High Co-Expression of GPAT4 and SLC7A11 as a Predictor of Platinum Resistance and Poor Prognosis in Patients with Epithelial Ovarian Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Patient Characteristics
- For ovarian cancer staging surgery or tumor cell reduction in our hospital, all samples were collected from the initial cytoreductive surgery in the ovary.
- Patients had a histological diagnosis of EOC, and pathological tissue sections were obtained.
- Imaging was performed before surgery, and patients had intraoperatively measurable lesions.
- Postoperative pathology revealed epithelial ovarian cancer, and platinum-based chemotherapy was performed after surgery.
- There was no history of other malignant tumors.
- Patients had complete clinical data.
- Complete follow-up information was available for all included patients.
- The quality of the pathological tissue sections was not good.
- Postoperative routine pathology revealed other types of tumors rather than ovarian epithelial carcinoma.
- Regular chemotherapy was not performed after surgery.
- The patient had other basic diseases that affect the survival of patients.
- The patient received neoadjuvant chemotherapy.
- The patient was pregnant or breastfeeding.
2.2. 5hmC Library Construction and High-Throughput Sequencing
2.3. Immunohistochemistry
2.4. Cell Culture
2.5. The Cell Viability Was Assessed by IncuCyte Live-Cell Assays
2.6. Statistical Analysis
3. Results
3.1. Screening of GPAT4
3.2. Association of GPAT4 and SLC7A11 Expression with Clinical Features and Platinum Resistance
3.3. Role of SLC7A11 and GPAT4 Expression in the Prediction of Drug Resistance in Ovarian Cancer
3.4. Effects of GPAT4 and SLC7A11 Expression on the Prognosis of Ovarian Cancer
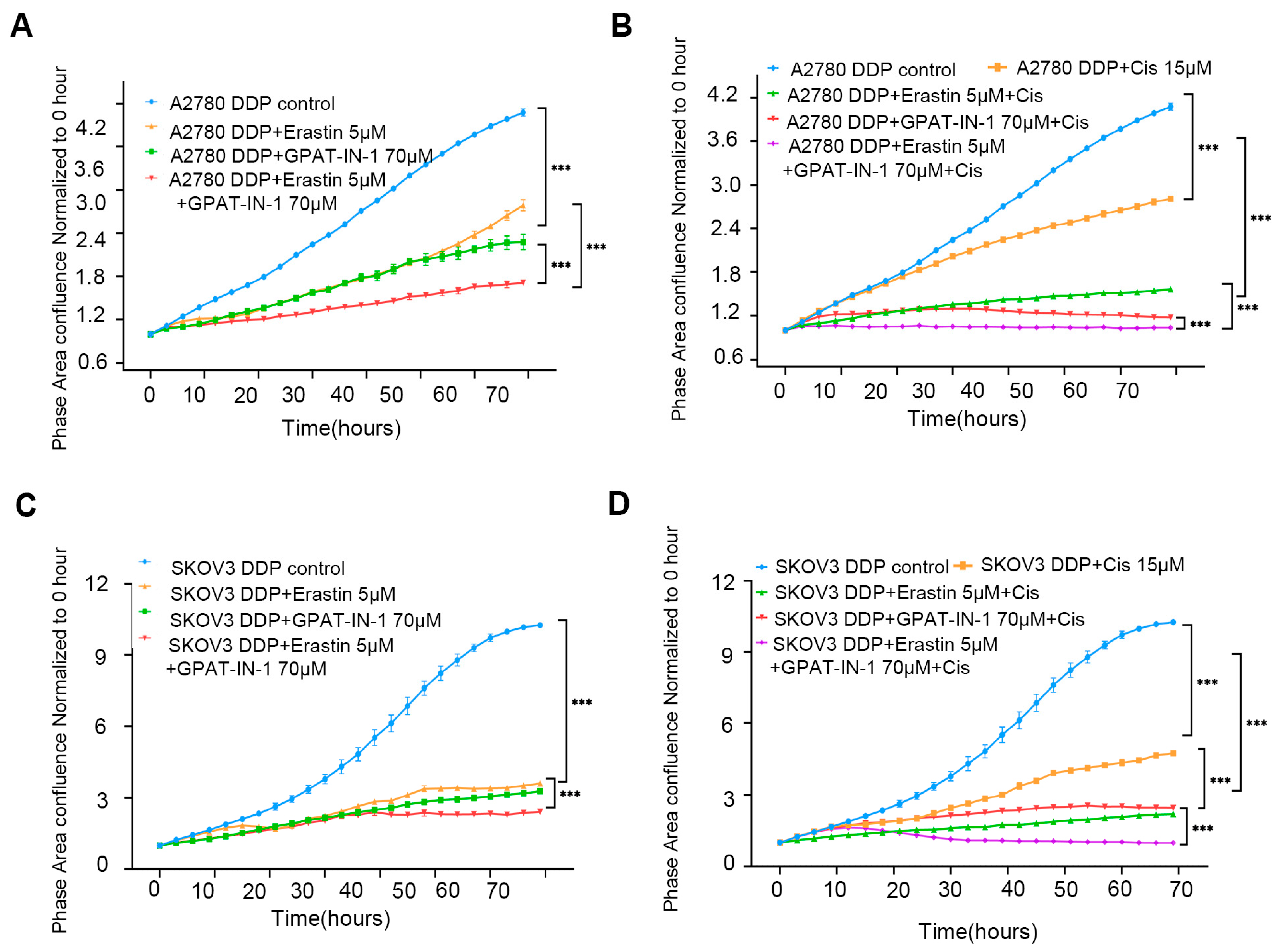
3.5. Effect of GPAT4 and SLC7A11 Inhibitors on Ovarian Cancer Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- DiSilvestro, P.; Banerjee, S.; Colombo, N.; Scambia, G.; Kim, B.G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; et al. Overall Survival with Maintenance Olaparib at a 7-Year Follow-Up in Patients with Newly Diagnosed Advanced Ovarian Cancer and a BRCA Mutation: The SOLO1/GOG 3004 Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2023, 41, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Ray-Coquard, I.; Pautier, P.; Pignata, S.; Pérol, D.; González-Martín, A.; Berger, R.; Fujiwara, K.; Vergote, I.; Colombo, N.; Mäenpää, J.; et al. Olaparib plus Bevacizumab as First-Line Maintenance in Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2416–2428. [Google Scholar] [CrossRef]
- Coleman, R.L.; Brady, M.F.; Herzog, T.J.; Sabbatini, P.; Armstrong, D.K.; Walker, J.L.; Kim, B.G.; Fujiwara, K.; Tewari, K.S.; O’Malley, D.M.; et al. Bevacizumab and paclitaxel-carboplatin chemotherapy and secondary cytoreduction in recurrent, platinum-sensitive ovarian cancer (NRG Oncology/Gynecologic Oncology Group study GOG-0213): A multicentre, open-label, randomised, phase 3 trial. Lancet Oncol. 2017, 18, 779–791. [Google Scholar] [CrossRef] [PubMed]
