Artificial Intelligence and ECG: A New Frontier in Cardiac Diagnostics and Prevention
Abstract
1. Introduction
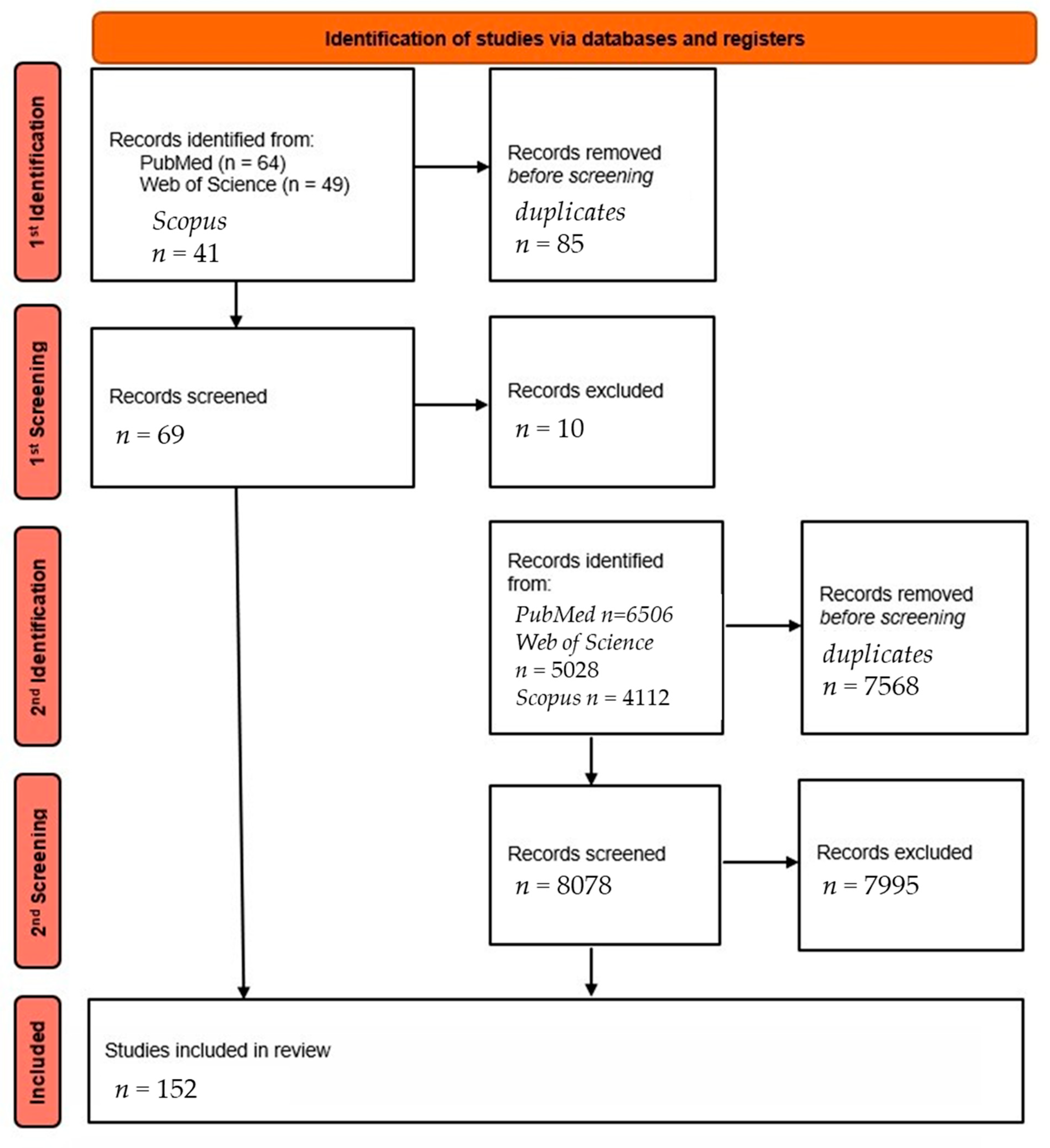
2. Materials and Methods
3. AI in ECG Analysis
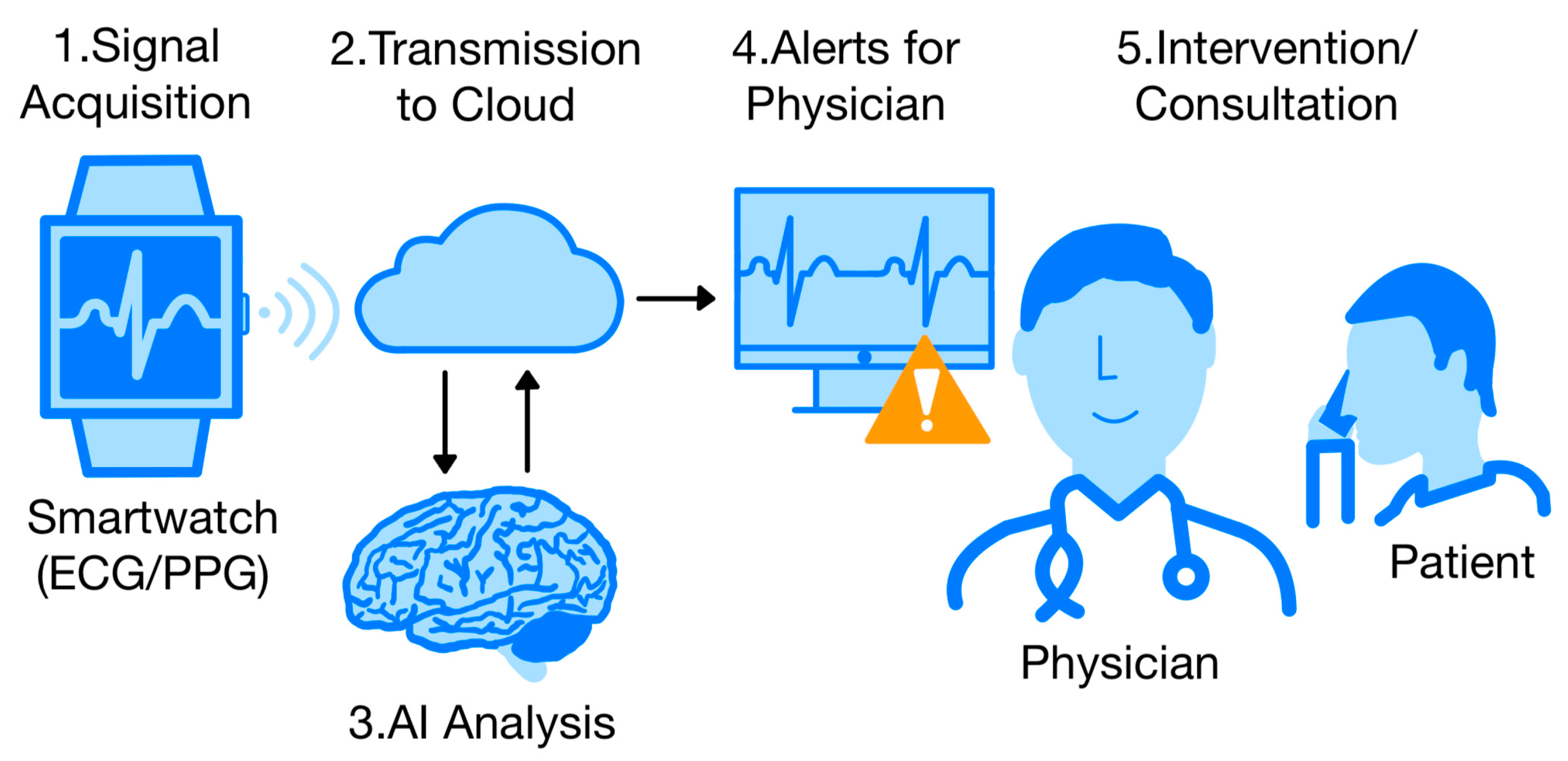
4. The Use of Wearable Devices Combined with Artificial Intelligence (AI)
4.1. Detection of Atrial Fibrillation (AF)
4.2. Assessment of Ejection Fraction (EF) and Heart Failure (HF)
4.3. Assessment of QTc Distance and Electrolytic Disturbance
4.4. Sleep Disorders and Detection of ECG Pathology in Children
4.5. Advanced ECG Signal Reconstruction and Asynchronous Measurements
5. Large Language Models (LLMs) in Cardiology
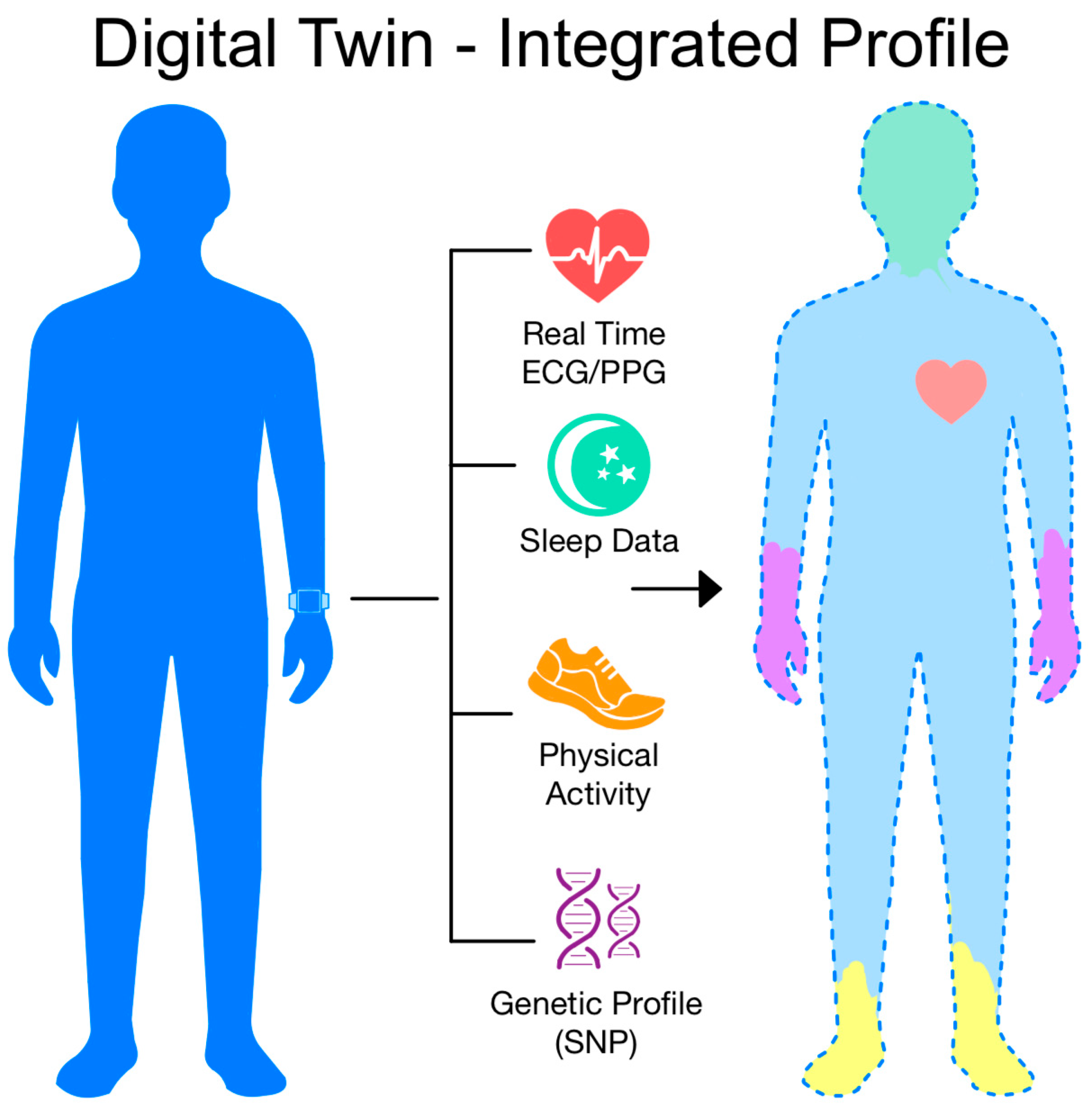
6. Future Directions and Innovative Concepts
7. Integration with the Healthcare System
8. Challenges and Current Limitations
8.1. Regulation and Data Safety
8.2. Technological Limitations
8.3. Algorithmic and Quality Problems
8.4. Bias and Generalizability
8.5. Lack of Hard Clinical Evidence
8.6. Differences in Adoption of Wearable Devices
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Heart Report 2025. Available online: https://world-heart-federation.org/report2025/ (accessed on 3 June 2025).
- Shimizu, M.; Misumi, M.; Yamada, M.; Ohishi, W.; Yamamoto, H.; Kihara, Y. Choice Reaction Time and Grip Strength as Predictors of Cardiovascular Mortality in Middle-Aged and Elderly Japanese: From the Radiation Effects Research Foundation Adult Health Study. Intern. Med. J. 2018, 48, 1331–1336. [Google Scholar] [CrossRef] [PubMed]
- Ukil, A.; Bandyopadhyay, S. Automated Cardiac Health Screening Using Smartphone and Wearable Sensors through Anomaly Analytics. In Mobile Solutions and Their Usefulness in Everyday Life, 1st ed.; Sara, P., Ed.; Springer: Cham, Switzerland, 2019; pp. 145–172. [Google Scholar] [CrossRef]
- Hempel, P.; Bender, T.; Spicher, N. Enhancing Explainability in ECG Analysis through Evidence-Based AI Interpretability. In Proceedings of the 2024 46th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Orlando, FL, USA, 15–19 July 2024. [Google Scholar] [CrossRef]
- Di Costanzo, A.; Spaccarotella, C.A.M.; Esposito, G.; Indolfi, C. An Artificial Intelligence Analysis of Electrocardiograms for the Clinical Diagnosis of Cardiovascular Diseases: A Narrative Review. J. Clin. Med. 2024, 13, 1033. [Google Scholar] [CrossRef]
- Kolhar, M.; Kazi, R.N.A.; Mohapatra, H.; Al Rajeh, A.M. AI-Driven Real-Time Classification of ECG Signals for Cardiac Monitoring Using i-AlexNet Architecture. Diagnostics 2024, 14, 1344. [Google Scholar] [CrossRef]
- Hong, S.; Heo, J.; Park, K.S. Signal Quality Index Based on Template Cross-Correlation in Multimodal Biosignal Chair for Smart Healthcare. Sensors 2021, 21, 7564. [Google Scholar] [CrossRef]
- Geweid, G.G.N.; Chen, J.D.Z. Automatic Classification of Atrial Fibrillation from Short Single-Lead ECG Recordings Using a Hybrid Approach of Dual Support Vector Machine. Expert Syst. Appl. 2022, 198, 116848. [Google Scholar] [CrossRef]
- He, K.; Liang, W.; Liu, S.; Bian, L.; Xu, Y.; Luo, C.; Li, Y.; Yue, H.; Yang, C.; Wu, Z. Long-Term Single-Lead Electrocardiogram Monitoring to Detect New-Onset Postoperative Atrial Fibrillation in Patients after Cardiac Surgery. Front. Cardiovasc. Med. 2022, 9, 1001883. [Google Scholar] [CrossRef]
- Hsu, C.-Y.; Liu, P.-Y.; Liu, S.-H.; Kwon, Y.; Lavie, C.J.; Lin, G.-M. Machine Learning for Electrocardiographic Features to Identify Left Atrial Enlargement in Young Adults: CHIEF Heart Study. Front. Cardiovasc. Med. 2022, 9, 840585. [Google Scholar] [CrossRef]
- Zheng, Z.C.; Yuan, W.; Wang, N.; Jiang, B.; Ma, C.P.; Ai, H.; Wang, X.; Nie, S.P. Exploring the Feasibility of Machine Learning to Predict Risk Stratification Within 3 Months in Chest Pain Patients with Suspected NSTE-ACS. Biomed. Environ. Sci. 2023, 36, 625–634. [Google Scholar] [CrossRef]
- Guo, L.; Gao, C.; Yang, W.; Ma, Z.; Zhou, M.; Liu, J.; Shao, H.; Wang, B.; Hu, G.; Zhao, H.; et al. Derivation and Validation of a Screening Model for Hypertrophic Cardiomyopathy Based on Electrocardiogram Features. Front. Cardiovasc. Med. 2022, 9, 889523. [Google Scholar] [CrossRef]
- Zheng, J.; Fu, G.; Struppa, D.; Abudayyeh, I.; Contractor, T.; Anderson, K.; Chu, H.; Rakovski, C. A High Precision Machine Learning-Enabled System for Predicting Idiopathic Ventricular Arrhythmia Origins. Front. Cardiovasc. Med. 2022, 9, 809027. [Google Scholar] [CrossRef]
