Serum Levels of Nε-(Carboxymethyl)-Lysine in Chronic Kidney Disease and Type 2 Diabetes Mellitus
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Patients and Sample Collection
2.3. Measurement of Serum CML
2.4. Statistical Analysis
3. Results
3.1. Characteristics of the Three Groups of Patients
3.1.1. Age
3.1.2. Sex
3.1.3. Fasting Glucose
3.2. Characteristics of the CDK Patients with and Without DM
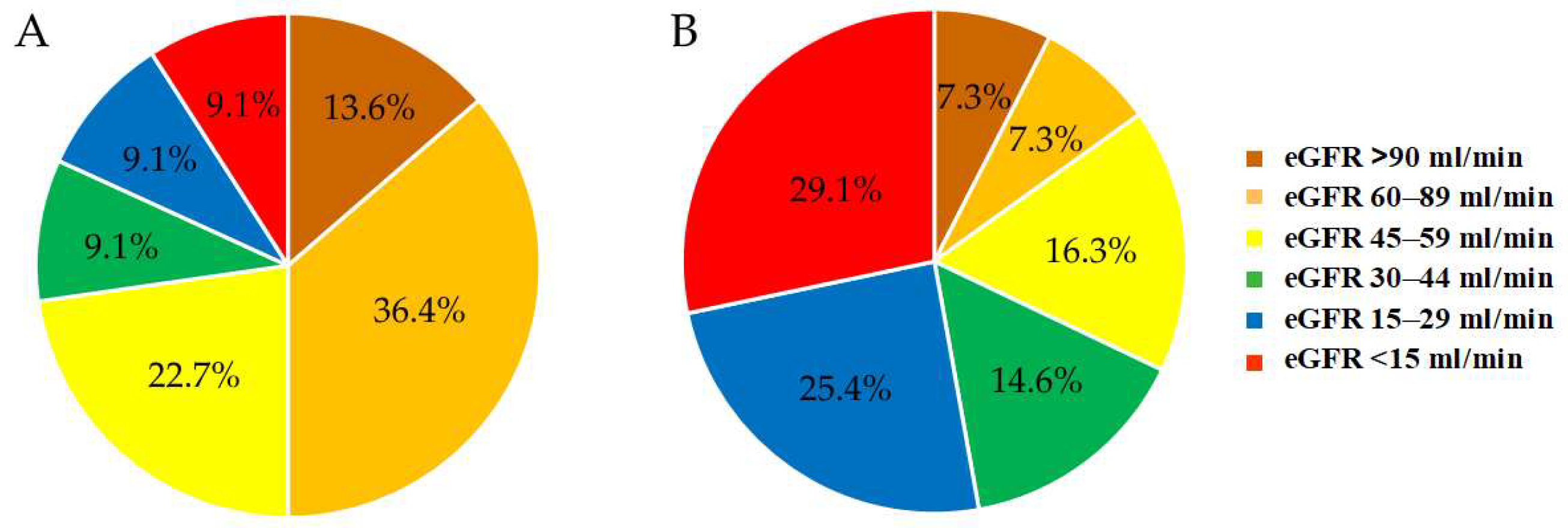
3.2.1. eGFR
3.2.2. Albuminuria
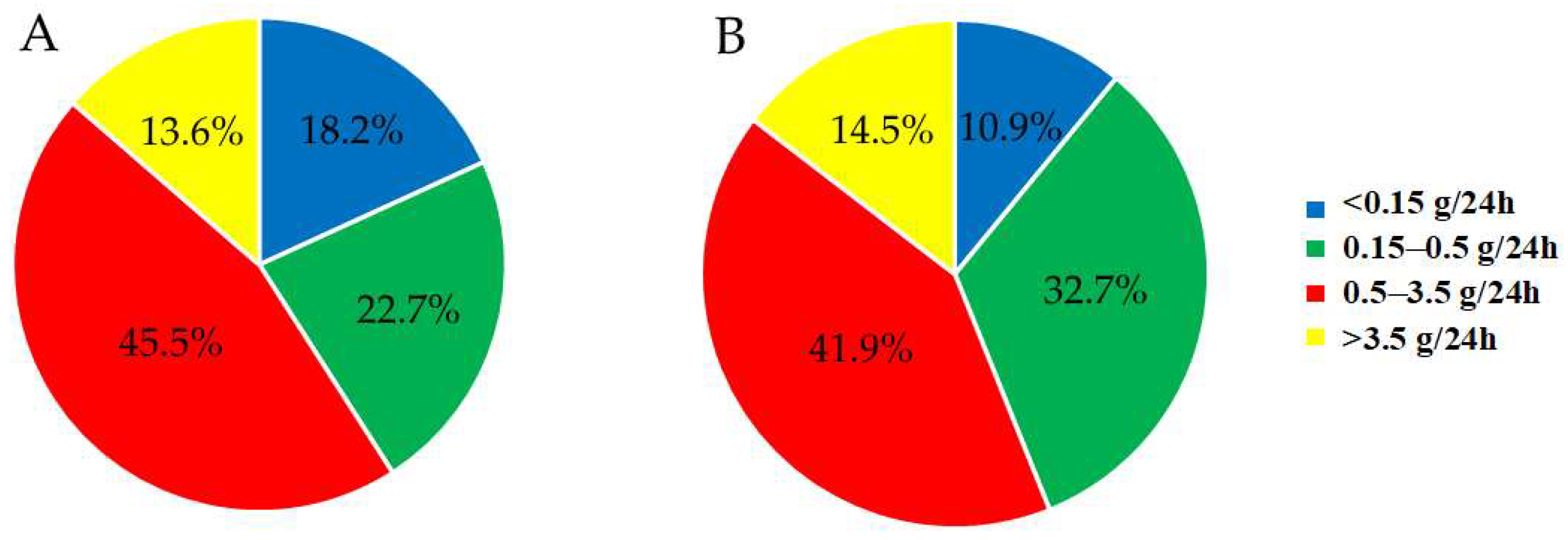
3.2.3. Proteinuria
3.3. Characteristics of the T2DM Patients with and Without CKD
3.3.1. Duration of Diabetes
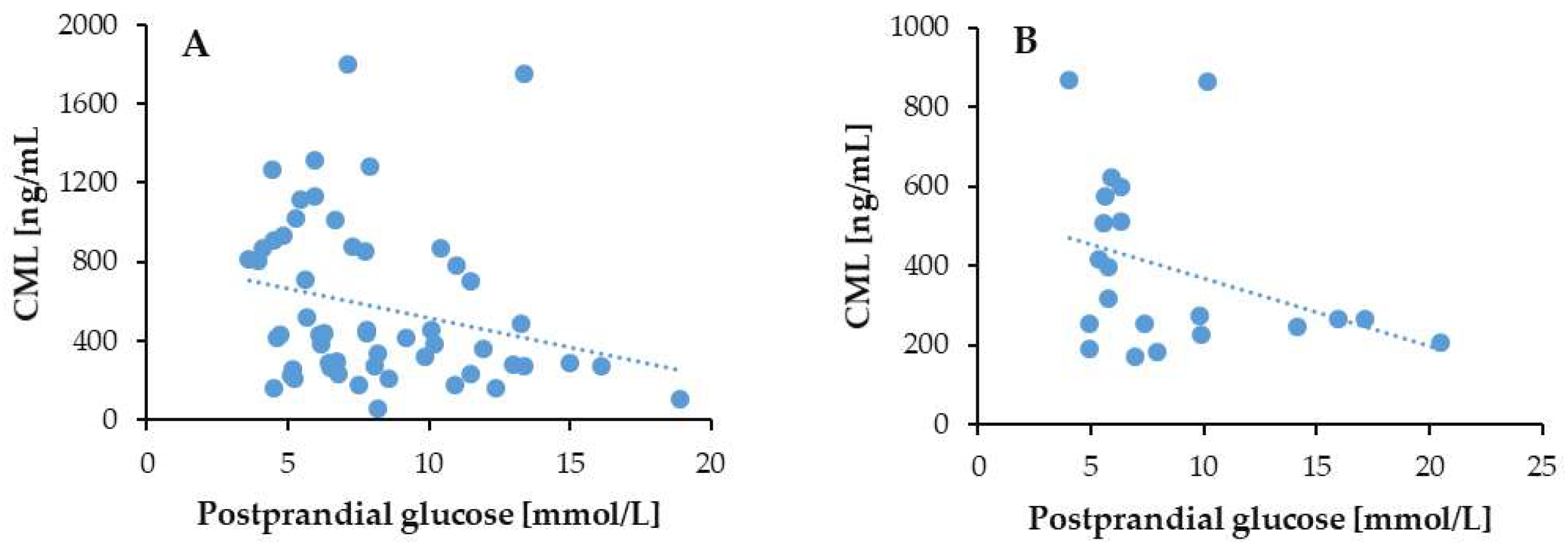
3.3.2. Postprandial Glucose
3.4. Power Analysis
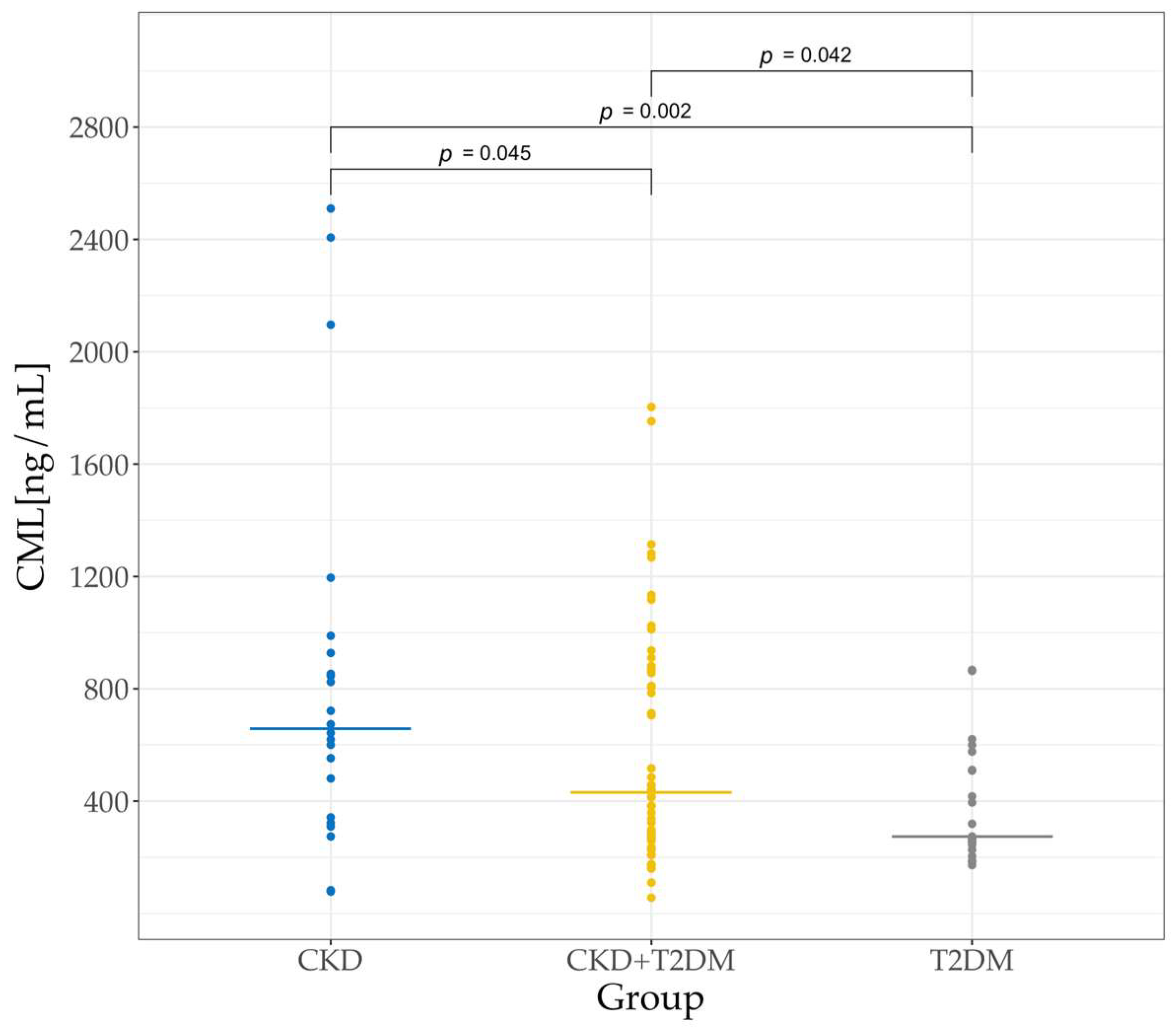
3.5. Serum CML Levels in the Three Groups of Patients
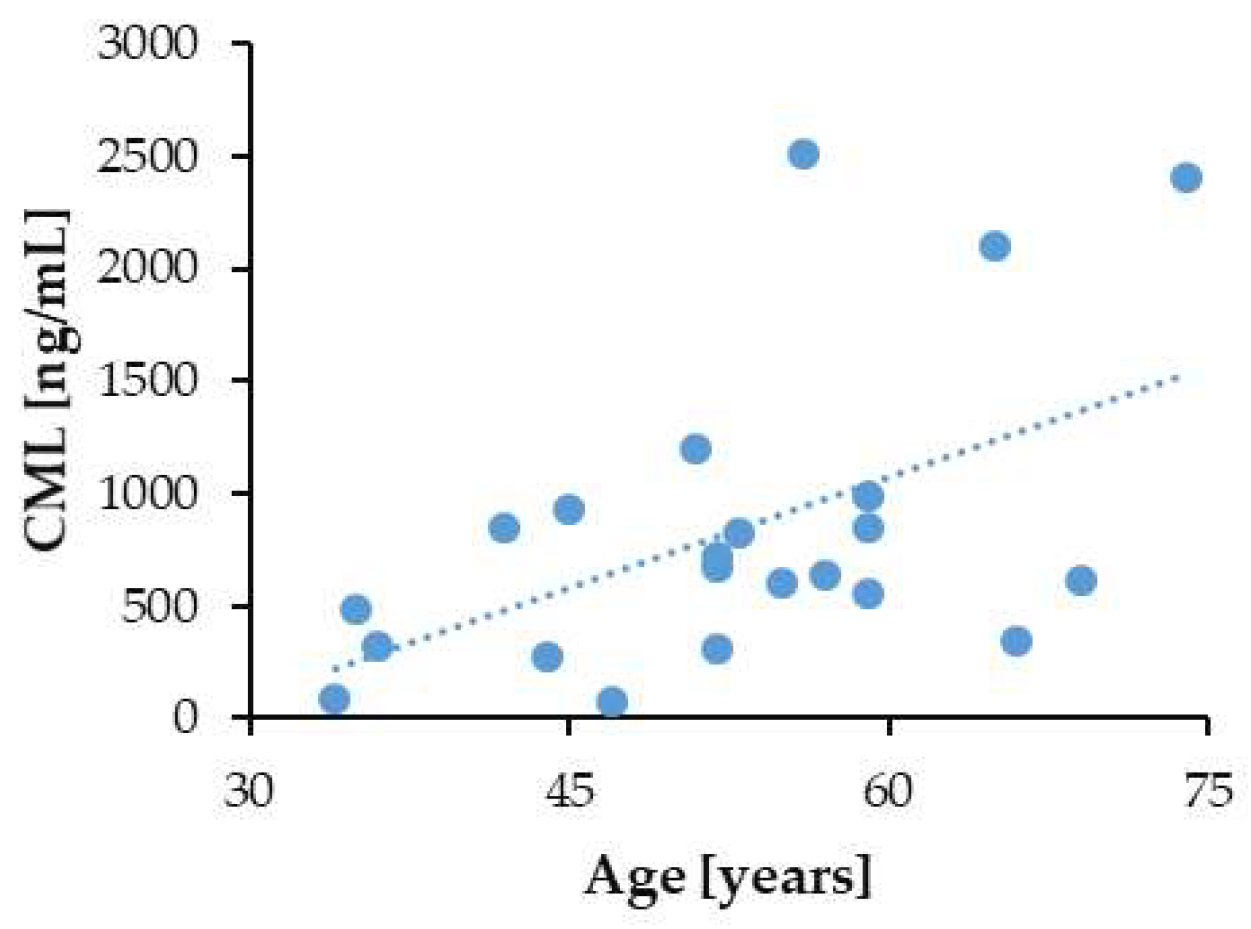
3.6. Correlation of Serum CML Levels with Patients’ Age and Markers of Kidney Disease
3.7. Correlation of Serum CML Levels with Duration of Diabetes and Glycemic Markers
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kovesdy, C.P. Epidemiology of chronic kidney disease: An update 2022. Kidney Int. Suppl. 2022, 12, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Levin, A.; Stevens, P.E.; Bilous, R.W.; Coresh, J.; De Francisco, A.L.; De Jong, P.E. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int. Suppl. 2013, 3, 1–150. [Google Scholar]
- Jha, V.; Al-Ghamdi, S.M.G.; Li, G.; Wu, M.S.; Stafylas, P.; Retat, L.; Card-Gowers, J.; Barone, S.; Cabrera, C.; Garcia Sanchez, J.J. Global economic burden associated with chronic kidney disease: A pragmatic review of medical costs for the inside CKD research programme. Adv. Ther. 2023, 40, 4405–4420. [Google Scholar] [CrossRef] [PubMed]
- Chadban, S.; Arıcı, M.; Power, A.; Wud, M.S.; Menninie, F.S.; Álvarez, A.; Sanchez, J.J.G.; Barone, S.; Card-Gowers, J.; Martin, A.; et al. Projecting the economic burden of chronic kidney disease at the patient level (Inside CKD): A microsimulation modelling study. eClinicalMedicine 2024, 72, 102615. [Google Scholar] [CrossRef]
- Wan, E.Y.F.; Chin, W.Y.; Yu, E.Y.T.; Wong, I.C.K.; Chan, E.W.Y.; Li, S.X.; Cheung, N.K.L.; Wang, Y.; Lam, C.L.K. The impact of cardiovascular disease and chronic kidney disease on life expectancy and direct medical cost in a 10-year diabetes cohort study. Diabetes Care 2020, 43, 1750–1758. [Google Scholar] [CrossRef]
- Webster, A.C.; Nagler, E.V.; Morton, R.L.; Masson, P. Chronic Kidney Disease. Lancet 2017, 389, 1238–1252. [Google Scholar] [CrossRef]
