatakis, I.; Pantou, M.P.; Gourzi, P.; Bazoukis, G.; Mililis, P.; Saplaouras, A.; Vlachos, K.; Prappa, E.; Degiannis, D.; Efremidis, M.; et al. KCNE2 gene mutation and Brugada syndrome. J. Electrocardiol. 2021, 65, 143–145. [Google Scholar] [CrossRef] [PubMed]
- Abbott, G.W. KCNE4 and KCNE5: K+ channel regulation and cardiac arrhythmogenesis. Gene 2016, 593, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Ohno, S.; Zankov, D.P.; Ding, W.-G.; Itoh, H.; Makiyama, T.; Doi, T.; Shizuta, S.; Hattori, T.; Miyamoto, A.; Naiki, N.; et al. KCNE5 (KCNE1L) variants are novel modulators of Brugada syndrome and idiopathic ventricular fibrillation. Circ. Arrhythm. Electrophysiol. 2011, 4, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Giudicessi, J.R.; Ye, D.; Tester, D.J.; Crotti, L.; Mugione, A.; Nesterenko, V.V.; Albertson, R.M.; Antzelevitch, C.; Schwartz, P.J.; Ackerman, M.J. Transient outward current (I(to)) gain-of-function mutations in the KCND3-encoded Kv4.3 potassium channel and Brugada syndrome. Heart Rhythm 2011, 8, 1024–1032. [Google Scholar] [CrossRef] [PubMed]
- Perrin, M.J.; Adler, A.; Green, S.; Al-Zoughool, F.; Doroshenko, P.; Orr, N.; Uppal, S.; Healey, J.S.; Birnie, D.; Sanatani, S.; et al. Evaluation of genes encoding for the transient outward current (Ito) identifies the KCND2 gene as a cause of J-wave syndrome associated with sudden cardiac death. Circ. Cardiovasc. Genet. 2014, 7, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Medeiros-Domingo, A.; Tan, B.-H.; Crotti, L.; Tester, D.J.; Eckhardt, L.; Cuoretti, A.; Kroboth, S.L.; Song, C.; Zhou, Q.; Kopp, D.; et al. Gain-of-function mutation S422L in the KCNJ8-encoded cardiac K(ATP) channel Kir6.1 as a pathogenic substrate for J-wave syndromes. Heart Rhythm 2010, 7, 1466–1471. [Google Scholar] [CrossRef] [PubMed]
- Antzelevitch, C.; Barajas-Martinez, H. A gain-of-function IK-ATP mutation and its role in sudden cardiac death associated with J-wave syndromes. Heart Rhythm 2010, 7, 1472–1474. [Google Scholar] [CrossRef] [PubMed]
- Barajas-Martínez, H.; Hu, D.; Ferrer, T.; Onetti, C.G.; Wu, Y.; Burashnikov, E.; Boyle, M.; Surman, T.; Urrutia, J.; Veltmann, C.; et al. Molecular genetic and functional association of Brugada and early repolarization syndromes with S422L missense mutation in KCNJ8. Heart Rhythm 2012, 9, 548–555. [Google Scholar] [CrossRef] [PubMed]
- Wilde, A.A. “J-wave syndromes” bring the ATP-sensitive potassium channel back in the spotlight. Heart Rhythm 2012, 9, 556–557. [Google Scholar] [CrossRef] [PubMed]
- Juang, J.M.; Lu, T.P.; Lai, L.C.; Ho, C.C.; Liu, Y.B.; Tsai, C.T.; Lin, L.Y.; Yu, C.C.; Chen, W.J.; Chiang, F.T.; et al. Disease-targeted sequencing of ion channel genes identifies de novo mutations in patients with non-familial Brugada syndrome. Sci. Rep. 2014, 4, 6733. [Google Scholar] [CrossRef] [PubMed]
