Targeting p-FGFR1Y654 Enhances CD8+ T Cells Infiltration and Overcomes Immunotherapy Resistance in Esophageal Squamous Cell Carcinoma by Regulating the CXCL8–CXCR2 Axis
Abstract
1. Introduction
2. Methods
2.1. Patient Information and Public Database Sources
2.2. Immunohistochemistry
2.3. Cell Culture and Reagents
2.4. CCK-8 and BrdU Assay
2.5. Transwell Migration Assay
2.6. Mouse Model
2.7. Flow Cytometry Analysis
2.8. RNA-Sequencing (RNA-seq) Data
2.9. Western Blotting (WB) Analysis
2.10. Statistical Analysis
2.11. Ethics
3. Results
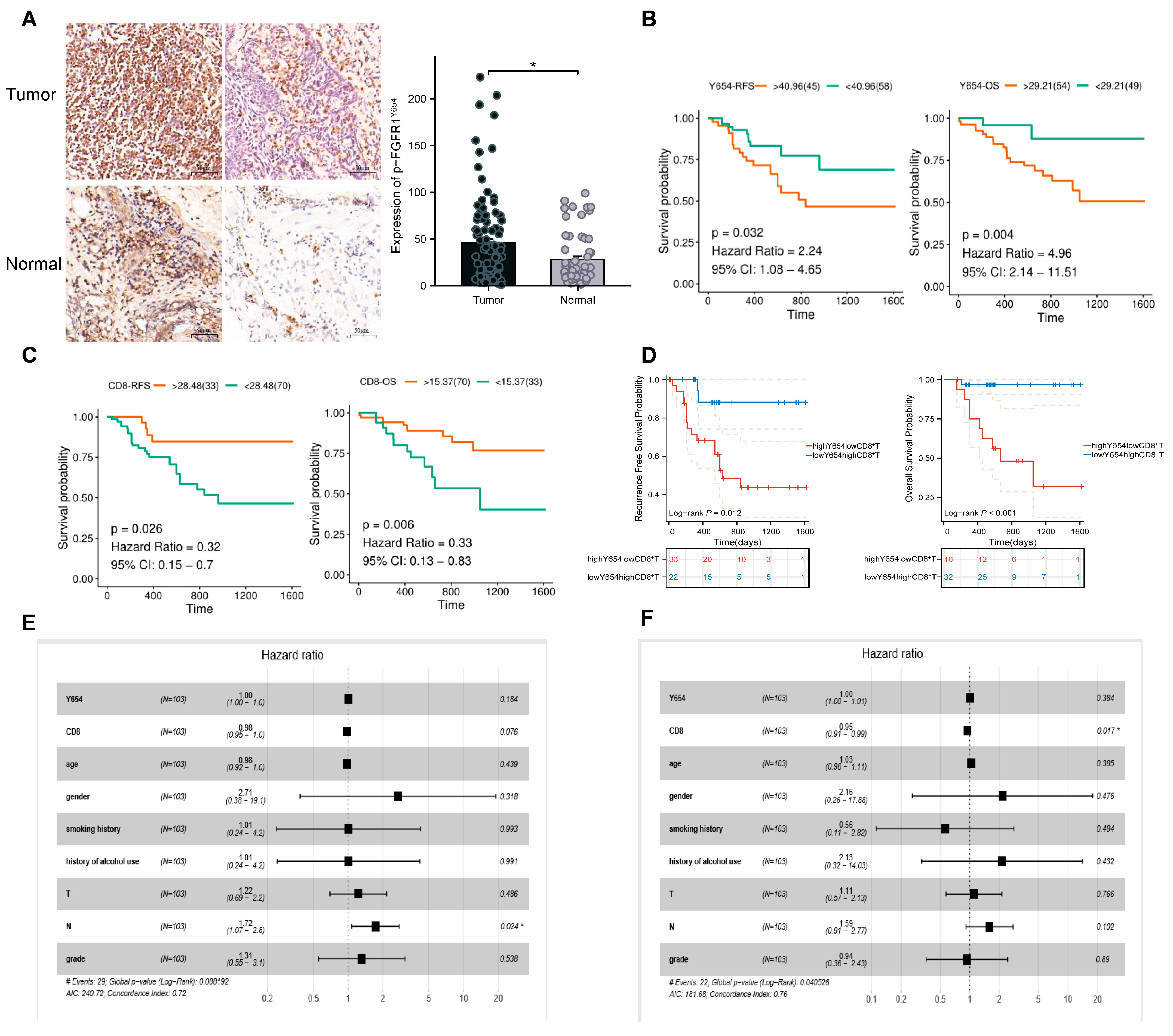
3.1. Expression of p-FGFR1Y654 and Infiltration of CD8+ T Cells Correlate with Patient Prognosis
3.2. AZD4547 Inhibits p-FGFR1Y654 Expression and ESCC Cell Proliferation and Migration In Vitro
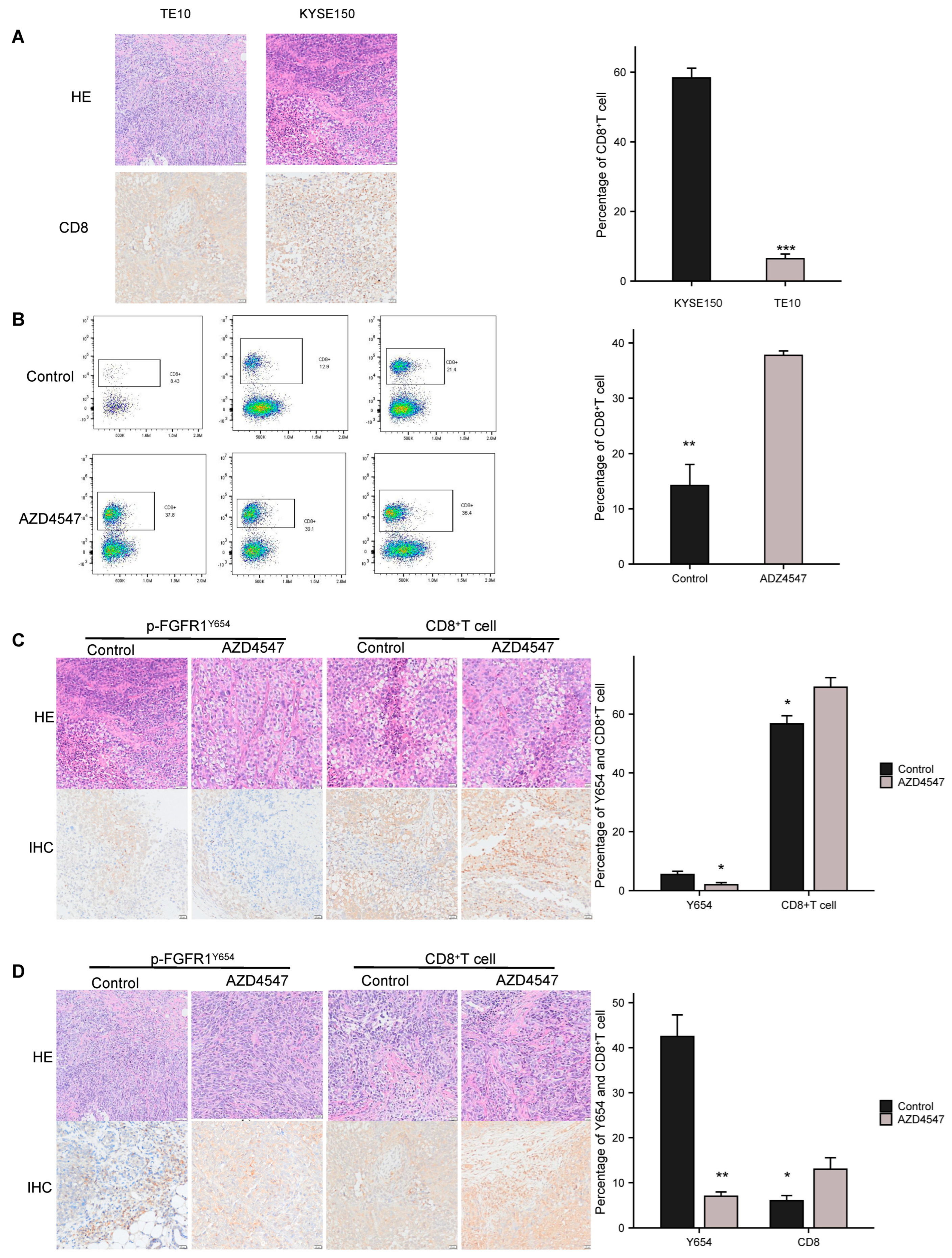
3.3. Inhibition of p-FGFR1Y654 Expression Enhances CD8+ T Cell Infiltration in ESCC
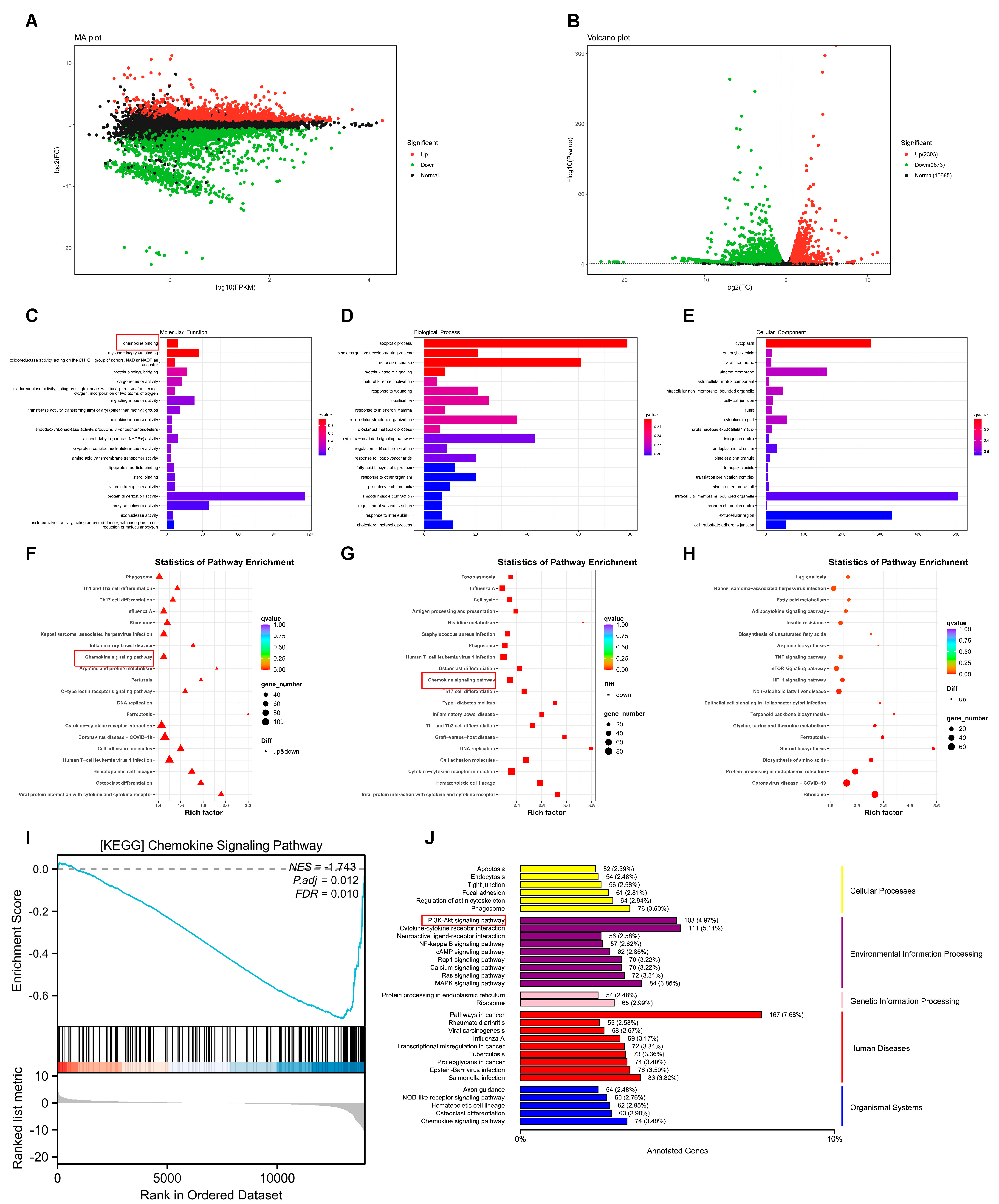
3.4. RNA-seq Reveals the Potential Mechanism of p-FGFR1Y654 Inhibition by AZD4547 in ESCC
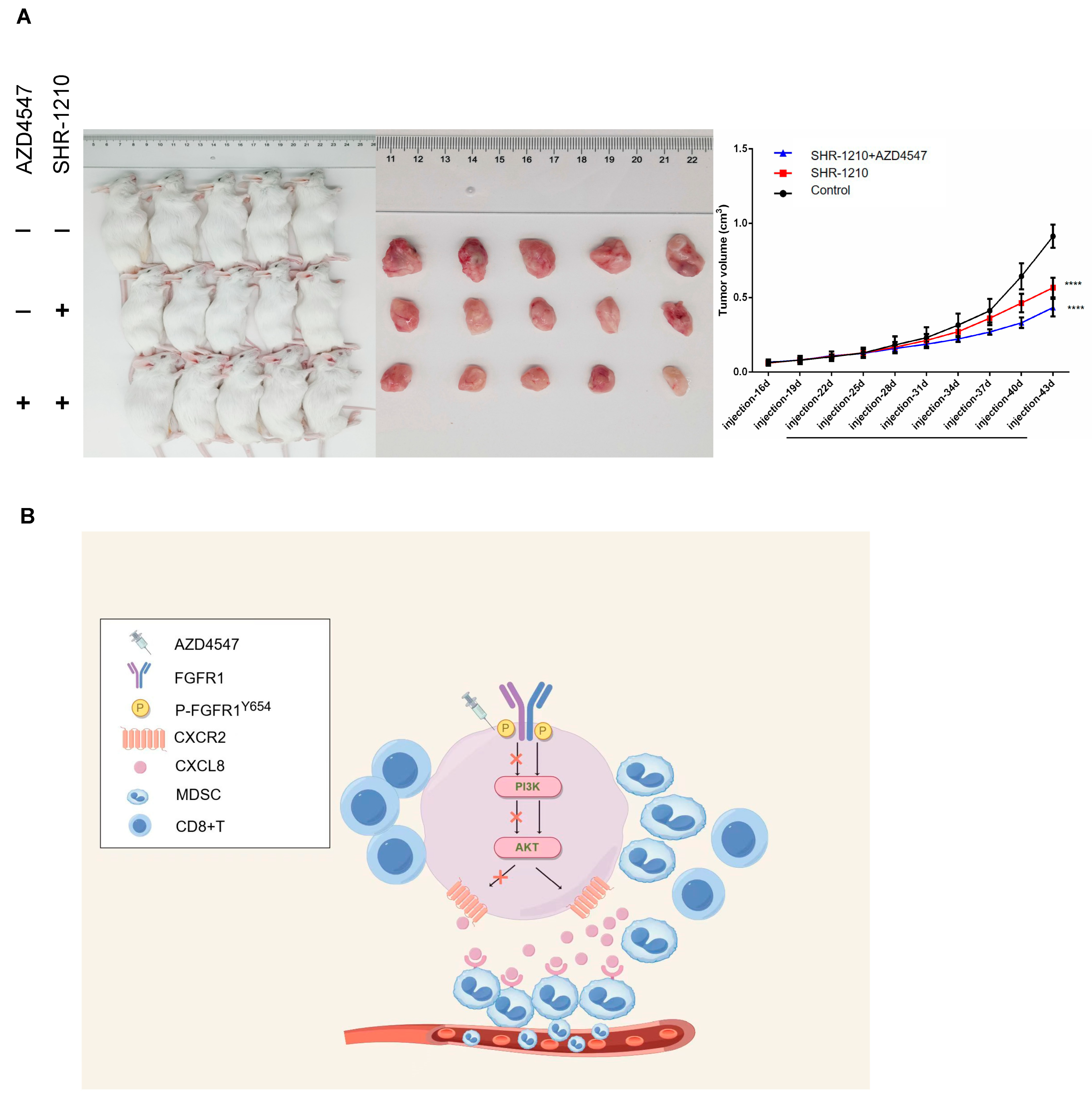
3.5. p-FGFR1Y654 Recruits MDSCs in ESCC Through the CXCL8–CXCR2 Signaling Axis
3.6. In Vivo Validation of Tumor Suppression Effect of AZD4547 Combined with SHR-1210
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AUC | Area Under the Curve |
| CI | Confidence Interval |
| CSCs | Cell-Like Cell Populations |
| DEGs | Differentially Expressed Genes |
| EMT | Epithelial–Mesenchymal Transition |
| ESCC | Esophageal Squamous Cell Carcinoma |
| GO enrichment | Gene Ontology Enrichment |
| GSCA | Gene Set Cancer Analysis |
| HR | Hazard Ratio |
| hu-PBMC | Human Peripheral Blood Mononuclear Cells |
| IHC | Immunohistochemistry |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| MDSCs | Myeloid-Derived Suppressor Cells |
| OS | Overall Survival |
| p-FGFR1Y654 | FGFR1 autophosphorylation at the Y654 site |
| RFS | Relapse-Free Survival |
| RTK | Receptor Tyrosine Kinase |
| TCGA | The Cancer Genome Atlas |
| WB | Western Blotting |
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Chen, W.; Zheng, R.; Zhang, S.; Ji, J.S.; Zou, X.; Xia, C.; Sun, K.; Yang, Z.; Li, H.; et al. Changing cancer survival in China during 2003–2015: A pooled analysis of 17 population-based cancer registries. Lancet Glob. Health 2018, 6, e555–e567. [Google Scholar] [CrossRef] [PubMed]
