Sex-Related Differences in Glioblastoma: A Single-Center Retrospective Cohort Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Statistical Analysis
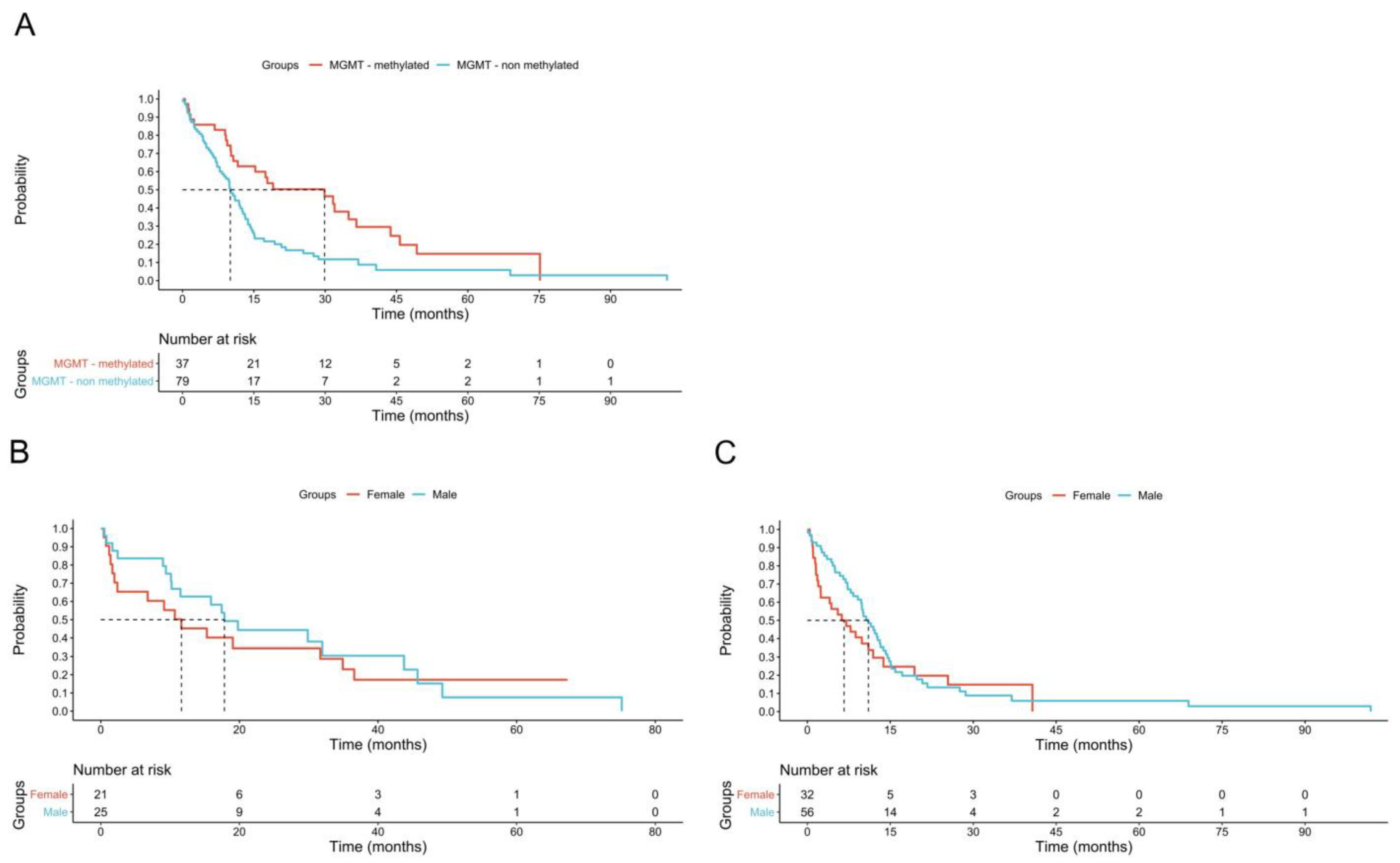
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, T.; Plutynski, A.; Ward, S.; Rubin, J.B. An integrative view on sex differences in brain tumors. Cell. Mol. Life Sci. CMLS 2015, 72, 3323–3342. [Google Scholar] [CrossRef] [PubMed]
- Kabat, G.C.; Etgen, A.M.; Rohan, T.E. Do steroid hormones play a role in the etiology of glioma? Cancer Epidemiol. Biomark. Prev. 2010, 19, 2421–2427. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Cioffi, G.; Gittleman, H.; Patil, N.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. Cbtrus statistical report: Primary brain and other central nervous system tumors diagnosed in the united states in 2012–2016. Neuro-Oncol. 2019, 21, v1–v100. [Google Scholar] [CrossRef] [PubMed]
- Ah-Pine, F.; Khettab, M.; Bedoui, Y.; Slama, Y.; Daniel, M.; Doray, B.; Gasque, P. On the origin and development of glioblastoma: Multifaceted role of perivascular mesenchymal stromal cells. Acta Neuropathol. Commun. 2023, 11, 104. [Google Scholar] [CrossRef]
- Carrano, A.; Juarez, J.J.; Incontri, D.; Ibarra, A.; Guerrero Cazares, H. Sex-specific differences in glioblastoma. Cells 2021, 10, 1783. [Google Scholar] [CrossRef]
- Komori, T. The 2021 who classification of tumors, 5th edition, central nervous system tumors: The 10 basic principles. Brain Tumor Pathol. 2022, 39, 47–50. [Google Scholar] [CrossRef]
- Sareen, H.; Ma, Y.; Becker, T.M.; Roberts, T.L.; de Souza, P.; Powter, B. Molecular biomarkers in glioblastoma: A systematic review and meta-analysis. Int. J. Mol. Sci. 2022, 23, 8835. [Google Scholar] [CrossRef]
- Franceschi, E.; Tosoni, A.; Minichillo, S.; Depenni, R.; Paccapelo, A.; Bartolini, S.; Michiara, M.; Pavesi, G.; Urbini, B.; Crisi, G.; et al. The prognostic roles of gender and O6-methylguanine-DNA methyltransferase methylation status in glioblastoma patients: The female power. World Neurosurg. 2018, 112, e342–e347. [Google Scholar] [CrossRef]
- Olympios, N.; Gilard, V.; Marguet, F.; Clatot, F.; Di Fiore, F.; Fontanilles, M. TERT promoter alterations in glioblastoma: A systematic review. Cancers 2021, 13, 1147. [Google Scholar] [CrossRef]