- Kurtz, J.E.; Pujade-Lauraine, E.; Oaknin, A.; Belin, L.; Leitner, K.; Cibula, D.; Denys, H.; Rosengarten, O.; Rodrigues, M.; de Gregorio, N.; et al. Atezolizumab Combined With Bevacizumab and Platinum-Based Therapy for Platinum-Sensitive Ovarian Cancer: Placebo-Controlled Randomized Phase III ATALANTE/ENGOT-ov29 Trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2023, 41, 4768–4778. [Google Scholar] [CrossRef]
- Ray-Coquard, I.; Leary, A.; Pignata, S.; Cropet, C.; González-Martín, A.; Marth, C.; Nagao, S.; Vergote, I.; Colombo, N.; Mäenpää, J.; et al. Olaparib plus bevacizumab first-line maintenance in ovarian cancer: Final overall survival results from the PAOLA-1/ENGOT-ov25 trial. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2023, 34, 681–692. [Google Scholar] [CrossRef]
- Compadre, A.J.; van Biljon, L.N.; Valentine, M.C.; Llop-Guevara, A.; Graham, E.; Fashemi, B.; Herencia-Ropero, A.; Kotnik, E.N.; Cooper, I.; Harrington, S.P.; et al. RAD51 Foci as a Biomarker Predictive of Platinum Chemotherapy Response in Ovarian Cancer. Clin. Cancer Res. 2023, 29, 2466–2479. [Google Scholar] [CrossRef] [PubMed]
- Qiu, B.; Zandkarimi, F.; Bezjian, C.T.; Reznik, E.; Soni, R.K.; Gu, W.; Jiang, X.; Stockwell, B.R. Phospholipids with two polyunsaturated fatty acyl tails promote ferroptosis. Cell 2024, 187, 1177–1190.e18. [Google Scholar] [CrossRef]
- Ouyang, S.; Li, H.; Lou, L.; Huang, Q.; Zhang, Z.; Mo, J.; Li, M.; Lu, J.; Zhu, K.; Chu, Y.; et al. Inhibition of STAT3-ferroptosis negative regulatory axis suppresses tumor growth and alleviates chemoresistance in gastric cancer. Redox Biol. 2022, 52, 102317. [Google Scholar] [CrossRef]
- Zheng, H.; Liu, J.; Cheng, Q.; Zhang, Q.; Zhang, Y.; Jiang, L.; Huang, Y.; Li, W.; Zhao, Y.; Chen, G.; et al. Targeted activation of ferroptosis in colorectal cancer via LGR4 targeting overcomes acquired drug resistance. Nat. Cancer 2024, 5, 572–589. [Google Scholar] [CrossRef] [PubMed]
- Qi, R.; Bai, Y.; Li, K.; Liu, N.; Xu, Y.; Dal, E.; Wang, Y.; Lin, R.; Wang, H.; Liu, Z.; et al. Cancer-associated fibroblasts suppress ferroptosis and induce gemcitabine resistance in pancreatic cancer cells by secreting exosome-derived ACSL4-targeting miRNAs. Drug Resist. Updates Rev. Comment. Antimicrob. Anticancer. Chemother. 2023, 68, 100960. [Google Scholar] [CrossRef]
- Fan, R.; Deng, A.; Lin, R.; Zhang, S.; Cheng, C.; Zhuang, J.; Hai, Y.; Zhao, M.; Yang, L.; Wei, G. A platinum(IV)-artesunate complex triggers ferroptosis by boosting cytoplasmic and mitochondrial lipid peroxidation to enhance tumor immunotherapy. MedComm 2024, 5, e570. [Google Scholar] [CrossRef] [PubMed]
