Advances in Pathophysiology and Novel Therapeutic Strategies for Coronary No-Reflow Phenomenon
Abstract
1. Introduction
2. Animal Models of Coronary No-Reflow
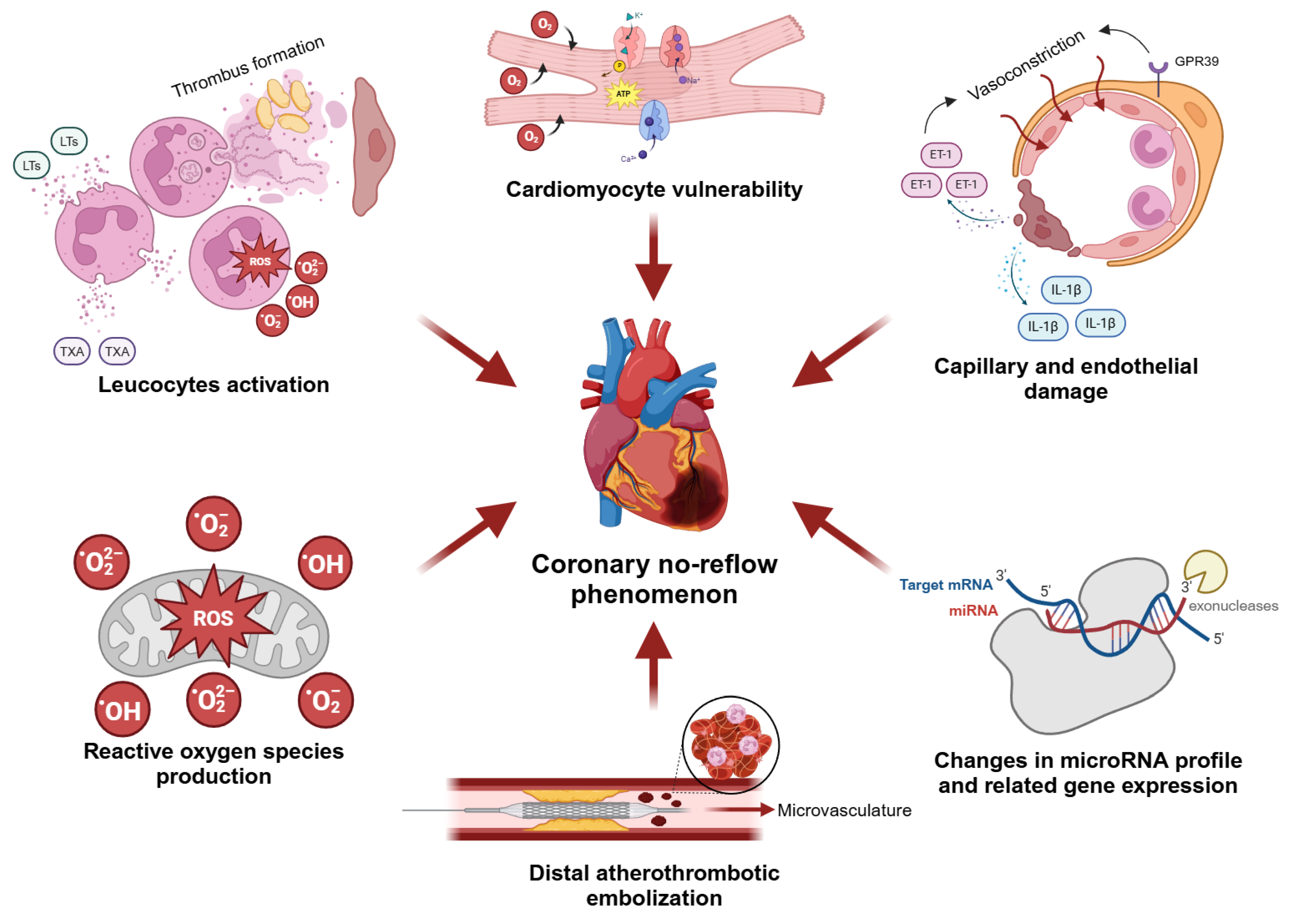
3. Pathophysiology of Coronary No-Reflow
3.1. Cardiomyocyte Vulnerability
3.2. Distal Atherothrombotic Embolization
3.3. Capillary and Endothelial Damage
3.4. Role of Leucocytes
3.5. Role of Reactive Oxygen Species
3.6. Role of microRNAs
4. Novel Therapeutic Strategies for Coronary No-Reflow—Preclinical Studies
| Drug/Therapeutic Intervention | Molecular Mechanisms of Action | Pharmacological Effects | Ref. |
|---|---|---|---|
| OP2113 | Inhibition of ROS formation at site IQ of complex I of the mitochondrial respiratory chain; Increased ATP production; Increased mitochondrial affinity to oxygen. | Reduced no-reflow zone size and MI area; No effect on blood pressure and heart rate. | [27,131] |
| Anisodamine | Opening mitochondrial KATP channels; Reduced ROS generation; Increased ATP production; Improved ultrastructure of myocardial tissue. | Increased LVDP and coronary flow; Reduced MI area; Reduced occurrence of ventricular reperfusion arrhythmias after I/R. | [132] |
| Pinacidil | Nonselective opening of KATP channels; Inhibition of calcium overload-induced mitochondrial dysfunction; Reduced cardiomyocyte and endothelial apoptosis; Increased NO activity and reduced levels of ET-1. | Reduced no-reflow zone size and MI area; Maintained endothelial barrier integrity; Improved left ventricular function; Attenuated left ventricular remodeling. | [134] |
| Tongmai Yangxin pill | Activation of cAMP/PKA and NO/cGMP signaling pathways; Activation of Nrf2/HO-1 and inhibition of p38-MAPK signaling pathways; Increased NO activity; Reduced ROS generation; Reduced cardiomyocyte apoptosis. | Relaxation of coronary microvessels; Reduced no-reflow zone size and MI area; Reduced infiltration of inflammatory cells and interstitial edema; Improved left ventricular function. | [136,137] |
| Post-reperfusion therapeutic hypothermia | Reduced no-reflow zone size; No impact on MI area. | [138,139] | |
| Hydrogen gas inhalation | Inhibition of oxidative stress and NLRP3-mediated endothelial pyroptosis; Improved ultrastructure of myocardial tissue. | Reduced no-reflow zone size and MI area; Improved left ventricular function. | [140] |
| Luteolin | Inhibition of TLR4/NF-κB/NLRP3 inflammasome pathway; Activation of Wnt/β-catenin pathway; Reduced ROS generation; Attenuated cardiac inflammatory response. | Reduced no-reflow zone size and MI area; Increased R-amplitude in ECG; Improved left ventricular function; Maintained endothelial barrier integrity. | [39,142] |
| Adiponectin | Improvement in endothelium-dependent vasodilatation; Reduced levels of ET-1, ICAM-1 and VCAM-1. | Reduced no-reflow zone size; Improved left ventricular function. | [145] |
| Intracoronary administration of CDCs a | Formation of new cardiac tissue; Reduced cardiomyocyte apoptosis. | Reduced no-reflow zone size and MI area; Improved left ventricular function; Attenuated left ventricular remodeling. | [146,147,148,149] |
| BSF 461314, bosentan, tezosentan, BQ 123 | Endothelin receptors ET-A and ET-B antagonists; Attenuated cardiac inflammatory response; Inhibition of neutrophils adhesion and activation. | Reduced no-reflow zone size and MI area; Maintained endothelial barrier integrity; No impact on left ventricular function. | [79,83,150,151] |
5. Novel Therapeutic Strategies for Coronary No-Reflow—Clinical Studies
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mensah, G.A.; Fuster, V.; Murray, C.J.; Roth, G.A.; Abate, Y.H.; Abbasian, M.; Abd-Allah, F.; Abdollahi, A.; Abdollahi, M.; Abdulah, D.M.; et al. Global Burden of Cardiovascular Diseases and Risks, 1990–2022. J. Am. Coll. Cardiol. 2023, 82, 2350–2473. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Ben-ziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Salari, N.; Morddarvanjoghi, F.; Abdolmaleki, A.; Rasoulpoor, S.; Khaleghi, A.A.; Hezarkhani, L.A.; Shohaimi, S.; Mohammadi, M. The global prevalence of myocardial infarction: A systematic review and meta-analysis. BMC Cardiovasc. Disord. 2023, 23, 1–12. [Google Scholar] [CrossRef] [PubMed]
- A Byrne, R.; Rossello, X.; Coughlan, J.J.; Barbato, E.; Berry, C.; Chieffo, A.; Claeys, M.J.; Dan, G.-A.; Dweck, M.R.; Galbraith, M.; et al. 2023 ESC Guidelines for the management of acute coronary syndromes. Eur. Heart J. Acute Cardiovasc. Care 2023, 13, 55–161. [Google Scholar] [CrossRef]
- Mandelzweig, L.; Battler, A.; Boyko, V.; Bueno, H.; Danchin, N.; Filippatos, G.; Gitt, A.; Hasdai, D.; Hasin, Y.; Marrugat, J.; et al. The second Euro Heart Survey on acute coronary syndromes: Characteristics, treatment, and outcome of patients with ACS in Europe and the Mediterranean Basin in 2004. Eur. Heart J. 2006, 27, 2285–2293. [Google Scholar] [CrossRef]
- Menees, D.S.; Peterson, E.D.; Wang, Y.; Curtis, J.P.; Messenger, J.C.; Rumsfeld, J.S.; Gurm, H.S. Door-to-Balloon Time and Mortality among Patients Undergoing Primary PCI. N. Engl. J. Med. 2013, 369, 901–909. [Google Scholar] [CrossRef]
- Freitas, F.; Attwell, D. Pericyte-mediated constriction of renal capillaries evokes no-reflow and kidney injury following ischaemia. eLife 2022, 11, e74211. [Google Scholar] [CrossRef] [PubMed]
- Furnas, H.; Rosen, J.M. Monitoring in Microvascular Surgery. Ann. Plast. Surg. 1991, 26, 265–272. [Google Scholar] [CrossRef]
- Kloner, R.A.; King, K.S.; Harrington, M.G. No-reflow phenomenon in the heart and brain. Am. J. Physiol. Circ. Physiol. 2018, 315, H550–H562. [Google Scholar] [CrossRef]
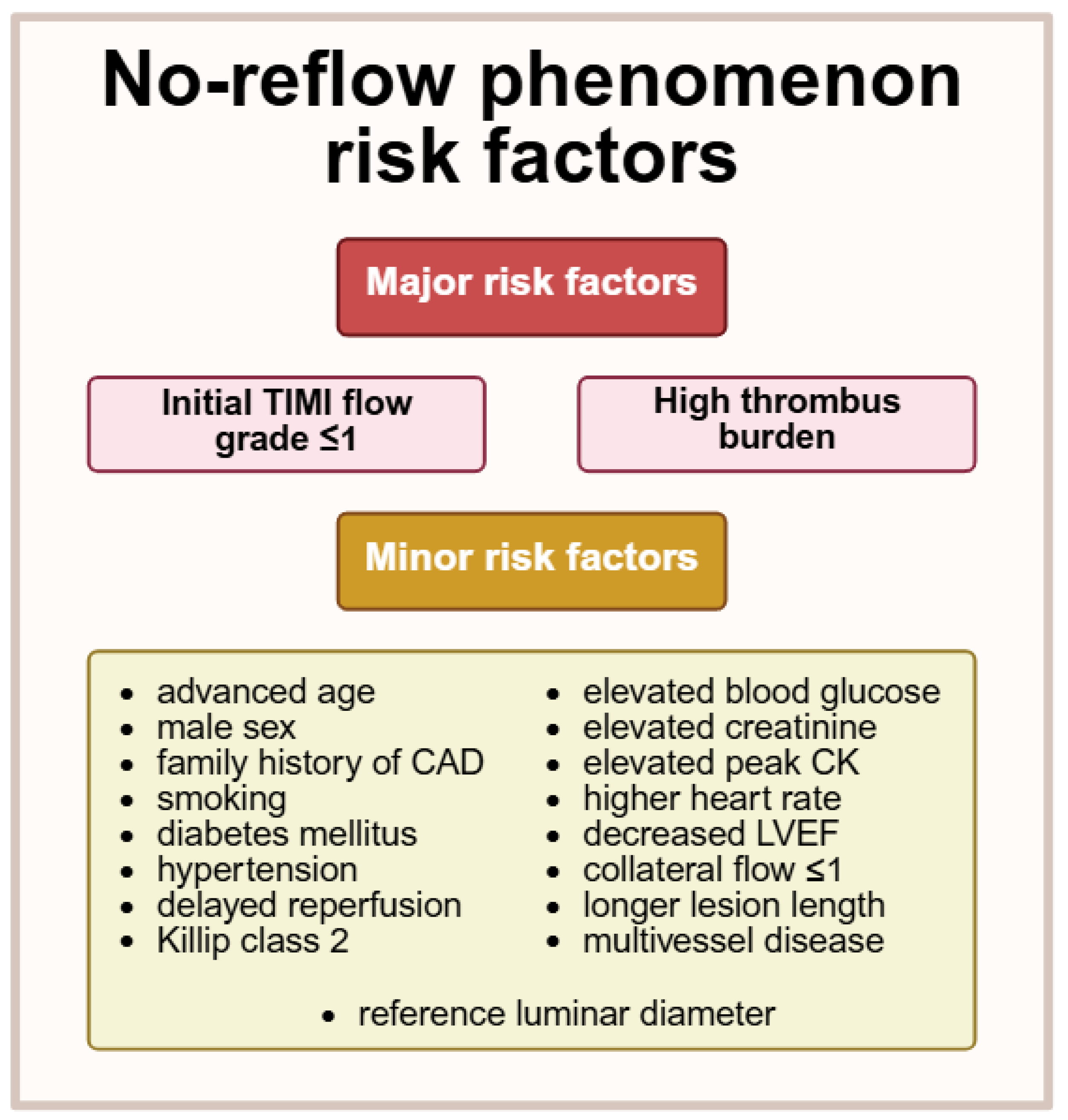
- Fajar, J.K.; Heriansyah, T.; Rohman, M.S. The predictors of no reflow phenomenon after percutaneous coronary intervention in patients with ST elevation myocardial infarction: A meta-analysis. Indian Heart J. 2018, 70, S406–S418. [Google Scholar] [CrossRef]
