Prospective Neuropsychological and Plasma Biomarker Changes in Treatment-Naïve People Living with HIV After Antiretroviral Treatment Initiation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
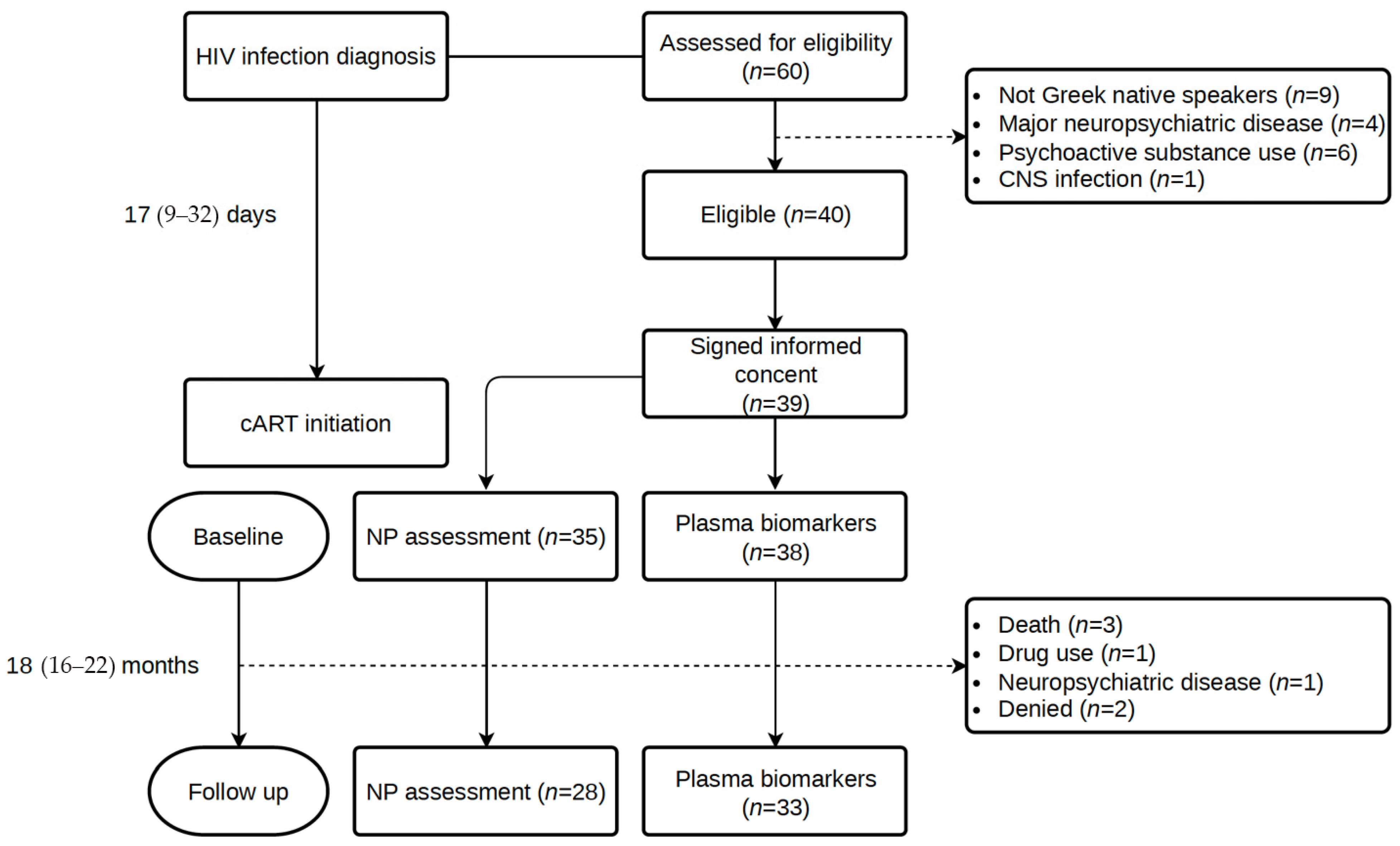
2.2. Participants
2.3. Study Procedures
2.3.1. Demographics and Routine Laboratory Exams
2.3.2. Neuropsychological Assessment
2.3.3. Plasma Biomarkers
2.4. Statistical Analysis
3. Results
3.1. Participant Characteristics
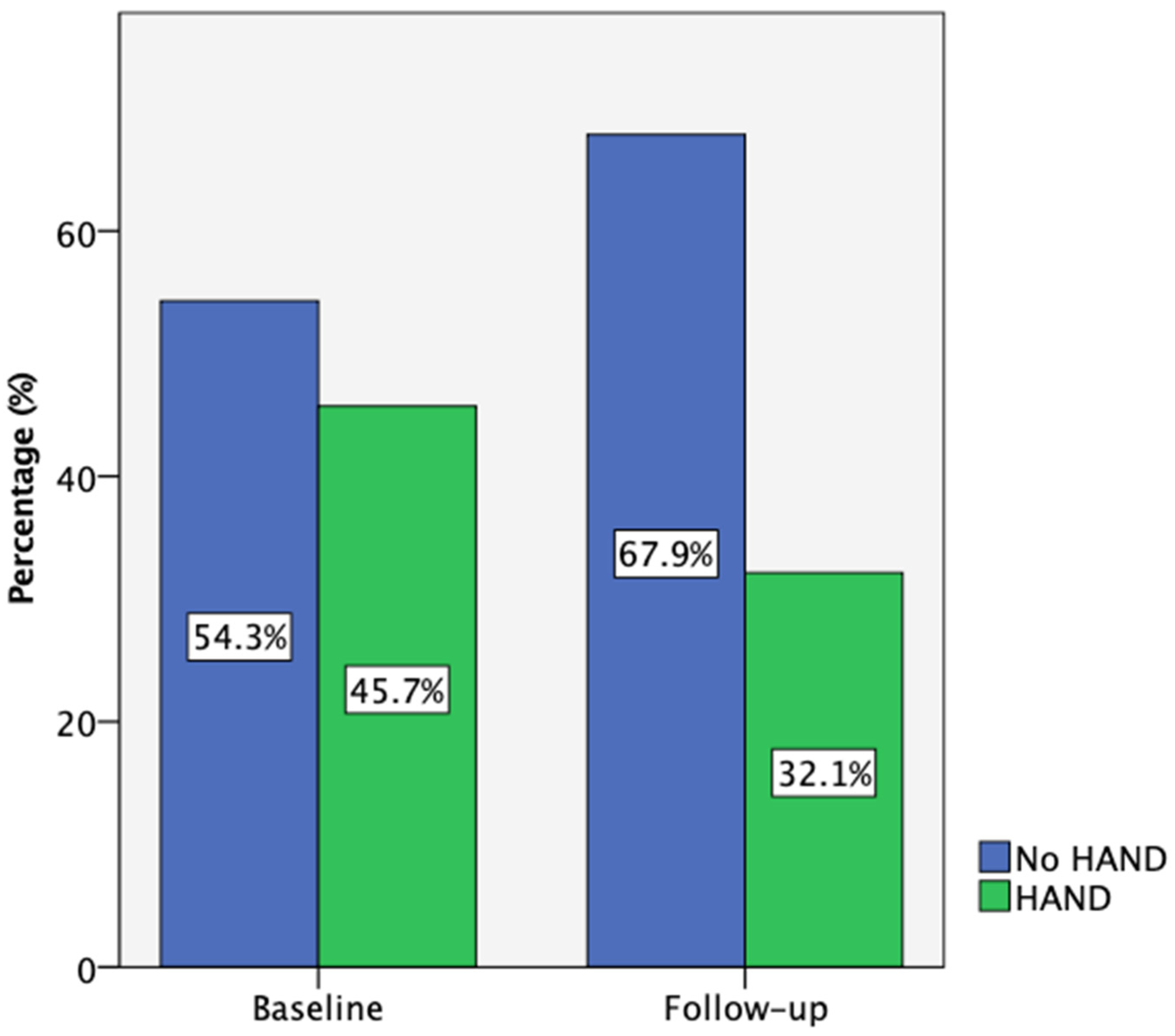
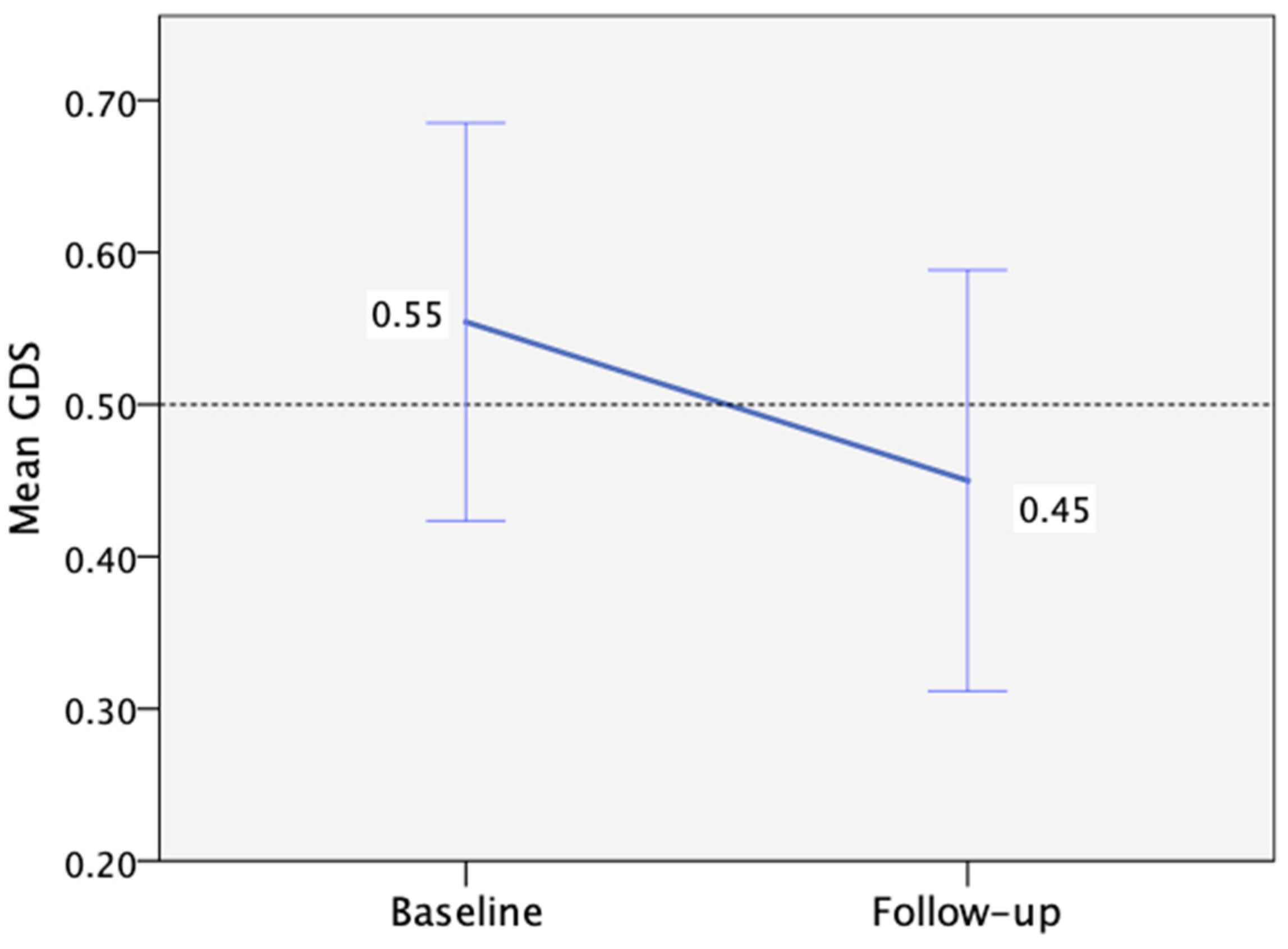
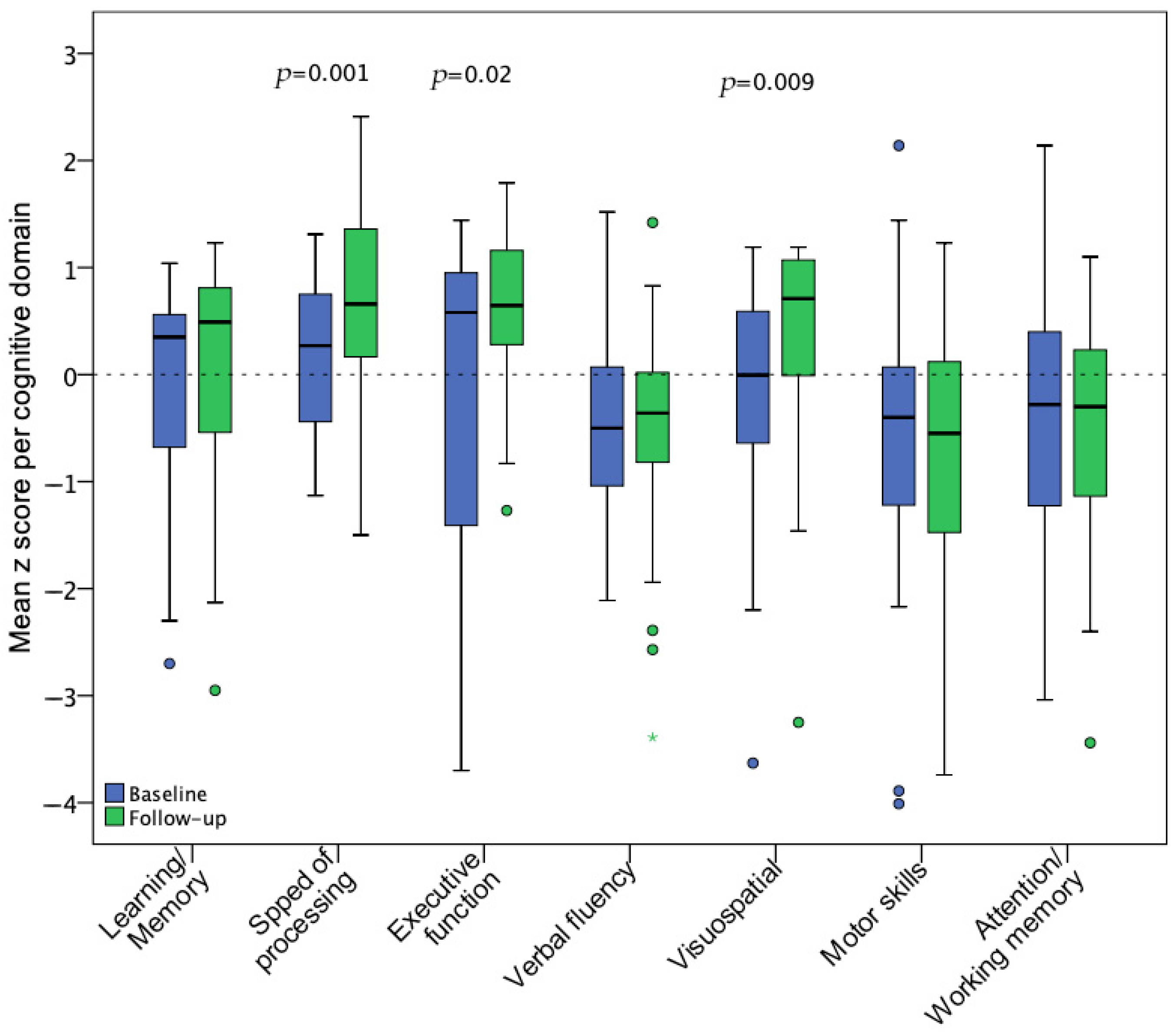
3.2. Neuropsychological Assessment
3.3. Plasma Biomarkers
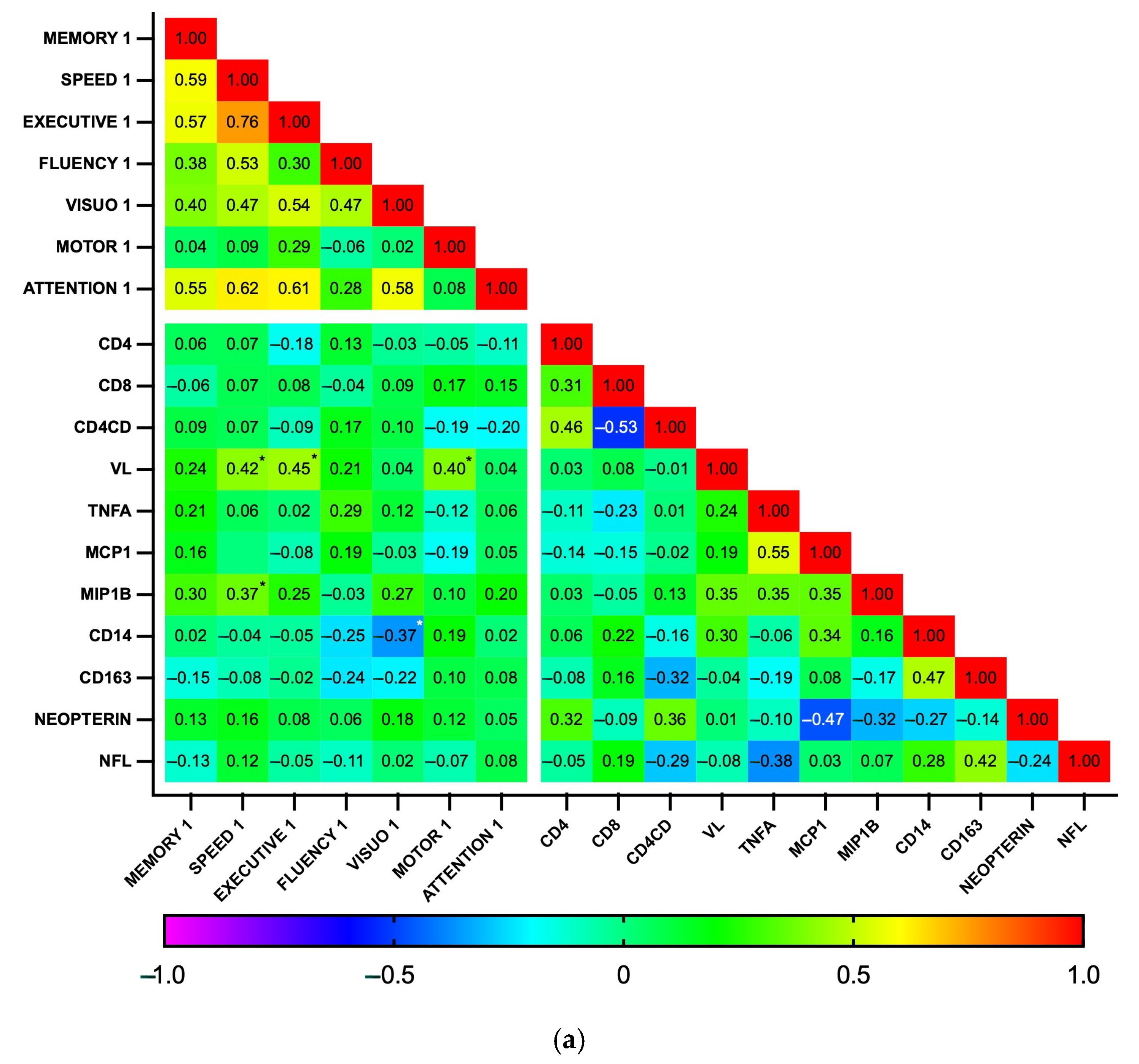
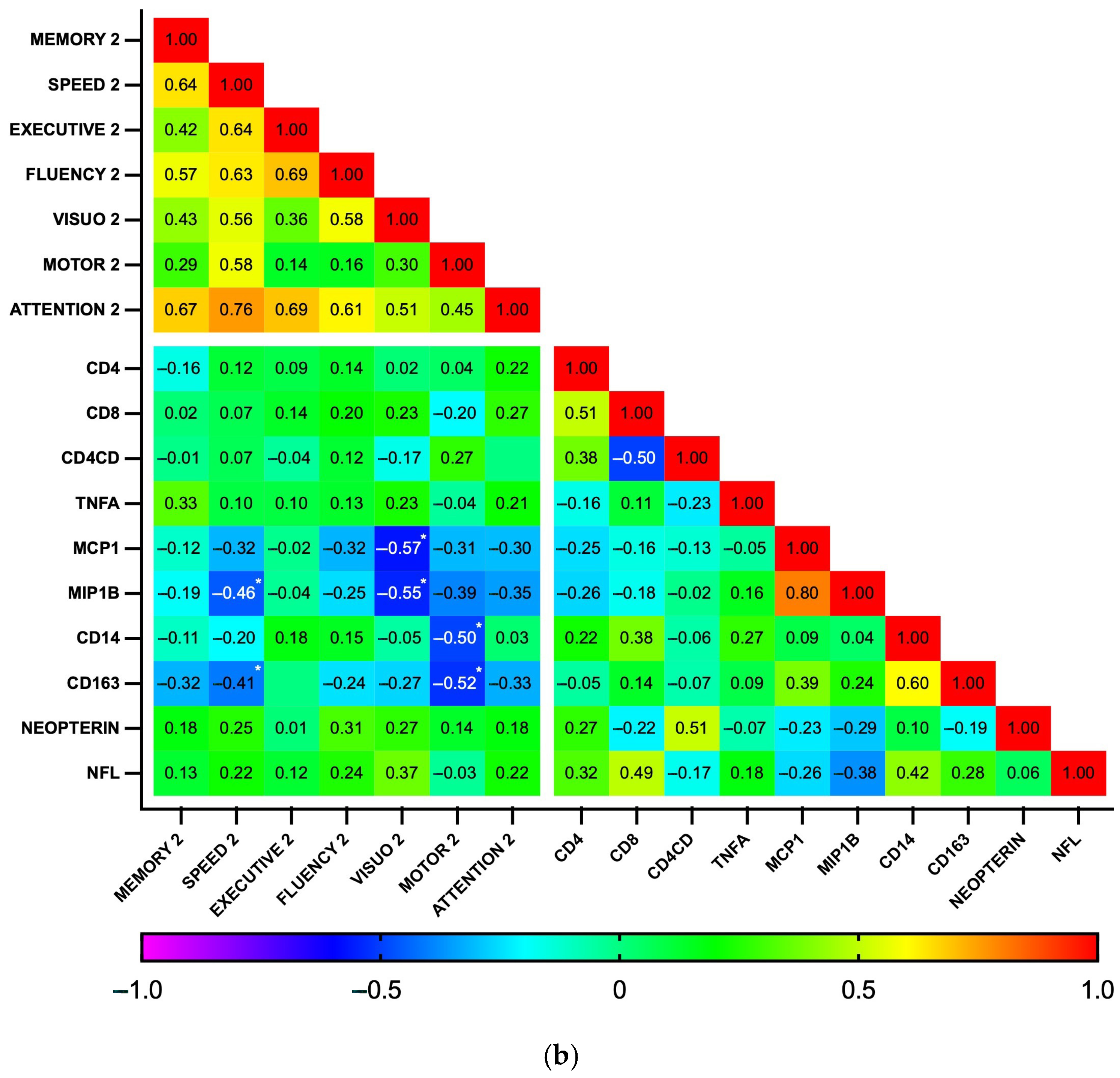
3.4. Associations Between Plasma Biomarker Levels and Cognitive Performance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chan, P.; Spudich, S. Central Nervous System Effects of Early HIV Infection and Consequences of Antiretroviral Therapy Initiation during Acute HIV. Viruses 2024, 16, 1082. [Google Scholar] [CrossRef] [PubMed]
- Killingsworth, L.; Spudich, S. Neuropathogenesis of HIV-1: Insights from across the Spectrum of Acute through Long-Term Treated Infection. Semin. Immunopathol. 2022, 44, 709–724. [Google Scholar] [CrossRef] [PubMed]
- Moschopoulos, C.D.; Alford, K.; Antoniadou, A.; Vera, J.H. Cognitive Impairment in People Living with HIV: Mechanisms, Controversies, and Future Perspectives. Trends Mol. Med. 2024, 30, 1076–1089. [Google Scholar] [CrossRef]
- Antinori, A.; Arendt, G.; Becker, J.T.; Brew, B.J.; Byrd, D.A.; Cherner, M.; Clifford, D.B.; Cinque, P.; Epstein, L.G.; Goodkin, K.; et al. Updated Research Nosology for HIV-Associated Neurocognitive Disorders. Neurology 2007, 69, 1789–1799. [Google Scholar] [CrossRef] [PubMed]
- Keng, L.D.; Winston, A.; Sabin, C.A. The Global Burden of Cognitive Impairment in People with HIV. AIDS 2023, 37, 61–70. [Google Scholar] [CrossRef]
- Wei, J.; Hou, J.; Su, B.; Jiang, T.; Guo, C.; Wang, W.; Zhang, Y.; Chang, B.; Wu, H.; Zhang, T. The Prevalence of Frascati-Criteria-Based HIV-Associated Neurocognitive Disorder (HAND) in HIV-Infected Adults: A Systematic Review and Meta-Analysis. Front. Neurol. 2020, 11, 581346. [Google Scholar] [CrossRef]
- Bandera, A.; Taramasso, L.; Bozzi, G.; Muscatello, A.; Robinson, J.A.; Burdo, T.H.; Gori, A. HIV-Associated Neurocognitive Impairment in the Modern ART Era: Are We Close to Discovering Reliable Biomarkers in the Setting of Virological Suppression? Front. Aging Neurosci. 2019, 11, 187. [Google Scholar] [CrossRef]
- Williams, M.E.; Stein, D.J.; Joska, J.A.; Naudé, P.J.W. Cerebrospinal Fluid Immune Markers and HIV-Associated Neurocognitive Impairments: A Systematic Review. J. Neuroimmunol. 2021, 358, 577649. [Google Scholar] [CrossRef]