- Ni, M.; Zhou, J.; Zhu, Z.; Xu, Q.; Yin, Z.; Wang, Y.; Zheng, Z.; Zhao, H. Shikonin and cisplatin synergistically overcome cisplatin resistance of ovarian cancer by inducing ferroptosis via upregulation of HMOX1 to promote Fe2+ accumulation. Phytomedicine 2023, 112, 154701. [Google Scholar] [CrossRef]
- Kim, J.W.; Kim, M.J.; Han, T.H.; Lee, J.Y.; Kim, S.; Kim, H.; Oh, K.J.; Kim, W.K.; Han, B.S.; Bae, K.H.; et al. FSP1 confers ferroptosis resistance in KEAP1 mutant non-small cell lung carcinoma in NRF2-dependent and -independent manner. Cell Death Dis. 2023, 14, 567. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, Y.; Wang, Z.; Jin, Y.; Gu, W. Ferroptosis as a new tool for tumor suppression through lipid peroxidation. Commun. Biol. 2024, 7, 1475. [Google Scholar] [CrossRef]
- Li, D.; Li, Y. The interaction between ferroptosis and lipid metabolism in cancer. Signal Transduct. Target. Ther. 2020, 5, 108. [Google Scholar] [CrossRef]
- Kim, J.W.; Lee, J.Y.; Oh, M.; Lee, E.W. An integrated view of lipid metabolism in ferroptosis revisited via lipidomic analysis. Exp. Mol. Med. 2023, 55, 1620–1631. [Google Scholar] [CrossRef]
- Thürmer, M.; Gollowitzer, A.; Pein, H.; Neukirch, K.; Gelmez, E.; Waltl, L.; Wielsch, N.; Winkler, R.; Löser, K.; Grander, J.; et al. PI(18:1/18:1) is a SCD1-derived lipokine that limits stress signaling. Nat. Commun. 2022, 13, 2982. [Google Scholar] [CrossRef]
- Sen, U.; Coleman, C.; Sen, T. Stearoyl coenzyme A desaturase-1: Multitasker in cancer, metabolism, and ferroptosis. Trends Cancer 2023, 9, 480–489. [Google Scholar] [CrossRef]
- Shiozaki, Y.; Miyazaki-Anzai, S.; Okamura, K.; Keenan, A.L.; Masuda, M.; Miyazaki, M. GPAT4-Generated Saturated LPAs Induce Lipotoxicity through Inhibition of Autophagy by Abnormal Formation of Omegasomes. iScience 2020, 23, 101105. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.G.; Nicholson Puthenveedu, S.; Shen, Y.; La, K.; Ozlu, C.; Wang, T.; Klompstra, D.; Gultekin, Y.; Chi, J.; Fidelin, J.; et al. CHP1 Regulates Compartmentalized Glycerolipid Synthesis by Activating GPAT4. Mol. Cell 2019, 74, 45–58.e7. [Google Scholar] [CrossRef]
- Ha, J.H.; Radhakrishnan, R.; Jayaraman, M.; Yan, M.; Ward, J.D.; Fung, K.M.; Moxley, K.; Sood, A.K.; Isidoro, C.; Mukherjee, P.; et al. LPA Induces Metabolic Reprogramming in Ovarian Cancer via a Pseudohypoxic Response. Cancer Res. 2018, 78, 1923–1934. [Google Scholar] [CrossRef]
- Ojasalu, K.; Lieber, S.; Sokol, A.M.; Nist, A.; Stiewe, T.; Bullwinkel, I.; Finkernagel, F.; Reinartz, S.; Müller-Brüsselbach, S.; Grosse, R.; et al. The lysophosphatidic acid-regulated signal transduction network in ovarian cancer cells and its role in actomyosin dynamics, cell migration and entosis. Theranostics 2023, 13, 1921–1948. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Oon, C.; Diaz, L.; Sandborg, H.; Stempinski, E.S.; Saoi, M.; Morgan, T.K.; López, C.S.; Cross, J.R.; Sherman, M.H. Autotaxin-lysolipid signaling suppresses a CCL11-eosinophil axis to promote pancreatic cancer progression. Nat. Cancer 2024, 5, 283–298. [Google Scholar] [CrossRef] [PubMed]