- Annibali, G.; Scrocca, I.; Aranzulla, T.C.; Meliga, E.; Maiellaro, F.; Musumeci, G. “No-Reflow” Phenomenon: A Contemporary Review. J. Clin. Med. 2022, 11, 2233. [Google Scholar] [CrossRef] [PubMed]
- Armillotta, M.; Bergamaschi, L.; Paolisso, P.; Belmonte, M.; Angeli, F.; Sansonetti, A.; Stefanizzi, A.; Bertolini, D.; Bodega, F.; Amicone, S.; et al. Prognostic Relevance of Type 4a Myocardial Infarction and Periprocedural Myocardial Injury in Patients With Non–ST-Segment–Elevation Myocardial Infarction. Circulation 2025, 151, 760–772. [Google Scholar] [CrossRef] [PubMed]
- Fahrni, G.; Wolfrum, M.; De Maria, G.L.; Cuculi, F.; Dawkins, S.; Alkhalil, M.; Patel, N.; Forfar, J.C.; Prendergast, B.D.; Choudhury, R.P.; et al. Index of Microcirculatory Resistance at the Time of Primary Percutaneous Coronary Intervention Predicts Early Cardiac Complications: Insights From the OxAMI (Oxford Study in Acute Myocardial Infarction) Cohort. J. Am. Heart Assoc. 2017, 6, 11. [Google Scholar] [CrossRef]
- Giugliano, R.P.; Sabatine, M.S.; Gibson, C.; Roe, M.T.; A Harrington, R.; A Murphy, S.; A Morrow, D.; Antman, E.M.; Braunwald, E. Combined assessment of thrombolysis in myocardial infarction flow grade, myocardial perfusion grade, and ST-segment resolution to evaluate epicardial and myocardial reperfusion. Am. J. Cardiol. 2004, 93, 1362–1367. [Google Scholar] [CrossRef]
- Rezkalla, S.H.; Stankowski, R.V.; Hanna, J.; Kloner, R.A. Management of No-Reflow Phenomenon in the Catheterization Laboratory. JACC Cardiovasc. Interv. 2017, 10, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Schröder, R. Prognostic Impact of Early ST-Segment Resolution in Acute ST-Elevation Myocardial Infarction. Circulation 2004, 110, e506–e5010. [Google Scholar] [CrossRef]
- Bulluck, H.; Dharmakumar, R.; Arai, A.E.; Berry, C.; Hausenloy, D.J. Cardiovascular Magnetic Resonance in Acute ST-Segment–Elevation Myocardial Infarction. Circulation 2018, 137, 1949–1964. [Google Scholar] [CrossRef]
- Nijveldt, R.; Hofman, M.B.M.; Hirsch, A.; Beek, A.M.; Umans, V.A.W.M.; Algra, P.R.; Piek, J.J.; van Rossum, A.C. Assessment of Microvascular Obstruction and Prediction of Short-term Remodeling after Acute Myocardial Infarction: Cardiac MR Imaging Study. Radiology 2009, 250, 363–370. [Google Scholar] [CrossRef]
- Weir, R.A.; Murphy, C.A.; Petrie, C.J.; Martin, T.N.; Balmain, S.; Clements, S.; Steedman, T.; Wagner, G.S.; Dargie, H.J.; McMurray, J.J. Microvascular Obstruction Remains a Portent of Adverse Remodeling in Optimally Treated Patients With Left Ventricular Systolic Dysfunction After Acute Myocardial Infarction. Circ. Cardiovasc. Imaging 2010, 3, 360–367. [Google Scholar] [CrossRef]
- Yildirim, A.; Coskun, M.; Demirtas, A.O. The Predictive Value of ALBI Score for No-Reflow in Non-ST Elevation Acute Coronary Syndrome. J. Clin. Med. 2025, 14, 3035. [Google Scholar] [CrossRef]
- Dawson, L.P.; Rashid, M.; Dinh, D.T.; Brennan, A.; Bloom, J.E.; Biswas, S.; Lefkovits, J.; Shaw, J.A.; Chan, W.; Clark, D.J.; et al. No-Reflow Prediction in Acute Coronary Syndrome During Percutaneous Coronary Intervention: The NORPACS Risk Score. Circ. Cardiovasc. Interv. 2024, 17, e013738. [Google Scholar] [CrossRef] [PubMed]
- Gui, S.; Fu, G.; Jia, M.; Liu, S.; Jia, X.; Jian, L. Prediction of no reflow phenomenon in percutaneous coronary intervention with optical coherence tomography and analysis of risk factors. Heart Surg. Forum 2023, 26, E051–E055. [Google Scholar] [CrossRef] [PubMed]
- Galasso, G.; Schiekofer, S.; D’aNna, C.; Di Gioia, G.; Piccolo, R.; Niglio, T.; De Rosa, R.; Strisciuglio, T.; Cirillo, P.; Piscione, F.; et al. No-Reflow Phenomenon. Angiology 2013, 65, 180–189. [Google Scholar] [CrossRef] [PubMed]
- Ndrepepa, G.; Kastrati, A. Coronary No-Reflow after Primary Percutaneous Coronary Intervention—Current Knowledge on Pathophysiology, Diagnosis, Clinical Impact and Therapy. J. Clin. Med. 2023, 12, 5592. [Google Scholar] [CrossRef]
- Reffelmann, T.; Hale, S.L.; Dow, J.S.; Kloner, R.A. No-Reflow Phenomenon Persists Long-Term After Ischemia/Reperfusion in the Rat and Predicts Infarct Expansion. Circulation 2003, 108, 2911–2917. [Google Scholar] [CrossRef]
- Dai, W.; Shi, J.; Siddarth, P.; Zhao, L.; Carreno, J.; Kleinman, M.T.; Herman, D.A.; Arechavala, R.J.; Renusch, S.; Hasen, I.; et al. Effects of Electronic Cigarette Exposure on Myocardial Infarction and No-Reflow, and Cardiac Function in a Rat Model. J. Cardiovasc. Pharmacol. Ther. 2023, 28, 10742484231155992. [Google Scholar] [CrossRef]
- Dai, W.; Amoedo, N.D.; Perry, J.; Le Grand, B.; Boucard, A.; Carreno, J.; Zhao, L.; Brown, D.A.; Rossignol, R.; Kloner, R.A. Effects of OP2113 on Myocardial Infarct Size and No Reflow in a Rat Myocardial Ischemia/Reperfusion Model. Cardiovasc. Drugs Ther. 2021, 36, 217–227. [Google Scholar] [CrossRef]
- Li, Q.; Guo, Z.; Wu, C.; Tu, Y.; Wu, Y.; Xie, E.; Yu, C.; Sun, W.; Li, X.; Zheng, J.; et al. Ischemia preconditioning alleviates ischemia/reperfusion injury-induced coronary no-reflow and contraction of microvascular pericytes in rats. Microvasc. Res. 2022, 142, 104349. [Google Scholar] [CrossRef]
- Gao, J.; Ren, J.; Ma, X.; Zhang, Y.; Song, L.; Liu, J.; Shi, D.; Ma, X. Ligustrazine prevents coronary microcirculation dysfunction in rats via suppression of miR-34a-5p and promotion of Sirt1. Eur. J. Pharmacol. 2022, 929, 175150. [Google Scholar] [CrossRef]
- Su, Q.; Lv, X.; Ye, Z.; Sun, Y.; Kong, B.; Qin, Z.; Li, L. The mechanism of miR-142-3p in coronary microembolization-induced myocardiac injury via regulating target gene IRAK-1. Cell Death Dis. 2019, 10, 1–15. [Google Scholar] [CrossRef]
- Kleinbongard, P.; Lieder, H.; Skyschally, A.; Heusch, G. No sex-related differences in infarct size, no-reflow, and protection by ischaemic pre-conditioning in Göttingen minipigs. Cardiovasc. Res. 2022, 119, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-T.; Wu, X.-D.; Lu, Y.-X.; Sun, Y.-H.; Zhu, H.-H.; Liang, J.-B.; He, W.-K.; Zeng, Z.-Y.; Li, L. Potential Involvement of MiR-30e-3p in Myocardial Injury Induced by Coronary Microembolization via Autophagy Activation. Cell. Physiol. Biochem. 2017, 44, 1995–2004. [Google Scholar] [CrossRef]