- Verkerk, A.O.; Wilders, R.; Schulzebahr, E.; Beekman, L.; Bhuiyan, Z.A.; Bertrand, J.; Eckardt, L.; Lin, D.; Borggrefe, M.; Breithardt, G.; et al. Role of sequence variations in the human ether-a-go-go-related gene (HERG, KCNH2) in the Brugada syndrome. Cardiovasc. Res. 2005, 68, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Abramochkin, D.; Li, B.; Zhang, H.; Kravchuk, E.; Nesterova, T.; Glukhov, G.; Shestak, A.; Zaklyazminskaya, E.; Sokolova, O.S. Novel Gain-of-Function Mutation in the Kv11.1 Channel Found in the Patient with Brugada Syndrome and Mild QTc Shortening. Biochemistry 2024, 89, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Ueda, K.; Hirano, Y.; Higashiuesato, Y.; Aizawa, Y.; Hayashi, T.; Inagaki, N.; Tana, T.; Ohya, Y.; Takishita, S.; Muratani, H.; et al. Role of HCN4 channel in preventing ventricular arrhythmia. J. Hum. Genet. 2009, 54, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Biel, S.; Aquila, M.; Hertel, B.; Berthold, A.; Neumann, T.; DiFrancesco, D.; Moroni, A.; Thiel, G.; Kauferstein, S. Mutation in S6 domain of HCN4 channel in patient with suspected Brugada syndrome modifies channel function. Pflug. Arch. Eur. J. Physiol. 2016, 468, 1663–1671. [Google Scholar] [CrossRef] [PubMed]
- Verkerk, A.O.; Wilders, R. The Action Potential Clamp Technique as a Tool for Risk Stratification of Sinus Bradycardia Due to Loss-of-Function Mutations in HCN4: An In Silico Exploration Based on In Vitro and In Vivo Data. Biomedicines 2023, 11, 2447. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Barajas-Martínez, H.; Terzic, A.; Park, S.; Pfeiffer, R.; Burashnikov, E.; Wu, Y.; Borggrefe, M.; Veltmann, C.; Schimpf, R.; et al. ABCC9 is a novel Brugada and early repolarization syndrome susceptibility gene. Int. J. Cardiol. 2014, 171, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Portero, V.; Le Scouarnec, S.; Es-Salah-Lamoureux, Z.; Burel, S.; Gourraud, J.-B.; Bonnaud, S.; Lindenbaum, P.; Simonet, F.; Violleau, J.; Baron, E.; et al. Dysfunction of the Voltage-Gated K+ Channel β2 Subunit in a Familial Case of Brugada Syndrome. J. Am. Heart Assoc. 2016, 5, e003122. [Google Scholar] [CrossRef] [PubMed]
- Boczek, N.J.; Ye, D.; Johnson, E.K.; Wang, W.; Crotti, L.; Tester, D.J.; Dagradi, F.; Mizusawa, Y.; Torchio, M.; Alders, M.; et al. Characterization of SEMA3A-encoded semaphorin as a naturally occurring Kv4.3 protein inhibitor and its contribution to Brugada syndrome. Circ. Res. 2014, 115, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Friedrich, C.; Rinné, S.; Zumhagen, S.; Kiper, A.K.; Silbernagel, N.; Netter, M.F.; Stallmeyer, B.; Schulze-Bahr, E.; Decher, N. Gain-of-function mutation in TASK-4 channels and severe cardiac conduction disorder. EMBO Mol. Med. 2014, 6, 937–951. [Google Scholar] [CrossRef] [PubMed]
- Di Mauro, V.; Ceriotti, P.; Lodola, F.; Salvarani, N.; Modica, J.; Bang, M.-L.; Mazzanti, A.; Napolitano, C.; Priori, S.G.; Catalucci, D. Peptide-Based Targeting of the L-Type Calcium Channel Corrects the Loss-of-Function Phenotype of Two Novel Mutations of the CACNA1 Gene Associated with Brugada Syndrome. Front Physiol. 2020, 11, 616819. [Google Scholar] [CrossRef] [PubMed]
