Brazilian Green Propolis Carried in Lipid-Based Nanostructures: A Potent Adjuvant Therapy to Non-Surgical Periodontal Treatment in the Management of Experimental Periodontitis
Abstract
1. Introduction
2. Materials and Methods
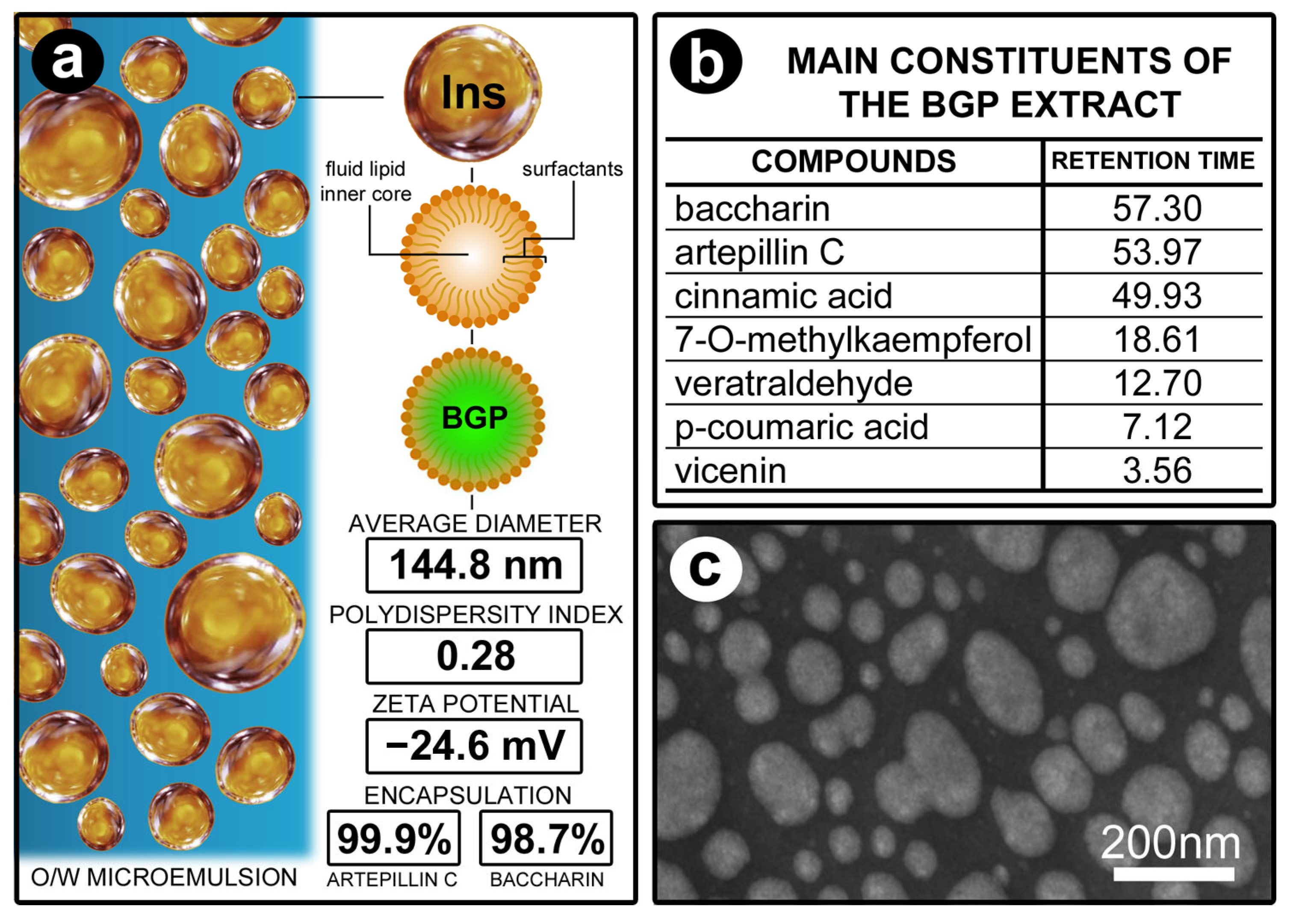
2.1. Preparation of Ethanolic Extract of BGP and BGPlns
2.2. Animals, Ethical Aspects, and Sample Calculation
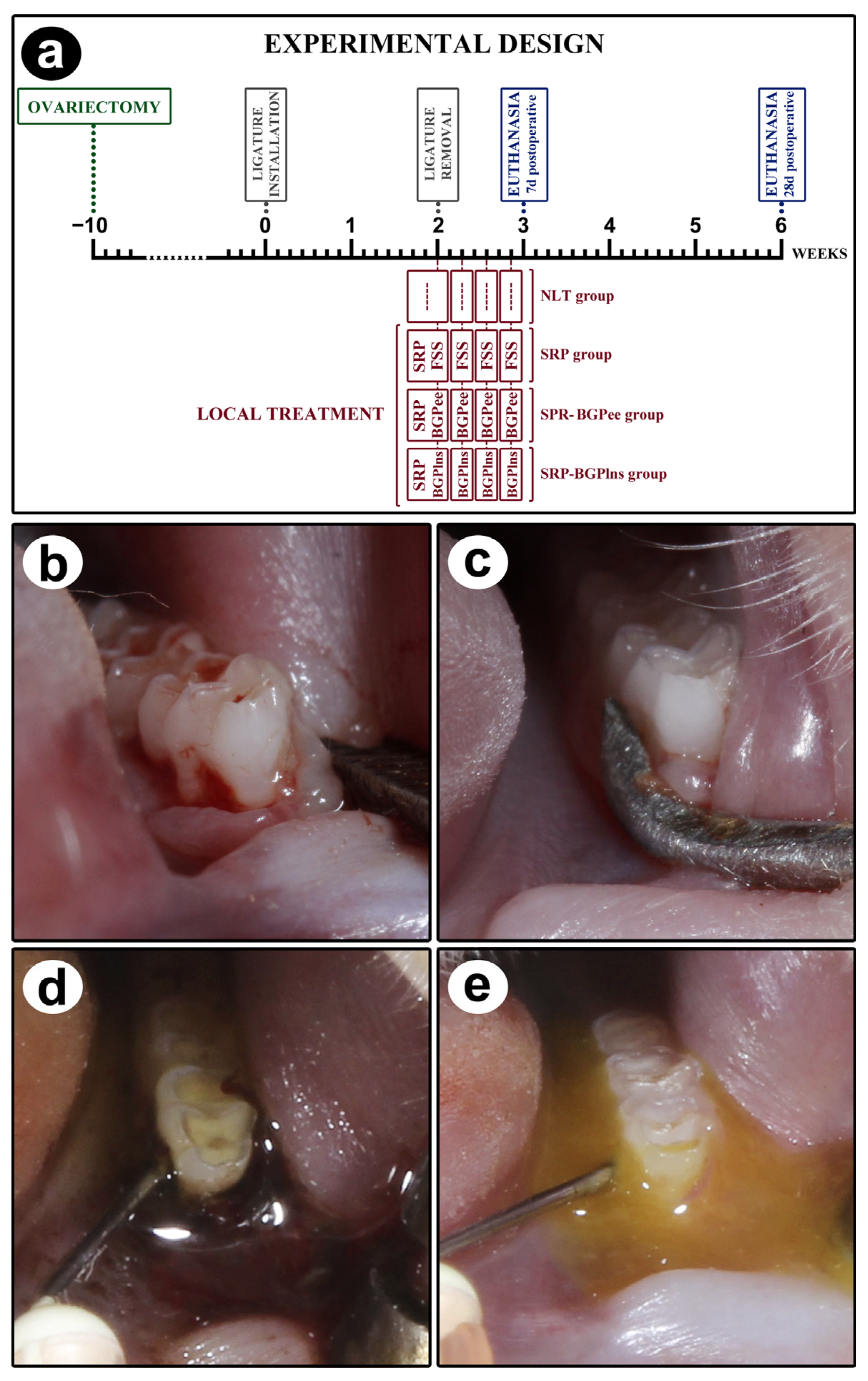
2.3. Experimental Procedures Performed on Animals
2.4. Analysis of General Health Condition and Intraoral Examination
2.5. Computerized Microtomography of Samples
2.6. Histological and Immunohistochemical Processing of Samples
2.7. Histopathological and Histometric Analysis of the PBT Analyses
2.8. Immunolabeling Pattern of TRAP, TGF-β1, and OCN in the Furcation Region of the First Lower Molar
2.9. Statistical Analysis
3. Results
3.1. Characterization of the Physicochemical Properties of Lipid-Based Nanostructures Containing BGP
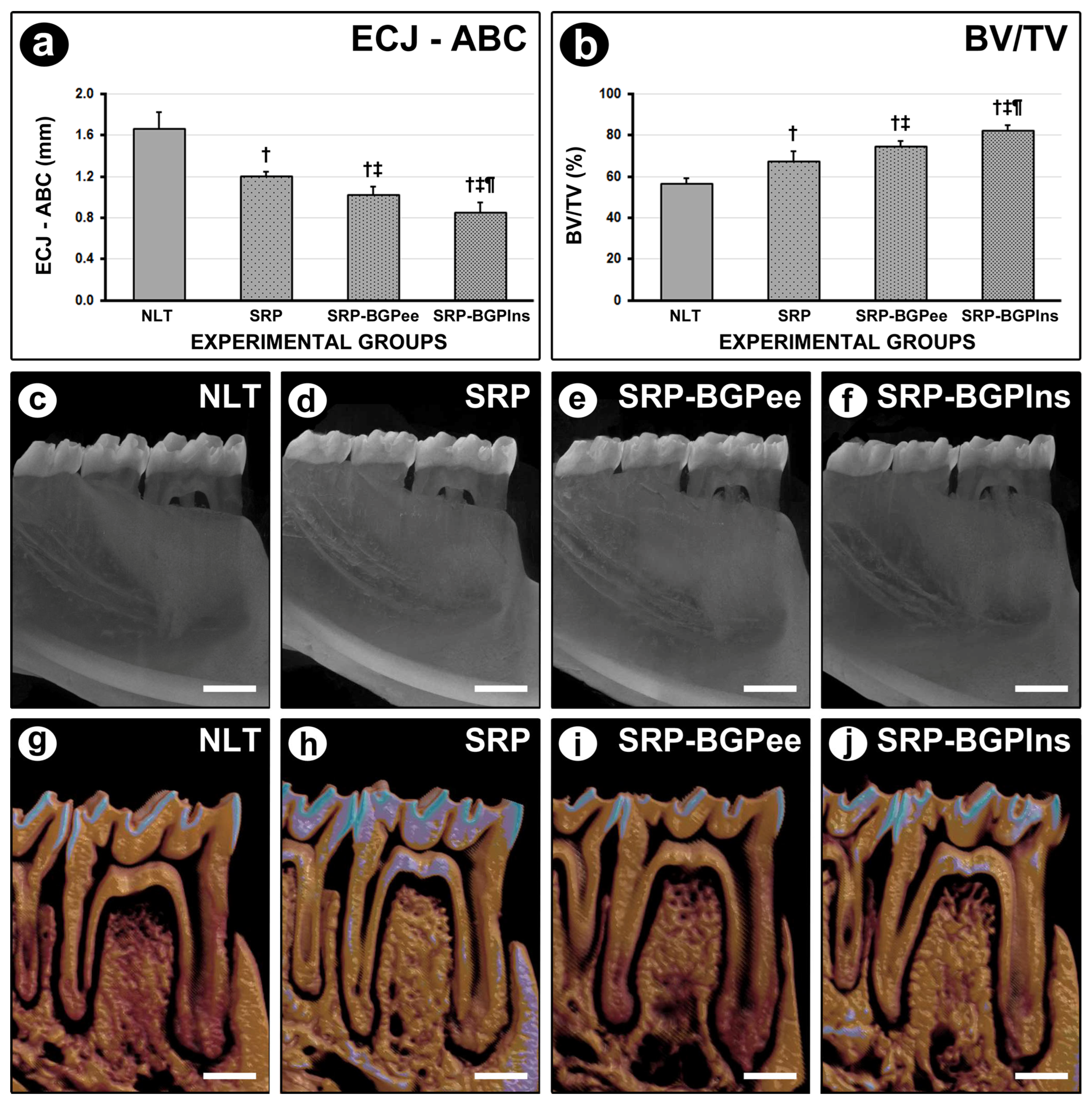
3.2. ECJ–ABC
3.3. BV/TV in the Furcation Region of the First Lower Molar
3.4. PBT in the Furcation Region
3.5. Histological Analysis of Periodontal Tissues
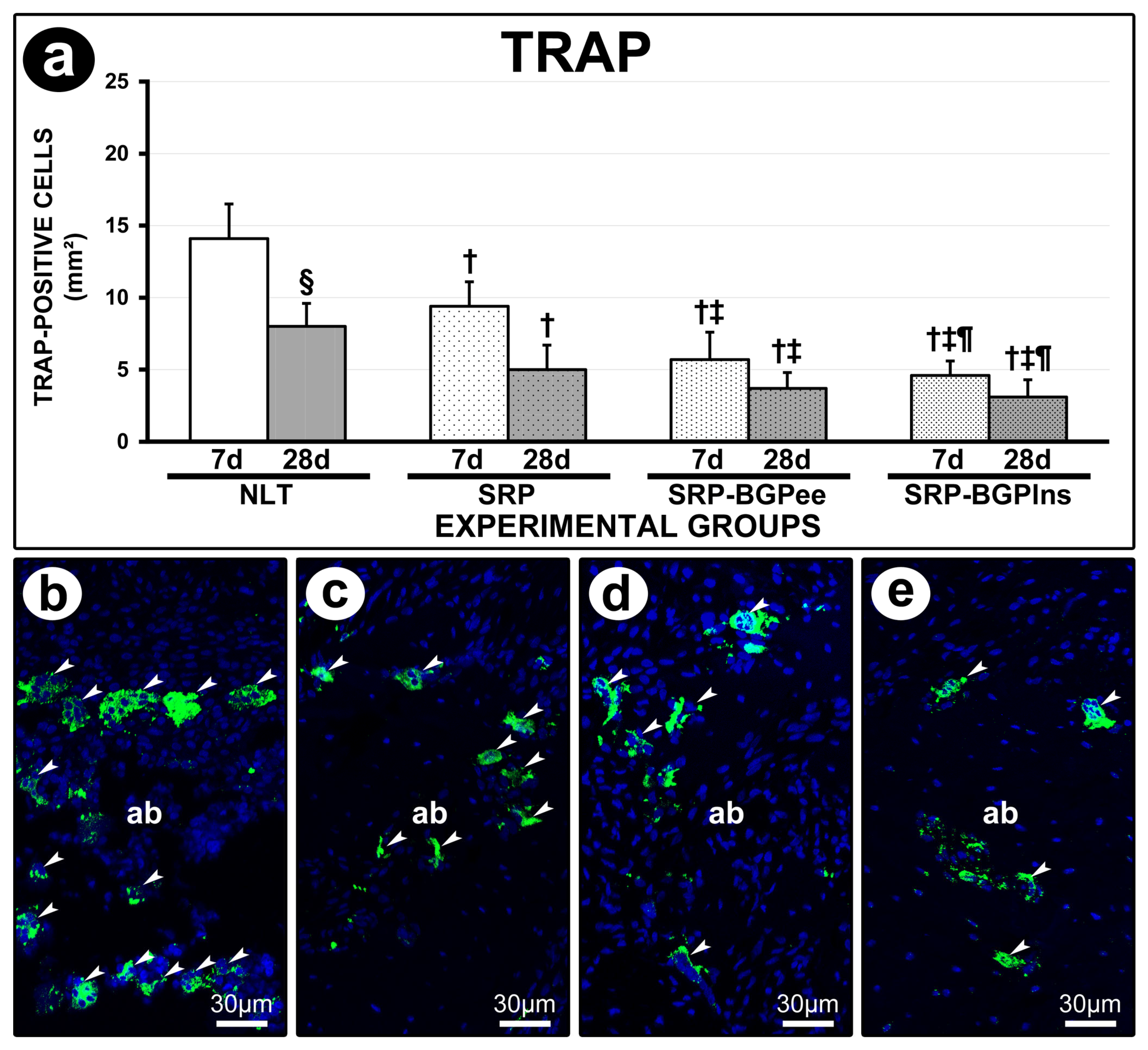
3.6. TRAP Immunolabeling
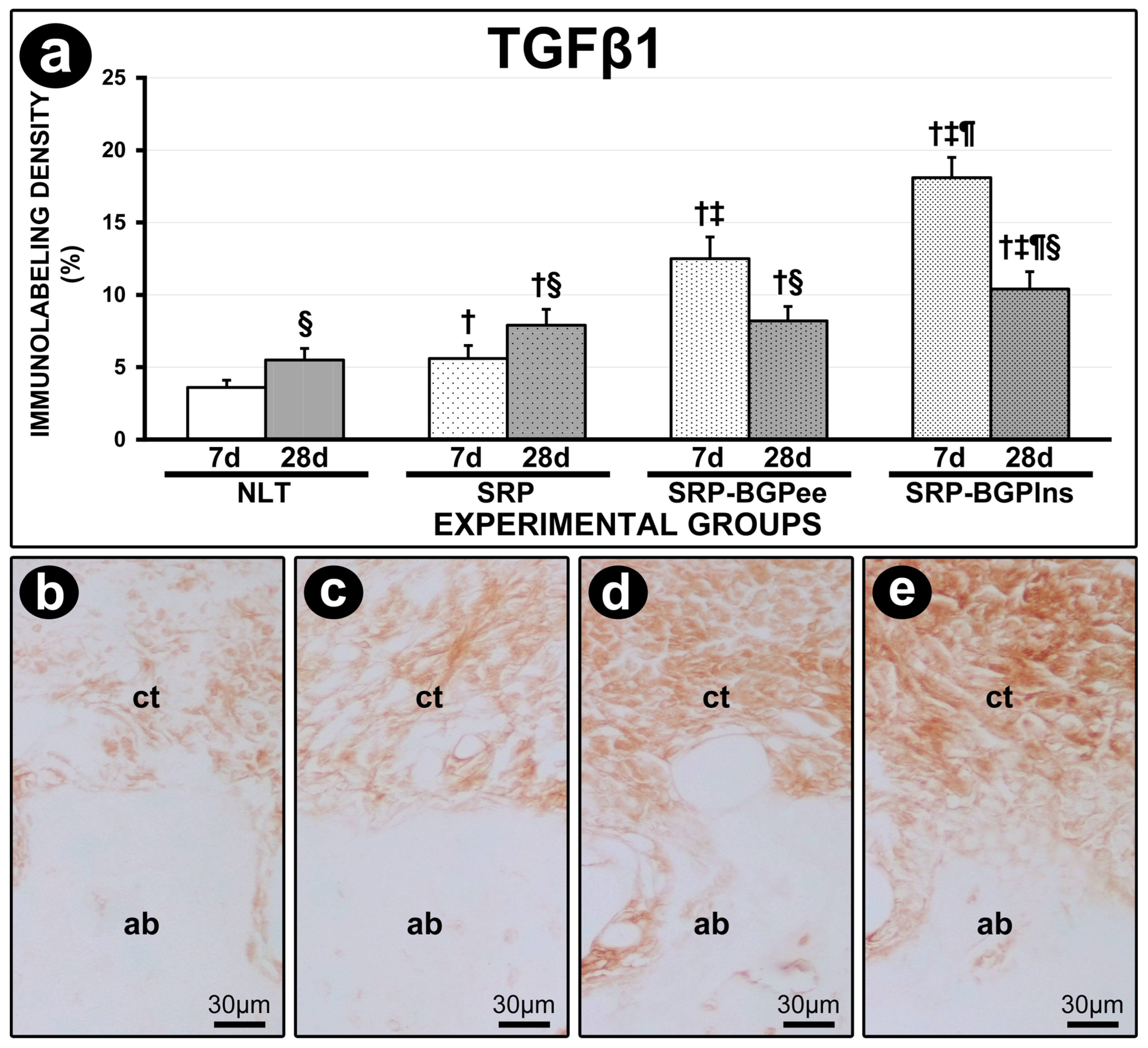
3.7. TGFβ1 Immunolabeling
3.8. OCN Immunolabeling
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Villoria, G.E.M.; Fischer, R.G.; Tinoco, E.M.B.; Meyle, J.; Loos, B.G. Periodontal disease: A systemic condition. Periodontol. 2000 2024, 96, 7–19. [Google Scholar] [CrossRef] [PubMed]
- Sedghi, L.M.; Bacino, M.; Kapila, Y.L. Periodontal disease: The good, the bad, and the unknown. Front. Cell. Infect. Microbiol. 2021, 11, 766944. [Google Scholar] [CrossRef]
- Caton, J.G.; Armitage, G.; Berglundh, T.; Chapple, I.L.C.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.N.; Sanz, M.; Tonetti, M.S. A new classification scheme for periodontal and peri-implant diseases and conditions—Introduction and key changes from the 1999 classification. J. Clin. Periodontol. 2018, 45 (Suppl. 20), S1–S8. [Google Scholar] [CrossRef]
- Kassebaum, N.J.; Smith, A.G.C.; Bernabé, E.; Fleming, T.D.; Reynolds, A.E.; Vos, T.; Murray, C.J.L.; Marcenes, W.; GBD 2015 Oral Health Collaborators. Global, Regional, and National Prevalence, Incidence, and Disability-Adjusted Life Years for Oral Conditions for 195 Countries, 1990–2015: A Systematic Analysis for the Global Burden of Diseases, Injuries, and Risk Factors. J. Dent. Res. 2017, 96, 380–387. [Google Scholar] [CrossRef]
- Pannuti, C.M.; Alarcón, M.A.; Ramírez Lemus, G.M.; Yunes Fragoso, P.; Retamal-Valdes, B.S.; Cornejo-Ovalle, M.; Duarte, P.M.; Leite, F.R.M.; Gimenez, X. Risk factors of periodontal disease: Latin America and the Caribbean Consensus 2024. Braz. Oral. Res. 2024, 38 (Suppl. 1), e118. [Google Scholar] [CrossRef] [PubMed]
- Reeves, J. The impact of female hormones on the periodontium—A narrative review. Int. J. Dent. Hyg. 2025. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, D.V.; Ferreira, C.L.; Nunes, C.M.M.; Tricoly, T.D.S.; de Moura, N.B.; Santamaria, M.P.; De Marco, A.C.; Jardini, M.A.N. Effects of the Pulsed Electromagnetic Fields on Experimental Periodontitis and Estrogen Deficiency. Bioelectromagnetics 2022, 43, 426–437. [Google Scholar] [CrossRef]
- Xu, X.C.; Chen, H.; Zhang, X.; Zhai, Z.J.; Liu, X.Q.; Zheng, X.Y.; Zhang, J.; Qin, A.; Lu, E.Y. Effects of oestrogen deficiency on the alveolar bone of rats with experimental periodontitis. Mol. Med. Rep. 2015, 12, 3494–3502. [Google Scholar] [CrossRef]