- Zhu, H.; Zhao, Y.; Pan, Y.; Xie, H.; Wu, F.; Huan, R. Robust Heartbeat Classification for Wearable Single-Lead ECG via Extreme Gradient Boosting. Sensors 2021, 21, 5290. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Chaudhary, R.; Bliden, K.P.; Tantry, U.S.; Gurbel, P.A.; Visweswaran, S.; Harinstein, M.E. Meta-Analysis of the Performance of AI-Driven ECG Interpretation in the Diagnosis of Valvular Heart Diseases. Am. J. Cardiol. 2024, 213, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Khan Mamun, M.M.R.; Elfouly, T. Detection of Cardiovascular Disease from Clinical Parameters Using a One-Dimensional Convolutional Neural Network. Bioengineering 2023, 10, 796. [Google Scholar] [CrossRef]
- Li, Y.; Qian, R.; Li, K. Inter-Patient Arrhythmia Classification with Improved Deep Residual Convolutional Neural Network. Comput. Methods Programs Biomed. 2022, 214, 106582. [Google Scholar] [CrossRef]
- Sawano, S.; Kodera, S.; Katsushika, S.; Nakamoto, M.; Ninomiya, K.; Shinohara, H.; Higashikuni, Y.; Nakanishi, K.; Nakao, T.; Seki, T.; et al. Deep Learning Model to Detect Significant Aortic Regurgitation Using Electrocardiography. J. Cardiol. 2022, 79, 334–341. [Google Scholar] [CrossRef]
- Che, C.; Zhang, P.; Zhu, M.; Qu, Y.; Jin, B. Constrained Transformer Network for ECG Signal Processing and Arrhythmia Classification. BMC Med. Inform. Decis. Mak. 2021, 21, 184. [Google Scholar] [CrossRef]
- Hua, Q.; Yaqin, Y.; Wan, B.; Chen, B.; Zhong, Y.; Pan, J. An Interpretable Model for ECG Data Based on Bayesian Neural Networks. IEEE Access 2021, 9, 57001–57009. [Google Scholar] [CrossRef]
- Hua, J.; Rao, J.; Peng, Y.; Liu, J.; Tang, J. Deep Compressive Sensing on ECG Signals with Modified Inception Block and LSTM. Entropy 2022, 24, 1024. [Google Scholar] [CrossRef]
- Shyam Kumar, P.; Ramasamy, M.; Kallur, K.R.; Rai, P.; Varadan, V.K. Personalized LSTM Models for ECG Lead Transformations Led to Fewer Diagnostic Errors Than Generalized Models: Deriving 12-Lead ECG from Lead II, V2, and V6. Sensors 2023, 23, 1389. [Google Scholar] [CrossRef]
- Khurshid, S.; Friedman, S.; Reeder, C.; Di Achille, P.; Diamant, N.; Singh, P.; Harrington, L.X.; Wang, X.; Al-Alusi, M.A.; Sarma, G.; et al. ECG-Based Deep Learning and Clinical Risk Factors to Predict Atrial Fibrillation. Circulation 2022, 145, 122–133. [Google Scholar] [CrossRef]
- Attia, Z.I.; Noseworthy, P.A.; Lopez-Jimenez, F.; Asirvatham, S.J.; Deshmukh, A.J.; Gersh, B.J.; Carter, R.E.; Yao, X.; Rabinstein, A.A.; Erickson, B.J.; et al. An Artificial Intelligence-Enabled ECG Algorithm for the Identification of Patients with Atrial Fibrillation during Sinus Rhythm: A Retrospective Analysis of Outcome Prediction. Lancet 2019, 394, 861–867. [Google Scholar] [CrossRef] [PubMed]
- Cersosimo, A.; Zito, E.; Pierucci, N.; Matteucci, A.; La Fazia, V.M. A Talk with ChatGPT: The Role of Artificial Intelligence in Shaping the Future of Cardiology and Electrophysiology. J. Pers. Med. 2025, 15, 205. [Google Scholar] [CrossRef]
- Svennberg, E.; Han, J.K.; Caiani, E.G.; Engelhardt, S.; Ernst, S.; Friedman, P.; Garcia, R.; Ghanbari, H.; Hindricks, G.; Man, S.H.; et al. State of the Art of Artificial Intelligence in Clinical Electrophysiology in 2025: A Scientific Statement of the European Heart Rhythm Association (EHRA) of the ESC, the Heart Rhythm Society (HRS), and the ESC Working Group on E-Cardiology. Europace 2025, 27, euaf071. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Razeghi, O.; Kapoor, R.; Alhusseini, M.I.; Fazal, M.; Rogers, A.J.; Bort, M.R.; Clopton, P.; Wang, P.; Rubin, D.; et al. Machine Learning-Enabled Multimodal Fusion of Intra-Atrial and Body Surface Signals in Prediction of Atrial Fibrillation Ablation Outcomes. Circ. Arrhythm. Electrophysiol. 2022, 15, e010850. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-S.; Liu, W.-T.; Tsai, D.-J.; Lou, Y.-S.; Chang, C.-H.; Lee, C.-C.; Fang, W.-H.; Wang, C.-C.; Chen, Y.-Y.; Lin, W.-S.; et al. AI-Enabled Electrocardiography Alert Intervention and All-Cause Mortality: A Pragmatic Randomized Clinical Trial. Nat. Med. 2024, 30, 1461–1470. [Google Scholar] [CrossRef]
- Yao, X.; McCoy, R.G.; Friedman, P.A.; Shah, N.D.; Barry, B.A.; Behnken, E.M.; Inselman, J.W.; Attia, Z.I.; Noseworthy, P.A. ECG AI-Guided Screening for Low Ejection Fraction (EAGLE): Rationale and Design of a Pragmatic Cluster Randomized Trial. Am. Heart. J. 2020, 219, 31–36. [Google Scholar] [CrossRef]
- Yao, X.; Rushlow, D.R.; Inselman, J.W.; McCoy, R.G.; Thacher, T.D.; Behnken, E.M.; Bernard, M.E.; Rosas, S.L.; Akfaly, A.; Misra, A.; et al. Artificial Intelligence-Enabled Electrocardiograms for Identification of Patients with Low Ejection Fraction: A Pragmatic, Randomized Clinical Trial. Nat. Med. 2021, 27, 815–819. [Google Scholar] [CrossRef]
- Chokshi, S.; Tologonova, G.; Calixte, R.; Yadav, V.; Razvi, N.; Lazar, J.; Kachnowski, S. Comparison Between QT and Corrected QT Interval Assessment by an Apple Watch With the AccurBeat Platform and by a 12-Lead Electrocardiogram With Manual Annotation: Prospective Observational Study. JMIR Form. Res. 2022, 6, e41241. [Google Scholar] [CrossRef]