- Trickey, A.; Sabin, C.A.; Burkholder, G.; Crane, H.; d’Arminio Monforte, A.; Egger, M.; Gill, M.J.; Grabar, S.; Guest, J.L.; Jarrin, I.; et al. Life Expectancy after 2015 of Adults with HIV on Long-Term Antiretroviral Therapy in Europe and North America: A Collaborative Analysis of Cohort Studies. Lancet HIV 2023, 10, e295–e307. [Google Scholar] [CrossRef]
- Moschopoulos, C.D.; Stanitsa, E.; Protopapas, K.; Kavatha, D.; Papageorgiou, S.G.; Antoniadou, A.; Papadopoulos, A. Multimodal Approach to Neurocognitive Function in People Living with HIV in the CART Era: A Comprehensive Review. Life 2024, 14, 508. [Google Scholar] [CrossRef]
- McLaurin, K.A.; Booze, R.M.; Mactutus, C.F. Diagnostic and Prognostic Biomarkers for HAND. J. Neurovirol. 2019, 25, 686–701. [Google Scholar] [CrossRef] [PubMed]
- Woods, S.P.; Rippeth, J.D.; Frol, A.B.; Levy, J.K.; Ryan, E.; Soukup, V.M.; Hinkin, C.H.; Lazzaretto, D.; Cherner, M.; Marcotte, T.D.; et al. Interrater Reliability of Clinical Ratings and Neurocognitive Diagnoses in HIV. J. Clin. Exp. Neuropsychol. 2004, 26, 759–778. [Google Scholar] [CrossRef] [PubMed]
- Carey, C.L.; Woods, S.P.; Gonzalez, R.; Conover, E.; Marcotte, T.D.; Grant, I.; Heaton, R.K. Predictive Validity of Global Deficit Scores in Detecting Neuropsychological Impairment in HIV Infection. J. Clin. Exp. Neuropsychol. 2004, 26, 307–319. [Google Scholar] [CrossRef] [PubMed]
- Konstantopoulos, K.; Vogazianos, P.; Doskas, T. Normative Data of the Montreal Cognitive Assessment in the Greek Population and Parkinsonian Dementia. Arch. Clin. Neuropsychol. 2016, 31, 246–253. [Google Scholar] [CrossRef]
- Kosmidis, M.H.; Vlahou, C.H.; Panagiotaki, P.; Kiosseoglou, G. The Verbal Fluency Task in the Greek Population: Normative Data, and Clustering and Switching Strategies. J. Int. Neuropsychol. Soc. 2004, 10, 164–172. [Google Scholar] [CrossRef]
- Benedict, R.H.B.; Schretlen, D.; Groninger, L.; Dobraski, M.; Shpritz, B. Revision of the Brief Visuospatial Memory Test: Studies of Normal Performance, Reliability, and Validity. Psychol. Assess. 1996, 8, 145–153. [Google Scholar] [CrossRef]
- Zalonis, I.; Kararizou, E.; Triantafyllou, N.I.; Kapaki, E.; Papageorgiou, S.; Sgouropoulos, P.; Vassilopoulos, D. A Normative Study of the Trail Making Test A and B in Greek Adults. Clin. Neuropsychol. 2008, 22, 842–850. [Google Scholar] [CrossRef]
- Smith, A. Symbol Digit Modalities Test; Western Psychological Services: Los Angeles, CA, USA, 1982. [Google Scholar]