- Herring, M.J.; Dai, W.; Hale, S.L.; Kloner, R.A.; Leor, J. Rapid Induction of Hypothermia by the ThermoSuit System Profoundly Reduces Infarct Size and Anatomic Zone of No Reflow Following Ischemia–Reperfusion in Rabbit and Rat Hearts. J. Cardiovasc. Pharmacol. Ther. 2014, 20, 193–202. [Google Scholar] [CrossRef]
- Kloner, R.A.; Ganote, C.E.; Jennings, R.B. The “No-Reflow” Phenomenon after Temporary Coronary Occlusion in the Dog. J. Clin. Investig. 1974, 54, 1496–1508. [Google Scholar] [CrossRef]
- Ambrosio, G.; Weisman, H.F.; A Mannisi, J.; Becker, L.C. Progressive impairment of regional myocardial perfusion after initial restoration of postischemic blood flow. Circulation 1989, 80, 1846–1861. [Google Scholar] [CrossRef]
- Reffelmann, T.; Kloner, R.A. Microvascular reperfusion injury: Rapid expansion of anatomic no reflow during reperfusion in the rabbit. Am. J. Physiol. Circ. Physiol. 2002, 283, H1099–H1107. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, D.-H.; Qu, N.; Wen, W.-M.; Huang, W.-Q. The Role of ERK1/2 Signaling Pathway in Coronary Microembolization-Induced Rat Myocardial Inflammation and Injury. Cardiology 2010, 117, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-T.; Lu, Y.-X.; Sun, Y.-H.; He, W.-K.; Liang, J.-B.; Li, L. TAK-242 Protects Against Apoptosis in Coronary Microembolization-Induced Myocardial Injury in Rats by Suppressing TLR4/NF-κB Signaling Pathway. Cell. Physiol. Biochem. 2017, 41, 1675–1683. [Google Scholar] [CrossRef]
- Qin, X.; Qin, H.; Li, Z.; Xue, S.; Huang, B.; Liu, X.; Wang, D. Luteolin alleviates ischemia/reperfusion injury-induced no-reflow by regulating Wnt/β-catenin signaling in rats. Microvasc. Res. 2021, 139, 104266. [Google Scholar] [CrossRef]
- Quan, X.; Liu, X.; Qin, X.; Wang, Y.; Sun, T.; Li, Z.; Zhu, L.; Chen, J.; Zhou, Y.; Singh, S.; et al. The role of LR-TIMAP/PP1c complex in the occurrence and development of no-reflow. EBioMedicine 2021, 65, 103251. [Google Scholar] [CrossRef]
- Deng, J. Advanced research on the regulated necrosis mechanism in myocardial ischemia-reperfusion injury. Int. J. Cardiol. 2021, 334, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Del Re, D.P.; Amgalan, D.; Linkermann, A.; Liu, Q.; Kitsis, R.N. Fundamental Mechanisms of Regulated Cell Death and Implications for Heart Disease. Physiol. Rev. 2019, 99, 1765–1817. [Google Scholar] [CrossRef]
- Dong, Y.; Chen, H.; Gao, J.; Liu, Y.; Li, J.; Wang, J. Molecular machinery and interplay of apoptosis and autophagy in coronary heart disease. J. Mol. Cell. Cardiol. 2019, 136, 27–41. [Google Scholar] [CrossRef] [PubMed]
- Lillo-Moya, J.; Rojas-Solé, C.; Muñoz-Salamanca, D.; Panieri, E.; Saso, L.; Rodrigo, R. Targeting Ferroptosis against Ischemia/Reperfusion Cardiac Injury. Antioxidants 2021, 10, 667. [Google Scholar] [CrossRef]
- Vasques-Nóvoa, F.; Angélico-Gonçalves, A.; Alvarenga, J.M.; Nobrega, J.; Cerqueira, R.J.; Mancio, J.; Leite-Moreira, A.F.; Roncon-Albuquerque, R. Myocardial oedema: Pathophysiological basis and implications for the failing heart. ESC Heart Fail. 2022, 9, 958–976. [Google Scholar] [CrossRef]
- A Laine, G.; Allen, S.J. Left ventricular myocardial edema. Lymph flow, interstitial fibrosis, and cardiac function. Circ. Res. 1991, 68, 1713–1721. [Google Scholar] [CrossRef]
- Steenbergen, C.; Hill, M.L.; Jennings, R.B. Volume regulation and plasma membrane injury in aerobic, anaerobic, and ischemic myocardium in vitro. Effects of osmotic cell swelling on plasma membrane integrity. Circ. Res. 1985, 57, 864–875. [Google Scholar] [CrossRef]
- Feigl, E.O. Coronary physiology. Physiol. Rev. 1983, 63, 1–205. [Google Scholar] [CrossRef] [PubMed]
- Barry, W.H. Mechanisms of Myocardial Cell Injury during Ischemia and Reperfusion. J. Card. Surg. 1987, 2, 375–383. [Google Scholar] [CrossRef]
- He, J.; Liu, D.; Zhao, L.; Zhou, D.; Rong, J.; Zhang, L.; Xia, Z. Myocardial ischemia/reperfusion injury: Mechanisms of injury and implications for management (Review). Exp. Ther. Med. 2022, 23, 1–11. [Google Scholar] [CrossRef]
- Hochachka, P.W. Defense Strategies Against Hypoxia and Hypothermia. Science 1986, 231, 234–241. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Jiménez, R.; Sánchez-González, J.; Agüero, J.; García-Prieto, J.; López-Martín, G.J.; García-Ruiz, J.M.; Molina-Iracheta, A.; Rosselló, X.; Fernández-Friera, L.; Pizarro, G.; et al. Myocardial Edema After Ischemia/Reperfusion Is Not Stable and Follows a Bimodal Pattern. J. Am. Coll. Cardiol. 2015, 65, 315–323. [Google Scholar] [CrossRef]
- Fernández-Jiménez, R.; García-Prieto, J.; Sánchez-González, J.; Agüero, J.; López-Martín, G.J.; Galán-Arriola, C.; Molina-Iracheta, A.; Doohan, R.; Fuster, V.; Ibáñez, B. Pathophysiology Underlying the Bimodal Edema Phenomenon After Myocardial Ischemia/Reperfusion. J. Am. Coll. Cardiol. 2015, 66, 816–828. [Google Scholar] [CrossRef]
- Kalogeris, T.; Baines, C.P.; Krenz, M.; Korthuis, R.J. Ischemia/Reperfusion. Compr. Physiol. 2016, 7, 113–170. [Google Scholar] [CrossRef]
- Schäfer, C. Role of the reverse mode of the Na+/Ca2+ exchanger in reoxygenation-induced cardiomyocyte injury. Cardiovasc. Res. 2001, 51, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Heusch, G.; Skyschally, A.; Kleinbongard, P. Coronary microembolization and microvascular dysfunction. Int. J. Cardiol. 2018, 258, 17–23. [Google Scholar] [CrossRef]
- El-Maraghi, N.; Genton, E. The relevance of platelet and fibrin thromboembolism of the coronary microcirculation, with special reference to sudden cardiac death. Circulation 1980, 62, 936–944. [Google Scholar] [CrossRef] [PubMed]
- Alkarithi, G.; Duval, C.; Shi, Y.; Macrae, F.L.; Ariëns, R.A. Thrombus Structural Composition in Cardiovascular Disease. Arter. Thromb. Vasc. Biol. 2021, 41, 2370–2383. [Google Scholar] [CrossRef]