- Kudo, T.; Hamamoto, Y.; Kato, K.; Ura, T.; Kojima, T.; Tsushima, T.; Hironaka, S.; Hara, H.; Satoh, T.; Iwasa, S.; et al. Nivolumab treatment for oesophageal squamous-cell carcinoma: An open-label, multicentre, phase 2 trial. Lancet Oncol. 2017, 18, 631–639. [Google Scholar] [CrossRef] [PubMed]
- Kojima, T.; Shah, M.A.; Muro, K.; Francois, E.; Adenis, A.; Hsu, C.; Doi, T.; Moriwaki, T.; Kim, S.; Lee, S.; et al. Randomized Phase III KEYNOTE-181 Study of Pembrolizumab Versus Chemotherapy in Advanced Esophageal Cancer. J. Clin. Oncol. 2020, 38, 4138–4148. [Google Scholar] [CrossRef]
- Wang, Q.; Wu, X. Primary and acquired resistance to PD-1/PD-L1 blockade in cancer treatment. Int. Immunopharmacol. 2017, 46, 210–219. [Google Scholar] [CrossRef]
- Jiang, M.; Hu, Y.; Lin, G.; Chen, C.; Li, H. Radiotherapy combined with immune checkpoint inhibitors in locally advanced/metastatic esophageal squamous cell carcinoma: Clinical trials, efficacy and future directions. Front. Immunol. 2023, 14, 1177085. [Google Scholar] [CrossRef]
- Luo, H.; Lu, J.; Bai, Y.; Mao, T.; Wang, J.; Fan, Q.; Zhang, Y.; Zhao, K.; Chen, Z.; Gao, S.; et al. Effect of Camrelizumab vs Placebo Added to Chemotherapy on Survival and Progression-Free Survival in Patients with Advanced or Metastatic Esophageal Squamous Cell Carcinoma. JAMA 2021, 326, 916. [Google Scholar] [CrossRef]
- Sun, J.; Shen, L.; Shah, M.A.; Enzinger, P.; Adenis, A.; Doi, T.; Kojima, T.; Metges, J.; Li, Z.; Kim, S.; et al. Pembrolizumab plus chemotherapy versus chemotherapy alone for first-line treatment of advanced oesophageal cancer (KEYNOTE-590): A randomised, placebo-controlled, phase 3 study. Lancet 2021, 398, 759–771. [Google Scholar] [CrossRef]
- Bandla, S.; Pennathur, A.; Luketich, J.D.; Beer, D.G.; Lin, L.; Bass, A.J.; Godfrey, T.E.; Litle, V.R. Comparative Genomics of Esophageal Adenocarcinoma and Squamous Cell Carcinoma. Ann. Thorac. Surg. 2012, 93, 1101–1106. [Google Scholar] [CrossRef]
- Chioni, A.; Grose, R.P. Biological Significance and Targeting of the FGFR Axis in Cancer. Cancers 2021, 13, 5681. [Google Scholar] [CrossRef]
- Krook, M.A.; Reeser, J.W.; Ernst, G.; Barker, H.; Wilberding, M.; Li, G.; Chen, H.; Roychowdhury, S. Fibroblast growth factor receptors in cancer: Genetic alterations, diagnostics, therapeutic targets and mechanisms of resistance. Br. J. Cancer 2021, 124, 880–892. [Google Scholar] [CrossRef]