- Gewin, L.; Zent, R.; Pozzi, A. Progression of chronic kidney disease: Too much cellular talk causes damage. Kidney Int. 2017, 91, 552–560. [Google Scholar] [CrossRef]
- Schelling, J.R. Tubular atrophy in the pathogenesis of chronic kidney disease progression. Pediatr. Nephrol. 2016, 31, 693–706. [Google Scholar] [CrossRef]
- Xu, L.; Guo, J.; Moledina, D.G.; Cantley, L.G. Immune-mediated tubule atrophy promotes acute kidney injury to chronic kidney disease transition. Nat. Commun. 2022, 13, 4892. [Google Scholar] [CrossRef]
- Lusco, M.A.; Fogo, A.B.; Najafian, B.; Alpers, C.E. AJKD Atlas of Renal Pathology: Tubular Atrophy. Am. J. Kidney Dis. 2016, 67, e33–e34. [Google Scholar] [CrossRef]
- Farris, A.B.; Colvin, R.B. Renal interstitial fibrosis: Mechanisms and evaluation. Curr. Opin. Nephrol. Hypertens. 2012, 21, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Fu, P.; Ma, L. Kidney fibrosis: From mechanisms to therapeutic medicines. Sig. Transduct. Target Ther. 2023, 8, 129. [Google Scholar] [CrossRef]
- Fowler, M.J. Microvascular and macrovascular complications of diabetes. Clin. Diabetes 2008, 26, 77–82. [Google Scholar] [CrossRef]
- Yan, S.; Ramasamy, R.; Schmidt, A. Mechanisms of Disease: Advanced glycation end-products and their receptor in inflammation and diabetes complications. Nat. Rev. Endocrinol. 2008, 4, 285–293. [Google Scholar] [CrossRef]
- Gasser, A.; Forbes, J.M. Advanced glycation: Implications in tissue damage and disease. Protein Pept. Lett. 2008, 15, 385–391. [Google Scholar] [CrossRef]
- Schrijvers, B.F.; De Vriese, A.S.; Flyvbjerg, A. From hyperglycemia to diabetic kidney disease: The role of metabolic, hemodynamic, intracellular factors and growth factors/cytokines. Endocr. Rev. 2004, 25, 971–1010. [Google Scholar] [CrossRef]
- Tanji, N.; Markowitz, G.S.; Fu, C.; Kislinger, T.; Taguchi, A.; Pischetsrieder, M.; Stern, D.; Schmidt, A.M.; D’AGati, V.D. Expression of advanced glycation end products and their cellular receptor RAGE in diabetic nephropathy and nondiabetic renal disease. J. Am. Soc. Nephrol. 2000, 11, 1656–1666. [Google Scholar] [CrossRef]
- Mallipattu, S.K.; Uribarri, J. Advanced glycation end product accumulation: A new enemy to target in chronic kidney disease? Curr. Opin. Nephrol. Hypertens. 2014, 23, 547–554. [Google Scholar] [CrossRef]
- Busch, M.; Franke, S.; Rüster, C.; Wolf, G. Advanced glycation end-products and the kidney. Eur. J. Clin. Investig. 2010, 40, 742–755. [Google Scholar] [CrossRef]
- Twarda-Clapa, A.; Olczak, A.; Białkowska, A.M.; Koziołkiewicz, M. Advanced glycation end-products (AGEs): Formation, chemistry, classification, receptors, and diseases related to AGEs. Cells 2022, 11, 1312. [Google Scholar] [CrossRef]