- Zalonis, I.; Christidi, F.; Bonakis, A.; Kararizou, E.; Triantafyllou, N.I.; Paraskevas, G.; Kapaki, E.; Vasilopoulos, D. The Stroop Effect in Greek Healthy Population: Normative Data for the Stroop Neuropsychological Screening Test. Arch. Clin. Neuropsychol. 2009, 24, 81–88. [Google Scholar] [CrossRef]
- Benton, A.L.; Hamsher, K.; Varney, N.; Spreen, O. Judgement of Line Orientation Test; University of Iowa: Iowa City, IA, USA, 1983. [Google Scholar]
- Ruff, R.M.; Parker, S.B. Gender- and Age-Specific Changes in Motor Speed and Eye-Hand Coordination in Adults: Normative Values for the Finger Tapping and Grooved Pegboard Tests. Percept. Mot. Skills 1993, 76, 1219–1230. [Google Scholar] [CrossRef]
- Wechsler, D. WAIS-III: Wechsler Adult Intelligence Scale; Pearson: San Antonio, TX, USA, 1997. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef]
- Lawton, M.P.; Brody, E.M.; Médecin, U. Instrumental Activities of Daily Living (IADL). Gerontologist 1969, 9, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, R.I.; Kurosaki, T.T.; Harrah, C.H.; Chance, J.M.; Filos, S. Measurement of Functional Activities in Older Adults in the Community. J. Gerontol. 1982, 37, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Gisslén, M.; Price, R.W.; Andreasson, U.; Norgren, N.; Nilsson, S.; Hagberg, L.; Fuchs, D.; Spudich, S.; Blennow, K.; Zetterberg, H. Plasma Concentration of the Neurofilament Light Protein (NFL) Is a Biomarker of CNS Injury in HIV Infection: A Cross-Sectional Study. EBioMedicine 2016, 3, 135–140. [Google Scholar] [CrossRef]
- Cysique, L.A.; Franklin, D.; Abramson, I.; Ellis, R.J.; Letendre, S.; Collier, A.; Clifford, D.; Gelman, B.; McArthur, J.; Morgello, S.; et al. Normative Data and Validation of a Regression Based Summary Score for Assessing Meaningful Neuropsychological Change. J. Clin. Exp. Neuropsychol. 2011, 33, 505–522. [Google Scholar] [CrossRef]
- Webb, K.L.; Ryan, J.; Wolfe, R.; Woods, R.L.; Shah, R.C.; Murray, A.M.; Orchard, S.G.; Storey, E. Test-Retest Reliability and Minimal Detectable Change of Four Cognitive Tests in Community-Dwelling Older Adults. J. Alzheimer’s Dis. 2022, 87, 1683–1693. [Google Scholar] [CrossRef]
- Duff, K.; Beglinger, L.J.; Schultz, S.K.; Moser, D.J.; McCaffrey, R.J.; Haase, R.F.; Westervelt, H.J.K.; Langbehn, D.R.; Paulsen, J.S.; Huntington’s Study Group. Practice Effects in the Prediction of Long-Term Cognitive Outcome in Three Patient Samples: A Novel Prognostic Index. Arch. Clin. Neuropsychol. 2007, 22, 15–24. [Google Scholar] [CrossRef]
- Calamia, M.; Markon, K.; Tranel, D. Scoring Higher the Second Time Around: Meta-Analyses of Practice Effects in Neuropsychological Assessment. Clin. Neuropsychol. 2012, 26, 543–570. [Google Scholar] [CrossRef]
