Vestibular Versus Cochlear Stimulation on the Relief of Phantom Pain After Traumatic Finger Amputation
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Procedures
2.2.1. Preliminary Evaluations
2.2.2. Questionnaires
- The Douleur Neuropathique 4 Questions (DN4) questionnaire [29], which includes 10 items: 7 on the quality of pain (burning, painful cold, and electric shocks) and abnormal sensations (tingling, pins and needles, numbness, and itching), and 3 on neurological signs in the painful area (touch hypoesthesia, pinprick hypoesthesia, and tactile allodynia). This instrument has 83% sensibility and 90% specificity for the diagnosis of neuropathic pain [29] and an intraclass correlation of 0.71 [30].
- A visual analog scale (VAS) was used to mark pain intensity as a point on a 100 mm horizontal line, representing “no pain” at the left end and the “most severe pain imaginable” at the right end, with an intraclass correlation > 0.8 [32]. At the first evaluation of the study, the repeatability coefficient was estimated as 11.8 mm [33], which is similar to the minimum change of 13 mm considered as clinically significant in patients with acute pain [34]. Therefore, in this study, a clinically significant change was considered when >12 mm.
- The Hospital Anxiety and Depression Scale (HADS) [35] comprises 14 items, including 7 for anxiety and 7 for depression, which are rated on a 4-point scale (from 0 to 3). Subscores were obtained by summing the ratings for the seven items of each subscale (range of 0–21), and a total score was obtained by summing the ratings of all items (range of 0–42), with Cronbach’s alpha ranging from 0.67 to 0.93 [36].
- The Dissociative Experiences Scale [37], which comprises 28 items, was used to assess disturbances in memory, identity, cognition, absorption, imaginative involvement, and feelings of derealization and depersonalization. Each item score ranges from 0%, “This never happens to you”, to 100%, “This always happens to you”, in multiples of ten. A total score was calculated by dividing the sum of the individual scores by 28 (range of 0 to 100%), with a mean Cronbach’s alpha of 0.93 and test–retest reliability of 0.78–0.93 [38].
- The Depersonalization/Derealization Inventory [39] that was designed to assess depersonalization/derealization (DD) symptoms in clinically anxiety states, enabling correlation with concurrent neurophysiological variables. It comprises 28 items rated on a 5-point scale (from 0 to 4). A total score was obtained by summing up the ratings of all items (range of 0–112), with a Cronbach’s alpha of 0.95 [39].
2.2.3. Stimuli
- Unilateral centrifuge (I-Portal NOTC, Neuro Kinetics, Pittsburgh, PA, USA) was applied either to the right or to the left (3.85 cm at 300°/s peak velocity). The chair accelerated to 300°/s in 60 s. After 60 s at full-speed rotation, it moved from the center position to the right/left over 30 s. It remained in the offset position for 60 s, then moved from the right/left position to the center over 30 s. Finally, it decelerated from 300°/s to 0°/s in 60 s.
- Transient evoked otoacoustic emissions (TOAEs) (1.5 to 4 kHz, 64 s averaging, clicks at 83 dB SPL peak equivalent) (OtoRead, Interacoustics, Assens, Denmark) were delivered monoaurally to either the right or the left ear.