- Kobori, T.; Ganesh, D.; Kumano-Kuramochi, M.; Torigoe, K.; Machida, S. Assay for advanced glycation end products generating intracellular oxidative stress through binding to its receptor. Anal. Biochem. 2020, 611, 114018. [Google Scholar] [CrossRef] [PubMed]
- Prasad, C.; Davis, K.E.; Imrhan, V.; Juma, S.; Vijayagopal, P. Advanced glycation end products and risks for chronic diseases: Intervening through lifestyle modification. Am. J. Lifestyle Med. 2017, 13, 384–404. [Google Scholar] [CrossRef] [PubMed]
- Linden, E.; Cai, W.; He, J.C.; Xue, C.; Li, Z.; Winston, J.; Vlassara, H.; Uribarri, J. Endothelial dysfunction in patients with chronic kidney disease results from advanced glycation end products (AGE)-mediated inhibition of endothelial nitric oxide synthase through RAGE activation. Clin. J. Am. Soc. Nephrol. 2008, 3, 691–698. [Google Scholar] [CrossRef]
- Steenbeke, M.; Speeckaert, R.; Desmedt, S.; Glorieux, G.; Delanghe, J.R.; Speeckaert, M.M. The role of advanced glycation end products and its soluble receptor in kidney diseases. Int. J. Mol. Sci. 2022, 23, 3439. [Google Scholar] [CrossRef]
- Fukami, K.; Taguchi, K.; Yamagishi, S.; Okuda, S. Receptor for advanced glycation endproducts and progressive kidney disease. Curr. Opin. Nephrol. Hypertens. 2015, 24, 54–60. [Google Scholar] [CrossRef]
- Lappas, M.; Permezel, M.; Rice, G.E. Advanced glycation endproducts mediate pro-inflammatory actions in human gestational tissues via nuclear factor-κB and extracellular signal-regulated kinase ½. J. Endocrinol. 2007, 193, 269–277. [Google Scholar] [CrossRef]
- Ott, C.; Jacobs, K.; Haucke, E.; Santos, A.N.; Grune, T.; Simm, A. Role of advanced glycation end products in cellular signaling. Redox Biol. 2014, 2, 411–429. [Google Scholar] [CrossRef]
- Shrivastav, D.; Singh, D.D.; Mir, R.; Mehra, P.; Mehta, V.; Dabla, P.K. Comparative analysis of Nε-carboxymethyl-lysine and inflammatory markers in diabetic and non-diabetic coronary artery disease patients. World J. Diabetes 2023, 14, 1754–1765. [Google Scholar] [CrossRef]
- Bastaa, G.; Schmidtb, A.M.; De Caterina, R. Advanced glycation end products and vascular inflammation: Implications for accelerated atherosclerosis in diabetes. Cardiovasc. Res. 2004, 63, 582–592. [Google Scholar] [CrossRef]
- Dunn, J.A.; McCance, D.R.; Thorpe, S.R.; Lyons, T.J.; Baynes, J.W. Age-dependent accumulation of Nε-(carboxymethyl)lysine and Nε-(carboxymethyl)hydroxylysine in human skin collagen. Biochemistry 1991, 30, 1205–1210. [Google Scholar] [CrossRef]