- Amadei, S.U.; Souza, D.M.; Brandão, A.A.; Rocha, R.F. Influence of different durations of estrogen deficiency on alveolar bone loss in rats. Braz. Oral Res. 2011, 25, 538–543. [Google Scholar] [CrossRef]
- Anbinder, A.L.; Prado, M.A.; Spalding, M.; Balducci, I.; Carvalho, Y.R.; Rocha, R.F. Estrogen deficiency and periodontal condition in rats: A radiographic and macroscopic study. Braz. Dent. J. 2006, 17, 201–207. [Google Scholar] [CrossRef]
- Duarte, P.M.; Gonçalves, P.F.; Sallum, A.W.; Sallum, E.A.; Casati, M.Z.; Nociti, F.H., Jr. Effect of an estrogen-deficient state and its therapy on bone loss resulting from an experimental periodontitis in rats. J. Periodontal Res. 2004, 39, 107–110. [Google Scholar] [CrossRef] [PubMed]
- Park, K.Y.; Kim, M.H.; Choi, S.H.; Pang, E.K. Association of periodontitis with menopause and hormone replacement therapy: A hospital cohort study using a common data model. J. Periodontal Implant Sci. 2023, 53, 184–193. [Google Scholar] [CrossRef]
- Yu, B.; Wang, C.Y. Osteoporosis and periodontal diseases—An update on their association and mechanistic links. Periodontol. 2000 2022, 89, 99–113. [Google Scholar] [CrossRef]
- Ciesielska, A.; Kusiak, A.; Ossowska, A.; Grzybowska, M.E. Changes in the Oral Cavity in Menopausal Women—A Narrative Review. Int. J. Environ. Res. Public Health 2021, 19, 253. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, R.A.; Payne, J.B.; Maze, C.A.; Patil, K.D.; Gallagher, S.J.; Mattson, J.S. Influence of estrogen and osteopenia/osteoporosis on clinical periodontitis in postmenopausal women. J. Periodontol. 1999, 70, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Genco, R.J.; Grossi, S.G. Is estrogen deficiency a risk factor for periodontal disease? Compend. Contin. Educ. Dent. Suppl. 1998, 22, S23–S29. [Google Scholar]
- Cobb, C.M.; Sottosanti, J.S. A re-evaluation of scaling and root planing. J. Periodontol. 2021, 92, 1370–1378. [Google Scholar] [CrossRef]
- Suvan, J.; Leira, Y.; Moreno Sancho, F.M.; Graziani, F.; Derks, J.; Tomasi, C. Subgingival instrumentation for treatment of periodontitis. A systematic review. J. Clin. Periodontol. 2020, 47, 155–175. [Google Scholar] [CrossRef]
- Fang, H.; Han, M.; Li, Q.-L.; Cao, C.-Y.; Xia, R.; Zhang, Z.-H. Comparison of full-mouth disinfection and quadrant-wise scaling in the treatment of adult chronic periodontitis: A systematic review and meta-analysis. J. Periodontal Res. 2016, 51, 417–430. [Google Scholar] [CrossRef]
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Beglundh, T.; Sculean, A.; Tonetti, M.S.; EFP Workshop Participants and Methodological Consultants. Treatment of stage I–III periodontitis—The EFP S3 level clinical practice guideline. J. Clin. Periodontol. 2020, 47 (Suppl. 22), 4–60. [Google Scholar] [CrossRef]
- Herrera, D.; Sanz, M.; Kebschull, M.; Jepsen, S.; Sculean, A.; Berglundh, T.; Papapanou, P.N.; Chapple, I.; Tonetti, M.S.; EFP Workshop Participants and Methodological Consultant. Treatment of stage IV periodontitis: The EFP S3 level clinical practice guideline. J. Clin. Periodontol. 2022, 49 (Suppl. 24), 4–71. [Google Scholar] [CrossRef] [PubMed]
- Ożarowski, M.; Karpiński, T.M.; Alam, R.; Łochyńska, M. Antifungal Properties of Chemically Defined Propolis from Various Geographical Regions. Microorganisms 2022, 10, 364. [Google Scholar] [CrossRef]