- Popat, A.; Yadav, S.; Obholz, J.; Hwang, E.A.; Rehman, A.U.; Sharma, P. The Efficacy of Artificial Intelligence in the Detection and Management of Atrial Fibrillation. Cureus 2025, 17, e77135. [Google Scholar] [CrossRef]
- Raissi Dehkordi, N.; Raissi Dehkordi, N.; Karimi Toudeshki, K.; Farjoo, M.H. Artificial Intelligence in Diagnosis of Long QT Syndrome: A Review of Current State, Challenges, and Future Perspectives. Mayo. Clin. Proc. Digit. Health 2024, 2, 21–31. [Google Scholar] [CrossRef]
- Nearing, B.D.; Verrier, R.L. Novel Application of Convolutional Neural Networks for Artificial Intelligence-Enabled Modified Moving Average Analysis of P-, R-, and T-Wave Alternans for Detection of Risk for Atrial and Ventricular Arrhythmias. J. Electrocardiol. 2024, 83, 12–20. [Google Scholar] [CrossRef]
- Ortega-Martorell, S.; Olier, I.; Ohlsson, M.; Lip, G.Y.H. TARGET Consortium Advancing Personalised Care in Atrial Fibrillation and Stroke: The Potential Impact of AI from Prevention to Rehabilitation. Trends. Cardiovasc. Med. 2025, 35, 205–211. [Google Scholar] [CrossRef]
- Lee, H.S.; Han, G.I.; Kim, K.-H.; Kang, S.; Jang, J.-H.; Jo, Y.-Y.; Son, J.M.; Lee, M.S.; Kwon, J.-M.; Oh, B.-H. Electrocardiographic-Driven Artificial Intelligence Model: A New Approach to Predicting One-Year Mortality in Heart Failure with Reduced Ejection Fraction Patients. Int. J. Med. Inform. 2025, 197, 105843. [Google Scholar] [CrossRef]
- Devkota, A.; Prajapati, R.; El-Wakeel, A.; Adjeroh, D.; Patel, B.; Gyawali, P. AI Analysis for Ejection Fraction Estimation from 12-Lead ECG. Sci. Rep. 2025, 15, 13502. [Google Scholar] [CrossRef]
- Love, C.J.; Lampert, J.; Huneycutt, D.; Musat, D.L.; Shah, M.; Enciso, J.E.S.; Doherty, B.; Gentry, J.L.; Kwan, M.D.; Carter, E.C.; et al. Clinical Implementation of an AI-Enabled ECG for Hypertrophic Cardiomyopathy Detection. Heart 2024. [Google Scholar] [CrossRef]
- Lee, M.S.; Shin, T.G.; Lee, Y.; Kim, D.H.; Choi, S.H.; Cho, H.; Lee, M.J.; Jeong, K.Y.; Kim, W.Y.; Min, Y.G.; et al. Artificial Intelligence Applied to Electrocardiogram to Rule out Acute Myocardial Infarction: The ROMIAE Multicentre Study. Eur. Heart. J. 2025, 46, 1917–1929. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Zeng, X.; Wu, F.; Chu, Y.; Wei, W.; Fan, M.; Pang, C.; Hu, X. Build a Bridge between ECG and EEG Signals for Atrial Fibrillation Diagnosis Using AI Methods. Comput. Biol. Med. 2023, 166, 107429. [Google Scholar] [CrossRef]
- Chakshu, N.K.; Nithiarasu, P. Orbital Learning: A Novel, Actively Orchestrated Decentralised Learning for Healthcare. Sci. Rep. 2024, 14, 10459. [Google Scholar] [CrossRef]
- Baumgartner, M.; Veeranki, S.P.K.; Hayn, D.; Schreier, G. Introduction and Comparison of Novel Decentral Learning Schemes with Multiple Data Pools for Privacy-Preserving ECG Classification. J. Healthc. Inform. Res. 2023, 7, 291–312. [Google Scholar] [CrossRef]
- Tölle, M.; Burger, L.; Kelm, H.; André, F.; Bannas, P.; Diller, G.; Frey, N.; Garthe, P.; Groß, S.; Hennemuth, A.; et al. Multi-Modal Dataset Creation for Federated Learning with DICOM-Structured Reports. Int. J. Comput. Assist. Radiol. Surg. 2025, 20, 485–495. [Google Scholar] [CrossRef]
- Selder, J.L.; Te Kolste, H.J.; Twisk, J.; Schijven, M.; Gielen, W.; Allaart, C.P. Accuracy of a Standalone Atrial Fibrillation Detection Algorithm Added to a Popular Wristband and Smartwatch: Prospective Diagnostic Accuracy Study. J. Med. Internet Res. 2023, 25, e44642. [Google Scholar] [CrossRef] [PubMed]
- Sowiński, P.; Rachwał, K.; Danilenka, A.; Bogacka, K.; Kobus, M.; Dąbrowska, A.; Paszkiewicz, A.; Bolanowski, M.; Ganzha, M.; Paprzycki, M. Frugal Heart Rate Correction Method for Scalable Health and Safety Monitoring in Construction Sites. Sensors 2023, 23, 6464. [Google Scholar] [CrossRef]
- Polak, A.G.; Klich, B.; Saganowski, S.; Prucnal, M.A.; Kazienko, P. Processing Photoplethysmograms Recorded by Smartwatches to Improve the Quality of Derived Pulse Rate Variability. Sensors 2022, 22, 7047. [Google Scholar] [CrossRef] [PubMed]
- Baca, H.A.H.; Toia, A.M.D.C.; Torres, J.A.S.; Montesinos, R.C.; Salas, L.A.C.; Herrera, S.C.C. Detection of Arrhythmias Using Heart Rate Signals from Smartwatches. Appl. Sci. 2024, 14, 7233. [Google Scholar] [CrossRef]
- Tang, Q.; Chen, Z.; Ward, R.; Menon, C.; Elgendi, M. PPG2ECGps: An End-to-End Subject-Specific Deep Neural Network Model for Electrocardiogram Reconstruction from Photoplethysmography Signals without Pulse Arrival Time Adjustments. Bioengineering 2023, 10, 630. [Google Scholar] [CrossRef]