- Xu, H.; Zong, H.; Ma, C.; Ming, X.; Shang, M.; Li, K.; He, X.; Du, H.; Cao, L. Epidermal growth factor receptor in glioblastoma. Oncol. Lett. 2017, 14, 512–516. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Kinnersley, B.; Wrensch, M.R.; Eckel-Passow, J.E.; Armstrong, G.; Rice, T.; Chen, Y.; Wiencke, J.K.; McCoy, L.S.; Hansen, H.M.; et al. Sex-specific glioma genome-wide association study identifies new risk locus at 3p21.31 in females, and finds sex-differences in risk at 8q24.21. Sci. Rep. 2018, 8, 7352. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, X.; Liu, J.; Chen, W.; Guo, X.; Wang, Y.; Wang, Y.; Xing, H.; Liang, T.; Shi, Y.; et al. Clinical roles of egfr amplification in diffuse gliomas: A real-world study using the 2021 who classification of cns tumors. Front. Neurosci. 2024, 18, 1308627. [Google Scholar] [CrossRef] [PubMed]
- Gittleman, H.; Ostrom, Q.T.; Stetson, L.C.; Waite, K.; Hodges, T.R.; Wright, C.H.; Wright, J.; Rubin, J.B.; Berens, M.E.; Lathia, J.; et al. Sex is an important prognostic factor for glioblastoma but not for nonglioblastoma. Neuro-Oncol. Pract. 2019, 6, 451–462. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Rubin, J.B.; Lathia, J.D.; Berens, M.E.; Barnholtz-Sloan, J.S. Females have the survival advantage in glioblastoma. Neuro-Oncol. 2018, 20, 576–577. [Google Scholar] [CrossRef]
- Barnett, A.E.; Ozair, A.; Bamashmos, A.S.; Li, H.; Bosler, D.S.; Yeaney, G.; Ali, A.; Peereboom, D.M.; Lathia, J.D.; Ahluwalia, M.S. MGMT Methylation and Differential Survival Impact by Se in Glioblastoma. Cancers 2024, 16, 1374. [Google Scholar] [CrossRef]
- Sipos, T.C.; Kövecsi, A.; Ovidiu-Ioan, Ș.; Zsuzsánna, P. General clinico-pathological characteristics in glioblastomas in correlation with p53 and ki67. Medicina 2023, 59, 1918. [Google Scholar] [CrossRef]
- World Health Organization Classification of Tumours Editorial Board. Central nervous system tumours. In WHO Classification of Tumours, 5th ed.; International Agency for Research on Cancer: Lyon, France, 2021; Volume 6. [Google Scholar]
- Wen, P.Y.; van den Bent, M.; Youssef, G.; Cloughesy, T.F.; Ellingson, B.M.; Weller, M.; Galanis, E.; Barboriak, D.P.; de Groot, J.; Gilbert, M.R.; et al. Rano 2.0: Update to the response assessment in neuro-oncology criteria for high- and low-grade gliomas in adults. J. Clin. Oncol. 2023, 41, 5187–5199. [Google Scholar] [CrossRef]
- Ghaferi, A.A.; Schwartz, T.A.; Pawlik, T.M. Strobe reporting guidelines for observational studies. JAMA Surg. 2021, 156, 577–578. [Google Scholar] [CrossRef]