- Hossain, R.; Quispe, C.; Khan, R.A.; Saikat, A.S.M.; Ray, P.; Ongalbek, D.; Yeskaliyeva, B.; Jain, D.; Smeriglio, A.; Trombetta, D.; et al. Propolis: An update on its chemistry and pharmacological applications. Chin. Med. 2022, 17, 100. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, T.; Kumazawa, S. Propolis: Chemical Constituents, Plant Origin, and Possible Role in the Prevention and Treatment of Obesity and Diabetes. J. Agric. Food Chem. 2021, 69, 15484–15494. [Google Scholar] [CrossRef]
- Stojanović, S.; Najman, S.J.; Bogdanova-Popov, B.; Najman, S.S. Propolis: Chemical composition, biological and pharmacological activity—A review. Acta Medica Median. 2020, 59, 108–113. [Google Scholar] [CrossRef]
- Martinotti, S.; Bonsignore, G.; Ranzato, E. Propolis: A natural substance with multifaceted properties and activities. Int. J. Mol. Sci. 2025, 26, 1519. [Google Scholar] [CrossRef]
- Zullkiflee, N.; Taha, H.; Usman, A. Propolis: Its Role and Efficacy in Human Health and Diseases. Molecules 2022, 27, 6120. [Google Scholar] [CrossRef]
- Salatino, A. Perspectives for uses of propolis in therapy against infectious diseases. Molecules 2022, 27, 4594. [Google Scholar] [CrossRef]
- Sforcin, J.M. Biological Properties and Therapeutic Applications of Propolis. Phytother. Res. 2016, 30, 894–905. [Google Scholar] [CrossRef]
- Beserra, F.P.; Gushiken, L.F.S.; Hussni, M.F.; Ribeiro, V.P.; Bonamin, F.; Jackson, C.J.; Pellizzon, C.H.; Bastos, J.K. Artepillin C as an outstanding phenolic compound of Brazilian green propolis for disease treatment: A review on pharmacological aspects. Phytother. Res. 2021, 35, 2274–2286. [Google Scholar] [CrossRef]
- Moise, A.R.; Bobiş, O. Baccharis dracunculifolia and Dalbergia ecastophyllum, Main Plant Sources for Bioactive Properties in Green and Red Brazilian Propolis. Plants 2020, 9, 1619. [Google Scholar] [CrossRef]
- Figueiredo, L.C.; Figueiredo, N.F.; Cruz, D.F.d.; Baccelli, G.T.; Sarachini, G.E.; Bueno, M.R.; Feres, M.; Bueno-Silva, B. Propolis, Aloe Vera, Green Tea, Cranberry, Calendula, Myrrha and Salvia Properties against Periodontal Microorganisms. Microorganisms 2022, 10, 2172. [Google Scholar] [CrossRef] [PubMed]
- Stähli, A.; Schröter, H.; Bullitta, S.; Serralutzu, F.; Dore, A.; Nietzsche, S.; Milia, E.; Sculean, A.; Eick, S. In Vitro Activity of Propolis on Oral Microorganisms and Biofilms. Antibiotics 2021, 10, 1045. [Google Scholar] [CrossRef] [PubMed]
- Feres, M.; Figueiredo, L.C.; Barreto, I.M.; Coelho, M.H.; Araujo, M.W.; Cortelli, S.C. In vitro antimicrobial activity of plant extracts and propolis in saliva samples of healthy and periodontally-involved subjects. J. Int. Acad. Periodontol. 2005, 7, 90–96. [Google Scholar]
- Ali, K.M.; Saleh, Z.; Jalal, J. Effect of local propolis irrigation in experimental periodontitis in rats on inflammatory markers (IL-1β and TNF-α) and oxidative stress. Indian J. Dent. Res. 2020, 31, 893–898. [Google Scholar] [CrossRef]
- Toker, H.; Ozan, F.; Ozer, H.; Ozdemir, H.; Eren, K.; Yeler, H. A morphometric and histopathologic evaluation of the effects of propolis on alveolar bone loss in experimental periodontitis in rats. J. Periodontol. 2008, 79, 1089–1094. [Google Scholar] [CrossRef] [PubMed]
- Eghbali Zarch, R.; Askari, M.; Boostani, H.; Mirzaii-Dizgah, I. Effect of propolis extract on clinical parameters and salivary level of matrix metalloproteinase 8 in periodontitis patients: A randomized controlled clinical trial. J. Adv. Periodontol. Implant Dent. 2021, 13, 84–89. [Google Scholar] [CrossRef]
