Translating Basic Science to Clinical Applications: A Narrative Review of Repurposed Pharmacological Agents in Preclinical Models of Diabetic Neuropathy
Abstract
1. Introduction
2. Materials and Methods
3. Pathogenesis of DN
3.1. Metabolic Dysfunction and Microvascular Damage
3.2. Oxidative Stress
3.3. Neuroinflammation
3.4. Ion Channel Modulation and Neuronal Hyperexcitability
3.5. Mitochondrial Dysfunction
3.6. Central Sensitization
4. Substances with Potential Effectiveness in Treating DN
5. Discussion
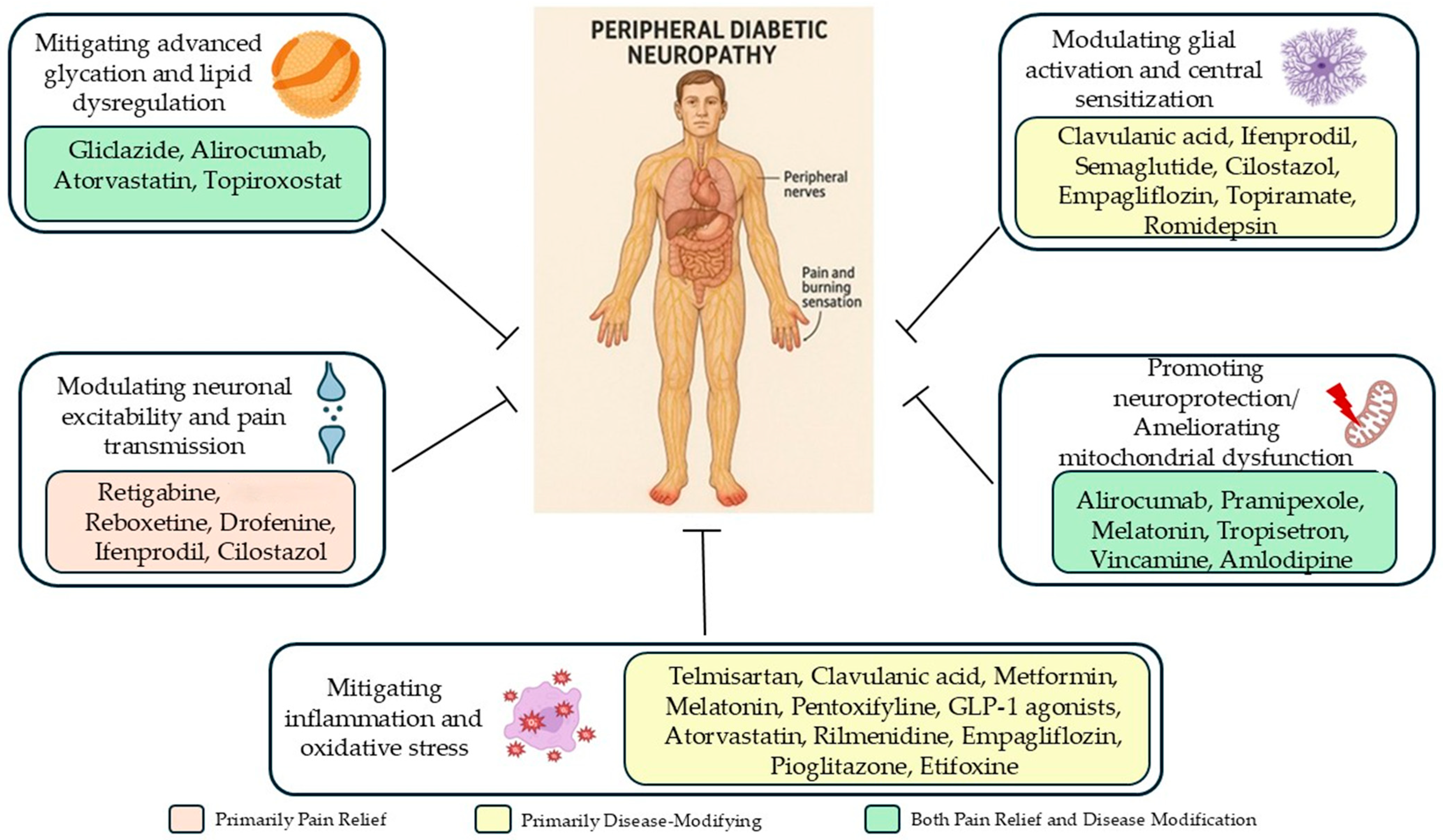
5.1. Modulating Neuronal Hyperexcitability
5.2. Mitigating Inflammation and Oxidative Stress
5.3. Promoting Neuroprotection, Remyelination, and Mitochondrial Function
5.4. Modulating Glial Activation and Central Sensitization
5.5. Mitigating Advanced Glycation and Metabolic Dysregulation
5.6. Multi-Targeted Approaches
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feldman, E.L.; Callaghan, B.C.; Pop-Busui, R.; Zochodne, D.W.; Wright, D.E.; Bennett, D.L.; Bril, V.; Russell, J.W.; Viswanathan, V. Diabetic Neuropathy. Nat. Rev. Dis. Prim. 2019, 5, 41. [Google Scholar] [CrossRef] [PubMed]
- Terminology|International Association for the Study of Pain. Available online: https://www.iasp-pain.org/resources/terminology/ (accessed on 17 October 2022).
- Colloca, L.; Ludman, T.; Bouhassira, D.; Baron, R.; Dickenson, A.H.; Yarnitsky, D.; Freeman, R.; Truini, A.; Attal, N.; Finnerup, N.B.; et al. Neuropathic Pain. Nat. Rev. Dis. Prim. 2017, 3, 17002. [Google Scholar] [CrossRef]
- Hicks, C.W.; Selvin, E. Epidemiology of Peripheral Neuropathy and Lower Extremity Disease in Diabetes. Curr. Diab. Rep. 2019, 19, 86. [Google Scholar] [CrossRef]
- Bates, D.; Schultheis, B.C.; Hanes, M.C.; Jolly, S.M.; Chakravarthy, K.V.; Deer, T.R.; Levy, R.M.; Hunter, C.W. A Comprehensive Algorithm for Management of Neuropathic Pain. Pain Med. 2019, 20 (Suppl. 1), S2–S12. [Google Scholar] [CrossRef] [PubMed]
- Attal, N. Pharmacological Treatments of Neuropathic Pain: The Latest Recommendations. Rev. Neurol. 2019, 175, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Moisset, X.; Bouhassira, D.; Avez Couturier, J.; Alchaar, H.; Conradi, S.; Delmotte, M.H.; Lanteri-Minet, M.; Lefaucheur, J.P.; Mick, G.; Piano, V.; et al. Pharmacological and Non-Pharmacological Treatments for Neuropathic Pain: Systematic Review and French Recommendations. Rev. Neurol. 2020, 176, 325–352. [Google Scholar] [CrossRef]
- Hennemann-Krause, L.; Sredni, S. Systemic Drug Therapy for Neuropathic Pain. Rev. Dor 2016, 17, 91–94. [Google Scholar] [CrossRef]
- Rowbotham, M.C. Tricyclic Antidepressants and Opioids-Better Together? Pain 2015, 156, 1373–1374. [Google Scholar] [CrossRef]
- Shinu, P.; Morsy, M.A.; Nair, A.B.; Al Mouslem, A.K.; Venugopala, K.N.; Goyal, M.; Bansal, M.; Jacob, S.; Deb, P.K. Novel Therapies for the Treatment of Neuropathic Pain: Potential and Pitfalls. J. Clin. Med. 2022, 11, 3002. [Google Scholar] [CrossRef]
- Kopsky, D.J.; Keppel Hesselink, J.M. High Doses of Topical Amitriptyline in Neuropathic Pain: Two Cases and Literature Review. Pain Pract. 2012, 12, 148–153. [Google Scholar] [CrossRef]
- Gabriel, M.; Sharma, V. Antidepressant Discontinuation Syndrome. CMAJ 2017, 189, E747. [Google Scholar] [CrossRef] [PubMed]
- Maloney, J.; Pew, S.; Wie, C.; Gupta, R.; Freeman, J.; Strand, N. Comprehensive Review of Topical Analgesics for Chronic Pain. Curr. Pain Headache Rep. 2021, 25, 7. [Google Scholar] [CrossRef]
- Attal, N.; de Andrade, D.C.; Adam, F.; Ranoux, D.; Teixeira, M.J.; Galhardoni, R.; Raicher, I.; Üçeyler, N.; Sommer, C.; Bouhassira, D. Safety and Efficacy of Repeated Injections of Botulinum Toxin A in Peripheral Neuropathic Pain (BOTNEP): A Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Neurol. 2016, 15, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Finnerup, N.B.; Attal, N.; Haroutounian, S.; McNicol, E.; Baron, R.; Dworkin, R.H.; Gilron, I.; Haanpää, M.; Hansson, P.; Jensen, T.S.; et al. Pharmacotherapy for Neuropathic Pain in Adults: A Systematic Review and Meta-Analysis. Lancet Neurol. 2015, 14, 162–173. [Google Scholar] [CrossRef]
- Cao, X.J.; Wu, R.; Qian, H.Y.; Chen, X.; Zhu, H.Y.; Xu, G.Y.; Sun, Y.Z.; Zhang, P.A. Metformin Attenuates Diabetic Neuropathic Pain via AMPK/NF-ΚB Signaling Pathway in Dorsal Root Ganglion of Diabetic Rats. Brain Res. 2021, 1772, 147663. [Google Scholar] [CrossRef] [PubMed]
- Yalçın, M.B.; Bora, E.S.; Çakır, A.; Akbulut, S.; Erbaş, O. Autophagy and Anti-Inflammation Ameliorate Diabetic Neuropathy with Rilmenidine. Acta Cirúrgica Bras. 2023, 38, e387823. [Google Scholar] [CrossRef]
- Attia, M.A.; Soliman, N.; Eladl, M.A.; Bilasy, S.E.; El-Abaseri, T.B.; Ali, H.S.; Abbas, F.; Ibrahim, D.; Osman, N.M.S.; Hashish, A.A.; et al. Topiramate Affords Neuroprotection in Diabetic Neuropathy Model via Downregulating Spinal GFAP/Inflammatory Burden and Improving Neurofilament Production. Toxicol. Mech. Methods 2023, 33, 563–577. [Google Scholar] [CrossRef]
- Yang, Y.; Zhao, B.; Wang, Y.; Lan, H.; Liu, X.; Hu, Y.; Cao, P. Diabetic Neuropathy: Cutting-Edge Research and Future Directions. Signal Transduct. Target. Ther. 2025, 10, 132. [Google Scholar] [CrossRef]
- Tesfaye, S.; Boulton, A.J.M.; Dickenson, A.H. Mechanisms and Management of Diabetic Painful Distal Symmetrical Polyneuropathy. Diabetes Care 2013, 36, 2456–2465. [Google Scholar] [CrossRef]
- Feldman, E.L.; Nave, K.A.; Jensen, T.S.; Bennett, D.L.H. New Horizons in Diabetic Neuropathy: Mechanisms, Bioenergetics, and Pain. Neuron 2017, 93, 1296–1313. [Google Scholar] [CrossRef]
- Rosenberger, D.C.; Blechschmidt, V.; Timmerman, H.; Wolff, A.; Treede, R.-D. Challenges of Neuropathic Pain: Focus on Diabetic Neuropathy. J. Neural Transm. 2020, 127, 589–624. [Google Scholar] [CrossRef] [PubMed]
- Thrainsdottir, S.; Malik, R.A.; Dahlin, L.B.; Wiksell, P.; Eriksson, K.F.; Rosén, I.; Petersson, J.; Greene, D.A.; Sundkvist, G. Endoneurial Capillary Abnormalities Presage Deterioration of Glucose Tolerance and Accompany Peripheral Neuropathy in Man. Diabetes 2003, 52, 2615–2622. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.E.; Williams, P.E. Perineurial Cell Basement Membrane Thickening and Myelinated Nerve Fibre Loss in Diabetic and Nondiabetic Peripheral Nerve. J. Neurol. Sci. 2004, 217, 157–163. [Google Scholar] [CrossRef]
- Nukada, H. Ischemia and Diabetic Neuropathy. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2014; pp. 469–487. [Google Scholar]
- Cameron, N.E.; Eaton, S.E.M.; Cotter, M.A.; Tesfaye, S. Vascular Factors and Metabolic Interactions in the Pathogenesis of Diabetic Neuropathy. Diabetologia 2001, 44, 1973–1988. [Google Scholar] [CrossRef]
- Mizukami, H.; Osonoi, S. Collateral Glucose-Utlizing Pathwaya in Diabetic Polyneuropathy. Int. J. Mol. Sci. 2020, 22, 94. [Google Scholar] [CrossRef]