2.2.4. Pain Assessment
2.3. Analysis
3. Results
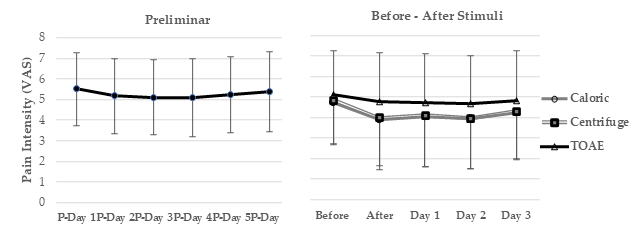
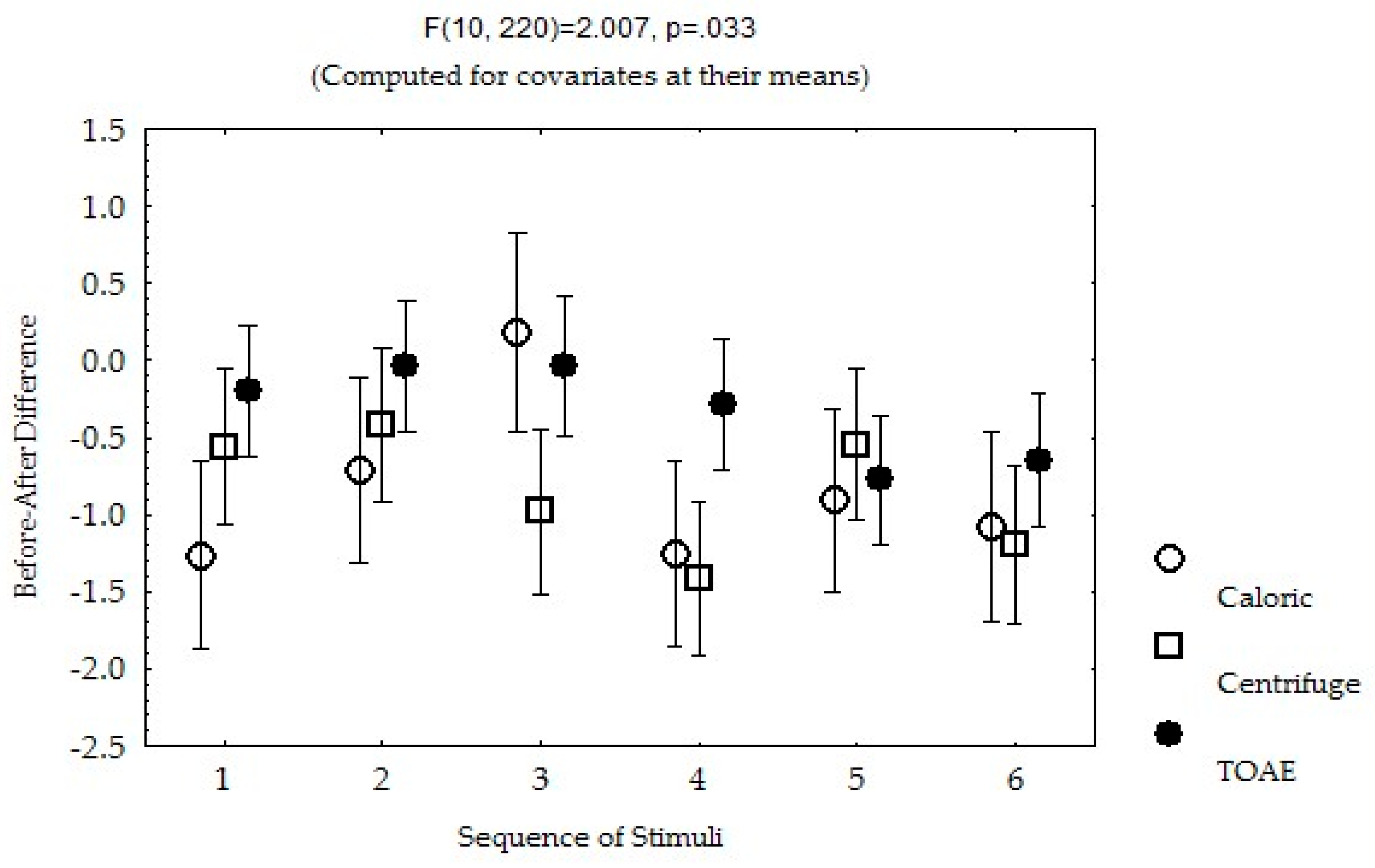
3.1. Bivariate Analysis of Pain Intensity
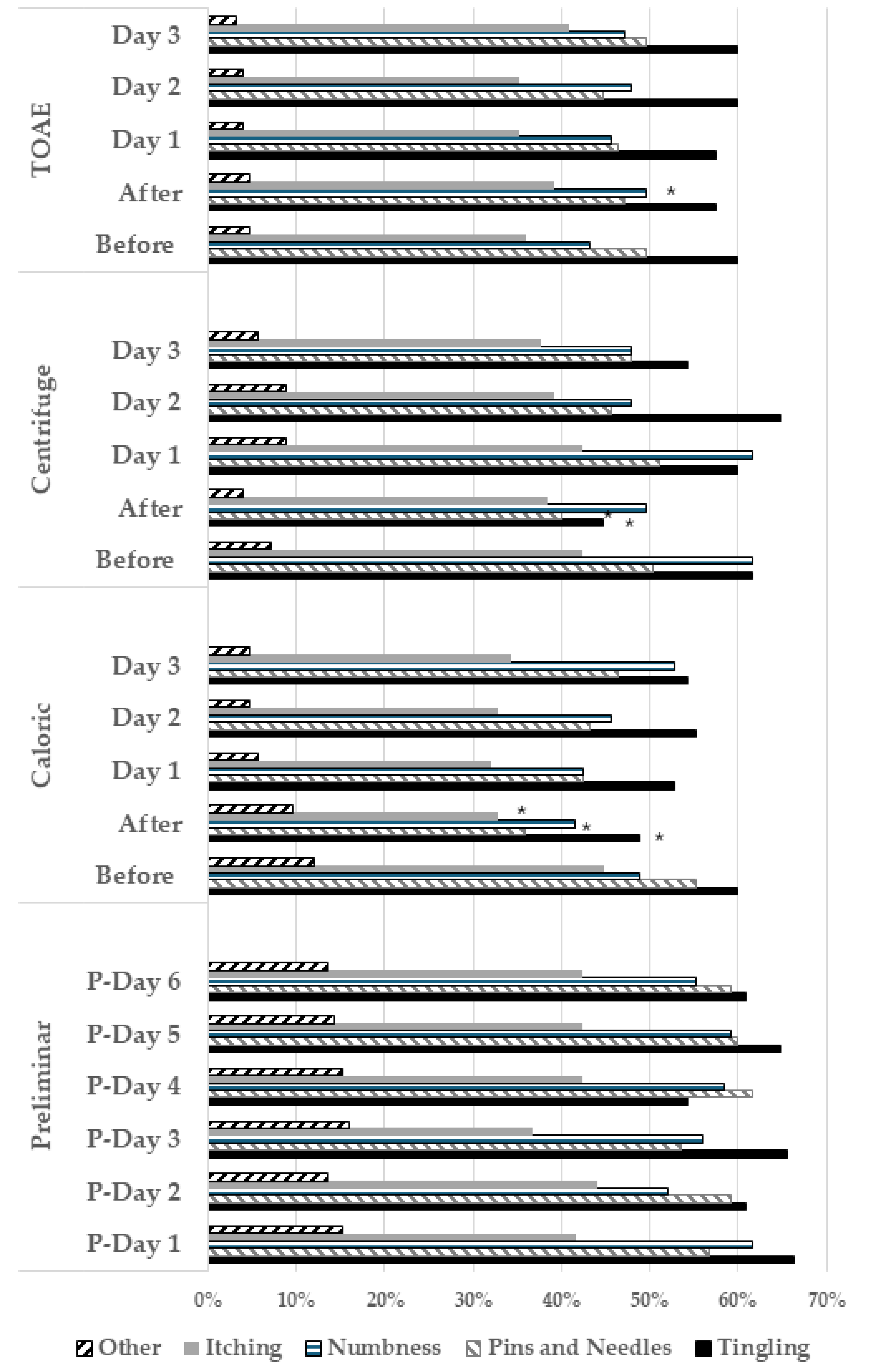
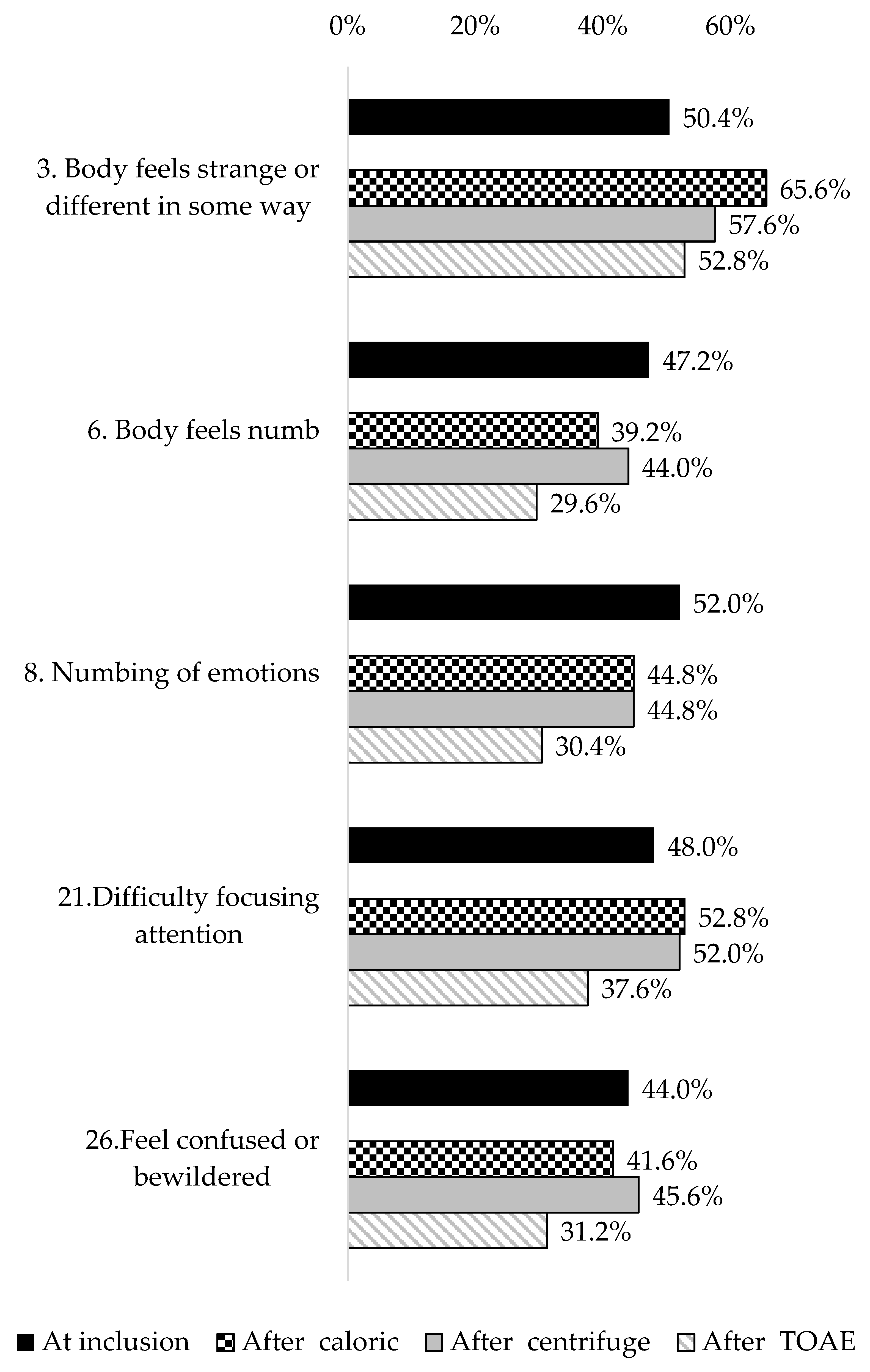
3.2. Bivariate Analysis on Depersonalization/Derealization Symptoms
3.3. Repeated Measures Multivariate Analysis of Pain Intensity
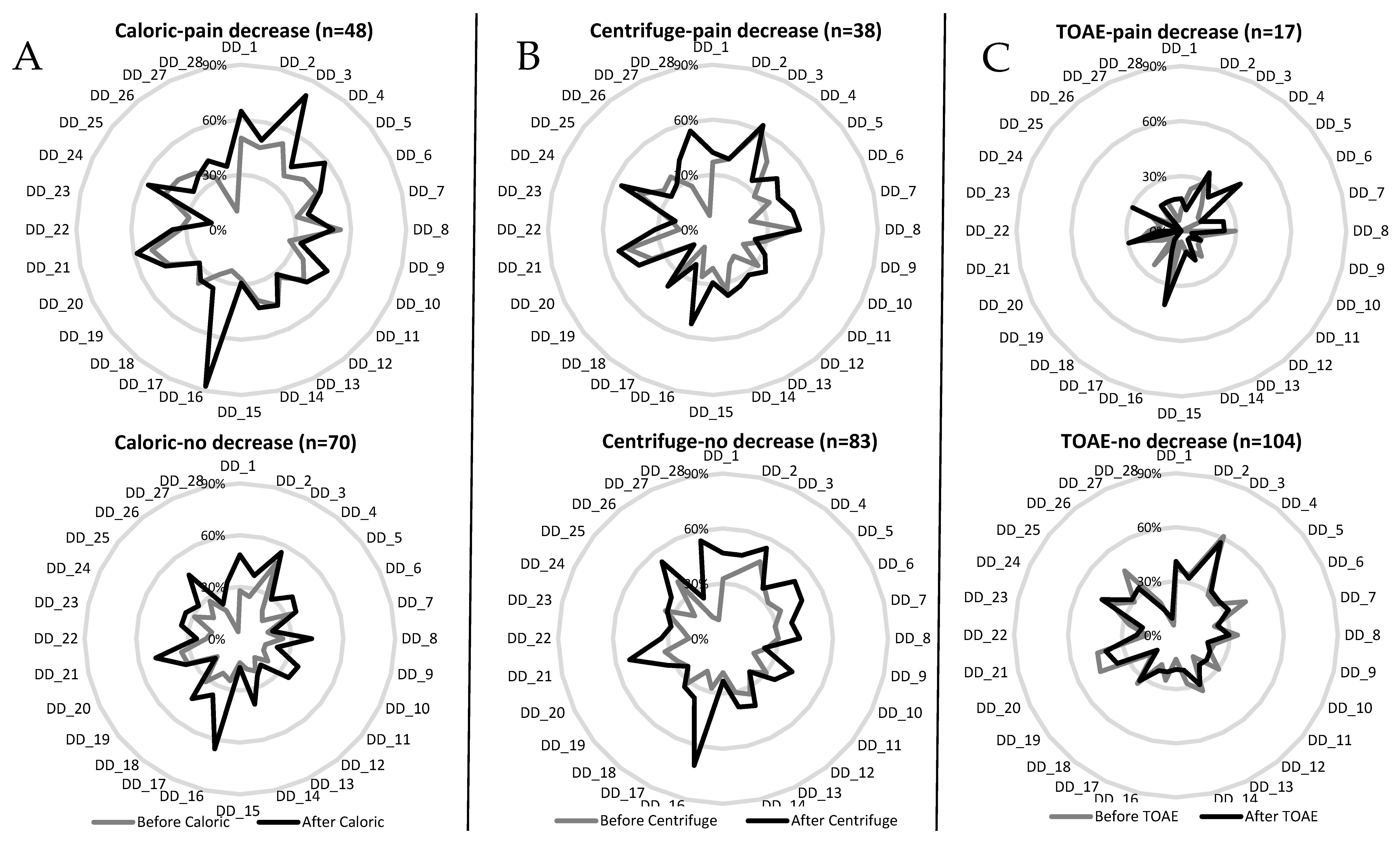
3.4. Repeated Measures Multivariate Analysis of Depersonalization/Derealization Symptoms
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| d.f. | Degrees of freedom |
| DD | Depersonalization/Derealization |
| S.D. | Standard deviation |
| T.H.S.D. | Tukey’s honest significant difference |
| TOAEs | Transient otoacoustic emissions |
| VAS | Visual analog scale |
| 95% C.I. | 95% confidence interval |
References
- Stankevicius, A.; Wallwork, S.B.; Summers, S.J.; Hordacre, B.; Stanton, T.R. Prevalence and incidence of phantom limb pain, phantom limb sensations and telescoping in amputees: A systematic rapid review. Eur. J. Pain 2021, 25, 23–38. [Google Scholar] [CrossRef]
- Hall, N.; Eldabe, S. Phantom limb pain: A review of pharmacological management. Br. J. Pain 2017, 12, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Byrne, K.P. Survey of phantom limb pain, phantom sensation and stump pain in Cambodian and New Zealand amputees. Pain Med. 2011, 12, 794–798. [Google Scholar] [CrossRef] [PubMed]