- Lew, E.D.; Furdui, C.M.; Anderson, K.S.; Schlessinger, J. The Precise Sequence of FGF Receptor Autophosphorylation Is Kinetically Driven and Is Disrupted by Oncogenic Mutations. Sci. Signal. 2009, 2, ra6. [Google Scholar] [CrossRef]
- Furdui, C.M.; Lew, E.D.; Schlessinger, J.; Anderson, K.S. Autophosphorylation of FGFR1 Kinase Is Mediated by a Sequential and Precisely Ordered Reaction. Mol. Cell 2006, 21, 711–717. [Google Scholar] [CrossRef]
- Spranger, S.; Gajewski, T.F. Impact of oncogenic pathways on evasion of antitumour immune responses. Nat. Rev. Cancer 2018, 18, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Gainor, J.F.; Shaw, A.T.; Sequist, L.V.; Fu, X.; Azzoli, C.G.; Piotrowska, Z.; Huynh, T.G.; Zhao, L.; Fulton, L.; Schultz, K.R.; et al. EGFR Mutations and ALK Rearrangements Are Associated with Low Response Rates to PD-1 Pathway Blockade in Non–Small Cell Lung Cancer: A Retrospective Analysis. Clin. Cancer Res. 2016, 22, 4585–4593. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Li, Y.; Wang, X.; Zhang, X.; Hu, Y.; Li, L.; Suo, D.; Ni, K.; Li, Z.; Zhan, J.; et al. Tumor Fibroblast–Derived FGF2 Regulates Expression of SPRY1 in Esophageal Tumor–Infiltrating T Cells and Plays a Role in T-cell Exhaustion. Cancer Res. 2020, 80, 5583–5596. [Google Scholar] [CrossRef]
- Kumar, V.; Patel, S.; Tcyganov, E.; Gabrilovich, D.I. The Nature of Myeloid-Derived Suppressor Cells in the Tumor Microenvironment. Trends Immunol. 2016, 37, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Molon, B.; Ugel, S.; Del Pozzo, F.; Soldani, C.; Zilio, S.; Avella, D.; De Palma, A.; Mauri, P.; Monegal, A.; Rescigno, M.; et al. Chemokine nitration prevents intratumoral infiltration of antigen-specific T cells. J. Exp. Med. 2011, 208, 1949–1962. [Google Scholar] [CrossRef]
- Blakely, C.M.; Watkins, T.B.K.; Wu, W.; Gini, B.; Chabon, J.J.; McCoach, C.E.; McGranahan, N.; Wilson, G.A.; Birkbak, N.J.; Olivas, V.R.; et al. Evolution and clinical impact of co-occurring genetic alterations in advanced-stage EGFR-mutant lung cancers. Nat. Genet. 2017, 49, 1693–1704. [Google Scholar] [CrossRef]