- Chae, C.S.; Sandoval, T.A.; Hwang, S.M.; Park, E.S.; Giovanelli, P.; Awasthi, D.; Salvagno, C.; Emmanuelli, A.; Tan, C.; Chaudhary, V.; et al. Tumor-Derived Lysophosphatidic Acid Blunts Protective Type I Interferon Responses in Ovarian Cancer. Cancer Discov. 2022, 12, 1904–1921. [Google Scholar] [CrossRef]
- Huang, Y.X.; Lin, K.H.; Chiang, J.C.; Chen, W.M.; Lee, H. Lysophosphatidic Acid Receptor 3 Activation Is Involved in the Regulation of Ferroptosis. Int. J. Mol. Sci. 2024, 25, 2315. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Zhang, P.; Liu, J.; Wang, R.; Kaufman, R.J.; Yaden, B.C.; Karin, M. ATF4 suppresses hepatocarcinogenesis by inducing SLC7A11 (xCT) to block stress-related ferroptosis. J. Hepatol. 2023, 79, 362–377. [Google Scholar] [CrossRef]
- Chen, S.J.; Zhang, J.; Zhou, T.; Rao, S.S.; Li, Q.; Xiao, L.Y.; Wei, S.T.; Zhang, H.F. Epigenetically upregulated NSUN2 confers ferroptosis resistance in endometrial cancer via m5C modification of SLC7A11 mRNA. Redox Biol. 2024, 69, 102975. [Google Scholar] [CrossRef]
- Huang, Y.; Dai, Z.; Barbacioru, C.; Sadée, W. Cystine-glutamate transporter SLC7A11 in cancer chemosensitivity and chemoresistance. Cancer Res. 2005, 65, 7446–7454. [Google Scholar] [CrossRef]
- Fantone, S.; Piani, F.; Olivieri, F.; Rippo, M.R.; Sirico, A.; Di Simone, N.; Marzioni, D.; Tossetta, G. Role of SLC7A11/xCT in Ovarian Cancer. Int. J. Mol. Sci. 2024, 25, 587. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Shen, S.; Qin, J.; Fei, W.; Fan, F.; Gu, J.; Shen, T.; Zhang, T.; Cheng, X. High co-expression of SLC7A11 and GPX4 as a predictor of platinum resistance and poor prognosis in patients with epithelial ovarian cancer. BJOG Int. J. Obstet. Gynaecol. 2022, 129 (Suppl. 2), 40–49. [Google Scholar] [CrossRef]
- Dong, C.; Chen, J.; Zheng, J.; Liang, Y.; Yu, T.; Liu, Y.; Gao, F.; Long, J.; Chen, H.; Zhu, Q.; et al. 5-Hydroxymethylcytosine signatures in circulating cell-free DNA as diagnostic and predictive biomarkers for coronary artery disease. Clin. Epigenetics 2020, 12, 17. [Google Scholar] [CrossRef] [PubMed]
- Xuan, Y.; Wang, H.; Yung, M.M.; Chen, F.; Chan, W.S.; Chan, Y.S.; Tsui, S.K.; Ngan, H.Y.; Chan, K.K.; Chan, D.W. SCD1/FADS2 fatty acid desaturases equipoise lipid metabolic activity and redox-driven ferroptosis in ascites-derived ovarian cancer cells. Theranostics 2022, 12, 3534–3552. [Google Scholar] [CrossRef]