- Hermans, A.N.L.; Isaksen, J.L.; Gawalko, M.; Pluymaekers, N.A.H.A.; van der Velden, R.M.J.; Snippe, H.; Evens, S.; De Witte, G.; Luermans, J.G.L.M.; Manninger, M.; et al. Accuracy of Continuous Photoplethysmography-Based 1 Min Mean Heart Rate Assessment during Atrial Fibrillation. Europace 2023, 25, 835–844. [Google Scholar] [CrossRef]
- Huette, P.; Beyls, C.; Diouf, M.; Ibrahima, A.; Haye, G.; Guilbart, M.; Lefebvre, T.; Bayart, G.; Lhotellier, F.; Radji, M.; et al. Study Protocol: Diagnosis of Atrial Fibrillation in Postoperative Thoracic Surgery Using a Smartwatch, an Open-Label Randomised Controlled Study (THOFAWATCH Trial). BMJ Open 2025, 15, e097765. [Google Scholar] [CrossRef]
- Badertscher, P.; Lischer, M.; Mannhart, D.; Knecht, S.; Isenegger, C.; Du Fay de Lavallaz, J.; Schaer, B.; Osswald, S.; Kühne, M.; Sticherling, C. Clinical Validation of a Novel Smartwatch for Automated Detection of Atrial Fibrillation. Heart Rhythm O2 2022, 3, 208–210. [Google Scholar] [CrossRef]
- Mannhart, D.; Lefebvre, B.; Gardella, C.; Henry, C.; Serban, T.; Knecht, S.; Kühne, M.; Sticherling, C.; Badertscher, P. Clinical Validation of an Artificial Intelligence Algorithm Offering Cross-Platform Detection of Atrial Fibrillation Using Smart Device Electrocardiograms. Arch. Cardiovasc. Dis. 2023, 116, 249–257. [Google Scholar] [CrossRef]
- Weidlich, S.; Mannhart, D.; Kennedy, A.; Doggart, P.; Serban, T.; Knecht, S.; Du Fay de Lavallaz, J.; Kühne, M.; Sticherling, C.; Badertscher, P. Reducing the Burden of Inconclusive Smart Device Single-Lead ECG Tracings via a Novel Artificial Intelligence Algorithm. Cardiovasc. Digit. Health J. 2024, 5, 29–35. [Google Scholar] [CrossRef]
- Ng, Y.; Liao, M.-T.; Chen, T.-L.; Lee, C.-K.; Chou, C.-Y.; Wang, W. Few-Shot Transfer Learning for Personalized Atrial Fibrillation Detection Using Patient-Based Siamese Network with Single-Lead ECG Records. Artif. Intell. Med. 2023, 144, 102644. [Google Scholar] [CrossRef] [PubMed]
- Jaeger, K.M.; Nissen, M.; Flaucher, M.; Graf, L.; Joanidopoulos, J.; Anneken, L.; Huebner, H.; Goossens, C.; Titzmann, A.; Pontones, C.; et al. Systematic Comparison of ECG Delineation Algorithm Performance on Smartwatch Data. IEEE Access 2024, 12, 160794–160804. [Google Scholar] [CrossRef]
- Fiorina, L.; Chemaly, P.; Cellier, J.; Said, M.A.; Coquard, C.; Younsi, S.; Salerno, F.; Horvilleur, J.; Lacotte, J.; Manenti, V.; et al. Artificial Intelligence-Based Electrocardiogram Analysis Improves Atrial Arrhythmia Detection from a Smartwatch Electrocardiogram. Eur. Heart. J. Digit. Health 2024, 5, 535–541. [Google Scholar] [CrossRef]
- Radke, P. Artificial Intelligence in Cardiovascular Medicine—Status and Perspectives. Akt. Kardiol. 2023, 12, 433–438. [Google Scholar] [CrossRef]
- Antiperovitch, P.; Mortara, D.; Barrios, J.; Avram, R.; Yee, K.; Khaless, A.N.; Cristal, A.; Tison, G.; Olgin, J. Continuous Atrial Fibrillation Monitoring From Photoplethysmography: Comparison Between Supervised Deep Learning and Heuristic Signal Processing. JACC Clin. Electrophysiol. 2024, 10, 334–345. [Google Scholar] [CrossRef]
- Pan, Y.; Chen, E.; Jie, S.; Huo, D.; Ding, Z.; Zhou, J.; Jiang, J.; Li, J.; Huo, Y. Continuous Atrial Fibrillation Monitoring Using a Wearable Smartwatch: Using Long-Term Holter as Reference. Digit. Health 2025, 11, 20552076251314105. [Google Scholar] [CrossRef]
- van Vliet, M.; Aalberts, J.J.J.; Hamelinck, C.; Hauer, A.D.; Hoftijzer, D.; Monnink, S.H.J.; Schipper, J.C.; Constandse, J.C.; Peters, N.S.; Lip, G.Y.H.; et al. Ambulatory Atrial Fibrillation Detection and Quantification by Wristworn AI Device Compared to Standard Holter Monitoring. NPJ Digit. Med. 2025, 8, 177. [Google Scholar] [CrossRef] [PubMed]
- Wasserlauf, J.; You, C.; Patel, R.; Valys, A.; Albert, D.; Passman, R. Smartwatch Performance for the Detection and Quantification of Atrial Fibrillation. Circ. Arrhythm. Electrophysiol. 2019, 12, e006834. [Google Scholar] [CrossRef] [PubMed]
- Attia, Z.I.; Harmon, D.M.; Dugan, J.; Manka, L.; Lopez-Jimenez, F.; Lerman, A.; Siontis, K.C.; Noseworthy, P.A.; Yao, X.; Klavetter, E.W.; et al. Prospective Evaluation of Smartwatch-Enabled Detection of Left Ventricular Dysfunction. Nat. Med. 2022, 28, 2497–2503. [Google Scholar] [CrossRef]
- Dhingra, L.S.; Aminorroaya, A.; Pedroso, A.F.; Khunte, A.; Sangha, V.; McIntyre, D.; Chow, C.K.; Asselbergs, F.W.; Brant, L.C.C.; Barreto, S.M.; et al. Artificial Intelligence-Enabled Prediction of Heart Failure Risk From Single-Lead Electrocardiograms. JAMA Cardiol. 2025, 10, e250492. [Google Scholar] [CrossRef]
- Lee, H.S.; Kang, S.; Jo, Y.-Y.; Son, J.M.; Lee, M.S.; Kwon, J.-M.; Kim, K.-H. AI-Enabled Smartwatch ECG: A Feasibility Study for Early Prediction and Prevention of Heart Failure Rehospitalization. JACC Basic Transl. Sci. 2025, 10, 250–252. [Google Scholar] [CrossRef] [PubMed]