- Dörge, H.; Neumann, T.; Behrends, M.; Skyschally, A.; Schulz, R.; Kasper, C.; Erbel, R.; Heusch, G.; Lindsey, M.L.; Bolli, R.; et al. Perfusion-contraction mismatch with coronary microvascular obstruction: Role of inflammation. Am. J. Physiol. Circ. Physiol. 2000, 279, H2587–H2592. [Google Scholar] [CrossRef]
- Ramaiola, I.; Padró, T.; Peña, E.; Juan-Babot, O.; Cubedo, J.; Martin-Yuste, V.; Sabate, M.; Badimon, L. Changes in thrombus composition and profilin-1 release in acute myocardial infarction. Eur. Heart J. 2014, 36, 965–975. [Google Scholar] [CrossRef]
- Thielmann, M.; Dörge, H.; Martin, C.; Belosjorow, S.; Schwanke, U.; van de Sand, A.; Konietzka, I.; Büchert, A.; Krüger, A.; Schulz, R.; et al. Myocardial Dysfunction With Coronary Microembolization. Circ. Res. 2002, 90, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Yunoki, K.; Naruko, T.; Inoue, T.; Sugioka, K.; Inaba, M.; Iwasa, Y.; Komatsu, R.; Itoh, A.; Haze, K.; Yoshiyama, M.; et al. Relationship of Thrombus Characteristics to the Incidence of Angiographically Visible Distal Embolization in Patients With ST-Segment Elevation Myocardial Infarction Treated With Thrombus Aspiration. JACC Cardiovasc. Interv. 2013, 6, 377–385. [Google Scholar] [CrossRef][Green Version]
- Okamura, A.; Ito, H.; Iwakura, K.; Kurotobi, T.; Koyama, Y.; Date, M.; Higuchi, Y.; Inoue, K.; Fujii, K. Clinical Implications of Distal Embolization During Coronary Interventional Procedures in Patients With Acute Myocardial Infarction. JACC Cardiovasc. Interv. 2008, 1, 268–276. [Google Scholar] [CrossRef]
- Stone, G.W.; Webb, J.; Cox, D.A.; Brodie, B.R.; Qureshi, M.; Kalynych, A.; Turco, M.; Schultheiss, H.P.; Dulas, D.; Rutherford, B.D.; et al. Distal Microcirculatory Protection During Percutaneous Coronary Intervention in Acute ST-Segment Elevation Myocardial InfarctionA Randomized Controlled Trial. JAMA 2005, 293, 1063–1072. [Google Scholar] [CrossRef]
- Nepper-Christensen, L.; Kelbæk, H.; A Ahtarovski, K.; E Høfsten, D.; Holmvang, L.; Pedersen, F.; Tilsted, H.-H.; Aarøe, J.; E Jensen, S.; Raungaard, B.; et al. Angiographic outcome in patients treated with deferred stenting after ST-segment elevation myocardial infarction—results from DANAMI-3-DEFER. Eur. Heart J. Acute Cardiovasc. Care 2022, 11, 742–748. [Google Scholar] [CrossRef]
- Olafsson, B.; Forman, M.B.; Puett, D.W.; Pou, A.; Cates, C.U.; Friesinger, G.C.; Virmani, R. Reduction of reperfusion injury in the canine preparation by intracoronary adenosine: Importance of the endothelium and the no-reflow phenomenon. Circulation 1987, 76, 1135–1145. [Google Scholar] [CrossRef] [PubMed]
- Levi, Y.; Sultan, A.; Alemayehu, M.; Wall, S.; Lavi, S. Association of endothelial dysfunction and no-reflow during primary percutaneous coronary intervention for ST-elevation myocardial infarction. Cardiovasc. Revascularization Med. 2016, 17, 552–555. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Yang, Y.; You, S.; Cui, C.; Gao, R. Carvedilol preserves endothelial junctions and reduces myocardial no-reflow after acute myocardial infarction and reperfusion. Int. J. Cardiol. 2007, 115, 334–341. [Google Scholar] [CrossRef]
- Li, Y.; Yao, Y.; Li, J.; Chen, Q.; Zhang, L.; Wang, Q.K. Losartan protects against myocardial ischemia and reperfusion injury via vascular integrity preservation. FASEB J. 2019, 33, 8555–8564. [Google Scholar] [CrossRef]
- Galaup, A.; Gomez, E.; Souktani, R.; Durand, M.; Cazes, A.; Monnot, C.; Teillon, J.; Le Jan, S.; Bouleti, C.; Briois, G.; et al. Protection Against Myocardial Infarction and No-Reflow Through Preservation of Vascular Integrity by Angiopoietin-Like 4. Circulation 2012, 125, 140–149. [Google Scholar] [CrossRef]
- Sun, W.; Lu, H.; Dong, S.; Li, R.; Chu, Y.; Wang, N.; Zhao, Y.; Zhang, Y.; Wang, L.; Sun, L.; et al. Beclin1 controls caspase-4 inflammsome activation and pyroptosis in mouse myocardial reperfusion-induced microvascular injury. Cell Commun. Signal. 2021, 19, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Liu, G.; Huang, B.; Liu, H.; Jiang, H.; Hu, Z.; Chen, J.; Liang, S. KDM3A Attenuates Myocardial Ischemic and Reperfusion Injury by Ameliorating Cardiac Microvascular Endothelial Cell Pyroptosis. Oxidative Med. Cell. Longev. 2022, 2022, 4622520. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. The fate and role of the pericytes in myocardial diseases. Eur. J. Clin. Investig. 2024, 54, e14204. [Google Scholar] [CrossRef] [PubMed]
- O’Farrell, F.M.; Mastitskaya, S.; Hammond-Haley, M.; Freitas, F.; Wah, W.R.; Attwell, D. Capillary pericytes mediate coronary no-reflow after myocardial ischaemia. eLife 2017, 6, e29280. [Google Scholar] [CrossRef]
- Methner, C.; Cao, Z.; Mishra, A.; Kaul, S. Mechanism and potential treatment of the “no reflow” phenomenon after acute myocardial infarction: Role of pericytes and GPR39. Am. J. Physiol. Circ. Physiol. 2021, 321, H1030–H1041. [Google Scholar] [CrossRef]
- Niccoli, G.; Lanza, G.A.; Shaw, S.; Romagnoli, E.; Gioia, D.; Burzotta, F.; Trani, C.; Mazzari, M.A.; Mongiardo, R.; De Vita, M.; et al. Endothelin-1 and acute myocardial infarction: A no-reflow mediator after successful percutaneous myocardial revascularization. Eur. Heart J. 2006, 27, 1793–1798. [Google Scholar] [CrossRef] [PubMed]
- Eitel, I.; Nowak, M.; Stehl, C.; Adams, V.; Fuernau, G.; Hildebrand, L.; Desch, S.; Schuler, G.; Thiele, H. Endothelin-1 release in acute myocardial infarction as a predictor of long-term prognosis and no-reflow assessed by contrast-enhanced magnetic resonance imaging. Am. Heart J. 2010, 159, 882–890. [Google Scholar] [CrossRef]
- Omland, T.; Lie, R.T.; Aakvaag, A.; Aarsland, T.; Dickstein, K. Plasma endothelin determination as a prognostic indicator of 1-year mortality after acute myocardial infarction. Circulation 1994, 89, 1573–1579. [Google Scholar] [CrossRef]