- Houghton, A.D.; Nicholls, G.; Houghton, A.L.; Saadah, E.; McColl, L. Phantom pain: Natural history and association with rehabilitation. Ann. R. Coll. Surg. Engl. 1994, 76, 22–25. [Google Scholar]
- van der Schans, C.P.; Geertzen, J.B.H.; Schoppen, T.; Dijkstra, P.U. Phantom Pain and Health-Related Quality of Life in Lower Limb Amputees. J. Pain. Symptom Manag. 2002, 24, 429–436. [Google Scholar] [CrossRef]
- Sparling, T.; Iyer, L.; Pasquina, P.; Petrus, E. Cortical Reorganization after Limb Loss: Bridging the Gap between Basic Science and Clinical Recovery. J. Neurosci. 2024, 44, e105123202. [Google Scholar] [CrossRef]
- Makin, T.R.; Filippini, N.; Duff, E.P.; Slater, D.H.; Tracey, I.; Johansen-Berg, H. Network-level reorganisation of functional connectivity following arm amputation. NeuroImage 2015, 114, 217–225. [Google Scholar] [CrossRef]
- Kikkert, S.; Mezue, M.; O’Shea, J.; Henderson Slater, D.; Johansen-Berg, H.; Tracey, I.; Makin, T.R. Neural basis of induced phantom limb pain relief. Ann. Neurol. 2019, 85, 59–73. [Google Scholar] [CrossRef]
- Pinto, C.B.; Pacheco-Barrios, K.; Saleh Velez, F.; Gunduz, M.E.; Munger, M.; Fregni, F. Detangling the structural neural correlates associated with resting versus dynamic phantom limb pain intensity using a voxel-based morphometry analysis. Pain Med. 2023, 24, 528–537. [Google Scholar] [CrossRef]
- Kieselbach, K.; Fauler, I.; Abberger, B. Patients With Chronic Pain: The Aspect Of Negative Body Image. Psychother. Psychosom. Med. Psychol. 2024, 74, 369–375. [Google Scholar] [CrossRef]