- Srutova, M.; Kremen, V.; Lhotska, L. Electrocardiographic Discrimination of Long QT Syndrome Genotypes: A Comparative Analysis and Machine Learning Approach. Sensors 2025, 25, 2253. [Google Scholar] [CrossRef] [PubMed]
- Hoek, L.J.; Brouwer, J.L.P.; Voors, A.A.; Maass, A.H. Smart Devices to Measure and Monitor QT Intervals. Front. Cardiovasc. Med. 2023, 10, 1172666. [Google Scholar] [CrossRef]
- Maille, B.; Wilkin, M.; Million, M.; Rességuier, N.; Franceschi, F.; Koutbi-Franceschi, L.; Hourdain, J.; Martinez, E.; Zabern, M.; Gardella, C.; et al. Smartwatch Electrocardiogram and Artificial Intelligence for Assessing Cardiac-Rhythm Safety of Drug Therapy in the COVID-19 Pandemic. The QT-Logs Study. Int. J. Cardiol. 2021, 331, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Chiu, I.-M.; Wu, P.-J.; Zhang, H.; Hughes, J.W.; Rogers, A.J.; Jalilian, L.; Perez, M.; Lin, C.-H.R.; Lee, C.-T.; Zou, J.; et al. Serum Potassium Monitoring Using AI-Enabled Smartwatch Electrocardiograms. JACC Clin. Electrophysiol. 2024, 10, 2644–2654. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.M.; Itti, L.; Sheth, B.R. Expert-Level Sleep Staging Using an Electrocardiography-Only Feed-Forward Neural Network. Comput. Biol. Med. 2024, 176, 108545. [Google Scholar] [CrossRef]
- Teich, L.; Franke, D.; Michaelis, A.; Daehnert, R.A.; Gebauer, R.A.; Markel, F.; Paech, C. Development of an AI Based Automated Analysis of Pediatric Apple Watch iECGs. Front. Pediatr. 2023, 11, 1185629. [Google Scholar] [CrossRef]
- Han, C.; Song, Y.; Lim, H.-S.; Tae, Y.; Jang, J.-H.; Lee, B.T.; Lee, Y.; Bae, W.; Yoon, D. Automated Detection of Acute Myocardial Infarction Using Asynchronous Electrocardiogram Signals-Preview of Implementing Artificial Intelligence With Multichannel Electrocardiographs Obtained From Smartwatches: Retrospective Study. J. Med. Internet Res. 2021, 23, e31129. [Google Scholar] [CrossRef]
- Behzadi, A.; Sepehri Shamloo, A.; Mouratis, K.; Hindricks, G.; Arya, A.; Bollmann, A. Feasibility and Reliability of SmartWatch to Obtain 3-Lead Electrocardiogram Recordings. Sensors 2020, 20, 5074. [Google Scholar] [CrossRef]
- Choi, J.; Kim, J.; Spaccarotella, C.; Esposito, G.; Oh, I.-Y.; Cho, Y.; Indolfi, C. Smartwatch ECG and Artificial Intelligence in Detecting Acute Coronary Syndrome Compared to Traditional 12-Lead ECG. Int. J. Cardiol. Heart Vasc. 2025, 56, 101573. [Google Scholar] [CrossRef]
- Presacan, O.; Dorobanţiu, A.; Isaksen, J.L.; Willi, T.; Graff, C.; Riegler, M.A.; Sridhar, A.R.; Kanters, J.K.; Thambawita, V. Evaluating the Feasibility of 12-Lead Electrocardiogram Reconstruction from Limited Leads Using Deep Learning. Commun. Med. 2025, 5, 139. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.-M.; Jo, Y.-Y.; Lee, S.Y.; Kang, S.; Lim, S.-Y.; Lee, M.S.; Kim, K.-H. Artificial Intelligence-Enhanced Smartwatch ECG for Heart Failure-Reduced Ejection Fraction Detection by Generating 12-Lead ECG. Diagnostics 2022, 12, 654. [Google Scholar] [CrossRef]
- Angelaki, E.; Barmparis, G.D.; Fragkiadakis, K.; Maragkoudakis, S.; Zacharis, E.; Plevritaki, A.; Kampanieris, E.; Kalomoirakis, P.; Kassotakis, S.; Kochiadakis, G.; et al. Diagnostic Performance of Single-Lead Electrocardiograms for Arterial Hypertension Diagnosis: A Machine Learning Approach. J. Hum. Hypertens. 2025, 39, 58–65. [Google Scholar] [CrossRef] [PubMed]
- ECG-Chat: A Large ECG-Language Model for Cardiac Disease Diagnosis. Available online: https://arxiv.org/abs/2408.08849 (accessed on 4 July 2025).
- Zhu, L.; Mou, W.; Wu, K.; Lai, Y.; Lin, A.; Yang, T.; Zhang, J.; Luo, P. Multimodal ChatGPT-4V for Electrocardiogram Interpretation: Promise and Limitations. J. Med. Internet Res. 2024, 26, e54607. [Google Scholar] [CrossRef] [PubMed]
- Zaboli, A.; Brigo, F.; Ziller, M.; Massar, M.; Parodi, M.; Magnarelli, G.; Brigiari, G.; Turcato, G. Exploring ChatGPT’s Potential in ECG Interpretation and Outcome Prediction in Emergency Department. Am. J. Emerg. Med. 2025, 88, 7–11. [Google Scholar] [CrossRef]
- Gendler, M.; Nadkarni, G.N.; Sudri, K.; Cohen-Shelly, M.; Glicksberg, B.S.; Efros, O.; Soffer, S.; Klang, E. Large Language Models in Cardiology: A Systematic Review. medRxiv 2024, 2024–09. [Google Scholar]
- Bachtiger, P.; Petri, C.F.; Scott, F.E.; Ri Park, S.; Kelshiker, M.A.; Sahemey, H.K.; Dumea, B.; Alquero, R.; Padam, P.S.; Hatrick, I.R.; et al. Point-of-Care Screening for Heart Failure with Reduced Ejection Fraction Using Artificial Intelligence during ECG-Enabled Stethoscope Examination in London, UK: A Prospective, Observational, Multicentre Study. Lancet Digit. Health 2022, 4, e117–e125. [Google Scholar] [CrossRef]
- 510(k) Premarket Notification. Available online: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpmn/pmn.cfm?ID=K233409 (accessed on 1 June 2025).