- Bansal, D.; Badhan, Y.; Gudala, K.; Schifano, F. Ruboxistaurin for the Treatment of Diabetic Peripheral Neuropathy: A Systematic Review of Randomized Clinical Trials. Diabetes Metab. J. 2013, 37, 375. [Google Scholar] [CrossRef] [PubMed]
- Coppey, L.J.; Davidson, E.P.; Rinehart, T.W.; Gellett, J.S.; Oltman, C.L.; Lund, D.D.; Yorek, M.A. ACE Inhibitor or Angiotensin II Receptor Antagonist Attenuates Diabetic Neuropathy in Streptozotocin-Induced Diabetic Rats. Diabetes 2006, 55, 341–348. [Google Scholar] [CrossRef]
- Yorek, M.A. Vascular Impairment of Epineurial Arterioles of the Sciatic Nerve: Implications for Diabetic Peripheral Neuropathy. Rev. Diabet. Stud. 2015, 12, 13–28. [Google Scholar] [CrossRef]
- Shillo, P.R.; Selvarajah, D.; Greig, M.; Rao, G.D.; Edden, R.A.E.; Wilkinson, I.D.; Tesfaye, S. Diabetes UK Type 2 Diabetes Research Award. Diabet. Med. 2016, 33, 15–16. [Google Scholar] [CrossRef]
- Dewanjee, S.; Das, S.; Das, A.K.; Bhattacharjee, N.; Dihingia, A.; Dua, T.K.; Kalita, J.; Manna, P. Molecular Mechanism of Diabetic Neuropathy and Its Pharmacotherapeutic Targets. Eur. J. Pharmacol. 2018, 833, 472–523. [Google Scholar] [CrossRef]
- Chen, J.-L.; Lu, J.-H.; Xie, C.-S.; Shen, Y.-J.; Wang, J.-W.; Ye, X.-Y.; Zhang, M.-B.; Jia, G.-L.; Tao, Y.-X.; Li, J.; et al. Caveolin-1 in Spinal Cord Modulates Type-2 Diabetic Neuropathic Pain through the Rac1/NOX2/NR2B Signaling Pathway. Am. J. Transl. Res. 2020, 12, 1714–1727. [Google Scholar] [CrossRef]
- Eid, S.A.; El Massry, M.; Hichor, M.; Haddad, M.; Grenier, J.; Dia, B.; Barakat, R.; Boutary, S.; Chanal, J.; Aractingi, S.; et al. Targeting the NADPH Oxidase-4 and Liver X Receptor Pathway Preserves Schwann Cell Integrity in Diabetic Mice. Diabetes 2020, 69, 448–464. [Google Scholar] [CrossRef]
- Giacco, F.; Brownlee, M. Oxidative Stress and Diabetic Complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [PubMed]
- Fernyhough, P.; Roy Chowdhury, S.K.; Schmidt, R.E. Mitochondrial Stress and the Pathogenesis of Diabetic Neuropathy. Expert Rev. Endocrinol. Metab. 2010, 5, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Vincent, A.M.; Hayes, J.M.; McLean, L.L.; Vivekanandan-Giri, A.; Pennathur, S.; Feldman, E.L. Dyslipidemia-Induced Neuropathy in Mice. Diabetes 2009, 58, 2376–2385. [Google Scholar] [CrossRef] [PubMed]
- Tesco, G.; Lomoio, S. Pathophysiology of Neurodegenerative Diseases: An Interplay among Axonal Transport Failure, Oxidative Stress, and Inflammation? Semin. Immunol. 2022, 59, 101628. [Google Scholar] [CrossRef]
- Vincent, A.M.; Kato, K.; McLean, L.L.; Soules, M.E.; Feldman, E.L. Sensory Neurons and Schwann Cells Respond to Oxidative Stress by Increasing Antioxidant Defense Mechanisms. Antioxid. Redox Signal. 2009, 11, 425–438. [Google Scholar] [CrossRef]
- Hinder, L.M.; Figueroa-Romero, C.; Pacut, C.; Hong, Y.; Vivekanandan-Giri, A.; Pennathur, S.; Feldman, E.L. Long-Chain Acyl Coenzyme A Synthetase 1 Overexpression in Primary Cultured Schwann Cells Prevents Long Chain Fatty Acid-Induced Oxidative Stress and Mitochondrial Dysfunction. Antioxid. Redox Signal. 2014, 21, 588–600. [Google Scholar] [CrossRef]
- Xu, J.; Wu, S.; Wang, J.; Wang, J.; Yan, Y.; Zhu, M.; Zhang, D.; Jiang, C.; Liu, T. Oxidative Stress Induced by NOX2 Contributes to Neuropathic Pain via Plasma Membrane Translocation of PKCε in Rat Dorsal Root Ganglion Neurons. J. Neuroinflamm. 2021, 18, 106. [Google Scholar] [CrossRef]
- Jiao, Y.; Zhang, Y.; Wang, C.; Yu, Y.; Li, Y.; Cui, W.; Li, Q.; Yu, Y. MicroRNA-7a-5p Ameliorates Diabetic Peripheral Neuropathy by Regulating VDAC1/JNK/C-JUN Pathway. Diabet. Med. 2023, 40, e14890. [Google Scholar] [CrossRef]
- Zhu, J.; Hu, Z.; Luo, Y.; Liu, Y.; Luo, W.; Du, X.; Luo, Z.; Hu, J.; Peng, S. Diabetic Peripheral Neuropathy: Pathogenetic Mechanisms and Treatment. Front. Endocrinol. 2024, 14, 1265372. [Google Scholar] [CrossRef] [PubMed]
- Nowicki, M.; Müller, K.; Serke, H.; Kosacka, J.; Vilser, C.; Ricken, A.; Spanel-Borowski, K. Oxidized Low-Density Lipoprotein (OxLDL)-Induced Cell Death in Dorsal Root Ganglion Cell Cultures Depends Not on the Lectin-like OxLDL Receptor-1 but on the Toll-like Receptor-4. J. Neurosci. Res. 2010, 88, 403–412. [Google Scholar] [CrossRef]
- Vincent, A.M.; Perrone, L.; Sullivan, K.A.; Backus, C.; Sastry, A.M.; Lastoskie, C.; Feldman, E.L. Receptor for Advanced Glycation End Products Activation Injures Primary Sensory Neurons via Oxidative Stress. Endocrinology 2007, 148, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Keller, J.N.; Hanni, K.B.; Markesbery, W.R. Oxidized Low-Density Lipoprotein Induces Neuronal Death: Implications for Calcium, Reactive Oxygen Species, and Caspases. J. Neurochem. 1999, 72, 2601–2609. [Google Scholar] [CrossRef]
- Cotter, M.A.; Cameron, N.E. Effect of the NAD(P)H Oxidase Inhibitor, Apocynin, on Peripheral Nerve Perfusion and Function in Diabetic Rats. Life Sci. 2003, 73, 1813–1824. [Google Scholar] [CrossRef] [PubMed]
- Herder, C.; Kannenberg, J.M.; Huth, C.; Carstensen-Kirberg, M.; Rathmann, W.; Koenig, W.; Heier, M.; Püttgen, S.; Thorand, B.; Peters, A.; et al. Proinflammatory Cytokines Predict the Incidence and Progression of Distal Sensorimotor Polyneuropathy: KORA F4/FF4 Study. Diabetes Care 2017, 40, 569–576. [Google Scholar] [CrossRef]
- Ji, R.-R.; Xu, Z.-Z.; Gao, Y.-J. Emerging Targets in Neuroinflammation-Driven Chronic Pain. Nat. Rev. Drug Discov. 2014, 13, 533–548. [Google Scholar] [CrossRef]
- Tang, W.; Lv, Q.; Chen, X.; Zou, J.; Liu, Z.; Shi, Y. CD8 + T Cell-Mediated Cytotoxicity toward Schwann Cells Promotes Diabetic Peripheral Neuropathy. Cell. Physiol. Biochem. 2013, 32, 827–837. [Google Scholar] [CrossRef]
- Vydyanathan, A.; Wu, Z.Z.; Chen, S.R.; Pan, H.L. A-Type Voltage-Gated K+ Currents Influence Firing Properties of Isolectin B4-Positive but Not Isolectin B4-Negative Primary Sensory Neurons. J. Neurophysiol. 2005, 93, 3401–3409. [Google Scholar] [CrossRef]
- Wang, Q.; Xie, Y.; Ma, S.; Luo, H.; Qiu, Y. Role of Microglia in Diabetic Neuropathic Pain. Front. Cell Dev. Biol. 2024, 12, 1421191. [Google Scholar] [CrossRef]
- Gao, Y.-J.; Zhang, L.; Samad, O.A.; Suter, M.R.; Yasuhiko, K.; Xu, Z.-Z.; Park, J.-Y.; Lind, A.-L.; Ma, Q.; Ji, R.-R. JNK-Induced MCP-1 Production in Spinal Cord Astrocytes Contributes to Central Sensitization and Neuropathic Pain. J. Neurosci. 2009, 29, 4096–4108. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Liu, X.; Xiong, H.; Wang, W.; Liu, Y.; Yin, L.; Tu, C.; Wang, H.; Xiang, X.; Xu, J.; et al. CXCL13/CXCR5 Signaling Contributes to Diabetes-Induced Tactile Allodynia via Activating PERK, PSTAT3, PAKT Pathways and pro-Inflammatory Cytokines Production in the Spinal Cord of Male Mice. Brain Behav. Immun. 2019, 80, 711–724. [Google Scholar] [CrossRef] [PubMed]