- Beisheim-Ryan, E.H.; Hicks, G.E.; Pohlig, R.T.; Medina, J.; Sions, J.M. Body Image and Perception among Adults with and without Phantom Limb Pain. PM&R 2023, 15, 278–290. [Google Scholar]
- Day, B.L.; Fitzpatrick, R.C. The vestibular system. Curr. Biol. 2005, 15, R583–R586. [Google Scholar] [CrossRef]
- Fife, T.D.; Tusa, R.J.; Furman, J.M.; Zee, D.S.; Frohman, E.; Baloh, R.W.; Hain, T.; Goebel, J.; Demer, J.; Eviatar, L. Assessment: Vestibular testing techniques in adults and children Report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2000, 55, 1431–1441. [Google Scholar]
- Hallpike, C.S. The Caloric Tests. J. Laryngol. Otol. 1956, 70, 15–28. [Google Scholar] [CrossRef]
- Clarke, A.H.; Schönfeld, U.; Helling, K. Unilateral examination of utricle and saccule function. J. Vestib. Res. 2003, 13, 215–225. [Google Scholar] [CrossRef] [PubMed]
- Utz, K.S.; Dimova, V.; Oppenländer, K.; Kerkhoff, G. Electrified minds: Transcranial direct current stimulation (tDCS) and Galvanic Vestibular Stimulation (GVS) as methods of non-invasive brain stimulation in neuropsychology—A review of current data and future implications. Neuropsychologia 2010, 48, 2789–2810. [Google Scholar] [CrossRef]
- Ferrè, E.R.; Haggard, P. Vestibular cognition: State-of-the-art and future directions. Cogn. Neuropsychol. 2020, 37, 413–420. [Google Scholar] [CrossRef]
- André, J.M.; Martinet, N.; Paysant, J.; Beis, J.M.; Le Chapelain, L. Temporary phantom limbs evoked by vestibular caloric stimulation in amputees. Cogn. Behav. Neurol. 2001, 14, 190–196. [Google Scholar]