- Joska, J.A.; Gouse, H.; Paul, R.H.; Stein, D.J.; Flisher, A.J. Does Highly Active Antiretroviral Therapy Improve Neurocognitive Function? A Systematic Review. J. Neurovirol. 2010, 16, 101–114. [Google Scholar] [CrossRef]
- Heaton, R.K.; Franklin, D.R.J.; Deutsch, R.; Letendre, S.; Ellis, R.J.; Casaletto, K.; Marquine, M.J.; Woods, S.P.; Vaida, F.; Atkinson, J.H.; et al. Neurocognitive Change in the Era of HIV Combination Antiretroviral Therapy: The Longitudinal CHARTER Study. Clin. Infect. Dis. 2015, 60, 473–480. [Google Scholar] [CrossRef]
- Damas, J.; Ledergerber, B.; Nadin, I.; Tarr, P.E.; Stoeckle, M.; Kunze, U.; Hauser, C.; Gutbrod, K.; Calmy, A.; Assal, F.; et al. Neurocognitive Course at 2-Year Follow-up in a Swiss Cohort of People with Well-Treated HIV. AIDS 2021, 35, 2469–2480. [Google Scholar] [CrossRef]
- Sacktor, N.; Skolasky, R.L.; Seaberg, E.; Munro, C.; Becker, J.T.; Martin, E.; Ragin, A.; Levine, A.; Miller, E. Prevalence of HIV-Associated Neurocognitive Disorders in the Multicenter AIDS Cohort Study. Neurology 2016, 86, 334–340. [Google Scholar] [CrossRef] [PubMed]
- Le, L.T.; Price, R.W.; Gisslén, M.; Zetterberg, H.; Emu, B.; Fabre, R.; Christian, P.; Andersen, S.; Spudich, S.; Vassallo, M. Correlation between CD4/CD8 Ratio and Neurocognitive Performance during Early HIV Infection. HIV Med. 2023, 24, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Wright, E.J.; Grund, B.; Robertson, K.R.; Cysique, L.; Brew, B.J.; Collins, G.L.; Poehlman-Roediger, M.; Vjecha, M.J.; Penalva de Oliveira, A.C.; Standridge, B.; et al. No Neurocognitive Advantage for Immediate Antiretroviral Treatment in Adults with Greater than 500 CD4+ T-Cell Counts. AIDS 2018, 32, 985–997. [Google Scholar] [CrossRef] [PubMed]
- Hileman, C.O.; Funderburg, N.T. Inflammation, Immune Activation, and Antiretroviral Therapy in HIV. Curr. HIV/AIDS Rep. 2017, 14, 93–100. [Google Scholar] [CrossRef]
- Anderson, A.M.; Easley, K.A.; Kasher, N.; Franklin, D.; Heaton, R.K.; Zetterberg, H.; Blennow, K.; Gisslen, M.; Letendre, S.L. Neurofilament Light Chain in Blood Is Negatively Associated with Neuropsychological Performance in HIV-Infected Adults and Declines with Initiation of Antiretroviral Therapy. J. Neurovirol. 2018, 24, 695–701. [Google Scholar] [CrossRef]
- Alagaratnam, J.; De Francesco, D.; Zetterberg, H.; Heslegrave, A.; Toombs, J.; Kootstra, N.A.; Underwood, J.; Gisslen, M.; Reiss, P.; Fidler, S.; et al. Correlation between Cerebrospinal Fluid and Plasma Neurofilament Light Protein in Treated HIV Infection: Results from the COBRA Study. J. Neurovirol. 2022, 28, 54–63. [Google Scholar] [CrossRef]
- Mellgren, A.; Price, R.W.; Hagberg, L.; Rosengren, L.; Brew, B.J.; Gisslén, M. Antiretroviral Treatment Reduces Increased CSF Neurofilament Protein (NFL) in HIV-1 Infection. Neurology 2007, 69, 1536–1541. [Google Scholar] [CrossRef]