- Nakao, R.; Senpuku, H.; Ohnishi, M.; Takai, H.; Ogata, Y. Effect of topical administration of propolis in chronic periodontitis. Odontology 2020, 108, 704–714. [Google Scholar] [CrossRef]
- Giammarinaro, E.; Marconcini, S.; Genovesi, A.; Poli, G.; Lorenzi, C.; Covani, U. Propolis as an adjuvant to non-surgical periodontal treatment: A clinical study with salivary anti-oxidant capacity assessment. Minerva Stomatol. 2018, 67, 183–188. [Google Scholar] [CrossRef]
- Andrade, D.P.; Carvalho, I.C.S.; Gadoi, B.H.; Rosa, L.C.L.; Barreto, L.M.R.C.; Pallos, D. Subgingival Irrigation with a Solution of 20% Propolis Extract as an Adjunct to Non-Surgical Periodontal Treatment: A Preliminary Study. J. Int. Acad. Periodontol. 2017, 19, 145–151. [Google Scholar]
- Sanghani, N.N.; Shivaprasad, B.; Savita, S. Health from the hive: Propolis as an adjuvant in the treatment of chronic periodontitis—A clinicomicrobiologic study. J. Clin. Diagn. Res. 2014, 8, ZC41–ZC44. [Google Scholar] [CrossRef] [PubMed]
- Gebaraa, E.C.; Pustiglioni, A.N.; de Lima, L.A.; Mayer, M.P. Propolis extract as an adjuvant to periodontal treatment. Oral Health Prev. Dent. 2003, 1, 29–35. [Google Scholar]
- Kustiawan, P.M.; Syaifie, P.H.; Al Khairy Siregar, K.A.; Ibadillah, D.; Mardliyati, E. New insights of propolis nanoformulation and its therapeutic potential in human diseases. ADMET DMPK 2024, 12, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Jaldin-Crespo, L.; Silva, N.; Martínez, J. From propolis to nanopropolis: An exemplary journey and a paradigm shift of a resinous substance produced by bees. Phytother. Res. 2022, 36, 2016–2041. [Google Scholar] [CrossRef]
- Jaldin-Crespo, L.; Silva, N.; Martínez, J. Nanomaterials Based on Honey and Propolis for Wound Healing—A Mini-Review. Nanomaterials 2022, 12, 4409. [Google Scholar] [CrossRef] [PubMed]
- Doguer, C.; Ceylan, F.D.; Capanoglu, E.; Adrar, N.; Bölük, E.; Atayoglu, A.T.; Uzun, S.; Palabiyik, I. Enhanced In Vitro Bioaccessibility and Anticancer Activity of Brazilian Propolis Extracted with L-Lactic Acid. Prev. Nutr. Food Sci. 2025, 30, 81–91. [Google Scholar] [CrossRef]
- Machado, B.A.; Silva, R.P.; Barreto Gde, A.; Costa, S.S.; Silva, D.F.; Brandão, H.N.; Rocha, J.L.; Dellagostin, O.A.; Henriques, J.A.; Umsza-Guez, M.A.; et al. Chemical Composition and Biological Activity of Extracts Obtained by Supercritical Extraction and Ethanolic Extraction of Brown, Green and Red Propolis Derived from Different Geographic Regions in Brazil. PLoS ONE 2016, 11, e0145954. [Google Scholar] [CrossRef]
- Oliveira da Silva, L.; Assunção Ferreira, M.R.; Lira Soares, L.A. Nanotechnology Formulations Designed with Herbal Extracts and Their Therapeutic Applications—A Review. Chem. Biodivers. 2023, 20, e202201241. [Google Scholar] [CrossRef]
- Jain, K.K. Role of Nanobiotechnology in Drug Delivery. Methods Mol. Biol. 2020, 2059, 55–73. [Google Scholar] [CrossRef]
- Simona, A.D.; Florina, A.; Rodica, C.A.; Evelyne, O.; Maria-Corina, S. Nanoscale Delivery Systems: Actual and Potential Applications in the Natural Products Industry. Curr. Pharm. Des. 2017, 23, 2414–2421. [Google Scholar] [CrossRef]
- Bilia, A.R.; Piazzini, V.; Guccione, C.; Risaliti, L.; Asprea, M.; Capecchi, G.; Bergonzi, M.C. Improving on Nature: The Role of Nanomedicine in the Development of Clinical Natural Drugs. Planta Med. 2017, 83, 366–381. [Google Scholar] [CrossRef] [PubMed]