- Ngo, T.; Barsdell, W.N.; Law, P.C.F.; Arnold, C.A.; Chou, M.J.; Nunn, A.K.; Brown, D.J.; Fitzgerald, P.B.; Gibson, S.J.; Miller, S.M. Bedside Neuromodulation of Persistent Pain and Allodynia with Caloric Vestibular Stimulation. Biomedicines 2024, 12, 2365. [Google Scholar] [CrossRef] [PubMed]
- Ferrè, E.R.; Haggard, P.; Bottini, G.; Iannetti, G.D. Caloric vestibular stimulation modulates nociceptive evoked potentials. Exp. Brain Res. 2015, 233, 3393–3401. [Google Scholar] [CrossRef] [PubMed]
- Aranda-Moreno, C.; Jáuregui-Renaud, K.; Reyes-Espinosa, J.; Andrade-Galicia, A.; Bastida-Segura, A.E.; González Carrazco, L.G. Stimulation of the Semicircular Canals or the Utricles by Clinical Tests Can Modify the Intensity of Phantom Limb Pain. Front. Neurol. 2019, 10, 117. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, K.; Perchet, C.; Frot, M.; Bastuji, H.; Garcia-Larrea, L. Cortical modulation of nociception by galvanic vestibular stimulation: A potential clinical tool? Brain Stimul. 2020, 13, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Lopez, C.; Blanke, O.; Mast, F.W. The human vestibular cortex revealed by coordinate-based activation likelihood estimation meta-analysis. Neuroscience 2012, 212, 159–179. [Google Scholar] [CrossRef]
- Mazzola, L.; Mauguière, F.; Isnard, J. Functional mapping of the human insula: Data from electrical stimulations. Rev. Neurol. 2019, 175, 150–156. [Google Scholar] [CrossRef]