- Hu, Y.; Domínguez, C.M.; Bauer, J.; Weigel, S.; Schipperges, A.; Oelschlaeger, C.; Willenbacher, N.; Keppler, S.; Bastmeyer, M.; Heißler, S.; et al. Carbon-nanotube reinforcement of DNA-silica nanocomposites yields programmable and cell-instructive biocoatings. Nat. Commun. 2019, 10, 5514–5522. [Google Scholar] [CrossRef]
- Martin, K.; Pritchett, J.; Llewellyn, J.; Mullan, A.F.; Athwal, V.S.; Dobie, R.; Harvey, E.; Zeef, L.; Farrow, S.; Streuli, C.; et al. PAK proteins and YAP-1 signalling downstream of integrin beta-1 in myofibroblasts promote liver fibrosis. Nat. Commun. 2016, 7, 12502. [Google Scholar] [CrossRef] [PubMed]
- Thakur, A.; Qiu, G.; Xu, C.; Han, X.; Yang, T.; NGSP; Chan, K.W.Y.; Wu, C.M.L.; Lee, Y. Label-free sensing of exosomal MCT1 and CD147 for tracking metabolic reprogramming and malignant progression in glioma. Sci. Adv. 2020, 6, eaaz6119. [Google Scholar] [CrossRef]
- Yago, T.; Liu, Z.; Ahamed, J.; McEver, R.P. Cooperative PSGL-1 and CXCR2 signaling in neutrophils promotes deep vein thrombosis in mice. Blood 2018, 132, 1426–1437. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Ma, X.; Xu, T.; Li, Y.; Hodge, A.; Zhang, Q.; Torline, J.; Huang, Y.; Zhao, J.; Ling, K.; et al. Axoneme polyglutamylation regulated by Joubert syndrome protein ARL13B controls ciliary targeting of signaling molecules. Nat. Commun. 2018, 9, 3310–3314. [Google Scholar] [CrossRef]
- Katoh, M. FGFR inhibitors: Effects on cancer cells, tumor microenvironment and whole-body homeostasis (Review). Int. J. Mol. Med. 2016, 38, 3–15. [Google Scholar] [CrossRef]
- Chae, Y.K.; Hong, F.; Vaklavas, C.; Cheng, H.H.; Hammerman, P.; Mitchell, E.P.; Zwiebel, J.A.; Ivy, S.P.; Gray, R.J.; Li, S.; et al. Phase II Study of AZD4547 in Patients with Tumors Harboring Aberrations in the FGFR Pathway: Results from the NCI-MATCH Trial (EAY131) Subprotocol W. J. Clin. Oncol. 2020, 38, 2407–2417. [Google Scholar] [CrossRef]
- Van Cutsem, E.; Bang, Y.J.; Mansoor, W.; Petty, R.D.; Chao, Y.; Cunningham, D.; Ferry, D.R.; Smith, N.R.; Frewer, P.; Ratnayake, J.; et al. A randomized, open-label study of the efficacy and safety of AZD4547 monotherapy versus paclitaxel for the treatment of advanced gastric adenocarcinoma with FGFR2 polysomy or gene amplification. Ann. Oncol. 2017, 28, 1316–1324. [Google Scholar] [CrossRef] [PubMed]