- Bamanna, A.; Rajora, A.; Nagpal, K. Enhancing Microemulsion-Based Therapeutic Drug Delivery: Exploring Surfactants, Co-Surfactants, and Quality-by-Design Strategies within Pseudoternary Phase Diagrams. Crit. Rev. Ther. Drug Carrier. Syst. 2025, 42, 35–71. [Google Scholar] [CrossRef] [PubMed]
- Rehman, M.; Tahir, N.; Sohail, M.F.; Qadri, M.U.; Duarte, S.O.D.; Brandão, P.; Esteves, T.; Javed, I.; Fonte, P. Lipid-Based Nanoformulations for Drug Delivery: An Ongoing Perspective. Pharmaceutics 2024, 16, 1376. [Google Scholar] [CrossRef]
- Alatawi, H.M.; Alhwiti, S.S.; Alsharif, K.A.; Albalawi, S.S.; Abusaleh, S.M.; Sror, G.K.; Qushawy, M. Nanostructured Lipid Carriers (NLCs) as Effective Drug Delivery Systems: Methods of Preparation and their Therapeutic Applications. Recent. Pat. Nanotechnol. 2024, 18, 179–189. [Google Scholar] [CrossRef]
- Nikolaev, B.; Yakovleva, L.; Fedorov, V.; Li, H.; Gao, H.; Shevtsov, M. Nano- and Microemulsions in Biomedicine: From Theory to Practice. Pharmaceutics 2023, 15, 1989. [Google Scholar] [CrossRef] [PubMed]
- Long, J.A.; Evans, H.M. The estrous cycle in the rat and its associated phenomena. In Memories of University of California; University of California Press: Berkeley, CA, USA, 1922. [Google Scholar]
- Silveira, G.R.C.; Ganzaroli, V.F.; Toro, L.F.; Lopes-Pereira, E.; Costa, L.L.D.; Mello-Neto, J.M.; Buchaim, R.L.; Garcia, V.G.; Theodoro, L.H.; Sforcin, J.M.; et al. Effectiveness of Local Use of Green Propolis-Loaded Lipid Nanoparticles as Adjuvant Therapy to Scaling and Root Planing in the Management of Periodontitis in Rats Treated with Zoledronate. Int. J. Mol. Sci. 2024, 25, 12443. [Google Scholar] [CrossRef]
- Souza, E.Q.M.; Toro, L.F.; Ganzaroli, V.F.; Freire, J.O.A.; Matsumoto, M.A.; Casatti, C.A.; Cintra, L.T.A.; Buchaim, R.L.; Issa, J.P.M.; Garcia, V.G.; et al. Peri-implantitis increases the risk of medication-related osteonecrosis of the jaws associated with osseointegrated implants in rats treated with zoledronate. Sci. Rep. 2024, 14, 627. [Google Scholar] [CrossRef]
- Garcia, V.G.; Gualberto Júnior, E.C.; Fernandes, L.A.; Bosco, A.F.; Hitomi Nagata, M.J.; Casatti, C.A.; Ervolino, E.; Theodoro, L.H. Adjunctive antimicrobial photodynamic treatment of experimentally induced periodontitis in rats with ovariectomy. J. Periodontol. 2013, 84, 556–565. [Google Scholar] [CrossRef]
- Aldana-Mejia, J.A.; Rezende Granzoto, M.; Maria de Melo, E.; Pena Ribeiro, V.; Ross, S.A.; Marcato, P.D.; Andrade Furtado, R.; Kenupp Bastos, J. Analgesic and anti-inflammatory effects of free and nanoencapsulated green propolis extract from the northeastern Brazilian Caatinga biome. Chem. Biodivers. 2025, 22, e202402896. [Google Scholar] [CrossRef]
- Shahinozzaman, M.; Basak, B.; Emran, R.; Rozario, P.; Obanda, D.N. Artepillin C: A comprehensive review of its chemistry, bioavailability, and pharmacological properties. Fitoterapia 2020, 147, 104775. [Google Scholar] [CrossRef]
- Paulino, N.; Abreu, S.R.; Uto, Y.; Koyama, D.; Nagasawa, H.; Hori, H.; Dirsch, V.M.; Vollmar, A.M.; Scremin, A.; Bretz, W.A. Anti-inflammatory effects of a bioavailable compound, Artepillin C, in Brazilian propolis. Eur. J. Pharmacol. 2008, 587, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Almeida-Junior, S.; de Oliveira, K.R.P.; Marques, L.P.; Martins, J.G.; Ubeda, H.; Santos, M.F.C.; Rodrigues, M.A.; Andrade, E.S.M.L.; Ambrósio, S.R.; Bastos, J.K.; et al. In vivo anti-inflammatory activity of baccharin from Brazilian green propolis. Fitoterapia 2024, 175, 105975. [Google Scholar] [CrossRef]