- Petzl, A.M.; Jabbour, G.; Cadrin-Tourigny, J.; Pürerfellner, H.; Macle, L.; Khairy, P.; Avram, R.; Tadros, R. Innovative Approaches to Atrial Fibrillation Prediction: Should Polygenic Scores and Machine Learning Be Implemented in Clinical Practice? Europace 2024, 26, euae201. [Google Scholar] [CrossRef]
- Kany, S.; Rämö, J.T.; Friedman, S.F.; Weng, L.-C.; Roselli, C.; Kim, M.S.; Fahed, A.C.; Lubitz, S.A.; Maddah, M.; Ellinor, P.T.; et al. Integrating Clinical, Genetic, and Electrocardiogram-Based Artificial Intelligence to Estimate Risk of Incident Atrial Fibrillation. medRxiv 2024. [Google Scholar] [CrossRef]
- Hayashi, K.; Tada, H.; Yamagishi, M. The Genetics of Atrial Fibrillation. Curr. Opin. Cardiol. 2017, 32, 10–16. [Google Scholar] [CrossRef]
- Al-Kasasbeh, A.H.; Khabour, O.F.; Almomani, R.; Ababneh, M.; Ibdah, R.; Jarrah, M.I.; Rawashdeh, S.I.; Seif, A.M. The Association Between the Rs2200733 SNP and Atrial Fibrillation Among Arabs: A Study from Jordan. Biologics 2024, 18, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Wass, S.Y.; Sun, H.; Tchou, G.; Liu, N.; Van Wagoner, D.R.; Chung, M.K.; Barnard, J.; Smith, J.D. Transcriptomic Insights into the Atrial Fibrillation Susceptibility Locus near the MYOZ1 and SYNPO2L Genes. Int. J. Mol. Sci. 2024, 25, 10309. [Google Scholar] [CrossRef]
- Ji, F.; Liu, Q.; Feng, Z.; Han, X.; Li, Z. Genetic Association between 1425G/A SNP in PRKCH and Hypertrophic Cardiomyopathy in a Chinese Population. Oncotarget 2017, 8, 114839–114844. [Google Scholar] [CrossRef]
- Napolitano, C.; Novelli, V.; Francis, M.D.; Priori, S.G. Genetic Modulators of the Phenotype in the Long QT Syndrome: State of the Art and Clinical Impact. Curr. Opin. Genet. Dev. 2015, 33, 17–24. [Google Scholar] [CrossRef] [PubMed]
- van Setten, J.; Verweij, N.; Mbarek, H.; Niemeijer, M.N.; Trompet, S.; Arking, D.E.; Brody, J.A.; Gandin, I.; Grarup, N.; Hall, L.M.; et al. Genome-Wide Association Meta-Analysis of 30,000 Samples Identifies Seven Novel Loci for Quantitative ECG Traits. Eur. J. Hum. Genet. 2019, 27, 952–962. [Google Scholar] [CrossRef]
- Sau, A.; Ribeiro, A.H.; McGurk, K.A.; Pastika, L.; Bajaj, N.; Gurnani, M.; Sieliwonczyk, E.; Patlatzoglou, K.; Ardissino, M.; Chen, J.Y.; et al. Prognostic Significance and Associations of Neural Network-Derived Electrocardiographic Features. Circ. Cardiovasc. Qual. Outcomes. 2024, 17, e010602. [Google Scholar] [CrossRef] [PubMed]
- Senapati, S.G.; Bhanushali, A.K.; Lahori, S.; Naagendran, M.S.; Sriram, S.; Ganguly, A.; Pusa, M.; Damani, D.N.; Kulkarni, K.; Arunachalam, S.P. Mapping of Neuro-Cardiac Electrophysiology: Interlinking Epilepsy and Arrhythmia. J. Cardiovasc. Dev. Dis. 2023, 10, 433. [Google Scholar] [CrossRef]
- Yang, Y.; Truong, N.D.; Maher, C.; Nikpour, A.; Kavehei, O. A Comparative Study of AI Systems for Epileptic Seizure Recognition Based on EEG or ECG. Annu. Int. Conf. IEEE Eng. Med. Biol. Soc. 2021, 2021, 2191–2196. [Google Scholar] [CrossRef]
- Soto, J.T.; Weston Hughes, J.; Sanchez, P.A.; Perez, M.; Ouyang, D.; Ashley, E.A. Multimodal Deep Learning Enhances Diagnostic Precision in Left Ventricular Hypertrophy. Eur. Heart J. Digit. Health 2022, 3, 380–389. [Google Scholar] [CrossRef]
- Kolk, M.Z.H.; Ruipérez-Campillo, S.; Allaart, C.P.; Wilde, A.A.M.; Knops, R.E.; Narayan, S.M.; Tjong, F.V.Y. DEEP RISK investigators Multimodal Explainable Artificial Intelligence Identifies Patients with Non-Ischaemic Cardiomyopathy at Risk of Lethal Ventricular Arrhythmias. Sci. Rep. 2024, 14, 14889. [Google Scholar] [CrossRef]
- Maturi, B.; Dulal, S.; Sayana, S.B.; Ibrahim, A.; Ramakrishna, M.; Chinta, V.; Sharma, A.; Ravipati, H. Revolutionizing Cardiology: The Role of Artificial Intelligence in Echocardiography. J. Clin. Med. 2025, 14, 625. [Google Scholar] [CrossRef]
- Li, L.; Camps, J.; Jenny Wang, Z.; Beetz, M.; Banerjee, A.; Rodriguez, B.; Grau, V. Toward Enabling Cardiac Digital Twins of Myocardial Infarction Using Deep Computational Models for Inverse Inference. IEEE Trans. Med. Imaging 2024, 43, 2466–2478. [Google Scholar] [CrossRef]
- Qian, S.; Ugurlu, D.; Fairweather, E.; Toso, L.D.; Deng, Y.; Strocchi, M.; Cicci, L.; Jones, R.E.; Zaidi, H.; Prasad, S.; et al. Developing Cardiac Digital Twin Populations Powered by Machine Learning Provides Electrophysiological Insights in Conduction and Repolarization. Nat. Cardiovasc. Res. 2025, 4, 624–636. [Google Scholar] [CrossRef]
- Li, L.; Camps, J.; Rodriguez, B.; Grau, V. Solving the Inverse Problem of Electrocardiography for Cardiac Digital Twins: A Survey. IEEE Rev. Biomed. Eng. 2025, 18, 316–336. [Google Scholar] [CrossRef] [PubMed]
- Thangaraj, P.M.; Benson, S.H.; Oikonomou, E.K.; Asselbergs, F.W.; Khera, R. Cardiovascular Care with Digital Twin Technology in the Era of Generative Artificial Intelligence. Eur. Heart J. 2024, 45, 4808–4821. [Google Scholar] [CrossRef]
- Wang, Z.; Stavrakis, S.; Yao, B. Hierarchical Deep Learning with Generative Adversarial Network for Automatic Cardiac Diagnosis from ECG Signals. Comput. Biol. Med. 2023, 155, 106641. [Google Scholar] [CrossRef]
- Qu, Z.; Shi, W.; Tiwari, P. Quantum Conditional Generative Adversarial Network Based on Patch Method for Abnormal Electrocardiogram Generation. Comput. Biol. Med. 2023, 166, 107549. [Google Scholar] [CrossRef]
- Berger, L.; Haberbusch, M.; Moscato, F. Generative Adversarial Networks in Electrocardiogram Synthesis: Recent Developments and Challenges. Artif. Intell. Med. 2023, 143, 102632. [Google Scholar] [CrossRef]