- Frot, M.; Faillenot, I.; Mauguière, F. Processing of nociceptive input from posterior to anterior insula in humans. Hum. Brain Mapp. 2014, 35, 5486–5499. [Google Scholar] [CrossRef] [PubMed]
- Kemp, D.T. Otoacoustic emissions, their origin in cochlear function, and use. Br. Med. Bull. 2002, 63, 223–241. [Google Scholar] [CrossRef]
- Oldfield, R.C. The assessment and analysis of handedness: The Edinburgh Inventory. Neuropsychologia 1971, 9, 97–113. [Google Scholar]
- Jáuregui-Renaud, K.; Gutiérrez, M.A.; Viveros, R.L.; Villanueva, P.L. Síntomas de inestabilidad corporal y enfermedad vestibular. Rev. Med. IMSS 2003, 41, 373–378. [Google Scholar]
- Bouhassira, D.; Attal, N.; Alchaar, H.J.; Boureau, F.; Brochet, B.; Bruxelle, J.; Cunin, G.; Fermanian, J.; Ginies, P.; Grun-Overdyking, A.; et al. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire. Pain 2005, 114, 29–36. [Google Scholar] [CrossRef]
- Perez, C.; Galvez, R.; Huelbes, S.; Insausti, J.; Bouhassira, D.; Diaz, S.; Rejas, J. Validity and reliability of the Spanish version of the DN4 (Douleur Neuropathique 4 questions) questionnaire for differential diagnosis of pain syndromes associated to a neuropathic or somatic component. Health Qual. Life Outcomes 2007, 5, 66. [Google Scholar] [CrossRef]
- González-Escalada, J.R.; Camba, A.; Muriel, C.; Rodríguez, M.; Contreras, D.; Barutell, C. Validación del índice de Lattinen para la evaluación del paciente con dolor crónico. Rev. Soc. Esp. Dolor 2012, 19, 181–188. [Google Scholar]