- Middleton, S.J.; Barry, A.M.; Comini, M.; Li, Y.; Ray, P.R.; Shiers, S.; Themistocleous, A.C.; Uhelski, M.L.; Yang, X.; Dougherty, P.M.; et al. Studying Human Nociceptors: From Fundamentals to Clinic. Brain 2021, 144, 1312–1335. [Google Scholar] [CrossRef]
- Craner, M.J.; Klein, J.P.; Renganathan, M.; Black, J.A.; Waxman, S.G. Changes of Sodium Channel Expression in Experimental Painful Diabetic Neuropathy. Ann. Neurol. 2002, 52, 786–792. [Google Scholar] [CrossRef]
- Hoeijmakers, J.G.J.; Faber, C.G.; Merkies, I.S.J.; Waxman, S.G. Channelopathies, Painful Neuropathy, and Diabetes: Which Way Does the Causal Arrow Point? Trends Mol. Med. 2014, 20, 544–550. [Google Scholar] [CrossRef]
- Andersen, S.T.; Witte, D.R.; Dalsgaard, E.-M.; Andersen, H.; Nawroth, P.; Fleming, T.; Jensen, T.M.; Finnerup, N.B.; Jensen, T.S.; Lauritzen, T.; et al. Risk Factors for Incident Diabetic Polyneuropathy in a Cohort With Screen-Detected Type 2 Diabetes Followed for 13 Years: ADDITION-Denmark. Diabetes Care 2018, 41, 1068–1075. [Google Scholar] [CrossRef] [PubMed]
- Bierhaus, A.; Fleming, T.; Stoyanov, S.; Leffler, A.; Babes, A.; Neacsu, C.; Sauer, S.K.; Eberhardt, M.; Schnölzer, M.; Lasitschka, F.; et al. Methylglyoxal Modification of Nav1.8 Facilitates Nociceptive Neuron Firing and Causes Hyperalgesia in Diabetic Neuropathy. Nat. Med. 2012, 18, 926–933. [Google Scholar] [CrossRef]
- Andersson, D.A.; Gentry, C.; Light, E.; Vastani, N.; Vallortigara, J.; Bierhaus, A.; Fleming, T.; Bevan, S. Methylglyoxal Evokes Pain by Stimulating TRPA1. PLoS ONE 2013, 8, e77986. [Google Scholar] [CrossRef]
- Düll, M.M.; Riegel, K.; Tappenbeck, J.; Ries, V.; Strupf, M.; Fleming, T.; Sauer, S.K.; Namer, B. Methylglyoxal Causes Pain and Hyperalgesia in Human through C-Fiber Activation. Pain 2019, 160, 2497–2507. [Google Scholar] [CrossRef]
- Hong, S.; Agresta, L.; Guo, C.; Wiley, J.W. The TRPV1 Receptor Is Associated with Preferential Stress in Large Dorsal Root Ganglion Neurons in Early Diabetic Sensory Neuropathy. J. Neurochem. 2008, 105, 1212–1222. [Google Scholar] [CrossRef]
- Jagodic, M.M.; Pathirathna, S.; Nelson, M.T.; Mancuso, S.; Joksovic, P.M.; Rosenberg, E.R.; Bayliss, D.A.; Jevtovic-Todorovic, V.; Todorovic, S.M. Cell-Specific Alterations of T-Type Calcium Current in Painful Diabetic Neuropathy Enhance Excitability of Sensory Neurons. J. Neurosci. 2007, 27, 3305–3316. [Google Scholar] [CrossRef] [PubMed]
- Tomiyama, M.; Furusawa, K.; Kamijo, M.; Kimura, T.; Matsunaga, M.; Baba, M. Upregulation of MRNAs Coding for AMPA and NMDA Receptor Subunits and Metabotropic Glutamate Receptors in the Dorsal Horn of the Spinal Cord in a Rat Model of Diabetes Mellitus. Mol. Brain Res. 2005, 136, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, J.M.; dos Santos, G.G.; Neves, A.F.; Athie, M.C.P.; Bonet, I.J.M.; Nishijima, C.M.; Farias, F.H.; Figueiredo, J.G.; Hernandez-Olmos, V.; Alshaibani, S.; et al. Diabetes-Induced Neuropathic Mechanical Hyperalgesia Depends on P2X4 Receptor Activation in Dorsal Root Ganglia. Neuroscience 2019, 398, 158–170. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Li, L.; Liu, H.; Li, H.; Liu, Z.; Li, Z. KCNQ2/3/5 Channels in Dorsal Root Ganglion Neurons Can Be Therapeutic Targets of Neuropathic Pain in Diabetic Rats. Mol. Pain 2018, 14, 1744806918793229. [Google Scholar] [CrossRef]
- Rumora, A.E.; Savelieff, M.G.; Sakowski, S.A.; Feldman, E.L. Disorders of Mitochondrial Dynamics in Peripheral Neuropathy: Clues from Hereditary Neuropathy and Diabetes. Int. Rev. Neurobiol. 2019, 145, 127–176. [Google Scholar]
- Fernyhough, P. Mitochondrial Dysfunction in Diabetic Neuropathy: A Series of Unfortunate Metabolic Events. Curr. Diab. Rep. 2015, 15, 89. [Google Scholar] [CrossRef]
- Russell, J.W.; Golovoy, D.; Vincent, A.M.; Mahendru, P.; Olzmann, J.A.; Mentzer, A.; Feldman, E.L. High Glucose-induced Oxidative Stress and Mitochondrial Dysfunction in Neurons. FASEB J. 2002, 16, 1738–1748. [Google Scholar] [CrossRef]
- Chowdhury, S.K.R.; Zherebitskaya, E.; Smith, D.R.; Akude, E.; Chattopadhyay, S.; Jolivalt, C.G.; Calcutt, N.A.; Fernyhough, P. Mitochondrial Respiratory Chain Dysfunction in Dorsal Root Ganglia of Streptozotocin-Induced Diabetic Rats and Its Correction by Insulin Treatment. Diabetes 2010, 59, 1082–1091. [Google Scholar] [CrossRef]
- Rumora, A.E.; Lentz, S.I.; Hinder, L.M.; Jackson, S.W.; Valesano, A.; Levinson, G.E.; Feldman, E.L. Dyslipidemia Impairs Mitochondrial Trafficking and Function in Sensory Neurons. FASEB J. 2018, 32, 195–207. [Google Scholar] [CrossRef]
- Viader, A.; Sasaki, Y.; Kim, S.; Strickland, A.; Workman, C.S.; Yang, K.; Gross, R.W.; Milbrandt, J. Aberrant Schwann Cell Lipid Metabolism Linked to Mitochondrial Deficits Leads to Axon Degeneration and Neuropathy. Neuron 2013, 77, 886–898. [Google Scholar] [CrossRef]
- Padilla, A.; Descorbeth, M.; Almeyda, A.L.; Payne, K.; De Leon, M. Hyperglycemia Magnifies Schwann Cell Dysfunction and Cell Death Triggered by PA-Induced Lipotoxicity. Brain Res. 2011, 1370, 64. [Google Scholar] [CrossRef] [PubMed]
- Legrand-Poels, S.; Esser, N.; L’Homme, L.; Scheen, A.; Paquot, N.; Piette, J. Free Fatty Acids as Modulators of the NLRP3 Inflammasome in Obesity/Type 2 Diabetes. Biochem. Pharmacol. 2014, 92, 131–141. [Google Scholar] [CrossRef]
- Xu, X.; Chen, H.; Ling, B.-Y.; Xu, L.; Cao, H.; Zhang, Y.-Q. Extracellular Signal-Regulated Protein Kinase Activation in Spinal Cord Contributes to Pain Hypersensitivity in a Mouse Model of Type 2 Diabetes. Neurosci. Bull. 2014, 30, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Shillo, P.; Yiangou, Y.; Donatien, P.; Greig, M.; Selvarajah, D.; Wilkinson, I.D.; Anand, P.; Tesfaye, S. Nerve and Vascular Biomarkers in Skin Biopsies Differentiate Painful From Painless Peripheral Neuropathy in Type 2 Diabetes. Front. Pain Res. 2021, 2, 731658. [Google Scholar] [CrossRef]
- Selvarajah, D.; Wilkinson, I.D.; Fang, F.; Sankar, A.; Davies, J.; Boland, E.; Harding, J.; Rao, G.; Gandhi, R.; Tracey, I.; et al. Structural and Functional Abnormalities of the Primary Somatosensory Cortex in Diabetic Peripheral Neuropathy: A Multimodal MRI Study. Diabetes 2019, 68, 796–806. [Google Scholar] [CrossRef]
- Teh, K.; Wilkinson, I.D.; Heiberg-Gibbons, F.; Awadh, M.; Kelsall, A.; Pallai, S.; Sloan, G.; Tesfaye, S.; Selvarajah, D. Somatosensory Network Functional Connectivity Differentiates Clinical Pain Phenotypes in Diabetic Neuropathy. Diabetologia 2021, 64, 1412–1421. [Google Scholar] [CrossRef] [PubMed]
- Segerdahl, A.R.; Themistocleous, A.C.; Fido, D.; Bennett, D.L.; Tracey, I. A Brain-Based Pain Facilitation Mechanism Contributes to Painful Diabetic Polyneuropathy. Brain 2018, 141, 357–364. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, B.; Zhu, L.; Hao, S. A Novel Improved Therapy Strategy for Diabetic Nephropathy. Organogenesis 2012, 8, 18–21. [Google Scholar] [CrossRef]