- Zouki, C.; Baron, C.; Fournier, A.; Filep, J.G. Endothelin-1 enhances neutrophil adhesion to human coronary artery endothelial cells: Role of ETA receptors and platelet-activating factor. Br. J. Pharmacol. 1999, 127, 969–979. [Google Scholar] [CrossRef]
- Tauber, S.; Menger, M.D.; Lehr, H.-A. Microvascular in vivo assessment of reperfusion injury: Significance of prostaglandin E1 and I2 in postischemic “no-reflow” and “reflow-paradox”. J. Surg. Res. 2004, 120, 1–11. [Google Scholar] [CrossRef]
- Bruegger, D.; Rehm, M.; Jacob, M.; Chappell, D.; Stoeckelhuber, M.; Welsch, U.; Conzen, P.; Becker, B.F. Exogenous nitric oxide requires an endothelial glycocalyx to prevent postischemic coronary vascular leak in guinea pig hearts. Crit. Care 2008, 12, R73. [Google Scholar] [CrossRef]
- Czarnowska, E.; Karwatowska-Prokopczuk, E. Ultrastructural demonstration of endothelial glycocalyx disruption in the reperfused rat heart. Involvement of oxygen free radicals. Basic Res. Cardiol. 1995, 90, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Kurzelewski, M.; Czarnowska, E.; Beręsewicz, A. Endothelin in the mechanism of endothelial injury and neutrophil adhesion in the post-ischemic guinea-pig heart. Eur. J. Pharmacol. 2002, 434, 95–107. [Google Scholar] [CrossRef]
- Engler, R.L.; Schmid-Schönbein, G.W.; Pavelec, R.S. Leukocyte capillary plugging in myocardial ischemia and reperfusion in the dog. Am. J. Pathol. 1983, 111, 98–111. [Google Scholar] [PubMed]
- Engler, R.L.; Dahlgren, M.D.; Morris, D.D.; Peterson, M.A.; Schmid-Schonbein, G.W. Role of leukocytes in response to acute myocardial ischemia and reflow in dogs. Am. J. Physiol. Circ. Physiol. 1986, 251, H314–H323. [Google Scholar] [CrossRef] [PubMed]
- Litt, M.R.; Jeremy, R.W.; Weisman, H.F.; A Winkelstein, J.; Becker, L.C. Neutrophil depletion limited to reperfusion reduces myocardial infarct size after 90 minutes of ischemia. Evidence for neutrophil-mediated reperfusion injury. Circulation 1989, 80, 1816–1827. [Google Scholar] [CrossRef] [PubMed]
- Mazzoni, M.C.; Borgström, P.; Warnke, K.; Skalak, T.; Intaglietta, M.; Arfors, K. Mechanisms and Implications of Capillary Endotheloal Swelling and Luminal Narrowing in Low-Flow Ischemias. Int. J. Microcirc. 1995, 15, 265–270. [Google Scholar] [CrossRef]
- Duilio, C.; Ambrosio, G.; Kuppusamy, P.; DiPaula, A.; Becker, L.C.; Zweier, J.L. Neutrophils are primary source of O2radicals during reperfusion after prolonged myocardial ischemia. Am. J. Physiol. Circ. Physiol. 2001, 280, H2649–H2657. [Google Scholar] [CrossRef]
- Ge, L.; Zhou, X.; Ji, W.-J.; Lu, R.-Y.; Zhang, Y.; Zhang, Y.-D.; Ma, Y.-Q.; Zhao, J.-H.; Li, Y.-M. Neutrophil extracellular traps in ischemia-reperfusion injury-induced myocardial no-reflow: Therapeutic potential of DNase-based reperfusion strategy. Am. J. Physiol. Circ. Physiol. 2015, 308, H500–H509. [Google Scholar] [CrossRef]
- Golino, P.; Maroko, P.R.; E Carew, T. Efficacy of platelet depletion in counteracting the detrimental effect of acute hypercholesterolemia on infarct size and the no-reflow phenomenon in rabbits undergoing coronary artery occlusion-reperfusion. Circulation 1987, 76, 173–180. [Google Scholar] [CrossRef]
- Zweier, J.L.; Flaherty, J.T.; Weisfeldt, M.L. Direct measurement of free radical generation following reperfusion of ischemic myocardium. Proc. Natl. Acad. Sci. USA 1987, 84, 1404–1407. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Kalogeris, T.; Korthuis, R.J. Reactive species-induced microvascular dysfunction in ischemia/reperfusion. Free Radic. Biol. Med. 2019, 135, 182–197. [Google Scholar] [CrossRef] [PubMed]
- Boueiz, A.; Damarla, M.; Hassoun, P.M. Xanthine oxidoreductase in respiratory and cardiovascular disorders. Am. J. Physiol. Cell. Mol. Physiol. 2008, 294, L830–L840. [Google Scholar] [CrossRef]
- Wang, J.; Toan, S.; Zhou, H. Mitochondrial quality control in cardiac microvascular ischemia-reperfusion injury: New insights into the mechanisms and therapeutic potentials. Pharmacol. Res. 2020, 156, 104771. [Google Scholar] [CrossRef]
- Zhou, H.; Shi, C.; Hu, S.; Zhu, H.; Ren, J.; Chen, Y. BI1 is associated with microvascular protection in cardiac ischemia reperfusion injury via repressing Syk–Nox2–Drp1-mitochondrial fission pathways. Angiogenesis 2018, 21, 599–615. [Google Scholar] [CrossRef] [PubMed]
- Cadenas, S. ROS and redox signaling in myocardial ischemia-reperfusion injury and cardioprotection. Free Radic. Biol. Med. 2018, 117, 76–89. [Google Scholar] [CrossRef]
- Chouchani, E.T.; Pell, V.R.; James, A.M.; Work, L.M.; Saeb-Parsy, K.; Frezza, C.; Krieg, T.; Murphy, M.P. A Unifying Mechanism for Mitochondrial Superoxide Production during Ischemia-Reperfusion Injury. Cell Metab. 2016, 23, 254–263. [Google Scholar] [CrossRef]
- Pisarenko, O.; Studneva, I.; Khlopkov, V.; Solomatina, E.; Ruuge, E. An assessment of anaerobic metabolism during ischemia and reperfusion in isolated guinea pig heart. Biochim. Biophys. Acta BBA -Bioenerg. 1988, 934, 55–63. [Google Scholar] [CrossRef]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef]
- Lesnefsky, E.J.; Chen, Q.; Tandler, B.; Hoppel, C.L. Mitochondrial Dysfunction and Myocardial Ischemia-Reperfusion: Implications for Novel Therapies. Annu. Rev. Pharmacol. Toxicol. 2017, 57, 535–565. [Google Scholar] [CrossRef]
- Lesnefsky, E.J.; Chen, Q.; Moghaddas, S.; Hassan, M.O.; Tandler, B.; Hoppel, C.L. Blockade of Electron Transport during Ischemia Protects Cardiac Mitochondria. J. Biol. Chem. 2004, 279, 47961–47967. [Google Scholar] [CrossRef]
- Bedard, K.; Krause, K.-H. The NOX Family of ROS-Generating NADPH Oxidases: Physiology and Pathophysiology. Physiol. Rev. 2007, 87, 245–313. [Google Scholar] [CrossRef] [PubMed]
- Sirker, A.; Zhang, M.; Shah, A.M. NADPH oxidases in cardiovascular disease: Insights from in vivo models and clinical studies. Basic Res. Cardiol. 2011, 106, 735–747. [Google Scholar] [CrossRef] [PubMed]