- Rodriguez-Lozano, D.C.; Pina Medina, A.G.; Hansberg-Pastor, V.; Bello-Alvarez, C.; Camacho-Arroyo, I. Testoterone promotes glioblastoma cell proliferation, migration, and invasion through androgen receptor activation. Front. Endocrinol. 2019, 10, 16. [Google Scholar] [CrossRef]
- Tian, M.; Ma, W.; Chen, Y.; Yu, Y.; Zhu, D.; Shi, J.; Zhang, Y. Impact of gender on the survival of patients with glioblastoma. Biosci. Rep. 2018, 38, BSR20180752. [Google Scholar] [CrossRef]
- Rubin, J.B. Gender and sex interactions are intrinsic components of cancer phenotypes. Nat. Rev. Cancer, 2025; online ahead of print. [Google Scholar] [CrossRef]
- Baro, V.; Cerretti, G.; Todoverto, M.; Della Puppa, A.; Chioffi, F.; Volpin, F.; Causin, F.; Busato, F.; Fiduccia, P.; Landi, A.; et al. Newly diagnosed multifocal gbm: A monocentric experience and literature review. Curr. Oncol. 2022, 29, 3472–3488. [Google Scholar] [CrossRef] [PubMed]
- Talasila, K.M.; Soentgerath, A.; Euskirchen, P.; Rosland, G.V.; Wang, J.; Huszthy, P.C.; Prestegarden, L.; Skaftnesmo, K.O.; Sakariassen, P.; Eskilsson, E.; et al. Egfr wild-type amplification and activation promote invasion and development of glioblastoma independent of angiogenesis. Acta Neuropathol. 2013, 125, 683–698. [Google Scholar] [CrossRef] [PubMed]
- Bilello, M.; Akbari, H.; Da, X.; Pisapia, J.M.; Mohan, S.; Wolf, R.L.; O’Rourke, D.M.; Martinez-Lage, M.; Davatzikos, C. Population-based mri atlases of spatial distribution are specific to patient and tumor characteristics in glioblastoma. NeuroImage Clin. 2016, 12, 34–40. [Google Scholar] [CrossRef]
- Del Moral-Morales, A.; Gonzalez-Orozco, J.C.; Hernandez-Vega, A.L.; Hernández-Ortega, K.; Peña-Gutiérrez, K.M.; Camacho-Arroyo, I. EZH2 mediates proliferation, migration and invasion promoted by estradiol in human glioblastoma cells. Front. Endocrinol. 2022, 13, 703733. [Google Scholar] [CrossRef] [PubMed]
- Aleman, O.R.; Quintero, J.C.; Camacho-Arroyo, I. The language of gloiblastoma: A tale of cytokines and sex hormones communication. Neuro-Oncol. Adv. 2025, 7, vadaf017. [Google Scholar] [CrossRef]
- Zawlik, I.; Vaccarella, S.; Kita, D.; Mittelbronn, M.; Franceschi, S.; Ohgaki, H. Promoter methylation and polymorphisms of the mgmt gene in glioblastomas: A population-based study. Neuroepidemiology 2009, 32, 21–29. [Google Scholar] [CrossRef]
- Rhome, R.; Fisher, R.; Hormigo, A.; Parikh, R.R. Disparities in receipt of modern concurrent chemoradiotherapy in glioblastoma. J. Neuro-Oncol. 2016, 128, 241–250. [Google Scholar] [CrossRef]