- Ferreira, J.C.; Reis, M.B.; Coelho, G.D.P.; Gastaldello, G.H.; Peti, A.P.F.; Rodrigues, D.M.; Bastos, J.K.; Campo, V.L.; Sorgi, C.A.; Faccioli, L.H.; et al. Baccharin and p-coumaric acid from green propolis mitigate inflammation by modulating the production of cytokines and eicosanoids. J. Ethnopharmacol. 2021, 278, 114255. [Google Scholar] [CrossRef]
- Zulhendri, F.; Lesmana, R.; Tandean, S.; Christoper, A.; Chandrasekaran, K.; Irsyam, I.; Suwantika, A.A.; Abdulah, R.; Wathoni, N. Recent update on the anti-inflammatory activities of propolis. Molecules 2022, 27, 8473. [Google Scholar] [CrossRef]
- Sobacchi, C.; Menale, C.; Crisafulli, L.; Ficara, F. Role of RANKL signaling in bone homeostasis. Physiology 2025, 40, 46–66. [Google Scholar] [CrossRef] [PubMed]
- Bertolucci, V.; Ninomiya, A.F.; Longato, G.B.; Kaneko, L.O.; Nonose, N.; Scariot, P.P.M.; Messias, L.H.D. Bioactive compounds from propolis on bone homeostasis: A narrative review. Antioxidants 2025, 14, 81. [Google Scholar] [CrossRef]
- Machado Velho, J.C.; França, T.A.; Malagutti-Ferreira, M.J.; Albuquerque, E.R.; Lívero, F.A.D.R.; Soares, M.R.; Soares, A.E.E.; Ribeiro-Paes, J.T. Use of propolis for skin wound healing: Systematic review and meta-analysis. Arch. Dermatol. Res. 2023, 315, 943–955. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Pi, A.; Yan, L.; Li, J.; Nan, S.; Zhang, J.; Hao, Y. Research progress on therapeutic effect and mechanism of propolis on wound healing. Evid. Based Complement. Alternat. Med. 2022, 2022, 5798941. [Google Scholar] [CrossRef]
- Stojko, M.; Wolny, D.; Włodarczyk, J. Nonwoven releasing propolis as a potential new wound healing method—A review. Molecules 2021, 26, 5701. [Google Scholar] [CrossRef]
- Meimandi-Parizi, A.; Oryan, A.; Sayahi, E.; Bigham-Sadegh, A. Propolis extract: A new reinforcement material in improving bone healing—An in vivo study. Int. J. Surg. 2018, 56, 94–101. [Google Scholar] [CrossRef]
- Guney, A.; Karaman, I.; Oner, M.; Yerer, M.B. Effects of propolis on fracture healing: An experimental study. Phytother. Res. 2011, 25, 1648–1652. [Google Scholar] [CrossRef] [PubMed]
- Poniatowski, Ł.A.; Wojdasiewicz, P.; Gasik, R.; Szukiewicz, D. Transforming growth factor beta family: Insight into the role of growth factors in regulation of fracture healing biology and potential clinical applications. Mediat. Inflamm. 2015, 2015, 137823. [Google Scholar] [CrossRef] [PubMed]
- Javelaud, D.; Mauviel, A. Mammalian transforming growth factor-betas: Smad signaling and physio-pathological roles. Int. J. Biochem. Cell Biol. 2004, 36, 1161–1165. [Google Scholar] [CrossRef]
- Javelaud, D.; Mauviel, A. Transforming growth factor-betas: Smad signaling and roles in physiopathology. Pathol. Biol. 2004, 52, 50–54. [Google Scholar] [CrossRef]
- Souza, S.L.; Andrade, P.F.; Silva, J.S.; Tristão, F.S.; Rocha, F.A.; Palioto, D.B.; Grisi, M.F.; Taba, M., Jr.; Novaes, A.B., Jr. Effects of antimicrobial photodynamic therapy on transforming growth factor-beta1 levels in the gingival crevicular fluid. Photomed. Laser Surg. 2013, 31, 65–71. [Google Scholar] [CrossRef]