- Goddard, G.; Karibe, H.; McNeill, C. Reproducibility of visual analog scale (VAS) pain scores to mechanical pressure. Cranio 2004, 22, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Bland, J.M.; Altman, D.G. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986, 8476, 307–310. [Google Scholar] [CrossRef]
- Gallagher, E.J.; Liebman, M.; Bijur, P.E. Prospective validation of clinically important changes in pain severity measured on a visual analog scale. Ann. Emerg. Med. 2001, 38, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psych. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef]
- Bjelland, I.; Dahl, A.A.; Haug, T.T.; Neckelmann, D. The validity of the Hospital Anxiety and Depression Scale: An updated literature review. J. Psychosom. Res. 2002, 52, 69–77. [Google Scholar] [CrossRef]
- Bernstein, E.M.; Putnam, F.W. Development, reliability, and validity of a dissociation scale. J. Nerv. Ment. Dis. 1986, 174, 727–735. [Google Scholar] [CrossRef]
- van Ijzendoorn, M.H.; Schuengel, C. The measurement of dissociation in normal and clinical populations: Meta-analytic validation of the Dissociative Experiences Scale (DES). Clin. Psychol. Rev. 1996, 16, 365–382. [Google Scholar] [CrossRef]
- Cox, B.J.; Swinson, R.P. Instrument to assess depersonalization-derealization in panic disorder. Depress. Anxiety 2002, 15, 172–175. [Google Scholar] [CrossRef]