- Bhol, N.K.; Bhanjadeo, M.M.; Singh, A.K.; Dash, U.C.; Ojha, R.R.; Majhi, S.; Duttaroy, A.K.; Jena, A.B. The Interplay between Cytokines, Inflammation, and Antioxidants: Mechanistic Insights and Therapeutic Potentials of Various Antioxidants and Anti-Cytokine Compounds. Biomed. Pharmacother. 2024, 178, 117177. [Google Scholar] [CrossRef]
- Nashtahosseini, Z.; Eslami, M.; Paraandavaji, E.; Haraj, A.; Dowlat, B.F.; Hosseinzadeh, E.; Oksenych, V.; Naderian, R. Cytokine Signaling in Diabetic Neuropathy: A Key Player in Peripheral Nerve Damage. Biomedicines 2025, 13, 589. [Google Scholar] [CrossRef]
- Duzhyy, D.E.; Voitenko, N.V.; Belan, P.V. Peripheral Inflammation Results in Increased Excitability of Capsaicin-Insensitive Nociceptive DRG Neurons Mediated by Upregulation of ASICs and Voltage-Gated Ion Channels. Front. Cell. Neurosci. 2021, 15, 723295. [Google Scholar] [CrossRef] [PubMed]
- Maingret, F.; Coste, B.; Padilla, F.; Clerc, N.; Crest, M.; Korogod, S.M.; Delmas, P. Inflammatory Mediators Increase Nav1.9 Current and Excitability in Nociceptors through a Coincident Detection Mechanism. J. Gen. Physiol. 2008, 131, 211–225. [Google Scholar] [CrossRef]
- Wu, J.; Hu, H.; Li, X. Spinal Neuron-Glial Crosstalk and Ion Channel Dysregulation in Diabetic Neuropathic Pain. Front. Immunol. 2025, 16, 1480534. [Google Scholar] [CrossRef]
- Ma, J.; Yu, H.; Liu, J.; Chen, Y.; Wang, Q.; Xiang, L. Metformin Attenuates Hyperalgesia and Allodynia in Rats with Painful Diabetic Neuropathy Induced by Streptozotocin. Eur. J. Pharmacol. 2015, 764, 599–606. [Google Scholar] [CrossRef]
- Liu, F.; You, F.; Yang, L.; Wang, S.; Xie, D. Metformin Improves Diabetic Neuropathy by Reducing Inflammation through Up-Regulating the Expression of MiR-146a and Suppressing Oxidative Stress. J. Diabetes Complicat. 2024, 38, 108737. [Google Scholar] [CrossRef] [PubMed]
- Haddad, M.; Eid, S.; Harb, F.; Massry, M.E.L.; Azar, S.; Sauleau, E.-A.; Eid, A.A. Activation of 20-HETE Synthase Triggers Oxidative Injury and Peripheral Nerve Damage in Type 2 Diabetic Mice. J. Pain 2022, 23, 1371–1388. [Google Scholar] [CrossRef] [PubMed]
- Abdelkader, N.F.; Elbaset, M.A.; Moustafa, P.E.; Ibrahim, S.M. Empagliflozin Mitigates Type 2 Diabetes-Associated Peripheral Neuropathy: A Glucose-Independent Effect through AMPK Signaling. Arch. Pharm. Res. 2022, 45, 475–493. [Google Scholar] [CrossRef]
- Lee, S.-O.; Kuthati, Y.; Huang, W.-H.; Wong, C.-S. Semaglutide Ameliorates Diabetic Neuropathic Pain by Inhibiting Neuroinflammation in the Spinal Cord. Cells 2024, 13, 1857. [Google Scholar] [CrossRef]
- Akbarian, R.; Chamanara, M.; Rashidian, A.; Abdollahi, A.; Ejtemaei Mehr, S.; Dehpour, A.R. Atorvastatin Prevents the Development of Diabetic Neuropathic Nociception by Possible Involvement of Nitrergic System. J. Appl. Biomed. 2021, 19, 48–56. [Google Scholar] [CrossRef]
- McIver, L.A.; Siddique, M.S. Atorvastatin; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Cui, N.; Feng, Y.; Wang, M.; Lu, X.; Huang, Y.; Chen, Y.; Shi, X. Protective Effect of Alirocumab, a PCSK9 Inhibitor, on the Sciatic Nerve of Rats with Diabetic Peripheral Neuropathy. Endocr. J. 2024, 71, EJ23–EJ0359. [Google Scholar] [CrossRef]
- Alirocumab: Uses, Interactions, Mechanism of Action|DrugBank Online. Available online: https://go.drugbank.com/drugs/DB09302 (accessed on 18 June 2025).
- Pecikoza, U.; Tomić, M.; Nastić, K.; Micov, A.; Stepanović-Petrović, R. Synergism between Metformin and Analgesics/Vitamin B12 in a Model of Painful Diabetic Neuropathy. Biomed. Pharmacother. 2022, 153, 113441. [Google Scholar] [CrossRef]
- Duloxetine: Uses, Interactions, Mechanism of Action|DrugBank Online. Available online: https://go.drugbank.com/drugs/DB00476 (accessed on 18 June 2025).
- Vitamin B12: Uses, Interactions, Mechanism of Action|DrugBank Online. Available online: https://go.drugbank.com/drugs/DB00115 (accessed on 18 June 2025).
- Alkhudhayri, S.; Sajini, R.; Alharbi, B.; Qabbani, J.; Al-Hindi, Y.; Fairaq, A.; Yousef, A. Investigating the Beneficial Effect of Aliskiren in Attenuating Neuropathic Pain in Diabetic Sprague-Dawley Rats. Endocrinol. Diabetes Metab. 2021, 4, e00209. [Google Scholar] [CrossRef] [PubMed]
- Gliclazide: Uses, Interactions, Mechanism of Action|DrugBank Online. Available online: https://go.drugbank.com/drugs/DB01120 (accessed on 18 June 2025).
- Aliskiren: Uses, Interactions, Mechanism of Action|DrugBank Online. Available online: https://go.drugbank.com/drugs/DB09026 (accessed on 18 June 2025).
- Elkholy, S.E.; Elaidy, S.M.; El-Sherbeeny, N.A.; Toraih, E.A.; El-Gawly, H.W. Neuroprotective Effects of Ranolazine versus Pioglitazone in Experimental Diabetic Neuropathy: Targeting Nav1.7 Channels and PPAR-γ. Life Sci. 2020, 250, 117557. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Q.; Bai, Y.; Zheng, H.; Ji, L.; Zhu, X.; Sun, W.; Liu, X.; Zhang, S.; Li, Y.; et al. Glycogen Synthesis Kinase-3β Involves in the Analgesic Effect of Liraglutide on Diabetic Neuropathic Pain. J. Diabetes Complicat. 2023, 37, 108416. [Google Scholar] [CrossRef]
- Gateva, P.; Hristov, M.; Ivanova, N.; Vasileva, D.; Ivanova, A.; Sabit, Z.; Bogdanov, T.; Apostolova, S.; Tzoneva, R. Antinociceptive Behavior, Glutamine/Glutamate, and Neopterin in Early-Stage Streptozotocin-Induced Diabetic Neuropathy in Liraglutide-Treated Mice under a Standard or Enriched Environment. Int. J. Mol. Sci. 2024, 25, 10786. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Mizukami, H.; Osonoi, S.; Ogasawara, S.; Hara, Y.; Kudoh, K.; Takeuchi, Y.; Sasaki, T.; Daimon, M.; Yagihashi, S. Inhibitory Effects of Xanthine Oxidase Inhibitor, Topiroxostat, on Development of Neuropathy in Db/Db Mice. Neurobiol. Dis. 2021, 155, 105392. [Google Scholar] [CrossRef]
- Topiroxostat: Uses, Interactions, Mechanism of Action|DrugBank Online. Available online: https://go.drugbank.com/drugs/DB01685 (accessed on 18 June 2025).
- Wei, Y.; Huang, Y.; Huang, R.; Ruan, Y.; Feng, T.; Zhou, F.; Zhang, W.; Lu, J.; Xie, S.; Yao, Y.; et al. Antihypertensive Drug Amlodipine Besylate Shows Potential in Alleviating Diabetic Peripheral Neuropathy. Diabetes 2025, 74, 983–997. [Google Scholar] [CrossRef]
- Amlodipine: Uses, Interactions, Mechanism of Action|DrugBank Online. Available online: https://go.drugbank.com/drugs/DB00381 (accessed on 18 June 2025).
- Nayak, B.P.; Minaz, N.; Pasha, K. Molsidomine Ameliorates Diabetic Peripheral Neuropathy Complications in Wistar Rats. Anim. Model. Exp. Med. 2021, 4, 243–248. [Google Scholar] [CrossRef]
- Molsidomine: Uses, Interactions, Mechanism of Action|DrugBank Online. Available online: https://go.drugbank.com/drugs/DB09282 (accessed on 18 June 2025).