- Henning, C.; Glomb, M.A. Pathways of the Maillard reaction under physiological conditions. Glycoconj. J. 2016, 33, 499–512. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.X.; Requena, J.R.; Jenkins, A.J.; Lyons, T.J.; Baynes, J.W.; Thorpe, S.R. The advanced glycation end product, Nepsilon-(carboxymethyl)lysine, is a product of both lipid peroxidation and glycoxidation reactions. J. Biol. Chem. 1996, 271, 9982–9986. [Google Scholar] [CrossRef] [PubMed]
- Thorpe, S.R.; Baynes, J.W. CML: A brief history. Int. Congr. Ser. 2002, 1245, 91–99. [Google Scholar] [CrossRef]
- Wagner, Z.; Wittmann, I.; Mazák, I.; Schinzel, R.; Heidland, A.; Kientsch-Engel, R.; Nagy, J. Nε-(carboxymethyl)lysine levels in patients with type 2 diabetes: Role of renal function. Am. J. Kidney Dis. 2001, 38, 785–791. [Google Scholar] [CrossRef]
- Ding, L.; Hou, Y.; Liu, J.; Wang, X.; Wang, Z.; Ding, W.; Zhao, K. Circulating concentrations of advanced glycation end products, carboxymethyl lysine and methylglyoxal are associated with renal function in individuals with diabetes. J. Ren. Nutr. 2024, 34, 154–160. [Google Scholar] [CrossRef]
- Shaw, J.N.; Baynes, J.W.; Thorpe, S.R. Nε-(carboxymethyl) lysine (CML) as a biomarker of oxidative stress in long-lived tissue proteins. Methods Mol. Biol. 2002, 186, 129–137. [Google Scholar] [CrossRef]
- Lieuw-A-Fa, M.L.; van Hinsbergh, V.W.; Teerlink, T.; Barto, R.; Twisk, J.; Stehouwer, C.D.; Schalkwijk, C.G. Increased levels of N(epsilon)-(carboxymethyl)lysine and N(epsilon)-(carboxyethyl)lysine in type 1 diabetic patients with impaired renal function: Correlation with markers of endothelial dysfunction. Nephrol. Dial. Transplant. 2004, 19, 631–636. [Google Scholar] [CrossRef]
- Dias, C.G.; Venkataswamy, L.; Balakrishna, S. Diabetic nephropathy patients show hyper-responsiveness to N6-carboxymethyllysine. Braz. J. Med. Biol. Res. 2022, 55, e11984. [Google Scholar] [CrossRef]
- Kumar, P.A.; Welsh, G.I.; Raghu, G.; Menon, R.K.; Saleem, M.A.; Reddy, G.B. Carboxymethyl lysine induces EMT in podocytes through transcription factor ZEB2: Implications for podocyte depletion and proteinuria in diabetes mellitus. Arch. Biochem. Biophys. 2016, 590, 10–19. [Google Scholar] [CrossRef]
- Yuan, Y.; Sun, H.; Sun, Z. Advanced glycation end products (AGEs) increase renal lipid accumulation: A pathogenic factor of diabetic nephropathy (DN). Lipids Health Dis. 2017, 16, 126. [Google Scholar] [CrossRef]