- Coombes, R.C.; Badman, P.D.; Lozano-Kuehne, J.P.; Liu, X.; Macpherson, I.R.; Zubairi, I.; Baird, R.D.; Rosenfeld, N.; Garcia-Corbacho, J.; Cresti, N.; et al. Results of the phase IIa RADICAL trial of the FGFR inhibitor AZD4547 in endocrine resistant breast cancer. Nat. Commun. 2022, 13, 3211–3246. [Google Scholar] [CrossRef]
- Maehara, O.; Suda, G.; Natsuizaka, M.; Ohnishi, S.; Komatsu, Y.; Sato, F.; Nakai, M.; Sho, T.; Morikawa, K.; Ogawa, K.; et al. Fibroblast growth factor-2–mediated FGFR/Erk signaling supports maintenance of cancer stem-like cells in esophageal squamous cell carcinoma. Carcinogenesis 2017, 38, 1073–1083. [Google Scholar] [CrossRef]
- Wang, S.; Liu, C.; Yang, C.; Jin, Y.; Cui, Q.; Wang, D.; Ge, T.; He, G.; Li, W.; Zhang, G.; et al. PI3K/AKT/mTOR and PD-1/CTLA-4/CD28 pathways as key targets of cancer immunotherapy (Review). Oncol. Lett. 2024, 28, 567. [Google Scholar] [CrossRef]
- Chen, H.; Sun, G.; Han, Z.; Wang, H.; Li, J.; Ye, H.; Song, C.; Zhang, J.; Wang, P. Anti-CXCL8 Autoantibody: A Potential Diagnostic Biomarker for Esophageal Squamous Cell Carcinoma. Medicina 2022, 58, 1480. [Google Scholar] [CrossRef] [PubMed]
- Bohrer, L.R.; Schwertfeger, K.L. Macrophages Promote Fibroblast Growth Factor Receptor-Driven Tumor Cell Migration and Invasion in a Cxcr2-Dependent Manner. Mol. Cancer Res. 2012, 10, 1294–1305. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, S.; Zhen, Y.; Gao, P.; Zhang, Z.; Guo, H.; Wang, Y. Circular RNA circFGFR1 Functions as an Oncogene in Glioblastoma Cells through Sponging to hsa-miR-224-5p. J. Immunol. Res. 2022, 2022, 7990251. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Ren, J.; Ten Dijke, P. Targeting TGFβ signal transduction for cancer therapy. Signal Transduct. Target. Ther. 2021, 6, 8–20. [Google Scholar] [CrossRef]
- Baba, Y.; Nomoto, D.; Okadome, K.; Ishimoto, T.; Iwatsuki, M.; Miyamoto, Y.; Yoshida, N.; Baba, H. Tumor immune microenvironment and immune checkpoint inhibitors in esophageal squamous cell carcinoma. Cancer Sci. 2020, 111, 3132–3141. [Google Scholar] [CrossRef] [PubMed]
- Lasser, S.A.; Ozbay Kurt, F.G.; Arkhypov, I.; Utikal, J.; Umansky, V. Myeloid-derived suppressor cells in cancer and cancer therapy. Nat. Rev. Clin. Oncol. 2024, 21, 147–164. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Su, N.; Yang, J.; Tan, Q.; Huang, S.; Jin, M.; Ni, Z.; Zhang, B.; Zhang, D.; Luo, F.; et al. FGF/FGFR signaling in health and disease. Signal Transduct. Target. Ther. 2020, 5, 181. [Google Scholar] [CrossRef] [PubMed]