- Anderson, A.M.; Jang, J.H.; Easley, K.A.; Fuchs, D.; Gisslen, M.; Zetterberg, H.; Blennow, K.; Ellis, R.J.; Franklin, D.; Heaton, R.K.; et al. Cognitive and Neuronal Link with Inflammation: A Longitudinal Study in People with and Without HIV Infection. JAIDS J. Acquir. Immune Defic. Syndr. 2020, 85, 617–625. [Google Scholar] [CrossRef]
- Jessen Krut, J.; Mellberg, T.; Price, R.W.; Hagberg, L.; Fuchs, D.; Rosengren, L.; Nilsson, S.; Zetterberg, H.; Gisslén, M. Biomarker Evidence of Axonal Injury in Neuroasymptomatic HIV-1 Patients. PLoS ONE 2014, 9, e88591. [Google Scholar] [CrossRef]
- Eisenhut, M. Neopterin in Diagnosis and Monitoring of Infectious Diseases. J. Biomark. 2013, 2013, 196432. [Google Scholar] [CrossRef]
- Krebs, S.J.; Slike, B.M.; Sithinamsuwan, P.; Allen, I.E.; Chalermchai, T.; Tipsuk, S.; Phanuphak, N.; Jagodzinski, L.; Kim, J.H.; Ananworanich, J.; et al. Sex Differences in Soluble Markers Vary before and after the Initiation of Antiretroviral Therapy in Chronically HIV-Infected Individuals. AIDS 2016, 30, 1533–1542. [Google Scholar] [CrossRef] [PubMed]
- Abufares, H.I.; Zenati, R.A.; Soares, N.C.; El-Huneidi, W.; Dahabiyeh, L.A.; Al-Hroub, H.M.; Alqudah, M.A.Y.; Abuhelwa, A.Y.; Alzoubi, K.H.; Abu-Gharbieh, E.; et al. A Non-Targeted Metabolomics Comparative Study on Plasma of Pfizer and Sinopharm COVID-19 Vaccinated Individuals, Assessed by (TIMS-QTOF) Mass Spectrometry. Heliyon 2024, 10, e35443. [Google Scholar] [CrossRef] [PubMed]
- Hagberg, L.; Cinque, P.; Gisslen, M.; Brew, B.J.; Spudich, S.; Bestetti, A.; Price, R.W.; Fuchs, D. Cerebrospinal Fluid Neopterin: An Informative Biomarker of Central Nervous System Immune Activation in HIV-1 Infection. AIDS Res. Ther. 2010, 7, 15. [Google Scholar] [CrossRef] [PubMed]
- Imp, B.M.; Rubin, L.H.; Tien, P.C.; Plankey, M.W.; Golub, E.T.; French, A.L.; Valcour, V.G. Monocyte Activation Is Associated with Worse Cognitive Performance in HIV-Infected Women with Virologic Suppression. J. Infect. Dis. 2017, 215, 114–121. [Google Scholar] [CrossRef]
- Burdo, T.H.; Weiffenbach, A.; Woods, S.P.; Letendre, S.; Ellis, R.J.; Williams, K.C. Elevated SCD163 in Plasma but Not Cerebrospinal Fluid Is a Marker of Neurocognitive Impairment in HIV Infection. AIDS 2013, 27, 1387–1395. [Google Scholar] [CrossRef]
- Xing, Y.; Shepherd, N.; Lan, J.; Li, W.; Rane, S.; Gupta, S.K.; Zhang, S.; Dong, J.; Yu, Q. MMPs/TIMPs Imbalances in the Peripheral Blood and Cerebrospinal Fluid Are Associated with the Pathogenesis of HIV-1-Associated Neurocognitive Disorders. Brain Behav. Immun. 2017, 65, 161–172. [Google Scholar] [CrossRef]