- Wang, L.; Wang, H.L.; Liu, T.T.; Lan, H.Y. TGF-beta as a master regulator of diabetic nephropathy. Int. J. Mol. Sci. 2021, 22, 7881. [Google Scholar] [CrossRef] [PubMed]
- Fukami, K.; Ueda, S.; Yamagishi, S.I.; Kato, S.; Inagaki, Y.; Takeuchi, M.; Motomiya, Y.; Bucala, R.; Iida, S.; Tamaki, K.; et al. AGEs activate mesangial TGF-β–Smad signaling via an angiotensin II type I receptor interaction. Kidney Int. 2004, 66, 2137–2147. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; He, W.; Liu, Z.; Xu, H.; Ma, G. The accumulation of the glycoxidation product N(ε)-carboxymethyllysine in cardiac tissues with age, diabetes mellitus and coronary heart disease. Tohoku J. Exp. Med. 2013, 230, 25–32. [Google Scholar] [CrossRef]
- Fuijkschot, W.W.; de Graaff, H.J.; Berishvili, E.; Kakabadze, Z.; Kupreishvili, K.; Meinster, E.; Houtman, M.; van Broekhoven, A.; Schalkwijk, C.G.; Vonk, A.B.; et al. Prevention of age-induced N(ε)-(carboxymethyl)lysine accumulation in the microvasculature. Eur. J. Clin. Investig. 2016, 46, 334–341. [Google Scholar] [CrossRef]
- Whitson, H.E.; Arnold, A.M.; Yee, L.M.; Mukamal, K.J.; Kizer, J.R.; Djousse, L.; Ix, J.H.; Siscovick, D.; Tracy, R.P.; Thielke, S.M.; et al. Serum carboxymethyl-lysine, disability, and frailty in older persons: The Cardiovascular Health Study. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, 710–716. [Google Scholar] [CrossRef]
- Yuan, X.; Zhai, R.; Bai, Y.; Zheng, M.; Xie, X.; Chen, T.; Huang, T.; Chen, Z.; Li, J. Comparison of pharmacokinetics, biodistribution, and excretion of free and bound Nε-carboxymethyllysine in rats by HPLC–MS/MS. Food Res. Int. 2023, 164, 112395. [Google Scholar] [CrossRef]
- Yuan, X.; Bai, Y.; Zhang, J.; Zhai, R.; Nie, C.; Tu, A.; Li, S.; Chen, Z.; Zhang, M.; Li, J. Comparison of tissue distribution of free and protein bound Nɛ-carboxymethyllysine after long-term oral administration to mice. Food Res. Int. 2022, 161, 111787. [Google Scholar] [CrossRef]
- Raupbach, J.; Ott, C.; Koenig, J.; Grune, T. Proteasomal degradation of glycated proteins depends on substrate unfolding: Preferred degradation of moderately modified myoglobin. Free Radic. Biol. Med. 2020, 152, 516–524. [Google Scholar] [CrossRef]
- Schmid, K.; Haslbeck, M.; Buchner, J.; Somoza, V. Induction of heat shock proteins and the proteasome system by casein-Nɛ-(carboxymethyl) lysine and Nɛ-(carboxymethyl) lysine in Caco-2 cells. Ann. N. Y. Acad. Sci. 2008, 1126, 257–261. [Google Scholar] [CrossRef]
- Haddad, M.; Perrotte, M.; Landri, S.; Lepage, A.; Fülöp, T.; Ramassamy, C. Circulating and extracellular vesicles levels of N-(1-carboxymethyl)-l-lysine (CML) differentiate early to moderate Alzheimer’s disease. J. Alzheimers Dis. 2019, 69, 751–762. [Google Scholar] [CrossRef] [PubMed]