- Braunersreuther, V.; Montecucco, F.; Ashri, M.; Pelli, G.; Galan, K.; Frias, M.; Burger, F.; Quinderé, A.L.G.; Montessuit, C.; Krause, K.-H.; et al. Role of NADPH oxidase isoforms NOX1, NOX2 and NOX4 in myocardial ischemia/reperfusion injury. J. Mol. Cell. Cardiol. 2013, 64, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Hoffmeyer, M.R.; Jones, S.P.; Ross, C.R.; Sharp, B.; Grisham, M.B.; Laroux, F.S.; Stalker, T.J.; Scalia, R.; Lefer, D.J. Myocardial Ischemia/Reperfusion Injury in NADPH Oxidase–Deficient Mice. Circ. Res. 2000, 87, 812–817. [Google Scholar] [CrossRef]
- Thirunavukkarasu, M.; Adluri, R.S.; Juhasz, B.; Samuel, S.M.; Zhan, L.; Kaur, A.; Maulik, G.; A Sanchez, J.; Hager, J.; Maulik, N. Novel role of NADPH oxidase in ischemic myocardium: A study with Nox2 knockout mice. Funct. Integr. Genom. 2011, 12, 501–514. [Google Scholar] [CrossRef]
- Richard, V.; Murry, C.; Jennings, R.; Reimer, K. Oxygen-derived free radicals and postischemic myocardial reperfusion: Therapeutic implications. Fundam. Clin. Pharmacol. 1990, 4, 85–103. [Google Scholar] [CrossRef]
- Cote, C.G.; Yu, F.S.; Zulueta, J.J.; Vosatka, R.J.; Hassoun, P.M. Regulation of intracellular xanthine oxidase by endothelial-derived nitric oxide. Am. J. Physiol. Cell. Mol. Physiol. 1996, 271, L869–L874. [Google Scholar] [CrossRef]
- De Pascali, F.; Hemann, C.; Samons, K.; Chen, C.-A.; Zweier, J.L. Hypoxia and Reoxygenation Induce Endothelial Nitric Oxide Synthase Uncoupling in Endothelial Cells through Tetrahydrobiopterin Depletion and S-Glutathionylation. Biochemistry 2014, 53, 3679–3688. [Google Scholar] [CrossRef]
- Zhou, S.-S.; Jin, J.-P.; Wang, J.-Q.; Zhang, Z.-G.; Freedman, J.H.; Zheng, Y.; Cai, L. miRNAS in cardiovascular diseases: Potential biomarkers, therapeutic targets and challenges. Acta Pharmacol. Sin. 2018, 39, 1073–1084. [Google Scholar] [CrossRef]
- Jonas, S.; Izaurralde, E. Towards a molecular understanding of microRNA-mediated gene silencing. Nat. Rev. Genet. 2015, 16, 421–433. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.X.; Rothenberg, M.E. MicroRNA. J. Allergy Clin. Immunol. 2018, 141, 1202–1207. [Google Scholar] [CrossRef] [PubMed]
- Saliminejad, K.; Khorram Khorshid, H.R.; Soleymani Fard, S.; Ghaffari, S.H. An overview of microRNAs: Biology, functions, therapeutics, and analysis methods. J. Cell. Physiol. 2019, 234, 5451–5465. [Google Scholar] [CrossRef]
- Mohr, A.M.; Mott, J.L. Overview of MicroRNA Biology. Semin. Liver Dis. 2015, 35, 003–011. [Google Scholar] [CrossRef] [PubMed]
- Mo, B.; Wu, X.; Wang, X.; Xie, J.; Ye, Z.; Li, L. miR-30e-5p Mitigates Hypoxia-Induced Apoptosis in Human Stem Cell-Derived Cardiomyocytes by Suppressing Bim. Int. J. Biol. Sci. 2019, 15, 1042–1051. [Google Scholar] [CrossRef]
- Su, B.; Wang, X.; Sun, Y.; Long, M.; Zheng, J.; Wu, W.; Li, L.; Galea, N. miR-30e-3p Promotes Cardiomyocyte Autophagy and Inhibits Apoptosis via Regulating Egr-1 during Ischemia/Hypoxia. BioMed Res. Int. 2020, 2020, 7231243. [Google Scholar] [CrossRef]
- Yang, X.; Dai, R.; Qin, Z.; Cai, R.; Xu, Y.; Su, Q. LncRNA MALAT1 functions as a biomarker of no-reflow phenomenon in ST-segment elevation myocardial infarction patients receiving primary percutaneous coronary intervention. Sci. Rep. 2022, 12, 1–11. [Google Scholar] [CrossRef]
- Hu, C.-K.; Cai, R.-P.; He, L.; He, S.-R.; Liao, J.-Y.; Su, Q. A Nomogram model for predicting the occurrence of no-reflow phenomenon after percutaneous coronary intervention using the lncRNA TUG1/miR-30e/NPPB biomarkers. J. Thorac. Dis. 2022, 14, 2158–2168. [Google Scholar] [CrossRef]
- Su, Q.; Ye, Z.; Sun, Y.; Yang, H.; Li, L. Relationship between circulating miRNA-30e and no-reflow phenomenon in STEMI patients undergoing primary coronary intervention. Scand. J. Clin. Lab. Investig. 2018, 78, 318–324. [Google Scholar] [CrossRef]
- De Couto, G.; Jaghatspanyan, E.; DeBerge, M.; Liu, W.; Luther, K.; Wang, Y.; Tang, J.; Thorp, E.B.; Marbán, E. Mechanism of Enhanced MerTK-Dependent Macrophage Efferocytosis by Extracellular Vesicles. Arter. Thromb. Vasc. Biol. 2019, 39, 2082–2096. [Google Scholar] [CrossRef]
- Chen, Y.; Li, S.; Zhang, Y.; Wang, M.; Li, X.; Liu, S.; Xu, D.; Bao, Y.; Jia, P.; Wu, N.; et al. The lncRNA Malat1 regulates microvascular function after myocardial infarction in mice via miR-26b-5p/Mfn1 axis-mediated mitochondrial dynamics. Redox Biol. 2021, 41, 101910. [Google Scholar] [CrossRef] [PubMed]
- Santovito, D.; Egea, V.; Bidzhekov, K.; Natarelli, L.; Mourão, A.; Blanchet, X.; Wichapong, K.; Aslani, M.; Brunßen, C.; Horckmans, M.; et al. Autophagy unleashes noncanonical microRNA functions. Autophagy 2020, 16, 2294–2296. [Google Scholar] [CrossRef] [PubMed]
- Santovito, D.; Egea, V.; Bidzhekov, K.; Natarelli, L.; Mourão, A.; Blanchet, X.; Wichapong, K.; Aslani, M.; Brunßen, C.; Horckmans, M.; et al. Noncanonical inhibition of caspase-3 by a nuclear microRNA confers endothelial protection by autophagy in atherosclerosis. Sci. Transl. Med. 2020, 12, eaaz2294. [Google Scholar] [CrossRef]
- Su, Q.; Yang, H.; Li, L. Circulating miRNA-155 as a Potential Biomarker for Coronary Slow Flow. Dis. Markers 2018, 2018, 1–6. [Google Scholar] [CrossRef]
- Wagner, J.U.G.; Tombor, L.S.; Malacarne, P.F.; Kettenhausen, L.-M.; Panthel, J.; Kujundzic, H.; Manickam, N.; Schmitz, K.; Cipca, M.; Stilz, K.A.; et al. Aging impairs the neurovascular interface in the heart. Science 2023, 381, 897–906. [Google Scholar] [CrossRef]
- Hu, Y.; Xiong, J.; Wen, H.; Wei, H.; Zeng, X. MiR-98-5p promotes ischemia/reperfusion-induced microvascular dysfunction by targeting NGF and is a potential biomarker for microvascular reperfusion. Microcirculation 2020, 28, e12657. [Google Scholar] [CrossRef]
- Li, L.-L.; Mao, C.-D.; Wang, G.-P.; Wang, N.; Xue, A.-G. MiR-145-5p alleviates hypoxia/reoxygenation-induced cardiac microvascular endothelial cell injury in coronary heart disease by inhibiting Smad4 expression. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 5008–5017. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, S.; Zhang, X.; Zuo, C. Changes and Diagnostic Significance of miR-542-3p Expression in Patients with Myocardial Infarction. Mol. Biotechnol. 2024, 1–8. [Google Scholar] [CrossRef]