- von Loga, K.; Kohlhaussen, J.; Burkhardt, L.; Simon, R.; Steurer, S.; Burdak-Rothkamm, S.; Jacobsen, F.; Sauter, G.; Krech, T. FGFR1 Amplification Is Often Homogeneous and Strongly Linked to the Squamous Cell Carcinoma Subtype in Esophageal Carcinoma. PLoS ONE 2015, 10, e141867. [Google Scholar] [CrossRef]
- Chen, B.; Liu, S.; Gan, L.; Wang, J.; Hu, B.; Xu, H.; Tong, R.; Yang, H.; Cristina, I.; Xue, J.; et al. FGFR1 signaling potentiates tumor growth and predicts poor prognosis in esophageal squamous cell carcinoma patients. Cancer Biol. Ther. 2017, 19, 76–86. [Google Scholar] [CrossRef]
- Thar Min, A.K.; Okayama, H.; Saito, M.; Ashizawa, M.; Aoto, K.; Nakajima, T.; Saito, K.; Hayase, S.; Sakamoto, W.; Tada, T.; et al. Epithelial-mesenchymal transition-converted tumor cells can induce T-cell apoptosis through upregulation of programmed death ligand 1 expression in esophageal squamous cell carcinoma. Cancer Med. 2018, 7, 3321–3330. [Google Scholar] [CrossRef]
- Chen, J.; Liao, S.; Xiao, Z.; Pan, Q.; Wang, X.; Shen, K.; Wang, S.; Yang, L.; Guo, F.; Liu, H.; et al. The development and improvement of immunodeficient mice and humanized immune system mouse models. Front. Immunol. 2022, 13, 1007579. [Google Scholar] [CrossRef]
- Yaguchi, T.; Kobayashi, A.; Inozume, T.; Morii, K.; Nagumo, H.; Nishio, H.; Iwata, T.; Ka, Y.; Katano, I.; Ito, R.; et al. Human PBMC-transferred murine MHC class I/II-deficient NOG mice enable long-term evaluation of human immune responses. Cell Mol. Immunol. 2018, 15, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Mempel, T.R.; Lill, J.K.; Altenburger, L.M. How chemokines organize the tumour microenvironment. Nat. Rev. Cancer 2024, 24, 28–50. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhang, H.; Sui, Z.; Wang, Y.; Yu, Z. The biological role of the CXCL12/CXCR4 axis in esophageal squamous cell carcinoma. Cancer Biol. Med. 2021, 18, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Lu, Q.; Xu, Y.; Liu, C.; Sun, Q. Clinicopathologic significance of CXCR4 expressions in patients with esophageal squamous cell carcinoma. Pathol. Res. Pract. 2020, 216, 152787. [Google Scholar] [CrossRef]