- Greifenhagen, U.; Nguyen, V.D.; Moschner, J.; Giannis, A.; Frolov, A.; Hoffmann, R. Sensitive and site-specific identification of carboxymethylated and carboxyethylated peptides in tryptic digests of proteins and human plasma. J. Proteome Res. 2015, 14, 768–777. [Google Scholar] [CrossRef]
- Thornalley, P.J.; Battah, S.; Ahmed, N.; Karachalias, N.; Agalou, S.; Babaei-Jadidi, R.; Dawnay, A. Quantitative screening of advanced glycation endproducts in cellular and extracellular proteins by tandem mass spectrometry. Biochem. J. 2003, 375, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Somoza, V.; Wenzel, E.; Weiß, C.; Clawin-Rädecker, I.; Grübel, N.; Erbersdobler, H.F. Dose-dependent utilisation of casein-linked lysinoalanine, N (epsilon)-fructoselysine and N (epsilon)-carboxymethyllysine in rats. Mol. Nutr. Food Res. 2006, 50, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Cnossen, T.T.; Gladziwa, U.; Van De Kerkhof, J.J.; Schalkwijk, C.G.; Scheijen, J.; Van Amersfoort, J.; Moret, K.; Beerenhout, C.H.; Kooman, J.P. The influence of bicarbonate/lactate-buffered PD fluids on Nε-(carboxyethyl) lysine and Nε-(carboxymethyl) lysine in peritoneal effluent. Perit. Dial. Int. 2011, 31, 189–193. [Google Scholar] [CrossRef]
- Busch, M.; Franke, S.; Müller, A.; Wolf, M.; Gerth, J.; Ott, U.; Niwa, T.; Stein, G. Potential cardiovascular risk factors in chronic kidney disease: AGEs, total homocysteine and metabolites, and the C-reactive protein. Kidney Int. 2004, 66, 338–347. [Google Scholar] [CrossRef]
- Dwi, F.; Rahayu, M.; Rachmawati, B. Differences in AGEs-N-carboxymethyllysine and kidney injury molecule-1 in non-diabetic subjects, diabetic with and without diabetic nephropathy. Bali Med. J. 2021, 10, 325–330. [Google Scholar] [CrossRef]
- Hirata, K.; Kubo, K. Relationship between blood levels of N-carboxymethyl-lysine and pentosidine and the severity of microangiopathy in type 2 diabetes. Endocr. J. 2004, 51, 537–544. [Google Scholar] [CrossRef]
- Nishad, R.; Tahaseen, V.; Kavvuri, R.; Motrapu, M.; Singh, A.K.; Peddi, K.; Pasupulati, A.K. Advanced-glycation end-products induce podocyte injury and contribute to proteinuria. Front. Med. 2021, 8, 685447. [Google Scholar] [CrossRef]
- Wang, Y.X.; Xu, H.; Liu, X.; Liu, L.; Wu, Y.N.; Gong, Z.Y. Studies on mechanism of free Nε-(carboxymethyl) lysine-induced toxic injury in mice. J. Biochem. Mol. Toxicol. 2019, 33, e22322. [Google Scholar] [CrossRef]