- Aneja, S.; Khullar, D.; Yu, J.B. The influence of regional health system characteristics on the surgical management and receipt of post operative radiation therapy for glioblastoma multiforme. J. Neuro-Oncol. 2013, 112, 393–401. [Google Scholar] [CrossRef]
- Sherwood, P.R.; Dahman, B.A.; Donovan, H.S.; Mintz, A.; Given, C.W.; Bradley, C.J. Treatment disparities following the diagnosis of an astrocytoma. J. Neuro-Oncol. 2011, 101, 67–74. [Google Scholar] [CrossRef]
- Le Rhun, E.; Weller, M. Sex-specific aspects of epidemiology, molecular genetics and outcome: Primary brain tumours. ESMO Open 2020, 5, e001034. [Google Scholar] [CrossRef]
- Vera, R.; Juan-Vidal, O.; Safont-Aguilera, M.J.; de la Peña, F.A.; Del Alba, A.G. Sex differences in the diagnosis, treatment and prognosis of cancer: The rationale for an individualised approach. Clin. Transl. Oncol. 2023, 25, 2069–2076. [Google Scholar] [CrossRef] [PubMed]
- Cabral, M.; Dillender, M. Disparities in health care and medical evaluations by gender: A review of evidence and mechanisms: Gender disparities: Evidence on causes and implications. AEA Pap. Proc. Am. Econ. Assoc. 2021, 111, 159–163. [Google Scholar]
- Schiebinger, L. Women’s health and clinical trials. J. Clin. Investig. 2003, 112, 973–977. [Google Scholar] [PubMed][Green Version]
- Thunander Sundbom, L.; Bingefors, K.; Hedborg, K.; Isacson, D. Are men under-treated and women over-treated with antidepressants? Findings from a cross-sectional survey in Sweden. BJPsych Bull. 2017, 41, 145–150. [Google Scholar] [CrossRef]
- Stabellini, N.; Krebs, H.; Patil, N.; Waite, K.; Barnholtz-Sloan, J.S. Sex differences in time to treat and outcomes for gliomas. Front. Oncol. 2021, 11, 630597. [Google Scholar] [CrossRef]
- Pan, I.W.; Ferguson, S.D.; Lam, S. Patient and treatment factors associated with survival among adult glioblastoma patients: A USA population-based study from 2000–2010. J. Clin. Neurosci. 2015, 22, 1575–1581. [Google Scholar] [CrossRef]
- Tewari, S.; Tom, M.C.; Park, D.Y.J.; Wei, W.; Chao, S.T.; Yu, J.S.; Suh, J.H.; Kilic, S.; Peereboom, D.M.; Stevens, G.H.J.; et al. Sex-Specific Differences in Low-Grade Glioma Presentation and Outcome. Int. J. Radiat. Oncol. Biol. Phys. 2022, 114, 283–292. [Google Scholar] [CrossRef]
| Female (%) | Male (%) | All (%) | p Value | |
|---|---|---|---|---|
| Age, years (IQR) | 60 (45–71) | 61 (42–71) | 61 (43–71) | 0.55 |