- Weimann, K.; Conrad, T.O.F. Transfer Learning for ECG Classification. Sci Rep 2021, 11, 5251. [Google Scholar] [CrossRef]
- Chen, W.; Banerjee, T.; John, E. A Meta-Transfer Learning Approach to ECG Arrhythmia Detection. In Proceedings of the 2022 44th Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Glasgow, UK, 11–15 July 2022; pp. 1300–1305. [Google Scholar] [CrossRef]
- Kuang, R.; Wang, Z.; Ma, L.; Wang, H.; Chen, Q.; Junior, A.L.; Kumar, S.; Li, X.; Marques, C.; Min, R. Smart Photonic Wristband for Pulse Wave Monitoring. OES 2024, 3, 240009–240016. [Google Scholar] [CrossRef]
- Li, W.; Long, Y.; Yan, Y.; Xiao, K.; Wang, Z.; Zheng, D.; Leal-Junior, A.; Kumar, S.; Ortega, B.; Marques, C.; et al. Wearable Photonic Smart Wristband for Cardiorespiratory Function Assessment and Biometric Identification. OEA 2025, 8, 240254. [Google Scholar] [CrossRef]
- Ramakrishnaiah, Y.; Macesic, N.; Webb, G.I.; Peleg, A.Y.; Tyagi, S. EHR-ML: A Data-Driven Framework for Designing Machine Learning Applications with Electronic Health Records. Int. J. Med. Inform. 2025, 196, 105816. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, J.; Xu, N.Y.; Nguyen, K.T.; Klonoff, D.C. The Need for Data Standards and Implementation Policies to Integrate CGM Data into the Electronic Health Record. J. Diabetes. Sci. Technol. 2023, 17, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Yoo, R.M.; Viggiano, B.T.; Pundi, K.N.; Fries, J.A.; Zahedivash, A.; Podchiyska, T.; Din, N.; Shah, N.H. Scalable Approach to Consumer Wearable Postmarket Surveillance: Development and Validation Study. JMIR Med. Inform 2024, 12, e51171. [Google Scholar] [CrossRef]
- McGrath, C.; Chau, C.W.R.; Molina, G.F. Monitoring Oral Health Remotely: Ethical Considerations When Using AI among Vulnerable Populations. Front. Oral Health 2025, 6, 1587630. [Google Scholar] [CrossRef]
- Ayaz, M.; Pasha, M.F.; Alzahrani, M.Y.; Budiarto, R.; Stiawan, D. The Fast Health Interoperability Resources (FHIR) Standard: Systematic Literature Review of Implementations, Applications, Challenges and Opportunities. JMIR Med. Inform 2021, 9, e21929. [Google Scholar] [CrossRef]
- Sreejith, R.; Senthil, S. Smart Contract Authentication Assisted GraphMap-Based HL7 FHIR Architecture for Interoperable e-Healthcare System. Heliyon 2023, 9, e15180. [Google Scholar] [CrossRef]
- Pavão, J.; Bastardo, R.; Rocha, N.P. The Fast Health Interoperability Resources (FHIR) and Clinical Research, a Scoping Review. In Proceedings of the Information Systems and Technologies; Rocha, A., Adeli, H., Dzemyda, G., Moreira, F., Colla, V., Eds.; Springer Nature: Cham, Switzerland, 2024; pp. 409–418. [Google Scholar]
- Halilaj, M.; Vasil, S.; Kosta, A.; Lili, I. Interoperability within Healthcare Systems through FHIR, Artificial Intelligence and Cloud Integration. Int. J. Eng. Adv. Technol. 2025, 13, 22–28. [Google Scholar]
- Sardar, P.; Abbott, J.D.; Kundu, A.; Aronow, H.D.; Granada, J.F.; Giri, J. Impact of Artificial Intelligence on Interventional Cardiology: From Decision-Making Aid to Advanced Interventional Procedure Assistance. JACC Cardiovasc. Interv. 2019, 12, 1293–1303. [Google Scholar] [CrossRef]
- Xu, B.; Kocyigit, D.; Grimm, R.; Griffin, B.P.; Cheng, F. Applications of Artificial Intelligence in Multimodality Cardiovascular Imaging: A State-of-the-Art Review. Prog. Cardiovasc. Dis. 2020, 63, 367–376. [Google Scholar] [CrossRef] [PubMed]
- Poly, T.N.; Islam, M.M.; Muhtar, M.S.; Yang, H.-C.; Nguyen, P.A.; Li, Y.-C. Machine Learning Approach to Reduce Alert Fatigue Using a Disease Medication–Related Clinical Decision Support System: Model Development and Validation. JMIR Med. Inform. 2020, 8, e19489. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Jimenez, F.; Alger, H.M.; Attia, Z.I.; Barry, B.; Chatterjee, R.; Dolor, R.; Friedman, P.A.; Greene, S.J.; Greenwood, J.; Gundurao, V.; et al. A Multicenter Pragmatic Implementation Study of AI-ECG-Based Clinical Decision Support Software to Identify Low LVEF: Clinical Trial Design and Methods. Am. Heart J. Plus: Cardiol. Res. Pract. 2025, 54, 100528. [Google Scholar] [CrossRef]
- Turakhia, M.P.; Desai, M.; Hedlin, H.; Rajmane, A.; Talati, N.; Ferris, T.; Desai, S.; Nag, D.; Patel, M.; Kowey, P.; et al. Rationale and Design of a Large-Scale, App-Based Study to Identify Cardiac Arrhythmias Using a Smartwatch: The Apple Heart Study. Am. Heart J. 2019, 207. [Google Scholar] [CrossRef]
- Lee, T.T.; Kesselheim, A.S. U.S. Food and Drug Administration Precertification Pilot Program for Digital Health Software: Weighing the Benefits and Risks. Ann. Intern. Med. 2018, 168, 730–732. [Google Scholar] [CrossRef]
- Regulation—2017/745—EN—Medical Device Regulation—EUR-Lex. Available online: https://eur-lex.europa.eu/eli/reg/2017/745/oj/eng (accessed on 28 June 2025).
- Regulation - 2016/679 - EN - Gdpr - EUR-Lex. Available online: https://eur-lex.europa.eu/eli/reg/2016/679/oj/eng (accessed on 28 June 2025).
- Ioannidou, I.; Sklavos, N. On General Data Protection Regulation Vulnerabilities and Privacy Issues, for Wearable Devices and Fitness Tracking Applications. Cryptography 2021, 5, 29. [Google Scholar] [CrossRef]
- Taka, A.-M. A Deep Dive into Dynamic Data Flows, Wearable Devices, and the Concept of Health Data. Int. Data Priv. 2023, 13, 124–140. [Google Scholar] [CrossRef]
- Art. 4 GDPR—Definitions. General Data Protection Regulation (GDPR). Available online: https://gdpr-info.eu/art-4-gdpr/ (accessed on 10 March 2025).