- Zhang, J. Circulating miR-660-5p is associated with the no-reflow phenomenon in patients with ST segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Anatol. J. Cardiol. 2020, 25, 323–329. [Google Scholar] [CrossRef]
- Salama, A.M.; Khalil, W.A.; Al-Zaky, M.; Abdallah, S.H.; Kandil, N.T.; Abdelsabour, A.; Shaker, A.M.; Hasanein, M.T.; Luciani, G.B.; Azzazy, H.M.E. MicroRNA-208a: A Good Diagnostic Marker and a Predictor of no-Reflow in STEMI Patients Undergoing Primary Percutaneuos Coronary Intervention. J. Cardiovasc. Transl. Res. 2020, 13, 988–995. [Google Scholar] [CrossRef]
- Detaille, D.; Pasdois, P.; Sémont, A.; Dos Santos, P.; Diolez, P.; Lesnefsky, E.J. An old medicine as a new drug to prevent mitochondrial complex I from producing oxygen radicals. PLoS ONE 2019, 14, e0216385. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Wang, X.; Wu, H.; Chen, T.; Li, X.; Zhang, L.; Li, X.; Er, L.; Du, R. Cardioprotective effect of anisodamine against ischemia/reperfusion injury through the mitochondrial ATP-sensitive potassium channel. Eur. J. Pharmacol. 2021, 901, 174095. [Google Scholar] [CrossRef] [PubMed]
- Tinker, A.; Aziz, Q.; Thomas, A. The role of ATP-sensitive potassium channels in cellular function and protection in the cardiovascular system. Br. J. Pharmacol. 2013, 171, 12–23. [Google Scholar] [CrossRef]
- Liu, M.; Li, S.; Yin, M.; Li, Y.; Chen, J.; Chen, Y.; Zhou, Y.; Li, Q.; Xu, F.; Dai, C.; et al. Pinacidil ameliorates cardiac microvascular ischemia–reperfusion injury by inhibiting chaperone-mediated autophagy of calreticulin. Basic Res. Cardiol. 2024, 119, 113–131. [Google Scholar] [CrossRef] [PubMed]
- Tanano, I.; Nagaoka, T.; Omae, T.; Ishibazawa, A.; Kamiya, T.; Ono, S.; Yoshida, A. Dilation of Porcine Retinal Arterioles to Cilostazol: Roles of eNOS Phosphorylation via cAMP/Protein Kinase A and AMP-Activated Protein Kinase and Potassium Channels. Investig. Opthalmology Vis. Sci. 2013, 54, 1443–1449. [Google Scholar] [CrossRef]
- Chen, R.; Chen, T.; Wang, T.; Dai, X.; Zhang, S.; Jiang, D.; Meng, K.; Wang, Y.; Geng, T.; Xu, J.; et al. Tongmai Yangxin pill reduces myocardial No-reflow via endothelium-dependent NO-cGMP signaling by activation of the cAMP/PKA pathway. J. Ethnopharmacol. 2021, 267, 113462. [Google Scholar] [CrossRef]
- Cui, Y.; Li, C.; Zeng, C.; Li, J.; Zhu, Z.; Chen, W.; Huang, A.; Qi, X. Tongmai Yangxin pills anti-oxidative stress alleviates cisplatin-induced cardiotoxicity: Network pharmacology analysis and experimental evidence. Biomed. Pharmacother. 2018, 108, 1081–1089. [Google Scholar] [CrossRef]
- Dai, W.; Hale, S.; Kloner, R.A. Delayed therapeutic hypothermia protects against the myocardial no-reflow phenomenon independently of myocardial infarct size in a rat ischemia/reperfusion model. Int. J. Cardiol. 2017, 236, 400–404. [Google Scholar] [CrossRef]
- Hale, S.L.; Herring, M.J.; Kloner, R.A. Delayed Treatment With Hypothermia Protects Against the No-Reflow Phenomenon Despite Failure to Reduce Infarct Size. J. Am. Heart Assoc. 2013, 2, e004234. [Google Scholar] [CrossRef]
- Nie, C.; Ding, X.; A, R.; Zheng, M.; Li, Z.; Pan, S.; Yang, W. Hydrogen gas inhalation alleviates myocardial ischemia-reperfusion injury by the inhibition of oxidative stress and NLRP3-mediated pyroptosis in rats. Life Sci. 2021, 272, 119248. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, H.; Qi, W.; Zhang, Y.; Li, J.; Li, Z.; Lin, Y.; Bai, X.; Liu, X.; Chen, X.; et al. Nicotine promotes atherosclerosis via ROS-NLRP3-mediated endothelial cell pyroptosis. Cell Death Dis. 2018, 9, 1–12. [Google Scholar] [CrossRef]
- Zhang, X.; Du, Q.; Yang, Y.; Wang, J.; Dou, S.; Liu, C.; Duan, J. The protective effect of Luteolin on myocardial ischemia/reperfusion (I/R) injury through TLR4/NF-κB/NLRP3 inflammasome pathway. Biomed. Pharmacother. 2017, 91, 1042–1052. [Google Scholar] [CrossRef]
- Leon, B.M.; Maddox, T.M. Diabetes and cardiovascular disease: Epidemiology, biological mechanisms, treatment recommendations and future research. World J. Diabetes 2015, 6, 1246–1258. [Google Scholar] [CrossRef] [PubMed]
- Weyer, C.; Funahashi, T.; Tanaka, S.; Hotta, K.; Matsuzawa, Y.; Pratley, R.E.; Tataranni, P.A. Hypoadiponectinemia in Obesity and Type 2 Diabetes: Close Association with Insulin Resistance and Hyperinsulinemia. J. Clin. Endocrinol. Metab. 2001, 86, 1930–1935. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Wu, Y.; Liu, X.; Ma, L.; Lv, T.; Sun, Q.; Xu, W.; Zhang, S.; Wang, K.; Wang, W.; et al. Adiponectin improves coronary no-reflow injury by protecting the endothelium in rats with type 2 diabetes mellitus. Biosci. Rep. 2017, 37, BSR20170282. [Google Scholar] [CrossRef]
- Johnston, P.V.; Sasano, T.; Mills, K.; Evers, R.; Lee, S.-T.; Smith, R.R.; Lardo, A.C.; Lai, S.; Steenbergen, C.; Gerstenblith, G.; et al. Engraftment, Differentiation, and Functional Benefits of Autologous Cardiosphere-Derived Cells in Porcine Ischemic Cardiomyopathy. Circulation 2009, 120, 1075–1083. [Google Scholar] [CrossRef]
- Kanazawa, H.; Tseliou, E.; Malliaras, K.; Yee, K.; Dawkins, J.F.; De Couto, G.; Smith, R.R.; Kreke, M.; Seinfeld, J.; Middleton, R.C.; et al. Cellular Postconditioning. Circ. Heart Fail. 2015, 8, 322–332. [Google Scholar] [CrossRef] [PubMed]
- Sousonis, V.; Sfakianaki, T.; Ntalianis, A.; Nanas, I.; Kontogiannis, C.; Aravantinos, D.; Kapelios, C.; Katsaros, L.; Nana, M.; Sampaziotis, D.; et al. Intracoronary Administration of Allogeneic Cardiosphere-Derived Cells Immediately Prior to Reperfusion in Pigs With Acute Myocardial Infarction Reduces Infarct Size and Attenuates Adverse Cardiac Remodeling. J. Cardiovasc. Pharmacol. Ther. 2020, 26, 88–99. [Google Scholar] [CrossRef]