- Nightingale, S.; Ances, B.; Cinque, P.; Dravid, A.; Dreyer, A.J.; Gisslén, M.; Joska, J.A.; Kwasa, J.; Meyer, A.-C.; Mpongo, N.; et al. Cognitive Impairment in People Living with HIV: Consensus Recommendations for a New Approach. Nat. Rev. Neurol. 2023, 19, 424–433. [Google Scholar] [CrossRef]
| Test 1 | Test 2 | |
|---|---|---|
| General Cognitive Ability | MoCA [14] | - |
| Memory and Learning | GVLT [15] | BVMT [16] |
| Speed of Information Processing | TMT-A [17] | SDMT [18] |
| Executive Functions | Stroop Test [19] | TMT-B [17] |
| Verbal Fluency | Semantic tasks [15] | Phonemic tasks [15] |
| Visuospatial Perception | JLO [20] | - |
| Motor Speed and Dexterity | Grooved Pegboard [21] (dominant hand) | Grooved Pegboard (non-dominant hand) |
| Attention and Working Memory | LNS [22] | Spatial Span [22] (forward and backward) |
| All | Completed NP Follow-Up | Lost to Follow-Up | p-Value a | |
|---|---|---|---|---|
| Baseline Demographics, HIV Infection Characteristics, and Comorbidities | n = 39 | n = 28 | n = 9 | |
| Age (years) | 37.7 (±10.7) | 39.1 (±13.6) | 37.7 (±10.1) | 0.776 ^ |
| Male (%) | 37 (94.9) | 27 (96.4) | 8 (88.9) | |
| White (%) | 39 (100) | - | - | |
| Typical education (years) | 14.5 (12.3–16) | 12 (12–16) | 14.5 (14–16) | 0.30 # |
| MSM (%) Heterosexual (%) | 37 (94.9) 2 (5.1) | 27 (96.4) 1 (3.6) | 8 (88.9) 1 (11.1) | |
| CDC Classification, n (%) | 0.10 § | |||
| 0 | 9 (23.1) | 9 (32.1) | 1 (11.1) | |
| 1 | 5 (12.8) | 3 (10.7) | 3 (33.3) | |
| 2 | 19 (48.7) | 13 (46.4) | 2 (22.2) | |
| 3 | 6 (15.4) | 3 (10.7) | 3 (33.3) | |
| CD4 < 350 cells/μL, n (%) | 14 (35.9) | 8 (28.6) | 5 (55.5) | 0.229 § |
| CD4+ count (cells/μL) | 405 (260–506) | 429 (302–513) | 286 (139–500) | 0.160 # |
| CD8+ count (cells/μL) | 1024 (620–1397) | 1053 (621–1585) | 942 (602–1293) | 0.625 # |
| CD4+/CD8+ ratio | 0.34 (0.24–0.51) | 0.36 (0.30–0.56) | 0.32 (0.16–0.52) | 0.286 # |
| HIV RNA (log10 copies/mL) | 5.0 (4.4–5.7) | 5.0 (4.4–5.8) | 4.6 (4.2–5.9) | 0.648 # |
| INSTI-based cART, n (%) | 32 (82.1) | |||
| PI-based cART, n (%) | 7 (18.4) | |||
| Smoking (ex or current), n (%) | 26 (66.7) | 19 (67.9) | 7 (77.8) | 0.695 § |
| All Participants | Participants with Follow-Up | ||||
|---|---|---|---|---|---|
| Test | Baseline (n = 35) (mean, SD) | Baseline (n = 28) (mean, SD) | Follow-Up (n = 28) (mean, SD) | p a | Cohen’s d |
| Screening | |||||
| MoCA | 25 (3.1) | 25 (3.0) | 24.9 (4.7) | 0.84 | −0.04 |
| Learning/Memory | |||||
| GVLT Total Words | 56.9 (9.7) | 57.6 (10.0) | 55.5 (11.4) | 0.33 | −0.19 |
| BVMT-R Total Recall | 24.1 (9.1) | 25.2 (8.1) | 25.9 (8.1) | 0.70 | 0.08 |