- Singh, A.K.; Arya, R.K.; Trivedi, A.K.; Sanyal, S.; Baral, R.; Dormond, O.; Briscoe, D.M.; Datta, D. Chemokine receptor trio: CXCR3, CXCR4 and CXCR7 crosstalk via CXCL11 and CXCL12. Cytokine Growth Factor Rev. 2013, 24, 41–49. [Google Scholar] [CrossRef]
- Xiang, Z.; Zhou, Z.; Xia, G.; Zhang, X.; Wei, Z.; Zhu, J.; Yu, J.; Chen, W.; He, Y.; Schwarz, R.E.; et al. A positive crosstalk between CXCR4 and CXCR2 promotes gastric cancer metastasis. Oncogene 2017, 36, 5122–5133. [Google Scholar] [CrossRef]
- Joshi, S.; Sharabi, A. Targeting myeloid-derived suppressor cells to enhance natural killer cell-based immunotherapy. Pharmacol. Ther. 2022, 235, 108114. [Google Scholar] [CrossRef]
- Zhang, R.; Dong, M.; Tu, J.; Li, F.; Deng, Q.; Xu, J.; He, X.; Ding, J.; Xia, J.; Sheng, D.; et al. PMN-MDSCs modulated by CCL20 from cancer cells promoted breast cancer cell stemness through CXCL2-CXCR2 pathway. Signal Transduct. Target. Ther. 2023, 8, 97. [Google Scholar] [CrossRef]
- Zhou, X.; Fang, D.; Liu, H.; Ou, X.; Zhang, C.; Zhao, Z.; Zhao, S.; Peng, J.; Cai, S.; He, Y.; et al. PMN-MDSCs accumulation induced by CXCL1 promotes CD8+ T cells exhaustion in gastric cancer. Cancer Lett. 2022, 532, 215598. [Google Scholar] [CrossRef]
- Qi, L.; Wang, J.; Hou, S.; Liu, S.; Zhang, Q.; Zhu, S.; Liu, S.; Zhang, S. Unraveling the tumor microenvironment of esophageal squamous cell carcinoma through single-cell sequencing: A comprehensive review. Biochim. Biophys. Acta Rev. Cancer 2025, 1880, 189264. [Google Scholar] [CrossRef] [PubMed]


| Clinicopathological Characteristics | p-FGFR1Y654 | p | |
|---|---|---|---|
| Low Expression (n = 51) | High Expression (n = 52) | ||
| Age, n (%) | 0.754 | ||
| ≤60 | 21 (20.4%) | 23 (22.3%) | |
| >60 | 30 (29.1%) | 29 (28.2%) | |
| Gender, n (%) | 0.979 | ||
| Male | 47 (45.6%) | 49 (47.6%) | |
| Female | 4 (3.9%) | 3 (2.9) | |
| Smoking history, n (%) | 0.775 | ||
| Yes | 38 (36.9%) | 40 (38.8%) | |
| No | 13 (12.6%) | 12 (11.7%) | |
| History of alcohol, n (%) | 0.203 | ||
| Yes | 38 (36.9%) | 44 (42.7%) | |
| No | 13 (12.6%) | 8 (7.8%) | |
| Tumor size, cm (mean ± sd) | 3.880 ± 1.760 | 3.694 ± 1.801 | 0.530 |
| T Stage, n (%) | 0.514 | ||
| T1 | 3 (2.9%) | 6 (5.8%) | |
| T2 | 11 (10.7%) | 11 (10.7%) | |
| T3 | 32 (31.1%) | 33 (32.0%) | |
| T4 | 5 (4.9%) | 2 (1.9%) | |