| Gliomas | 81 (43) | 108 (57) | 189 | 0.18 |
| Glioblastoma | 49 (39) | 76 (61) | 125 | 0.17 |
| Astrocytoma | 22 (46) | 26 (54) | 48 | 0.74 |
| Oligodendroglioma | 10 (62.5) | 6 (37.5) | 16 | 0.12 |
| Glioblastoma | ||||
| Multifocality | 14 (28.5) | 8 (10.5) | 22 (17.5) | 0.01 |
| Molecular profile | ||||
| IDH1 wt | 45 (98) | 70 (97) | 115 (97.5) | 0.83 |
| MGMT unmethylated | 28 (62) | 51 (72) | 69 (68) | 0.28 |
| TERT mutation | 26 (84) | 34 (79) | 60 (81) | 0.6 |
| EGFR amplification | 20 (52.5) | 14 (25.5) | 34 (36.5) | <0.01 |
| ATRX expression | 22 (100) | 42 (87) | 64 (91.5) | 0.08 |
| Chemotherapy | 31 (63) | 60 (80) | 91 (73.5) | 0.04 |
| Radioteraphy | 33 (67) | 63 (84) | 96 (77.5) | 0.03 |
| TTF | 5 (10) | 11 (14.5) | 16 (13) | 0.48 |
| Biopsies | 15 (33.5) | 14 (19.5) | 29 (25) | 0.2 |
| Surgery | 46 (94) | 73 (96) | 119 (95) | 0.58 |
| Re-surgery | 7 (17) | 14 (23) | 21 (20.5) | 0.5 |
| Epilepsy | 19 (39.5) | 37 (50) | 56 (46) | 0.15 |
| MRI progression | 28 (61) | 51 (71) | 79 (67) | 0.25 |
| Mortality | 38 (78) | 65 (85) | 103 (82.5) | 0.25 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prosperetti, C.; Yenigün, M.; Pagnamenta, A.; Tabaee Damavandi, P.; Disanto, G.; Marchi, F.; Espeli, V.; Muoio, B.; Spina, P.; Pesce, G.; et al. Sex-Related Differences in Glioblastoma: A Single-Center Retrospective Cohort Study. Biomedicines 2025, 13, 1715. https://doi.org/10.3390/biomedicines13071715
Prosperetti C, Yenigün M, Pagnamenta A, Tabaee Damavandi P, Disanto G, Marchi F, Espeli V, Muoio B, Spina P, Pesce G, et al. Sex-Related Differences in Glioblastoma: A Single-Center Retrospective Cohort Study. Biomedicines. 2025; 13(7):1715. https://doi.org/10.3390/biomedicines13071715
Chicago/Turabian StyleProsperetti, Chiara, Meltem Yenigün, Alberto Pagnamenta, Payam Tabaee Damavandi, Giulio Disanto, Francesco Marchi, Vittoria Espeli, Barbara Muoio, Paolo Spina, Gianfranco Pesce, and et al. 2025. "Sex-Related Differences in Glioblastoma: A Single-Center Retrospective Cohort Study" Biomedicines 13, no. 7: 1715. https://doi.org/10.3390/biomedicines13071715
APA StyleProsperetti, C., Yenigün, M., Pagnamenta, A., Tabaee Damavandi, P., Disanto, G., Marchi, F., Espeli, V., Muoio, B., Spina, P., Pesce, G., & Agazzi, P. (2025). Sex-Related Differences in Glioblastoma: A Single-Center Retrospective Cohort Study. Biomedicines, 13(7), 1715. https://doi.org/10.3390/biomedicines13071715