- Kashiwa, A.; Makiyama, T.; Kohjitani, H.; Maurissen, T.L.; Ishikawa, T.; Yamamoto, Y.; Wuriyanghai, Y.; Gao, J.; Huang, H.; Imamura, T.; et al. Disrupted Ca(V)1.2 selectivity causes overlapping long QT and Brugada syndrome phenotypes in the CACNA1C-E1115K iPS cell model. Heart Rhythm 2023, 20, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Parthiban, N.; Sani, H.; Ang, R.; Sinning, C.; Bettin, M.; Demetriades, P.; Waight, M. Sudden cardiac arrest in a patient with malignant mitral valve prolapse with CACNB2 gene mutation: A simple coincidence or coexistence?—A case report. Eur. Heart J. Case Rep. 2023, 7, ytad196. [Google Scholar] [CrossRef] [PubMed]
- Blancard, M.; Debbiche, A.; Kato, K.; Cardin, C.; Sabrina, G.; Gandjbakhch, E.; Probst, V.; Haissaguerre, M.; Extramiana, F.; Hocini, M.; et al. An African loss-of-function CACNA1C variant p.T1787M associated with a risk of ventricular fibrillation. Sci. Rep. 2018, 8, 14619. [Google Scholar] [CrossRef] [PubMed]
- Venetucci, L.; Denegri, M.; Napolitano, C.; Priori, S.G. Inherited calcium channelopathies in the pathophysiology of arrhythmias. Nat. Rev. Cardiol. 2012, 9, 561–575. [Google Scholar] [CrossRef] [PubMed]
- Kamga, M.V.K.; Reppel, M.; Hescheler, J.; Nguemo, F. Modeling genetic cardiac channelopathies using induced pluripotent stem cells—Status quo from an electrophysiological perspective. Biochem. Pharmacol. 2021, 192, 114746. [Google Scholar] [CrossRef] [PubMed]
- Brohus, M.; Busuioc, A.-O.; Wimmer, R.; Nyegaard, M.; Overgaard, M.T. Calmodulin mutations affecting Gly114 impair binding to the NaV1.5 IQ-domain. Front. Pharmacol. 2023, 14, 1210140. [Google Scholar] [CrossRef] [PubMed]
- Ozhathil, L.C.; Rougier, J.-S.; Arullampalam, P.; Essers, M.C.; Ross-Kaschitza, D.; Abriel, H. Deletion of Trpm4 Alters the Function of the Nav1.5 Channel in Murine Cardiac Myocytes. Int. J. Mol. Sci. 2021, 22, 3401. [Google Scholar] [CrossRef] [PubMed]
- Musa, H.; Marcou, C.A.; Herron, T.J.; Makara, M.A.; Tester, D.J.; O’connell, R.P.; Rosinski, B.; Guerrero-Serna, G.; Milstein, M.L.; da Rocha, A.M.; et al. Abnormal myocardial expression of SAP97 is associated with arrhythmogenic risk. Am. J. Physiol. Circ. Physiol. 2020, 318, H1357–H1370. [Google Scholar] [CrossRef] [PubMed]
- Tafti, M.F.; Khatami, M.; Rezaei, S.; Heidari, M.M.; Hadadzadeh, M. Novel and heteroplasmic mutations in mitochondrial tRNA genes in Brugada syndrome. Cardiol. J. 2018, 25, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Stocchi, L.; Polidori, E.; Potenza, L.; Rocchi, M.B.L.; Calcabrini, C.; Busacca, P.; Capalbo, M.; Potenza, D.; Amati, F.; Mango, R.; et al. Mutational analysis of mitochondrial DNA in Brugada syndrome. Cardiovasc. Pathol. 2016, 25, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Behr, E.R. Genetic susceptibility and the Brugada syndrome. Eur. Heart J. 2019, 40, 3094–3096. [Google Scholar] [CrossRef] [PubMed]