- Bouderhem, R. Privacy and Regulatory Issues in Wearable Health Technology. Eng. Proc. 2023, 58, 87. [Google Scholar] [CrossRef]
- Hoyer, I.; Utz, A.; Hoog Antink, C.; Seidl, K. tinyHLS: A Novel Open Source High Level Synthesis Tool Targeting Hardware Accelerators for Artificial Neural Network Inference. Physiol. Meas. 2025, 13, 015002. [Google Scholar] [CrossRef]
- Hizem, M.; Bousbia, L.; Ben Dhiab, Y.; Aoueileyine, M.O.-E.; Bouallegue, R. Reliable ECG Anomaly Detection on Edge Devices for Internet of Medical Things Applications. Sensors 2025, 25, 2496. [Google Scholar] [CrossRef]
- Ortega-Martorell, S.; Olier, I.; Ohlsson, M.; Lip, G.Y.H.; on behalf of the TARGET Consortium. TARGET: A Major European Project Aiming to Advance the Personalised Management of Atrial Fibrillation-Related Stroke via the Development of Health Virtual Twins Technology and Artificial Intelligence. Thromb. Haemost. 2024, 125, 7–11. [Google Scholar] [CrossRef]
- Lee, S.-H.; Lee, D.-W.; Kim, M.S. A Deep Learning-Based Semantic Segmentation Model Using MCNN and Attention Layer for Human Activity Recognition. Sensors 2023, 23, 2278. [Google Scholar] [CrossRef] [PubMed]
- Noseworthy, P.A.; Attia, Z.I.; Brewer, L.P.C.; Hayes, S.N.; Yao, X.; Kapa, S.; Friedman, P.A.; Lopez-Jimenez, F. Assessing and Mitigating Bias in Medical Artificial Intelligence: The Effects of Race and Ethnicity on a Deep Learning Model for ECG Analysis. Circ. Arrhythm. Electrophysiol. 2020, 13, E007988. [Google Scholar] [CrossRef] [PubMed]
- Kaur, D.; Hughes, J.W.; Rogers, A.J.; Kang, G.; Narayan, S.M.; Ashley, E.A.; Perez, M.V. Race, Sex, and Age Disparities in the Performance of ECG Deep Learning Models Predicting Heart Failure. Circ. Heart Fail. 2024, 17, e010879. [Google Scholar] [CrossRef]
- Liu, M.; Ning, Y.; Ke, Y.; Shang, Y.; Chakraborty, B.; Ong, M.E.H.; Vaughan, R.; Liu, N. FAIM: Fairness-Aware Interpretable Modeling for Trustworthy Machine Learning in Healthcare. Patterns 2024, 5, 101059. [Google Scholar] [CrossRef]
- Sufian, M.A.; Alsadder, L.; Hamzi, W.; Zaman, S.; Sagar, A.S.M.S.; Hamzi, B. Mitigating Algorithmic Bias in AI-Driven Cardiovascular Imaging for Fairer Diagnostics. Diagnostics 2024, 14, 2675. [Google Scholar] [CrossRef]
- Adadi, A.; Berrada, M. Peeking Inside the Black-Box: A Survey on Explainable Artificial Intelligence (XAI). IEEE Access 2018, 6, 52138–52160. [Google Scholar] [CrossRef]
- Al-Zaiti, S.S.; Bond, R.R. Explainable-by-Design: Challenges, Pitfalls, and Opportunities for the Clinical Adoption of AI-Enabled ECG. J. Electrocardiol. 2023, 81, 292–294. [Google Scholar] [CrossRef] [PubMed]
- Kashou, A.H.; May, A.M.; Noseworthy, P.A. Comparison of Two Artificial Intelligence-Augmented ECG Approaches: Machine Learning and Deep Learning. J. Electrocardiol. 2023, 79, 75–80. [Google Scholar] [CrossRef]
- Siontis, K.C.; Suárez, A.B.; Sehrawat, O.; Ackerman, M.J.; Attia, Z.I.; Friedman, P.A.; Noseworthy, P.A.; Maanja, M. Saliency Maps Provide Insights into Artificial Intelligence-Based Electrocardiography Models for Detecting Hypertrophic Cardiomyopathy. J. Electrocardiol. 2023, 81, 286–291. [Google Scholar] [CrossRef]
- Jahmunah, V.; Ng, E.Y.K.; Tan, R.-S.; Oh, S.L.; Acharya, U.R. Explainable Detection of Myocardial Infarction Using Deep Learning Models with Grad-CAM Technique on ECG Signals. Comput. Biol. Med. 2022, 146, 105550. [Google Scholar] [CrossRef]
- Ao, R.; He, G. Image Based Deep Learning in 12-Lead ECG Diagnosis. Front. Artif. Intell. 2022, 5, 1087370. [Google Scholar] [CrossRef]
- Jekova, I.; Christov, I.; Krasteva, V. Atrioventricular Synchronization for Detection of Atrial Fibrillation and Flutter in One to Twelve ECG Leads Using a Dense Neural Network Classifier. Sensors 2022, 22, 6071. [Google Scholar] [CrossRef]
- Anand, A.; Kadian, T.; Shetty, M.K.; Gupta, A. Explainable AI Decision Model for ECG Data of Cardiac Disorders. Biomed. Signal Process. Control. 2022, 75, 103584. [Google Scholar] [CrossRef]
- Denysyuk, H.V.; Pinto, R.J.; Silva, P.M.; Duarte, R.P.; Marinho, F.A.; Pimenta, L.; Gouveia, A.J.; Gonçalves, N.J.; Coelho, P.J.; Zdravevski, E.; et al. Algorithms for Automated Diagnosis of Cardiovascular Diseases Based on ECG Data: A Comprehensive Systematic Review. Heliyon 2023, 9, e13601. [Google Scholar] [CrossRef] [PubMed]
- Lawin, D.; Kuhn, S.; Schulze Lammers, S.; Lawrenz, T.; Stellbrink, C. Use of Digital Health Applications for the Detection of Atrial Fibrillation. Herzschrittmacherther. Elektrophysiol. 2022, 33, 373–379. [Google Scholar] [CrossRef]
- Smith, S.; Maisrikrod, S. Wearable Electrocardiogram Technology: Help or Hindrance to the Modern Doctor? JMIR Cardio. 2025, 9, e62719. [Google Scholar] [CrossRef]
- Chandrasekaran, R.; Sadiq, T.M.; Moustakas, E. Usage Trends and Data Sharing Practices of Healthcare Wearable Devices Among US Adults: Cross-Sectional Study. J. Med. Internet Res. 2025, 27, e63879. [Google Scholar] [CrossRef]
- Nagappan, A.; Krasniansky, A.; Knowles, M. Patterns of Ownership and Usage of Wearable Devices in the United States, 2020-2022: Survey Study. J. Med. Internet Res. 2024, 26, e56504. [Google Scholar] [CrossRef]
- Chandrasekaran, R.; Sharma, P.; Moustakas, E. Exploring Disparities in Healthcare Wearable Use among Cardiovascular Patients: Findings from a National Survey. Rev. Cardiovasc. Med. 2023, 24, 307. [Google Scholar] [CrossRef]
- Pan, C.-C.; De Santis, K.K.; Muellmann, S.; Hoffmann, S.; Spallek, J.; Barnils, N.P.; Ahrens, W.; Zeeb, H.; Schüz, B. Sociodemographics and Digital Health Literacy in Using Wearables for Health Promotion and Disease Prevention: Cross-Sectional Nationwide Survey in Germany. J. Prev. 2024, 46, 371–391. [Google Scholar] [CrossRef]
- Veinot, T.C.; Mitchell, H.; Ancker, J.S. Good Intentions Are Not Enough: How Informatics Interventions Can Worsen Inequality. J. Am. Med. Inform. Assoc. 2018, 25, 1080–1088. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, K. Digital Health Equity. In Digital Health; Linwood, S.L., Ed.; Exon Publications: Brisbane, Australia, 2022; ISBN 13: 978-0-6453320-1-8. [Google Scholar]
| Category | Abbreviation | Full Name | Use in ECG Interpretation | Bibliography |
|---|---|---|---|---|
| ML | SVM | Support Vector Machine | Heart rhythm classification, AF detection (also based on 1-lead ECG), LAE detection with raw ECG data. | [8,9,10] |
| SVC | Support Vector Classification | Predicting MACEs and NSTE-ACS | [11] | |
| LASSO | Least Absolute Shrinkage and Selection Operator | ML technique for supervised learning. Used as a tool to select the best variables, among others, in HCM detection. | [12] | |
| MLP | Multilayer Perceptron | Detection of LAE when biological features are added. | [10] | |
| Extra Trees | Extremely Randomized Trees | Predicting the location of the source of ventricular arrhythmia. | [13] | |
| XGBoost | Extreme Gradient Boosting | Localization of the arrhythmia source (also based on 1-lead ECG). | [14] | |
| DL | CNN | Convolutional Neural Network | Detection of VHD, detection of CHD, HA, CHF, angina, cerebral stroke on the basis of array data (without chronology information). Automatic classification of arrhythmias (also on incomplete ECG data). AR detection. Acts as a “black box” | [15,16,17,18,19] |
| BNN | Bayesian Neural Network | Classification of arrhythmias (also on incomplete ECG data). Allows interpretation of the effect of each ECG feature on the outcome, shows decision uncertainty, which distinguishes it from CNNs. | [20] | |
| RNN:LSTM | Recurrent Neural Network: Long Short-Term Memory | ECG signal compression and reconstruction based on a limited number of leads or signal with noise. | [21,22] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartusik-Aebisher, D.; Rogóż, K.; Aebisher, D. Artificial Intelligence and ECG: A New Frontier in Cardiac Diagnostics and Prevention. Biomedicines 2025, 13, 1685. https://doi.org/10.3390/biomedicines13071685
Bartusik-Aebisher D, Rogóż K, Aebisher D. Artificial Intelligence and ECG: A New Frontier in Cardiac Diagnostics and Prevention. Biomedicines. 2025; 13(7):1685. https://doi.org/10.3390/biomedicines13071685
Chicago/Turabian StyleBartusik-Aebisher, Dorota, Kacper Rogóż, and David Aebisher. 2025. "Artificial Intelligence and ECG: A New Frontier in Cardiac Diagnostics and Prevention" Biomedicines 13, no. 7: 1685. https://doi.org/10.3390/biomedicines13071685
APA StyleBartusik-Aebisher, D., Rogóż, K., & Aebisher, D. (2025). Artificial Intelligence and ECG: A New Frontier in Cardiac Diagnostics and Prevention. Biomedicines, 13(7), 1685. https://doi.org/10.3390/biomedicines13071685