- Gölboyu, B.E.; Erdoğan, M.A.; Erbaş, O. Trimetazidine Provides Protection against Diabetic Polyneuropathy in Rats via Modulation of Soluble HMGB1. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 5854–5861. [Google Scholar] [CrossRef]
- Trimetazidine: Uses, Interactions, Mechanism of Action|DrugBank Online. Available online: https://go.drugbank.com/drugs/DB09069 (accessed on 30 January 2025).
- Xu, J.; Xu, X.; Ling, Y.; Wang, Y.; Huang, Y.; Yang, J.; Wang, J.; Shen, X. Vincamine as an Agonist of G-Protein-Coupled Receptor 40 Effectively Ameliorates Diabetic Peripheral Neuropathy in Mice. Acta Pharmacol. Sin. 2023, 44, 2388–2403. [Google Scholar] [CrossRef]
- Melatonin: Uses, Interactions, Mechanism of Action|DrugBank Online. Available online: https://go.drugbank.com/drugs/DB01065 (accessed on 18 June 2025).
- Che, H.; Li, H.; Li, Y.; Wang, Y.; Yang, Z.; Wang, R.; Wang, L. Melatonin Exerts Neuroprotective Effects by Inhibiting Neuronal Pyroptosis and Autophagy in STZ-induced Diabetic Mice. FASEB J. 2020, 34, 14042–14054. [Google Scholar] [CrossRef]
- Ghazipour, A.M.; Pourheydar, B.; Naderi, R. The Effect of Tropisetron on Peripheral Diabetic Neuropathy: Possible Protective Actions against Inflammation and Apoptosis. Cell Stress Chaperones 2022, 27, 513–521. [Google Scholar] [CrossRef]
- Tropisetron: Uses, Interactions, Mechanism of Action|DrugBank Online. Available online: https://go.drugbank.com/drugs/DB11699 (accessed on 18 June 2025).
- Topiramate: Uses, Interactions, Mechanism of Action|DrugBank Online. Available online: https://go.drugbank.com/drugs/DB00273 (accessed on 18 June 2025).
- Thakur, V.; Gonzalez, M.A.; Parada, M.; Martinez, R.D.; Chattopadhyay, M. Role of Histone Deacetylase Inhibitor in Diabetic Painful Neuropathy. Mol. Neurobiol. 2024, 61, 2283–2296. [Google Scholar] [CrossRef] [PubMed]
- Romidepsin: Uses, Interactions, Mechanism of Action|DrugBank Online. Available online: https://go.drugbank.com/drugs/DB06176 (accessed on 18 June 2025).
- Salama, R.A.M.; Raafat, F.A.; Hasanin, A.H.; Hendawy, N.; Saleh, L.A.; Habib, E.K.; Hamza, M.; Hassan, A.N.E. A Neuroprotective Effect of Pentoxifylline in Rats with Diabetic Neuropathy: Mitigation of Inflammatory and Vascular Alterations. Int. Immunopharmacol. 2024, 128, 111533. [Google Scholar] [CrossRef] [PubMed]
- Pentoxifylline: Uses, Interactions, Mechanism of Action|DrugBank Online. Available online: https://go.drugbank.com/drugs/DB00806 (accessed on 18 June 2025).
- Eisa, N.H.; Helmy, S.A.; El-Kashef, D.H.; El-Sherbiny, M.; Elsherbiny, N.M. Pramipexole Protects against Diabetic Neuropathy: Effect on Oxidative Stress, TLR4/IRAK-1/TRAF-6/NF-ΚB and Downstream Inflammatory Mediators. Int. Immunopharmacol. 2024, 128, 111514. [Google Scholar] [CrossRef]
- Al-Rejaie, S.S.; Abuohashish, H.M.; Ahmed, M.M.; Arrejaie, A.S.; Aleisa, A.M.; AlSharari, S.D. Telmisartan Inhibits Hyperalgesia and Inflammatory Progression in a Diabetic Neuropathic Pain Model of Wistar Rats. Neurosci. J. 2015, 20, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Kolahdouz, M.; Jafari, F.; Falanji, F.; Nazemi, S.; Mohammadzadeh, M.; Molavi, M.; Amin, B. Clavulanic Acid Attenuating Effect on the Diabetic Neuropathic Pain in Rats. Neurochem. Res. 2021, 46, 1759–1770. [Google Scholar] [CrossRef]
- Clavulanic Acid: Uses, Interactions, Mechanism of Action|DrugBank Online. Available online: https://go.drugbank.com/drugs/DB00766 (accessed on 18 June 2025).
- Turan Yücel, N.; Can, Ö.D.; Demir Özkay, Ü. Catecholaminergic and Opioidergic System Mediated Effects of Reboxetine on Diabetic Neuropathic Pain. Psychopharmacology 2020, 237, 1131–1145. [Google Scholar] [CrossRef]
- Ismail, C.A.N.; Suppian, R.; Ab Aziz, C.B.; Long, I. Ifenprodil Reduced Expression of Activated Microglia, BDNF and DREAM Proteins in the Spinal Cord Following Formalin Injection During the Early Stage of Painful Diabetic Neuropathy in Rats. J. Mol. Neurosci. 2021, 71, 379–393. [Google Scholar] [CrossRef]
- Ifenprodil: Uses, Interactions, Mechanism of Action|DrugBank Online. Available online: https://go.drugbank.com/drugs/DB08954 (accessed on 18 June 2025).
- Gazzo, G.; Salgado Ferrer, M.; Poisbeau, P. The Non-Benzodiazepine Anxiolytic Etifoxine Limits Mechanical Allodynia and Anxiety-like Symptoms in a Mouse Model of Streptozotocin-Induced Diabetic Neuropathy. PLoS ONE 2021, 16, e0248092. [Google Scholar] [CrossRef]
- Etifoxine: Uses, Interactions, Mechanism of Action|DrugBank Online. Available online: https://go.drugbank.com/drugs/DB08986 (accessed on 18 June 2025).
- Zhang, X.; Xia, L.; Xie, A.; Liao, O.; Ju, F.; Zhou, Y. Low Concentration of Bupivacaine Ameliorates Painful Diabetic Neuropathy by Mediating MiR-23a/PDE4B Axis in Microglia. Eur. J. Pharmacol. 2021, 891, 173719. [Google Scholar] [CrossRef] [PubMed]
- Bupivacaine: Uses, Interactions, Mechanism of Action|DrugBank Online. Available online: https://go.drugbank.com/drugs/DB00297 (accessed on 18 June 2025).
- Dastgheib, M.; Shetab-Boushehri, S.V.; Baeeri, M.; Gholami, M.; Karimi, M.Y.; Hosseini, A. Rolipram and Pentoxifylline Combination Ameliorates Experimental Diabetic Neuropathy through Inhibition of Oxidative Stress and Inflammatory Pathways in the Dorsal Root Ganglion Neurons. Metab. Brain Dis. 2022, 37, 2615–2627. [Google Scholar] [CrossRef]
- Rolipram: Uses, Interactions, Mechanism of Action|DrugBank Online. Available online: https://go.drugbank.com/drugs/DB01954 (accessed on 18 June 2025).
- Pușcașu, C.; Negreș, S.; Zbârcea, C.E.; Ungurianu, A.; Ștefănescu, E.; Blebea, N.M.; Chiriță, C. Evaluating the Antihyperalgesic Potential of Sildenafil–Metformin Combination and Its Impact on Biochemical Markers in Alloxan-Induced Diabetic Neuropathy in Rats. Pharmaceuticals 2024, 17, 783. [Google Scholar] [CrossRef] [PubMed]
- Sildenafil: Uses, Interactions, Mechanism of Action|DrugBank Online. Available online: https://go.drugbank.com/drugs/DB00203 (accessed on 18 June 2025).
- Pușcașu, C.; Ungurianu, A.; Șeremet, O.C.; Andrei, C.; Mihai, D.P.; Negreș, S. The Influence of Sildenafil–Metformin Combination on Hyperalgesia and Biochemical Markers in Diabetic Neuropathy in Mice. Medicina 2023, 59, 1375. [Google Scholar] [CrossRef]
- Alomar, S.Y.; El-Gheit, R.E.A.; Enan, E.T.; El-Bayoumi, K.S.; Shoaeir, M.Z.; Elkazaz, A.Y.; Al Thagfan, S.S.; Zaitone, S.A.; El-Sayed, R.M. Novel Mechanism for Memantine in Attenuating Diabetic Neuropathic Pain in Mice via Downregulating the Spinal HMGB1/TRL4/NF-KB Inflammatory Axis. Pharmaceuticals 2021, 14, 307. [Google Scholar] [CrossRef] [PubMed]
- Memantine: Uses, Interactions, Mechanism of Action|DrugBank Online. Available online: https://go.drugbank.com/drugs/DB01043 (accessed on 18 June 2025).
- Djouhri, L.; Malki, M.I.; Zeidan, A.; Nagi, K.; Smith, T. Activation of Kv7 Channels with the Anticonvulsant Retigabine Alleviates Neuropathic Pain Behaviour in the Streptozotocin Rat Model of Diabetic Neuropathy. J. Drug Target. 2019, 27, 1118–1126. [Google Scholar] [CrossRef]
- Ezogabine: Uses, Interactions, Mechanism of Action|DrugBank Online. Available online: https://go.drugbank.com/drugs/DB04953 (accessed on 18 June 2025).
- Tseng, K.-Y.; Wang, H.-C.; Wang, Y.-H.; Su, M.-P.; Cheng, K.-F.; Cheng, K.-I.; Chang, L.-L. Peripheral Nerve Denervation in Streptozotocin-Induced Diabetic Rats Is Reduced by Cilostazol. Medicina 2023, 59, 553. [Google Scholar] [CrossRef]
- Cilostazol: Uses, Interactions, Mechanism of Action|DrugBank Online. Available online: https://go.drugbank.com/drugs/DB01166 (accessed on 18 June 2025).