- Juang, J.J.; Binda, A.; Lee, S.J.; Hwang, J.J.; Chen, W.J.; Liu, Y.B.; Lin, L.Y.; Yu, C.C.; Ho, L.T.; Huang, H.C.; et al. GSTM3 variant is a novel genetic modifier in Brugada syndrome, a disease with risk of sudden cardiac death. eBioMedicine 2020, 57, 102843. [Google Scholar] [CrossRef] [PubMed]
- Di Resta, C.; Pietrelli, A.; Sala, S.; Della Bella, P.; De Bellis, G.; Ferrari, M.; Bordoni, R.; Benedetti, S. High-throughput genetic characterization of a cohort of Brugada syndrome patients. Hum. Mol. Genet. 2015, 24, 5828–5835. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Russo, V.; Papaccioli, G.; Maddaloni, V.; Caputo, A.; Pepe, N.; Rago, A.; Maiorino, M.; Golino, P.; Nigro, G. Case report: Lamin A/C gene mutation in patient with drug-induced type 1 Brugada syndrome at high arrhythmic risk. Front. Cardiovasc. Med. 2022, 9, 1099508. [Google Scholar] [CrossRef] [PubMed]
- Turker, I.; Makiyama, T.; Ueyama, T.; Shimizu, A.; Yamakawa, M.; Chen, P.-S.; Vatta, M.; Horie, M.; Ai, T. Telethonin variants found in Brugada syndrome, J-wave pattern ECG, and ARVC reduce peak Na(v) 1.5 currents in HEK-293 cells. Pacing Clin. Electrophysiol. 2020, 43, 838–846. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Tang, S.; Chen, Y.; Zhang, L.; Yin, K.; Wu, Y.; Zheng, J.; Wu, Q.; Makielski, J.C.; Cheng, J. Molecular pathological study on LRRC10 in sudden unexplained nocturnal death syndrome in the Chinese Han population. Int. J. Leg. Med. 2017, 131, 621–628. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rubio-Alarcón, M.; Cámara-Checa, A.; Dago, M.; Crespo-García, T.; Nieto-Marín, P.; Marín, M.; Merino, J.L.; Toquero, J.; Salguero-Bodes, R.; Tamargo, J.; et al. Zfhx3 Transcription Factor Represses the Expression of SCN5A Gene and Decreases Sodium Current Density (INa). Int. J. Mol. Sci. 2021, 22, 13031. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Wu, K.; Zhang, L.; Wang, Q.; Tang, S.; Wu, Q.; Jiang, P.; Lin, J.J.; Guo, J.; Wang, L.; et al. Critical Roles of Xirp Proteins in Cardiac Conduction and Their Rare Variants Identified in Sudden Unexplained Nocturnal Death Syndrome and Brugada Syndrome in Chinese Han Population. J. Am. Heart Assoc. 2018, 7, e006320. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, A.; Zankov, D.P.; Sato, A.; Komeno, M.; Toyoda, F.; Yamazaki, S.; Makita, T.; Noda, T.; Ikawa, M.; Asano, Y.; et al. Identification of transmembrane protein 168 mutation in familial Brugada syndrome. FASEB J. 2020, 34, 6399–6417. [Google Scholar] [CrossRef] [PubMed]
- Mango, R.; Luchetti, A.; Sangiuolo, R.; Ferradini, V.; Briglia, N.; Giardina, E.; Ferrè, F.; Citterich, M.H.; Romeo, F.; Novelli, G.; et al. Next Generation Sequencing and Linkage Analysis for the Molecular Diagnosis of a Novel Overlapping Syndrome Characterized by Hypertrophic Cardiomyopathy and Typical Electrical Instability of Brugada Syndrome. Circ. J. 2016, 80, 938–949. [Google Scholar] [CrossRef] [PubMed]
- Cordenier, A.; Flamez, A.; de Ravel, T.; Gheldof, A.; Pannone, L.; De Asmundis, C.; Pappaert, G.; Bissay, V. Case report: Coexistence of myotonia congenita and Brugada syndrome in one family. Front. Neurol. 2022, 13, 1011956. [Google Scholar] [CrossRef] [PubMed]