| N Stage, n (%) | 0.687 | ||
| N0 | 29 (28.2%) | 29 (28.2%) | |
| N1 | 14 (13.6%) | 12 (11.6%) | |
| N2 | 6 (5.8%) | 10 (9.7%) | |
| N3 | 2 (1.9%) | 1 (1.0%) | |
| Stage, n (%) | 0.270 | ||
| I | 4 (3.9%) | 10 (9.7%) | |
| II | 24 (23.3%) | 20 (19.4%) | |
| III | 22 (21.4%) | 22 (21.4%) | |
| IV | 1 (1.0%) | 0 (0%) | |
| Histological grade, n (%) | 0.165 | ||
| 1 | 6 (5.8%) | 8 (7.8%) | |
| 2 | 36 (35.0%) | 41 (39.8%) | |
| 3 | 9 (8.7%) | 3 (2.9%) | |
| Tumor site, n (%) | 0.113 | ||
| Upper | 0 (0%) | 2 (1.9%) | |
| Middle | 25 (24.3%) | 17 (16.5%) | |
| Lower | 26 (25.2%) | 33 (32.0%) | |
| RFS, n (%) | 0.056 | ||
| Recurrence | 10 (9.7%) | 19 (18.5%) | |
| No recurrence | 41 (39.8%) | 33 (32.0%) | |
| OS, n (%) | 0.005 | ||
| Alive | 46 (44.6%) | 35 (34.0%) | |
| Dead | 5 (4.9%) | 17 (16.5%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, H.; Wang, L.; Gao, H.; Zhou, D.; Qiu, Y.; Yang, L.; Li, J.; Du, D.; Huang, X.; Zhao, Y.; et al. Targeting p-FGFR1Y654 Enhances CD8+ T Cells Infiltration and Overcomes Immunotherapy Resistance in Esophageal Squamous Cell Carcinoma by Regulating the CXCL8–CXCR2 Axis. Biomedicines 2025, 13, 1667. https://doi.org/10.3390/biomedicines13071667
Luo H, Wang L, Gao H, Zhou D, Qiu Y, Yang L, Li J, Du D, Huang X, Zhao Y, et al. Targeting p-FGFR1Y654 Enhances CD8+ T Cells Infiltration and Overcomes Immunotherapy Resistance in Esophageal Squamous Cell Carcinoma by Regulating the CXCL8–CXCR2 Axis. Biomedicines. 2025; 13(7):1667. https://doi.org/10.3390/biomedicines13071667
Chicago/Turabian StyleLuo, Hong, Liwei Wang, Hui Gao, Daijun Zhou, Yu Qiu, Lijia Yang, Jing Li, Dan Du, Xiaoli Huang, Yu Zhao, and et al. 2025. "Targeting p-FGFR1Y654 Enhances CD8+ T Cells Infiltration and Overcomes Immunotherapy Resistance in Esophageal Squamous Cell Carcinoma by Regulating the CXCL8–CXCR2 Axis" Biomedicines 13, no. 7: 1667. https://doi.org/10.3390/biomedicines13071667
APA StyleLuo, H., Wang, L., Gao, H., Zhou, D., Qiu, Y., Yang, L., Li, J., Du, D., Huang, X., Zhao, Y., Qi, Z., Zhang, Y., Huang, X., Sun, L., Xu, T., & Li, D. (2025). Targeting p-FGFR1Y654 Enhances CD8+ T Cells Infiltration and Overcomes Immunotherapy Resistance in Esophageal Squamous Cell Carcinoma by Regulating the CXCL8–CXCR2 Axis. Biomedicines, 13(7), 1667. https://doi.org/10.3390/biomedicines13071667