- Vakrou, S.; Nana, M.A.; Nanas, I.A.; Nana-Leventaki, E.; Bonios, M.; Kapelios, C.; Nanas, J. Safety and efficacy of global intracoronary administration of cardiosphere-derived cells or conditioned medium immediately after coronary reperfusion in rats. Hell. J. Cardiol. 2019, 61, 256–261. [Google Scholar] [CrossRef]
- Hansen, A.; Bekeredjian, R.; Filusch, A.; Wolf, D.; Gross, M.-L.; Mueller, S.; Korosoglou, G.; Kuecherer, H.F. Cardioprotective Effects of the Novel Selective Endothelin-A Receptor Antagonist BSF 461314 in Ischemia–Reperfusion Injury. J. Am. Soc. Echocardiogr. 2005, 18, 1213–1220. [Google Scholar] [CrossRef]
- Goodwin, A. Inhibition of endogenous endothelin during cardioplegia improves low coronary reflow following prolonged hypothermic arrest. Eur. J. Cardio-Thoracic Surg. 1997, 11, 981–987. [Google Scholar] [CrossRef]
- Michaels, A.D.; Appleby, M.; Otten, M.H.; Dauterman, K.; A Ports, T.; Chou, T.M.; Gibson, C.M. Pretreatment with intragraft verapamil prior to percutaneous coronary intervention of saphenous vein graft lesions: Results of the randomized, controlled vasodilator prevention on no-reflow (VAPOR) trial. J. Invasive Cardiology 2002, 14, 299–302. [Google Scholar]
- Niccoli, G.; Rigattieri, S.; De Vita, M.R.; Valgimigli, M.; Corvo, P.; Fabbiocchi, F.; Romagnoli, E.; De Caterina, A.R.; La Torre, G.; Schiavo, P.L.; et al. Open-Label, Randomized, Placebo-Controlled Evaluation of Intracoronary Adenosine or Nitroprusside After Thrombus Aspiration During Primary Percutaneous Coronary Intervention for the Prevention of Microvascular Obstruction in Acute Myocardial Infarction. JACC Cardiovasc. Interv. 2013, 6, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cheng, Z.; Gu, Y.; Peng, D. Short-Term Effects of Verapamil and Diltiazem in the Treatment of No Reflow Phenomenon: A Meta-Analysis of Randomized Controlled Trials. BioMed Res. Int. 2015, 2015, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Grancini, L.; Diana, D.; Centola, A.; Monizzi, G.; Mastrangelo, A.; Olivares, P.; Montorsi, P.; Alushi, B.; Bartorelli, A.L.; Galassi, A.R. The SALINE Technique for the Treatment of the No-Reflow Phenomenon during Percutaneous Coronary Intervention in STEMI. J. Clin. Med. 2023, 12, 2405. [Google Scholar] [CrossRef] [PubMed]
- Osinalde, E.P.; Bastante, T.; Cecconi, A.; Muñiz, Á.M.; García-Guimaraes, M.; Rivero, F.; Rojas-González, A.; Olivera, M.J.; Salamanca, J.; de Isla, L.P.; et al. Intracoronary thrombus assessment with cardiac computed tomography angiography in a deferred stenting strategy: The MATURE prospective study (MSCT to Assess ThrombUs REsolution). Coron. Artery Dis. 2023, 34, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Skelding, K.A.; Goldstein, J.A.; Mehta, L.; Pica, M.C.; O’NEill, W.W. Resolution of refractory no-reflow with intracoronary epinephrine. Catheter. Cardiovasc. Interv. 2002, 57, 305–309. [Google Scholar] [CrossRef]
- Navarese, E.P.; Frediani, L.; Kandzari, D.E.; Caiazzo, G.; Cenname, A.M.; Cortese, B.; Piva, T.; Muçaj, A.; Tumscitz, C.; Paparoni, F.; et al. Efficacy and safety of intracoronary epinephrine versus conventional treatments alone in STEMI patients with refractory coronary no-reflow during primary PCI: The RESTORE observational study. Catheter. Cardiovasc. Interv. 2020, 97, 602–611. [Google Scholar] [CrossRef]
- Ryabov, V.; Dil, S.; Vyshlov, E.; Mochula, O.; Kercheva, M.; Baev, A.; Gergert, E.; Maslov, L. Efficiency and Safety of Intracoronary Epinephrine Administration in Patients With ST-Elevation Myocardial Infarction With Refractory Coronary No-Reflow. Am. J. Cardiol. 2024, 226, 118–127. [Google Scholar] [CrossRef]
- Khan, K.A.; Qamar, N.; Saghir, T.; Sial, J.A.; Kumar, D.; Kumar, R.; Qayyum, D.; Yasin, U.; Jalbani, J.; Karim, M. Comparison of Intracoronary Epinephrine and Adenosine for No-Reflow in Normotensive Patients with Acute Coronary Syndrome (COAR Trial). Circ. Cardiovasc. Interv. 2022, 15, 163–171. [Google Scholar] [CrossRef]
- Abu Arab, T.; Sedhom, R.; Gomaa, Y.; El Etriby, A. Intracoronary adenosine compared with adrenaline and verapamil in the treatment of no-reflow phenomenon following primary PCI in STEMI patients. Int. J. Cardiol. 2024, 410, 132228. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, J.M.; Gorlin, R. Effect of 1-Epinephrine on the Coronary Circulation in Human Subjects with and without Coronary Artery Disease. Circ. Res. 1967, 21, 919–924. [Google Scholar] [CrossRef] [PubMed]
- He, P.; Chen, H.; Yang, J.; Gao, L.; Guo, J.; Chen, Y.; Wang, Q. The efficacy of an intracoronary cocktail administration in preventing no-reflow during excimer laser coronary angioplasty in patients with in-stent restenosis: A pilot study. (ELCA- cocktail study). Int. J. Cardiol. 2024, 419, 132666. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, M.; Yang, Y.; Zeng, M. Efficacy and Safety of Alprostadil in Microcirculatory Disturbances During Emergency PCI: A Meta-Analysis of Randomized Controlled Trials. Am. J. Cardiovasc. Drugs 2024, 24, 547–556. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borzuta, H.; Kociemba, W.; Bochenek, O.; Jarowicz, M.; Wsół, A. Advances in Pathophysiology and Novel Therapeutic Strategies for Coronary No-Reflow Phenomenon. Biomedicines 2025, 13, 1716. https://doi.org/10.3390/biomedicines13071716
Borzuta H, Kociemba W, Bochenek O, Jarowicz M, Wsół A. Advances in Pathophysiology and Novel Therapeutic Strategies for Coronary No-Reflow Phenomenon. Biomedicines. 2025; 13(7):1716. https://doi.org/10.3390/biomedicines13071716
Chicago/Turabian StyleBorzuta, Hubert, Wiktor Kociemba, Oliwia Bochenek, Monika Jarowicz, and Agnieszka Wsół. 2025. "Advances in Pathophysiology and Novel Therapeutic Strategies for Coronary No-Reflow Phenomenon" Biomedicines 13, no. 7: 1716. https://doi.org/10.3390/biomedicines13071716
APA StyleBorzuta, H., Kociemba, W., Bochenek, O., Jarowicz, M., & Wsół, A. (2025). Advances in Pathophysiology and Novel Therapeutic Strategies for Coronary No-Reflow Phenomenon. Biomedicines, 13(7), 1716. https://doi.org/10.3390/biomedicines13071716