- Wang, C.K.; Chen, T.J.; Tan, G.Y.T.; Chang, F.P.; Sridharan, S.; Yu, C.A.; Chang, Y.H.; Chen, Y.J.; Cheng, L.T.; Hwang-Verslues, W.W. MEX3A Mediates p53 Degradation to Suppress Ferroptosis and Facilitate Ovarian Cancer Tumorigenesis. Cancer Res. 2023, 83, 251–263. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, G.; Condello, S.; Huang, H.; Cardenas, H.; Tanner, E.J.; Wei, J.; Ji, Y.; Li, J.; Tan, Y.; et al. Frizzled-7 Identifies Platinum-Tolerant Ovarian Cancer Cells Susceptible to Ferroptosis. Cancer Res. 2021, 81, 384–399. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Zhang, X.; Fang, C.; Zhu, M.; Wang, Z.; Jian, L.; Tan, W.; Wang, Y.; Li, H.; Xu, X.; et al. Progesterone Enhances Niraparib Efficacy in Ovarian Cancer by Promoting Palmitoleic-Acid-Mediated Ferroptosis. Research 2024, 7, 0371. [Google Scholar] [CrossRef]
- Niu, Y.F.; Wang, X.; Hu, D.X.; Balamurugan, S.; Li, D.W.; Yang, W.D.; Liu, J.S.; Li, H.Y. Molecular characterization of a glycerol-3-phosphate acyltransferase reveals key features essential for triacylglycerol production in Phaeodactylum tricornutum. Biotechnol. Biofuels 2016, 9, 60. [Google Scholar] [CrossRef]
- Meng, C.; Sun, Y.; Liu, G. Establishment of a prognostic model for ovarian cancer based on mitochondrial metabolism-related genes. Front. Oncol. 2023, 13, 1144430. [Google Scholar] [CrossRef]
- Huang, X.; Geng, H.; Liang, C.; Xiong, X.; Du, X.; Zhuan, Q.; Liu, Z.; Meng, L.; Zhou, D.; Zhang, L.; et al. Leonurine restrains granulosa cell ferroptosis through SLC7A11/GPX4 axis to promote the treatment of polycystic ovary syndrome. Free Radic. Biol. Med. 2025, 226, 330–347. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, P.; Shang, C.; Liu, Z.; Li, Y.; He, T.; Xue, Y.; Lin, J.; Li, Y.; Wu, Y.; Liu, T.; et al. High Co-Expression of GPAT4 and SLC7A11 as a Predictor of Platinum Resistance and Poor Prognosis in Patients with Epithelial Ovarian Cancer. Biomedicines 2025, 13, 1664. https://doi.org/10.3390/biomedicines13071664
Yu P, Shang C, Liu Z, Li Y, He T, Xue Y, Lin J, Li Y, Wu Y, Liu T, et al. High Co-Expression of GPAT4 and SLC7A11 as a Predictor of Platinum Resistance and Poor Prognosis in Patients with Epithelial Ovarian Cancer. Biomedicines. 2025; 13(7):1664. https://doi.org/10.3390/biomedicines13071664
Chicago/Turabian StyleYu, Ping, Chunliang Shang, Zhongyu Liu, Yuan Li, Tianhui He, Yuan Xue, Jian Lin, Yuan Li, Yu Wu, Tong Liu, and et al. 2025. "High Co-Expression of GPAT4 and SLC7A11 as a Predictor of Platinum Resistance and Poor Prognosis in Patients with Epithelial Ovarian Cancer" Biomedicines 13, no. 7: 1664. https://doi.org/10.3390/biomedicines13071664
APA StyleYu, P., Shang, C., Liu, Z., Li, Y., He, T., Xue, Y., Lin, J., Li, Y., Wu, Y., Liu, T., & Guo, H. (2025). High Co-Expression of GPAT4 and SLC7A11 as a Predictor of Platinum Resistance and Poor Prognosis in Patients with Epithelial Ovarian Cancer. Biomedicines, 13(7), 1664. https://doi.org/10.3390/biomedicines13071664