- Notartomaso, S.; Scarselli, P.; Mascio, G.; Liberatore, F.; Mazzon, E.; Mammana, S.; Gugliandolo, A.; Cruccu, G.; Bruno, V.; Nicoletti, F.; et al. N-Acetylcysteine Causes Analgesia in a Mouse Model of Painful Diabetic Neuropathy. Mol. Pain 2020, 16, 1744806920904292. [Google Scholar] [CrossRef]
- Acetylcysteine: Uses, Interactions, Mechanism of Action|DrugBank Online. Available online: https://go.drugbank.com/drugs/DB06151 (accessed on 18 June 2025).
- Jolivalt, C.G.; Frizzi, K.E.; Han, M.M.; Mota, A.J.; Guernsey, L.S.; Kotra, L.P.; Fernyhough, P.; Calcutt, N.A. Topical Delivery of Muscarinic Receptor Antagonists Prevents and Reverses Peripheral Neuropathy in Female Diabetic Mice. J. Pharmacol. Exp. Ther. 2020, 374, 44–51. [Google Scholar] [CrossRef]
- Pirenzepine: Uses, Interactions, Mechanism of Action|DrugBank Online. Available online: https://go.drugbank.com/drugs/DB00670 (accessed on 18 June 2025).
- Casselini, C.M.; Parson, H.K.; Frizzi, K.E.; Marquez, A.; Smith, D.R.; Guernsey, L.; Nemmani, R.; Tayarani, A.; Jolivalt, C.G.; Weaver, J.; et al. A Muscarinic Receptor Antagonist Reverses Multiple Indices of Diabetic Peripheral Neuropathy: Preclinical and Clinical Studies Using Oxybutynin. Acta Neuropathol. 2024, 147, 60. [Google Scholar] [CrossRef]
- Oxybutynin: Uses, Interactions, Mechanism of Action|DrugBank Online. Available online: https://go.drugbank.com/drugs/DB01062 (accessed on 18 June 2025).
- Atropine: Uses, Interactions, Mechanism of Action|DrugBank Online. Available online: https://go.drugbank.com/drugs/DB00572 (accessed on 18 June 2025).
- Xu, X.; Xu, X.; Hao, Y.; Zhu, X.; Lu, J.; Ouyang, X.; Lu, Y.; Huang, X.; Li, Y.; Wang, J.; et al. Antispasmodic Drug Drofenine as an Inhibitor of Kv2.1 Channel Ameliorates Peripheral Neuropathy in Diabetic Mice. iScience 2020, 23, 101617. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.F.S.; Donahue, R.R.; Laird, D.E.; Oliveira, M.C.G.; Taylor, B.K. The PPARγ Agonist Pioglitazone Produces a Female-Predominant Inhibition of Hyperalgesia Associated with Surgical Incision, Peripheral Nerve Injury, and Painful Diabetic Neuropathy. Neuropharmacology 2022, 205, 108907. [Google Scholar] [CrossRef]
- Ozcan, S.; Bulmus, O.; Ulker, N.; Canpolat, S.; Etem, E.O.; Oruc, S.; Yardimci, A.; Bulmus, F.G.; Ayar, A.; Kelestimur, H.; et al. Agomelatine Potentiates Anti-Nociceptive Effects of Morphine in a Mice Model for Diabetic Neuropathy: Involvement of NMDA Receptor Subtype NR1 within the Raphe Nucleus and Periaqueductal Grey. Neurol. Res. 2020, 42, 554–563. [Google Scholar] [CrossRef]
- Agomelatine: Uses, Interactions, Mechanism of Action|DrugBank Online. Available online: https://go.drugbank.com/drugs/DB06594 (accessed on 18 June 2025).
- Morphine: Uses, Interactions, Mechanism of Action|DrugBank Online. Available online: https://go.drugbank.com/drugs/DB00295 (accessed on 18 June 2025).
- Magar, A.; Devasani, K.; Majumdar, A. Melatonin Ameliorates Neuropathy in Diabetic Rats by Abating Mitochondrial Dysfunction and Metabolic Derangements. Endocr. Metab. Sci. 2020, 1, 100067. [Google Scholar] [CrossRef]
- Gabapentin: Uses, Interactions, Mechanism of Action|DrugBank Online. Available online: https://go.drugbank.com/drugs/DB00996 (accessed on 18 June 2025).
- Brock, C.; Hansen, C.S.; Karmisholt, J.; Møller, H.J.; Juhl, A.; Farmer, A.D.; Drewes, A.M.; Riahi, S.; Lervang, H.H.; Jakobsen, P.E.; et al. Liraglutide Treatment Reduced Interleukin-6 in Adults with Type 1 Diabetes but Did Not Improve Established Autonomic or Polyneuropathy. Br. J. Clin. Pharmacol. 2019, 85, 2512–2523. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Robinson, M.; Wong, N.; Chan, E.; Charles, M.A. The Effect of Pentoxifylline on Current Perception Thresholds in Patients with Diabetic Sensory Neuropathy. J. Diabetes Complicat. 1997, 11, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Carroll, D.G.; Kline, K.M.; Malnar, K.F. Role of Topiramate for the Treatment of Painful Diabetic Peripheral Neuropathy. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2004, 24, 1186–1193. [Google Scholar] [CrossRef]
- Alexandre, K.; Sh, A.A.; Nikoloz, G.; Ketevan, C. Clinical and Pharmacological Basis for the Use of Telmisartan in Patients with Diabetic Neuropathy Diabetes & Its Complications. Patients Diabet. Neuropathy Diabetes Complicat. 2018, 2, 1–7. [Google Scholar]
- Alexandre, K.; Sh, A.A.; Nikoloz, G.; Ketevan, C. 1 of 6 Diabetes Complications. Diabetes Mellit. Patients Peripher. Neuropathy Diabetes Complicat. 2018, 2, 1–6. [Google Scholar]
- Rosales, R.L.; Delgado-Delos Santos, M.M.S.; Mercado-Asis, L.B. Cilostazol: A Pilot Study on Safety and Clinical Efficacy in Neuropathies of Diabetes Mellitus Type 2 (ASCEND). Angiology 2011, 62, 625–635. [Google Scholar] [CrossRef]
- Dhanapalaratnam, R.; Issar, T.; Wang, L.L.; Tran, D.; Poynten, A.M.; Milner, K.-L.; Kwai, N.C.G.; Krishnan, A.V. Effect of Metformin on Peripheral Nerve Morphology in Type 2 Diabetes: A Cross-Sectional Observational Study. Diabetes 2024, 73, 1875–1882. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Yu, H.; Wu, J.; Chen, H.; Wang, M.; Wang, S.; Qin, X.; Wu, T.; Wu, Y.; Hu, Y. Metformin Treatment and Risk of Diabetic Peripheral Neuropathy in Patients with Type 2 Diabetes Mellitus in Beijing, China. Front. Endocrinol. 2023, 14, 1082720. [Google Scholar] [CrossRef] [PubMed]
- El-Haggar, S.M.; Hafez, Y.M.; El Sharkawy, A.M.; Khalifa, M. Effect of Empagliflozin in Peripheral Diabetic Neuropathy of Patients with Type 2 Diabetes Mellitus. Med. Clínica 2024, 163, 53–61. [Google Scholar] [CrossRef] [PubMed]
- ClinicalTrials.Gov. Study Details A Research Study to Investigate the Effects of CagriSema Compared to Placebo in People with Type 2 Diabetes and Painful Diabetic Peripheral Neuropathy|ClinicalTrials.Gov. Available online: https://clinicaltrials.gov/study/NCT06797869 (accessed on 23 June 2025).