- Kuru, L.; Griffiths, G.S.; Petrie, A.; Olsen, I. Changes in transforming growth factor-beta1 in gingival crevicular fluid following periodontal surgery. J. Clin. Periodontol. 2004, 31, 527–533. [Google Scholar] [CrossRef] [PubMed]
- Farhadi, F.; Khameneh, B.; Iranshahi, M.; Iranshahy, M. Antibacterial activity of flavonoids and their structure–activity relationship: An update review. Phytother. Res. 2019, 33, 13–40. [Google Scholar] [CrossRef]
- Przybyłek, I.; Karpiński, T.M. Antibacterial properties of propolis. Molecules 2019, 24, 2047. [Google Scholar] [CrossRef]
- de Sá Assis, M.A.; de Paula Ramos, L.; Abu Hasna, A.; de Queiroz, T.S.; Pereira, T.C.; Nagai de Lima, P.M.; Berretta, A.A.; Marcucci, M.C.; Talge Carvalho, C.A.; de Oliveira, L.D. Antimicrobial and antibiofilm effect of Brazilian green propolis aqueous extract against dental anaerobic bacteria. Molecules 2022, 27, 8128. [Google Scholar] [CrossRef]
- Santos, F.A.; Bastos, E.M.; Rodrigues, P.H.; de Uzeda, M.; de Carvalho, M.A.; Farias Lde, M.; Moreira, E.S. Susceptibility of Prevotella intermedia/Prevotella nigrescens (and Porphyromonas gingivalis) to propolis (bee glue) and other antimicrobial agents. Anaerobe 2002, 8, 9–15. [Google Scholar] [CrossRef]
- Yoshimasu, Y.; Ikeda, T.; Sakai, N.; Yagi, A.; Hirayama, S.; Morinaga, Y.; Furukawa, S.; Nakao, R. Rapid Bactericidal Action of Propolis against Porphyromonas gingivalis. J. Dent. Res. 2018, 97, 928–936. [Google Scholar] [CrossRef] [PubMed]
- Luque-Bracho, A.; Rosales, Y.; Vergara-Buenaventura, A. The benefits of propolis in periodontal therapy. A scoping review of preclinical and clinical studies. J. Ethnopharmacol. 2023, 303, 115926. [Google Scholar] [CrossRef] [PubMed]
- Assunção, M.; Carneiro, V.M.A.; Stefani, C.M.; de Lima, C.L. Clinical efficacy of subgingivally delivered propolis as an adjuvant to nonsurgical periodontal treatment of periodontitis: A systematic review and meta-analysis. Phytother. Res. 2021, 35, 5584–5595. [Google Scholar] [CrossRef] [PubMed]
- López-Valverde, N.; Pardal-Peláez, B.; López-Valverde, A.; Flores-Fraile, J.; Herrero-Hernández, S.; Macedo-de-Sousa, B.; Herrero-Payo, J.; Ramírez, J.M. Effectiveness of Propolis in the Treatment of Periodontal Disease: Updated Systematic Review with Meta-Analysis. Antioxidants 2021, 10, 269. [Google Scholar] [CrossRef]
- Freires, I.A.; Santaella, G.M.; de Cássia Orlandi Sardi, J.; Rosalen, P.L. The alveolar bone protective effects of natural products: A systematic review. Arch. Oral Biol. 2018, 87, 196–203. [Google Scholar] [CrossRef]


| Histopathological Analyses | ||||||||
|---|---|---|---|---|---|---|---|---|
| Parameters and Scores | Number of Samples | |||||||
| Experimental Groups | ||||||||
| NLT | SRP | SRP-BGPee | SRP-BGPlns | |||||
| 7 d | 28 d | 7 d | 28 d | 7 d | 28 d | 7 d | 28 d | |
| Intensity of Local Inflammatory Response | ||||||||
| (1) absence of inflammation | - | - | - | - | 1 | 3 | 2 | 6 |
| (2) small quantity of inflammatory cells (less than 1/3 of cells were inflammatory cells) | - | - | - | 5 | 4 | 4 | 5 | 1 |
| (3) moderate quantity of inflammatory cells (1/3 to 2/3 were inflammatory cells) | 1 | 4 | 6 | 2 | 2 | - | - | - |
| (4) large quantity of inflammatory cells (more than 2/3 were inflammatory cells) | 6 | 3 | 1 | - | - | - | - | - |
| Median | 4 | 3 | 3 | 2 † | 2 † | 2 † | 2 †‡ | 1 †‡ |
| Extension of Inflammatory Infiltrate | ||||||||
| (1) absence of inflammation | - | - | - | - | 1 | 3 | 2 | 6 |