- Strzelczyk, J.; Szumska, M.; Damasiewicz-Bodzek, A.; Krywult, A.; Długaszek, M.; Czubilińska, J.; Gawlik, K.; Synowiec, K.; Tyrpień-Golder, K.; Poczkaj, K.; et al. Determination of advanced glycation end-products and antibodies against them (anti-CML and anti-CEL) in the serum of Graves’ orbitopathy patients before and after methylprednisolone treatment. Endokrynol. Pol. 2016, 67, 383–389. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Reddy, S.; Bichler, J.; Wells-Knecht, K.J.; Thorpe, S.R.; Baynes, J.W. N epsilon-(carboxymethyl)lysine is a dominant advanced glycation end product (AGE) antigen in tissue proteins. Biochemistry 1995, 34, 10872–10878. [Google Scholar] [CrossRef] [PubMed]
- Shibayama, R.; Araki, N.; Nagai, R.; Horiuchi, S. Autoantibody against N(epsilon)-(carboxymethyl)lysine: An advanced glycation end product of the Maillard reaction. Diabetes 1999, 48, 1842–1849. [Google Scholar] [CrossRef]
- Vay, D.; Vidali, M.; Allochis, G.; Cusaro, C.; Rolla, R.; Mottaran, E.; Bellomo, G.; Albano, E. Antibodies against advanced glycation end product Nɛ-(carboxymethyl) lysine in healthy controls and diabetic patients. Diabetologia 2000, 43, 1385–1388. [Google Scholar] [CrossRef]
- Vasileva, B. Morphological and Laboratory Characteristics of Patients with Diabetes Mellitus and Chronic Kidney Disease. Ph.D. Thesis, Medical University, Sofa, Bulgaria, 2019. [Google Scholar]
- Schleicher, E.D.; Wagner, E.; Nerlich, A.G. Increased accumulation of the glycoxidation product N(epsilon)-(carboxymethyl)lysine in human tissues in diabetes and aging. J. Clin. Investig. 1997, 99, 457–468. [Google Scholar] [CrossRef]
- Horie, K.; Miyata, T.; Maeda, K.; Miyata, S.; Sugiyama, S.; Sakai, H.; De Strihou, C.V.; Monnier, V.M.; Witztum, J.L.; Kurokawa, K. Immunohistochemical colocalization of glycoxidation products and lipid peroxidation products in diabetic renal glomerular lesions. Implication for glycoxidative stress in the pathogenesis of diabetic nephropathy. J. Clin. Investig. 1997, 100, 2995–3004. [Google Scholar] [CrossRef]
- Mishra, N.; Saxena, S.; Shukla, R.K.; Singh, V.; Meyer, C.H.; Kruzliak, P.; Khanna, V.K. Association of serum Nε-Carboxy methyl lysine with severity of diabetic retinopathy. J. Diabetes Complicat. 2016, 30, 511–517. [Google Scholar] [CrossRef]
- Bora, S.; Adole, P.S.; Motupalli, N.; Pandit, V.R.; Vinod, K.V. Association between carbonyl stress markers and the risk of acute coronary syndrome in patients with type 2 diabetes mellitus–A pilot study. Diabetes Metab. Syndr. 2020, 14, 1751–1755. [Google Scholar] [CrossRef] [PubMed]