| Tested Substance | Approved Indication | Mode of Administration | Animal Strain | Results | Molecular Mechanism | Reference |
|---|---|---|---|---|---|---|
| Metformin | Type 2 diabetes mellitus | 200 mg/kg i.p. | Sprague–Dawley rats | Attenuated mechanical allodynia. | Down-regulation of NF-κB. Activation of AMPK signaling pathways in dorsal root ganglion. | [16] |
| 30, 200, and 500 mg/kg i.p. | Sprague–Dawley rats | Attenuated mechanical and heat hyperalgesia and cold allodynia. | ⇑ SOD ⇑ AMPK target genes in sciatic nerves ⇓ MDA ⇓ glycation end-products | [86] | ||
| 400 mg/kg orally | Sprague–Dawley rats | Ameliorated tactile hypersensitivity. | ⇑ expression of miRNA-146a in RSCs. ⇑ the number and structure of myelinated fibers in the SC. ⇑ SOD ⇑ MNCV ⇑ SNCV ⇓ TNF-α ⇓ p65 ⇓ NO | [87] | ||
| 150 mg/kg i.p. | MKR mice | Reduced thermal hypoalgesia, as shown by a significantly lower hind paw withdrawal latency, indicating restored thermal pain perception. Enhanced neuromuscular strength, evidenced by an increased grip strength and longer latency to fall. Exhibited better sensorimotor coordination and performance. | Activation of the AMPK signaling pathway. Restoration of neuronal integrity. ⇓ ROS production. Modulation of autophagic processes. | [88] | ||
| Empagliflozin | Type 2 diabetes mellitus | 3 mg/kg orally | Wistar rats | Reduced mechanical, heat, and cold sensitivity, along with improved motor performance. Additionally, axonal damage was minimized, endoneurial edema decreased, inflammatory cell infiltration reduced, and Schwann cells were preserved. | ⇑p-AMPK expression ⇓ reducing p-p38 MAPK expression ⇓ p-ERK1/2 ⇓ p-NF-κB p65 ⇓ TNF-α ⇓ IL-1β ⇑ SOD ⇓ MDA ⇓ mTOR ⇑ ULK1 ⇑ beclin-1 ⇓ miR-21 ⇑ RECK ⇓ MMP-2 | [89] |
| Semaglutide | Type 2 diabetes mellitus | 1.44 and 2.88 mg/kg orally | Wistar rats | Significantly reduced both mechanical allodynia and thermal hyperalgesia. | ⇓ activation of microglia and astrocytes in the dorsal horn ⇓ HbA1c ⇓ AGE ⇓ IL-1β ⇓ IL-6 ⇓ TNF-α | [90] |
| Atorvastatin | Several types of dyslipidemias; To reduce cardiovascular events in patients with risk factors and/or dyslipidemia. | 10, 20, and 40 mg/kg orally | Wistar rats | Higher dose significantly decreased the thermal sensitivity. | Modulation of nitrergic system. ⇓ NO ⇑ MNCV | [91,92] |
| Alirocumab | Adults with primary hyperlipidemia and homozygous familial hypercholesterolemia and pediatric patients aged 8 years and older with HeFH; To reduce cardiovascular events in patients with risk factors. | 10 mg/kg i.p. | Sprague–Dawley rats | Increased intraepidermal nerve fiber density. | Upregulation of the expression of myelin-related genes in sciatic nerve. ⇓ MDA ⇑ SOD ⇓ TNF-α ⇓ IL-1β ⇓ IL-6 ⇑ Sirt1 levels ⇑ NCV ⇓ TG ⇓ CHO ⇓ LDL ⇑ HDL | [93,94] |
| Drug combinations | ||||||
| Metformin OR Merformin + duloxetine/ oxycodone/ eslicarbazepine acetate OR Metformin + vitamin B12 | Metformin: type 2 diabetes mellitus Duloxetine: - Major depressive disorder; - Generalized anxiety disorder; - Diabetic peripheral neuropathy; - Fibromyalgia; - Relief of chronic musculoskeletal conditions; - Stress urinary incontinence in adult women. Vitamin B12: B12 deficiency due to various causes: - Pernicious anemia; - Celiac disease, gastrectomy; - Small bowel bacterial overgrowth; - Fish tapeworm infection; - Pancreatic or intestinal cancer. | 75–250 mg/kg/day orally OR 10–30 μg Intrathecal OR 10–30 μg Intra-plantarly 0.0625–0.5 mg/kg (orally) + 0.0625–0.5 mg/kg (orally) 0.0625–0.5 mg/kg (orally) + 0.15 mg/kg (i.p.) | C57BL/6 mice | Metformin produced dose-dependent antihyperalgic effects in diabetic mice. Combinations dose-dependently reduced mechanical hyperalgesia in a synergistic way. Metformin co-treatment increased analgesic efficacy and enabled the use of lower doses. | Spinal and peripheral mechanism. | [95,96,97] |
| Aliskiren or Gliclazide or Aliskiren + Gliclazide | Aliskiren: hypertension in adults and pediatric patients aged ≥6 years Glicazide: type 2 diabetes mellitus | 45 mg/kg or 25 mg/kg or 45 mg/kg + 25 mg/kg orally | Sprague–Dawley rats | Aliskiren alone and in combination effectively reduced thermal and mechanical hyperalgesia. Gliclazide alone did not produce a significant reduction in DN symptoms. | All the treatments: ⇓ glucose levels ⇓ TSOD ⇓ Mn-SOD ⇓ CAT ⇓ MDA ⇓ AR activities ⇑ GSH level | [98,99,100] |
| Pioglitazone or Ranolazine | Pioglitazone: type 2 diabetes mellitus Ranolazine: chronic angina | 10 mg/kg or 20, 50, and 100 mg/kg orally | Wistar rats | Decreased thermal hyperalgesia and mechanical allodynia, with ranolazine at a dose of 100 mg/kg demonstrating the most advantageous effects. | All treatments: ⇓ TNF-α ⇓ 1L-1B ⇓ levels of Nav 1.7 channels ⇑ spinal PPAR-γ gene expression | [101] |
| Tested Substance | Approved Indication | Mode of Administration | Animal Strain | Results | Molecular Mechanism | Reference |
|---|---|---|---|---|---|---|
| Liraglutide | Type 2 diabetes mellitus | 2 μg/2 μL into the right lateral ventricle | GSK3β(S9A) mice | Significantly ⇓ mechanical allodynia and thermal nociception in WT mice, while the analgesic effects were absent in GSK3β(S9A) mice. | GSK3β activation. Subsequent inhibition of NLRP3 inflammasome in brain microglia. | [102] |
| 0.4 mg/kg i.p. | ICR mice | Alleviated antinociceptive behaviors in the second phase of formalin test. Decreased mechanical sensitivity. | ⇓ neopterin levels ⇑ glutamine/glutamate ratio | [103] | ||
| Topiroxostat | Gout and hyperurcemia | 1 and 2 mg/kg orally | db/db mice | Preserved nerve conduction, intraepidermal nerve fiber density, and neurite outgrowth. Reduced inflammation and oxidative stress in a dose-dependent manner | Suppressed proinflammatory M1 macrophage polarization. ⇓ mRNA expression of TNF-α, IL-1β, and iNOS. ⇓ H2O2 and MDA levels in plasma and sciatic nerve. ⇓ 8-OHdG expression in Schwann cells, endothelial cells, and macrophages. | [104,105] |
| Amlodipine besylate | - Hypertension; - Coronary artery disease; - Chronic stable angina; - Vasospastic angina. | 2 and 4 mg/kg/day orally | C57BL/6N mice | Ameliorated 50% paw withdrawal threshold, thermal response latency, motor nerve conduction, and sensory nerve conduction velocity Improved intraepidermal nerve fiber loss and myelin sheath structural damage. | GPR40 activation. Suppressed neuroinflammation via the GPR40/β-arrestin2/NLRP3 pathway. Ameliorated mitochondrial dysfunction through the GPR40/LKB1/AMPK/SIRT1/PGC-1α pathway. | [106,107] |
| Rilmenidine | Hypertension | 0.1 and 0.2 mg/kg orally | Sprague–Dawley rats | Decreased perineural thickness and improved muscle activity as evidenced by EMG analysis in a dose-dependent manner | ⇓ TNF-α ⇓ HMGB-1 ⇓ MDA ⇑ NGF ⇑ LC-3 | [17] |
| Molsidomine | - Angina; - Ischemic heart disease; - Chronic heart failure; - Pulmonary hypertension; | 5 and 10 mg/kg orally | Wistar rats | Improved motor coordination, mechanical allodynia, and hyperalgesia. | Prevention of reduced GSH depletion in the sciatic nerve. ⇓ HbA1c ⇓ MDA ⇑ NCV | [108,109] |
| Trimetazidine | Stable angina | 10 and 20 mg/kg i.p. | Sprague–Dawley rats | Significant increase in CMAP amplitudes and a markedly shorter CMAP latency | ⇓ HMGB1 ⇓ pentraxin-3 ⇓ TGF-ß ⇓ MDA | [110,111] |
| Vincamine | Management and prevention of cerebrovascular diseases | 30 mg/kg i.p. | C57BL/6J mice | Improved neurological dysfunctions (as assayed against tactile allodynia, thermal response test, MNCV, and peripheral vascular dysfunctions). Ameliorated myelin sheath injury of sciatic nerve tissues. | Activated GPR40. Alleviated inflammation involving GPR40/β-arrestin2/NLRP3 and GPR40/β-arrestin2/IκBα/NF-κB/NLRP3 pathways. Improved mitochondrial respiration impairments in dorsal root ganglion neurons and mitochondrial dysfunction through GPR40/CaMKKβ/AMPK/SIRT1/PGC-1α pathway and oxidative stress through GPR40/Nrf2 pathway. | [112] |
| Melatonin | - Sleep disturbances - Circadian rhythm disruptions - Supportive role in benzodiazepine withdrawal syndrome | 10 mg/kg orally | KM mice | Significantly alleviated neuronal death and inhibited neuronal pyroptosis and excessive autophagy. | Modulated miR-214-3p/caspase-1 and miR-214-3p/ATG12 pathways. ⇓ levels of NLRP3 ⇓ cleaved caspase-1 ⇓ GSDMD-N ⇓ IL-1β ⇓ LC3, beclin1, and ATG12. | [113,114] |
| Tropisetron | Prevention of chemotherapy-induced and postoperative nausea and vomiting. | 3 mg/kg i.p. | Wistar rats | Reversed the thinning of nerve fibers and myelin sheaths and preserved the number of myelinated fibers. | ⇓ TNF-α ⇓ IL-1β ⇑ Bcl-2 expression ⇓ Bax expression. | [115,116] |
| Topiramate | - Partial-onset or generalized tonic–clonic seizures - Adjunctive treatment for Lennox–Gastaut syndrome; - Migraine prophylaxis | 10–30 mg/kg orally | Swiss mice | Increased hotplate latency time and von Frey test pain threshold. | ⇓ neuroinflammation, by inhibiting the activation of glial cells in the spinal cord. ⇑ the expression of GAP-43 and neurofilaments. | [18,117] |
| Romidepsin | Cutaneous T-cell lymphoma | 1 mg/kg i.p. | ob/ob mice | Alleviated thermal hyperalgesia and mechanical allodynia. | Inhibited histone deacetylase. Modulated immune-related markers such as neutrophil elastase, cfDNA, CitH3, and PADI4. Modulated the expression of GAP-43 and GLUT-4, promoting nerve regeneration. | [118,119] |
| Tested Substance | Approved Indication | Mode of Administration | Animal Strain | Results | Molecular Mechanism | Reference |
|---|---|---|---|---|---|---|