| (2) partial extension of connective tissue in the furcation region | - | - | - | 7 | 5 | 4 | 5 | 1 |
| (3) entire extension of connective tissue in the furcation region | 3 | 4 | 6 | - | 1 | - | - | - |
| (4) entire extension of connective tissue and bone tissue in the furcation region | 4 | 3 | 1 | - | - | - | - | - |
| Median | 4 | 3 | 3 | 2 †§ | 2 †‡ | 2 † | 2 †‡ | 1 † |
| Pattern of Structuration of the Connective Tissue in the Furcation Region | ||||||||
| (1) moderate number of fibroblasts and large amount of collagen fibers (dense connective tissue) | - | - | - | - | - | 3 | 2 | 6 |
| (2) moderate amount of both fibroblasts and collagen fibers | - | - | - | 6 | 3 | 4 | 5 | 1 |
| (3) small amount of both fibroblasts and collagen fibers | 3 | 6 | 6 | 1 | 4 | - | - | - |
| (4) severe tissue disorganization with necrosis areas | 4 | 1 | 1 | - | - | - | ||
| Median | 4 | 3 | 3 | 2 †§ | 3 | 2 †§ | 2 †‡ | 1 †‡ |
| Pattern of Structuration of the Alveolar Bone in the Furcation Region | ||||||||
| (1) bone trabeculae with regular contours coated with active osteoblasts, including areas of new bone formation | - | - | - | 1 | - | 1 | - | 7 |
| (2) bone trabeculae with predominantly vital bone tissue, with few areas comprising non-vital bone tissue | - | - | - | 6 | 4 | 6 | 7 | - |
| (3) irregularly contoured bone trabeculae filled with active osteoclasts | 7 | 7 | 7 | - | 3 | - | - | - |
| (4) bone trabeculae filled with active osteoclasts and areas of necrotic bone | - | - | - | - | - | - | - | - |
| Median | 3 | 3 | 3 | 2 † | 2 | 2 † | 2 †‡ | 1 † |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silveira, G.R.C.; Ganzaroli, V.F.; Toro, L.F.; da Costa, L.L.; Pereira, R.I.L.; da Silva, A.B.; Ferreira, I.R.S.; de Mello-Neto, J.M.; Garcia, V.G.; Theodoro, L.H.; et al. Brazilian Green Propolis Carried in Lipid-Based Nanostructures: A Potent Adjuvant Therapy to Non-Surgical Periodontal Treatment in the Management of Experimental Periodontitis. Biomedicines 2025, 13, 1643. https://doi.org/10.3390/biomedicines13071643
Silveira GRC, Ganzaroli VF, Toro LF, da Costa LL, Pereira RIL, da Silva AB, Ferreira IRS, de Mello-Neto JM, Garcia VG, Theodoro LH, et al. Brazilian Green Propolis Carried in Lipid-Based Nanostructures: A Potent Adjuvant Therapy to Non-Surgical Periodontal Treatment in the Management of Experimental Periodontitis. Biomedicines. 2025; 13(7):1643. https://doi.org/10.3390/biomedicines13071643
Chicago/Turabian StyleSilveira, Glauco Rodrigues Carmo, Vinícius Franzão Ganzaroli, Luan Felipe Toro, Leandro Lemes da Costa, Rodrigo Isaias Lopes Pereira, André Bueno da Silva, Iasmin Rosane Silva Ferreira, João Martins de Mello-Neto, Valdir Gouveia Garcia, Letícia Helena Theodoro, and et al. 2025. "Brazilian Green Propolis Carried in Lipid-Based Nanostructures: A Potent Adjuvant Therapy to Non-Surgical Periodontal Treatment in the Management of Experimental Periodontitis" Biomedicines 13, no. 7: 1643. https://doi.org/10.3390/biomedicines13071643
APA StyleSilveira, G. R. C., Ganzaroli, V. F., Toro, L. F., da Costa, L. L., Pereira, R. I. L., da Silva, A. B., Ferreira, I. R. S., de Mello-Neto, J. M., Garcia, V. G., Theodoro, L. H., Marcato, P. D., & Ervolino, E. (2025). Brazilian Green Propolis Carried in Lipid-Based Nanostructures: A Potent Adjuvant Therapy to Non-Surgical Periodontal Treatment in the Management of Experimental Periodontitis. Biomedicines, 13(7), 1643. https://doi.org/10.3390/biomedicines13071643