| GVLT Long Delay/Free Recall | 13.3 (3.0) | 13.6 (2.9) | 13.5 (2.6) | 0.81 | −0.05 |
| GVLT Long Delay/Cued Recall | 13.6 (2.5) | 13.8 (2.7) | 13.8 (2.7) | 0.90 | 0.02 |
| BVMT Delayed Recall | 10.1 (2.8) | 10.1 (3.0) | 9.8 (3.2) | 0.21 | −0.25 |
| Speed of information processing | |||||
| TMT-A | 32.2 (11.6) | 31.5 (11.3) | 28.0 (9.7) | 0.07 | −0.36 |
| SDMT | 49.8 (12.0) | 50.3 (12.3) | 53.8 (15.1) | 0.10 | 0.32 |
| Executive function | |||||
| TMT-B | 84.1 (59.7) | 78.6 (61.0) | 59.5 (31.3) | 0.06 | −0.39 |
| Stroop Color and Word | 95.6 (20.2) | 97.2 (19.1) | 102.4 (16.0) | 0.02 | 0.52 |
| Verbal fluency | |||||
| Phonemic Fluency | 32.6 (11.5) | 31.6 (11.9) | 35 (12.4) | 0.03 | 0.44 |
| Semantic Fluency | 49.5 (9.1) | 50.6 (9.8) | 48.2 (10.1) | 0.17 | −0.28 |
| Visuospatial ability | |||||
| Judgement of Line Orientation | 16.6 (2.7) | 16.8 (2.8) | 18.0 (2.2) | 0.007 | 0.56 |
| Motor dexterity | |||||
| Grooved Pegboard (dominant hand) | 72.4 (13.5) | 72.0 (12.4) | 69.2 (15.1) | 0.25 | −0.25 |
| Grooved Pegboard (non-dominant hand) | 79.5 (21.0) | 81.6 (23.4) | 77.8 (13.5) | 0.34 | −0.20 |
| Attention/Working memory | |||||
| Spatial Span Forward | 8.9 (2.6) | 9.0 (2.8) | 8.4 (1.9) | 0.15 | −0.28 |
| Spatial Span Backward | 7.4 (2.4) | 7.4 (2.6) | 7.3 (2.4) | 0.76 | −0.06 |
| Letter-Number Sequencing | 9.3 (2.8) | 9.4 (3.0) | 9.2 (2.9) | 0.63 | −0.09 |
| Mental health | |||||
| HADS Depression, median (IQR) Score > 7, n (%) Anxiety, median (IQR) Score > 7, n (%) | 2 (1–4.5) 2 (5.7) 4 (1–8) 9 (25.7) | 1 (1–5) 4 (14.3) 4 (1–7) 5 (17.9) | 0.67 # 0.43 # | ||
| Biomarker $ | LOD | <LOD (%) * | Baseline (n = 38) | Follow-Up (n = 33) | p # | r |
|---|---|---|---|---|---|---|
| IL-1b | 3.9 | 66.7 | - | - | - | - |
| IL-6 | 9.4 | 63.9 | - | - | - | - |
| IL-10 | 31.2 | 63.9 | - | - | - | - |
| TNFa | 15.6 | 25 | 12.6 (3.9–187.9) | 3.9 (3.9–37.6) | 0.005 | 0.50 |
| MCP1 | 15.6 | 13.9 | 49.8 (28.5–76.1) | 35 (28–47.5) | 0.006 | 0.47 |
| MIP-1b | 15.6 | 16.7 | 26.7 (16.3–48.4) | 33.4 (19.4–63.1) | 0.851 | 0.03 |
| sCD14 | 62.5 | 0 | 1620 (1196–2129) | 858 (553–1154) | <0.001 | 0.68 |
| sCD163 | 156 | 0 | 627 (506–830) | 288 (239–374) | <0.001 | 0.86 |
| S100B | 46.9 | 63.9 | - | - | - | - |
| Neopterin | 0.156 | 0 | 10.6 (7–13.9) | 13 (9.9–17) | 0.002 | 0.54 |
| NFL | 1.59 | 0 | 7.5 (5.5–10.6) | 7.2 (5.7–9.1) | 0.524 | 0.11 |
| Baseline | Follow-Up | |||||||
|---|---|---|---|---|---|---|---|---|
| Non-HAND | HAND | d/r ^ | p | Non-HAND | HAND | d/r | p | |
| Age (y) $ | 34.3 (10.4) | 41.2 (10.2) | 0.67 | 0.06 | 35.2 (9.9) | 42.9 (8.9) | 0.8 | 0.06 |
| Education (y) | 14.8 (1.9) | 13.7 (3.4) | 0.43 | 0.24 | 15.6 (1.8) | 12.2 (3.3) | 1.42 | 0.002 |