| Pentoxifylline | Intermittent claudication in peripheral arterial disease | 50, 100, and 200 mg/kg in drinking water | Wistar rats | Attenuated tactile allodynia dose-dependently. Prevented the reduction in epidermal thickness of footpad skin. | ⇓ TNFα ⇓ NF-κB ⇓ microglial Iba1 immunoreactivity | [120,121] |
| Pramipexole | Parkinson’s disease | 0.25 and 1 mg/kg orally | Sprague–Dawley rats | Improved survival rate and effectively restored normal structural characteristics of sciatic tissue in a dose-dependent manner. | ⇑ NGF ⇓ MDA ⇓ NO ⇑ reduced GSH levels ⇓ sciatic nerve tissue expression of TLR4/MyD88/IRAK-1/TRAF-6/NF-κB axis | [122] |
| Telmisartan | Hypertension | 5 and 10 mg/kg orally | Wistar rats | Reduced mechanical nociceptive threshold, motor coordination, and thermal nociceptive threshold. Prevented nerve degeneration. | ⇑ NGF ⇓ TNF-α ⇓ IL-1β ⇓ IL-6 | [123] |
| Clavulanic acid | Enhancement of antibiotic antibacterial efficacy. | 10, 20, and 40 mg/kg i.p. | Wistar rats | Reduced mechanical and cold allodynia, as well as thermal hyperalgesia, in both prophylactic and therapeutic regimens across all doses. | ⇑ GLT1 ⇓ iNOS ⇓ TNF-α ⇓ Bax/Bcl2 ratio | [124,125] |
| Reboxetine | Clinical depression | 8 and 16 mg/kg orally | Sprague–Dawley rats | Alleviated mechanical and thermal allodynia and thermal hyperalgesia. | Activation of β2-adrenoceptors. Modulation of D1, D2, and D3 dopaminergic and δ-opioid receptors. ⇑ noradrenaline levels | [126] |
| Ifenprodil | Neuropsychiatric conditions, notably dependence and depression | 0.5 and 1.0 μg intrathecally | Sprague–Dawley rats | Formalin-induced flinching and licking behavior significantly decreased in a dose-dependent manner. | Inhibition of microglia activation. Downregulation of DREAM protein expression. Reduction of BDNF expression. | [127,128] |
| Etifoxine | - Anxiety disorders - Enhancement of peripheral nerve repair | 50 mg/kg i.p. | C57BL6/J mice | Reduced STZ-induced mechanical allodynia. Analgesic effect persisted five weeks after the end of the treatment. | ⇓ in pro-inflammatory cytokine production, microglial activation, or neuroprotective properties. | [129,130] |
| Bupivacaine | Local or regional anesthesia | 0.01%, 0.1%, and 1%/400 μL through the dorsocaudal region of the spine. | C57BL/6 J mice | Ameliorated mechanical allodynia, thermal hyperalgesia, and thermal allodynia, demonstrating greater effectiveness at lower concentrations. | Regulation of miR-23a/PDE4B axis. ⇓ TNFα, IL-6, IL-1β, and MCP-1 (greater effect at lower concentration). | [131,132] |
| Drug combinations | ||||||
| Rolipram + Pentoxifylline | Rolipram: clinical depression Pentoxifylline: intermittent claudication in peripheral arterial disease | 1 mg/kg or 100 mg/kg or 0.5 mg/kg + 50 mg/kg | Sprague–Dawley rats | Monotherapy and association significantly attenuated motor function deficiency by modulating distance moved and velocity. They increased cAMP levels. The combination treatment demonstrated the maximum effectiveness. | All treatments: ⇓ LPO ⇓ ROS ⇓ TNF-α ⇓ NF-kB ⇓ COX2 ⇓ TAC ⇑ total thiol ⇑ CAT ⇑ SOD | [121,133,134] |
| Sildenafil + Metformin | Sildenafil: -Erectile dysfunction; -Pulmonary hypertension; Metformin: type 2 diabetes mellitus | 2, 2.5, and 3 mg/kg + 100, 300, and 500 mg/kg orally | Wistar rats | All combinations resulted in reduced thermal and tactile sensitivity. | ⇓ TNF-α ⇓ IL-6 levels ⇓ nitrite levels ⇑ total thiols concentration | [135,136] |
| 2, 2.5, and 3 mg/kg + 100, 300, and 500 mg/kg orally | NMRI mice | All combinations significantly ⇑ pain reaction latencies in the hot-plate and tail withdrawal tests in a dose-dependent manner. | ⇓ glucose levels. ⇓ nitrite levels in brain and liver. | [136,137] | ||
| Tested Substance | Approved Indication | Mode of Administration | Animal Strain | Results | Molecular Mechanism | Reference |
|---|---|---|---|---|---|---|
| Memantine | Alzheimer’s disease | 10 mg/kg orally | Swiss albino mice | Alleviated thermal hyperalgesia and mechanical allodynia. | Inhibited excessive activation of NMDAR1 receptors. ⇓ HMGB1/TLR4/NF-kB inflammatory pathway activity. ⇓ glutamate levels. ⇓ TNF-α and IL-1β in the spinal cord. | [138,139] |
| Retigabine | Adjunct therapy for partial-onset seizures | 15 mg/kg i.p. | Sprague–Dawley rats | Significantly attenuated mechanical, not heat, hypersensitivity. | Activation of Kv7 channels | [140,141] |
| Cilostazol | Intermittent claudication | 10, 30, and 100 mg/kg orally | Sprague–Dawley rats | Alleviated mechanical allodynia symptoms but had no effect on thermal analgesia. | 30 and 100 mg/kg: ⇓ PGP9.5 epidermal sensory nerve fiber disruptions in the hind paws. ⇓ P2X3, CGRP, and TRPV-1 expression in the hind paws. 100 mg/kg: Amelioration of microglial overreaction and restoration of the reduced astrocyte expression. | [142,143] |
| N-Acetylcysteine | - Mucolysis; - Paracetamol intoxication. | 50 and 100 mg/kg i.p. | C57BL/6 mice | Reduces mechanical nociceptive threshold at the dose of 100 mg/kg. | TRPV1, NMDAR, and mGlu5 antagonism. ⇓ RK1/2 phosphorylation ⇓ P2X7 receptor expression in the spinal cord | [144,145] |
| Pirenzepine | Peptic/gastric/ duodenal ulcer | 0.2, 1.0, 2.0, or 10% in 50 μL hydrogel locally (hind paw) | C57 BL/6J mice | 2.0%: Prevented paw tactile allodynia, heat hypoalgesia, and intraepidermal nerve fiber loss. 10%: Prevented paw tactile allodynia, heat hypoalgesia, and IENF loss. | Muscarinic M1 receptor antagonism | [146,147] |
| Oxybutynin | Overactive bladder | 3–10 mg/kg/day s.c./ 3% gel topical | Swiss Webster mice | Reversed paw heat hypoalgesia. Prevented nerve loss. | Muscarinic M1 receptor antagonism | [148,149] |
| Atropine | Systemic administration: muscarinic intoxication. Ophthalmic use: -Mydriasis; -Cycloplegia. | 2.0% in 50 μL hydrogel topically | Swiss Webster mice | Paw delivery: prevented MNCV slowing, heat hypoalgesia, and loss of IENF in the ipsilateral limb, but not in the contralateral. Ocular delivery: prevented or attenuated paw heat hypoalgesia in both hind paws, but not MNCV slowing, tactile allodynia, or loss of IENF. | Muscarinic M1 receptor antagonism | [146,150] |
| Drofenine | Visceral spastic pain | 10, 20 mg/kg i.p. | C57BL/6 mice | Attenuated nerve conduction deficiency, hypoalgesia, impairment in MNCV, enhanced sensitivity to mechanical stimuli, and augmented thermal response. | Kv2.1 inhibition | [151] |
| 10, 20 mg/kg i.p. | db/db mice | Attenuated nerve conduction deficiency, hypoalgesia, impairment in motor nerve conduction velocity, enhanced sensitivity to mechanical stimuli, and augmented thermal response. | ⇓ Kv2.1 expression, promoting neurite outgrowth. ⇓ IκBα/NF-κB signaling ⇓ apoptosis by regulating Bcl-2 and caspase-3 expression. Modulation of Kv2.1/CaMKKβ/AMPK/PGC1α pathway. | [151] | ||
| Pioglitazone | Type 2 diabetes mellitus | 100 mg/kg i.p., single dose or 0.3, 3, or 30 mg/kg/day in chow | db/db mice | Decreased heat hypersensitivity in db/db mice with greater efficacy in female mice after single i.p. dose. Neither 0.3, 3.0, nor 30 mg/kg/day decreased heat hypersensitivity in males or females. | PPARγ activation | [152] |
| Drug combinations | ||||||
| Agomelatine or Morphine or Agomelatine + Morphine | Agomelatine: major depressive episodes Morphine: chronic pain ranging from moderate to severe | 10 mg/kg, each i.p. | Balb/C mice | All the treatments attenuated thermal sensitivity. The combination produced a stronger and longer-lasting analgesic effect compared to morphine alone. Repeated exposure to morphine leads to a reduced analgesic effect, requiring higher doses to achieve the same level of pain relief. | Agomelatine: ⇓ GluN1 expression in the PAG and dorsal raphe, contributing to ⇓ morphine tolerance. | [153,154,155] |
| Melatonin or Melatonin + Gabapentin | Melatonin: - Sleep disturbances - Circadian rhythm disruptions, - Withdrawal symptoms from benzodiazepines and nicotine. Gabapentin: - Partial-onset seizures, - Peripheral neuropathic pain. | 25 and 50 mg/kg or 50 mg/kg + 100 mg/kg orally | Sprague–Dawley rats | The combination treatment significantly relieved cold allodynia, while both melatonin alone and the combination effectively alleviated hot allodynia. | Melatonin 25 mg/kg: ⇑ hepatic gene expression of PGC-1α and TFAM ⇑ SNCV Melatonin 50 mg/kg: ⇑ hepatic gene expression of PGC-1α and TFAM ⇑ SNCV ⇓ serum creatinine levels The combination: ⇑ SNCV | [113,156,157] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrei, C.; Șeremet, O.C.; Pușcașu, C.; Zanfirescu, A. Translating Basic Science to Clinical Applications: A Narrative Review of Repurposed Pharmacological Agents in Preclinical Models of Diabetic Neuropathy. Biomedicines 2025, 13, 1709. https://doi.org/10.3390/biomedicines13071709
Andrei C, Șeremet OC, Pușcașu C, Zanfirescu A. Translating Basic Science to Clinical Applications: A Narrative Review of Repurposed Pharmacological Agents in Preclinical Models of Diabetic Neuropathy. Biomedicines. 2025; 13(7):1709. https://doi.org/10.3390/biomedicines13071709
Chicago/Turabian StyleAndrei, Corina, Oana Cristina Șeremet, Ciprian Pușcașu, and Anca Zanfirescu. 2025. "Translating Basic Science to Clinical Applications: A Narrative Review of Repurposed Pharmacological Agents in Preclinical Models of Diabetic Neuropathy" Biomedicines 13, no. 7: 1709. https://doi.org/10.3390/biomedicines13071709
APA StyleAndrei, C., Șeremet, O. C., Pușcașu, C., & Zanfirescu, A. (2025). Translating Basic Science to Clinical Applications: A Narrative Review of Repurposed Pharmacological Agents in Preclinical Models of Diabetic Neuropathy. Biomedicines, 13(7), 1709. https://doi.org/10.3390/biomedicines13071709