| GENE | PROTEIN | MECHANISM | IMPACT ON BRS |
|---|---|---|---|
| SCN5A | NaV1.5 | Na+ channel | Primary cause of BrS |
| SCN10A | NaV1.8 | Na+ channel | Reduces INa |
| SCN1B | β1/β1b subunits | Na+ channel | Reduces INa |
| SCN2B | β2 subunit | Na+ channel | Reduces INa |
| SCN3B | β3 subunit | Na+ channel | Reduces INa |
| GPD1L | GPD1L | Na+ channel | Reduces INa |
| PKP2 | Plakophilin-2 | Na+ channel | Reduces INa |
| HEY2 | Hey2 | Transcription factor | Disrupts NaV1.5 expression |
| ANK2 | Ankyrin-B | Na+ channel | Reduces INa |
| FGF12 | FHF-1 | Na+ channel | Reduces INa |
| RANGRF | MOG1 | Na+ channel | Reduces INa |
| SLMAP | SLMAP | Na+ channel | Reduces INa |
| KCNE3 | MiRP2 | K+ channel | Increases Ito |
| KCND3 | KV4.3 | K+ channel | Increases Ito |
| KCNJ8 | Kir6.1 | K+ channel | Increases KATP current |
| KCNH2 | hERG | K+ channel | Increases IKr |
| HCN4 | HCN4 | Na+/K+ channel | Reduces If current |
| ABCC9 | SUR2A | K+ channel | Increases IK-ATP current |
| SEMA3A | Semaphorin 3A | K+ channel | Reduces Ito |
| CACNA1C | Cav1.2 | Ca2+ channel | Reduces ICa |
| CASQ2 | Calsequestrin 2 | Ca2+ handling | Reduces ICa |
| RYR2 | Ryanodine receptor 2 | Ca2+ release | Reduces ICa |
| CALM | Calmodulin | Ca2+ binding | Reduces ICa |
| TRPM4 | TRPM4 | Ca2+-activated cation channel | Increases K+ current |
| DLG1 | SAP97 | Regulatory protein | Affects ion channel localization |
| MTDNA | Mitochondrial DNA | Mitochondrial function | Mitochondrial dysfunction |
| RRAD | RRAD | Signaling protein | Affects ion channel function |
| GSTM3 | GSTM3 | Oxidative stress | Reduces GSTM3 levels |
| DSG2 | Desmoglein-2 | Cell junction | Reduces sodium current |
| LMNA | Lamin A/C | Nuclear envelope | Alters nuclear envelope |
| TCAP | Telethonin | Z-disk protein | Affects sodium channel |
| LRRC10 | LRRC10 | Regulatory protein | Affects ion channels |
| ZFHX3 | ZFHX3 | Transcription factor | Affects ion channels |
| XIRP | Xirp | Membrane protein | Affects ion channels |
| TMEM168 | TMEM168 | Contractile protein | Affects ion channels |
| TPM1 | TPM1 | Contractile protein | Affects contractile function |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, X.; Chen, Y.; Li, Z.; Sun, Y.; Chen, G. Genetic Basis of Brugada Syndrome. Biomedicines 2025, 13, 1740. https://doi.org/10.3390/biomedicines13071740
Xie X, Chen Y, Li Z, Sun Y, Chen G. Genetic Basis of Brugada Syndrome. Biomedicines. 2025; 13(7):1740. https://doi.org/10.3390/biomedicines13071740
Chicago/Turabian StyleXie, Xianghuan, Yanghui Chen, Zhiqiang Li, Yang Sun, and Guangzhi Chen. 2025. "Genetic Basis of Brugada Syndrome" Biomedicines 13, no. 7: 1740. https://doi.org/10.3390/biomedicines13071740
APA StyleXie, X., Chen, Y., Li, Z., Sun, Y., & Chen, G. (2025). Genetic Basis of Brugada Syndrome. Biomedicines, 13(7), 1740. https://doi.org/10.3390/biomedicines13071740