| CD4+ (cells/μL) | 432 (196) | 379 (170) | 0.28 | 0.41 | 809 (248) | 807 (458) | 0.01 | 0.99 |
| CD8+ (cells/μL) | 1296 (958) | 951 (460) | 0.46 | 0.20 | 1041 (451) | 959 (542) | 0.17 | 0.68 |
| CD4+/CD8+ ratio | 0.99 (0.4) | 0.85 (0.5) | 0.08 | 0.37 | 0.86 (0.35) | 0.90 (0.42) | 0.09 | 0.82 |
| HIV RNA (log10 copies/mL) | 5.15 (0.83) | 4.96 (1.3) | 0.19 | 0.61 | - | - | - | |
| TNFa # | 21.5 (6–189) | 11.1 (3–92) | 0.17 | 0.31 | 18 (3.9–158) | 3.9 (3.9–8) | 0.30 | 0.11 |
| MCP1 | 49.8 (28–76) | 52.1 (30–76) | 0 | 1.0 | 42 (31–49) | 27.6 (19–37) | 0.30 | 0.11 |
| MIP-1b | 35.5 (17–77) | 23.1 (12–31) | 0.25 | 0.15 | 38 (22–65) | 34 (19–102) | 0.02 | 0.88 |
| sCD14 | 1534 (1124–2091) | 1646 (1205–2590) | 0.13 | 0.45 | 787 (511–1167) | 858 (514–1133) | 0.05 | 0.79 |
| sCD163 | 626 (505–860) | 630 (519–821) | 0.06 | 0.72 | 277 (208–320) | 295 (262–402) | 0.17 | 0.36 |
| Neopterin | 11.2 (7–14) | 10 (7–14) | 0.01 | 0.97 | 13.3 (10–20) | 13 (10–17) | 0.08 | 0.68 |
| NFL | 7.6 (6–11) | 7.2 (6–11) | 0.03 | 0.85 | 7.7 (6–10) | 6.6 (5–9) | 0.23 | 0.23 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moschopoulos, C.D.; Stanitsa, E.; Protopapas, K.; Vatsi, A.; Galani, I.; Zetterberg, H.; Beratis, I.; Fragkou, P.C.; Tsiodras, S.; Kavatha, D.; et al. Prospective Neuropsychological and Plasma Biomarker Changes in Treatment-Naïve People Living with HIV After Antiretroviral Treatment Initiation. Biomedicines 2025, 13, 1704. https://doi.org/10.3390/biomedicines13071704
Moschopoulos CD, Stanitsa E, Protopapas K, Vatsi A, Galani I, Zetterberg H, Beratis I, Fragkou PC, Tsiodras S, Kavatha D, et al. Prospective Neuropsychological and Plasma Biomarker Changes in Treatment-Naïve People Living with HIV After Antiretroviral Treatment Initiation. Biomedicines. 2025; 13(7):1704. https://doi.org/10.3390/biomedicines13071704
Chicago/Turabian StyleMoschopoulos, Charalampos D., Evangelia Stanitsa, Konstantinos Protopapas, Akrivi Vatsi, Irene Galani, Henrik Zetterberg, Ion Beratis, Paraskevi C. Fragkou, Sotirios Tsiodras, Dimitra Kavatha, and et al. 2025. "Prospective Neuropsychological and Plasma Biomarker Changes in Treatment-Naïve People Living with HIV After Antiretroviral Treatment Initiation" Biomedicines 13, no. 7: 1704. https://doi.org/10.3390/biomedicines13071704
APA StyleMoschopoulos, C. D., Stanitsa, E., Protopapas, K., Vatsi, A., Galani, I., Zetterberg, H., Beratis, I., Fragkou, P. C., Tsiodras, S., Kavatha, D., Papadopoulos, A., Papageorgiou, S. G., & Antoniadou, A. (2025). Prospective Neuropsychological and Plasma Biomarker Changes in Treatment-Naïve People Living with HIV After Antiretroviral Treatment Initiation. Biomedicines, 13(7), 1704. https://doi.org/10.3390/biomedicines13071704