- Rahim, M.Z.M.; Azman, W.N.W.; Yaacob, N.M.; Wan Mohamed, W.M.I.; Shafii, N. The level of N-carboxymethyllysine and C-reactive protein in type 2 diabetes mellitus and it’s association with HbA1c in diabetic nephropathy. Malays. J. Med. Health Sci. 2023, 19, 282–289. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, J.; Ten, S.; Zhang, J.; Yuan, Y.; Yu, J.; An, X. Plasma heparanase is associated with blood glucose levels but not urinary microalbumin excretion in type 2 diabetic nephropathy at the early stage. Ren. Fail. 2017, 39, 698–701. [Google Scholar] [CrossRef][Green Version]
- Ahmed, K.A.; Muniandy, S.; Ismail, I.S. Role of Nε-(carboxymethyl)lysine in the development of ischemic heart disease in type 2 diabetes mellitus. J. Clin. Biochem. Nutr. 2007, 41, 97–105. [Google Scholar] [CrossRef] [PubMed]




| Study Group | FG < 6.1 mmol/L | FG 6.1–6.9 mmol/L | FG ≥ 7.0 mmol/L |
|---|---|---|---|
| CKD+ DM− | 86.36% (19) | 9.09% (2) | 4.55% (1) |
| CKD+ T2DM+ | 25.45% (14) | 14.55% (8) | 60% (33) |
| CKD− T2DM+ | 33.33% (7) | 19.05% (4) | 47.62% (10) |
| Study Group | PPG < 7.8 mmol/L | PPG 7.8–11.1 mmol/L | PPG ≥ 11.1 mmol/L |
|---|---|---|---|
| T2DM+ CKD+ | 56.36% (31) | 23.64% (13) | 20% (11) |
| T2DM+ CKD− | 61.9% (13) | 19.05% (4) | 19.05% (4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsekovska, R.; Gatev, E.; Mironova, R.; Kerezieva, S.; Ilieva, S.; Ilieva, T.; Vasileva, B.; Niwa, T.; Popova, D.; Vasilev, V. Serum Levels of Nε-(Carboxymethyl)-Lysine in Chronic Kidney Disease and Type 2 Diabetes Mellitus. Biomedicines 2025, 13, 1672. https://doi.org/10.3390/biomedicines13071672
Tsekovska R, Gatev E, Mironova R, Kerezieva S, Ilieva S, Ilieva T, Vasileva B, Niwa T, Popova D, Vasilev V. Serum Levels of Nε-(Carboxymethyl)-Lysine in Chronic Kidney Disease and Type 2 Diabetes Mellitus. Biomedicines. 2025; 13(7):1672. https://doi.org/10.3390/biomedicines13071672
Chicago/Turabian StyleTsekovska, Rositsa, Evan Gatev, Roumyana Mironova, Simona Kerezieva, Siyana Ilieva, Teodora Ilieva, Bilyana Vasileva, Toshimitsu Niwa, Daniela Popova, and Vasil Vasilev. 2025. "Serum Levels of Nε-(Carboxymethyl)-Lysine in Chronic Kidney Disease and Type 2 Diabetes Mellitus" Biomedicines 13, no. 7: 1672. https://doi.org/10.3390/biomedicines13071672
APA StyleTsekovska, R., Gatev, E., Mironova, R., Kerezieva, S., Ilieva, S., Ilieva, T., Vasileva, B., Niwa, T., Popova, D., & Vasilev, V. (2025). Serum Levels of Nε-(Carboxymethyl)-Lysine in Chronic Kidney Disease and Type 2 Diabetes Mellitus. Biomedicines, 13(7), 1672. https://doi.org/10.3390/biomedicines13071672