- Mast, F.W.; Preuss, N.; Hartmann, M.; Grabherr, L. Spatial cognition, body representation and affective processes: The role of vestibular information beyond ocular reflexes and control of posture. Front. Integr. Neurosci. 2014, 8, 44. [Google Scholar] [CrossRef]
- Dunbar, R.I.M.; Pearce, E.; Tarr, B.; Makdani, A.; Bamford, J.; Smith, S.; McGlone, F. Cochlear SGN neurons elevate pain thresholds in response to music. Sci. Rep. 2021, 11, 14547. [Google Scholar] [CrossRef] [PubMed]
- Grabherr, L.; Macauda, G.; Lenggenhager, B. The moving history of vestibular stimulation as a therapeutic intervention. Multisensory Res. 2015, 28, 653–687. [Google Scholar] [CrossRef] [PubMed]
- Mutschler, I.; Wankerl, J.; Seifritz, E.; Ball, T. The role of the human insular cortex in pain processing. Eur. Psychiatr. 2011, 26, 1001. [Google Scholar] [CrossRef]
- Brooks, J.C.; Nurmikko, T.J.; Bimson, W.E.; Singh, K.D.; Roberts, N. fMRI of thermal pain: Effects of stimulus laterality and attention. NeuroImage 2002, 15, 293–301. [Google Scholar] [CrossRef]
- Mandonnet, V.; Obaid, S.; Descoteaux, M.; St-Onge, E.; Devaux, B.; Levé, C.; Froelich, S.; Rheault, F.; Mandonnet, E. Electrostimulation of the white matter of the posterior insula and medial operculum: Perception of vibrations, heat, and pain. Pain 2022, 10, 10–97. [Google Scholar] [CrossRef]
- Lobel, E.; Kleine, J.F.; Leroy-Willig, A.; Van de Moortele, P.F.; Le Bihan, D.; Grüsser, O.J.; Berthoz, A. Functional MRI of galvanic vestibular stimulation. J. Neurophysiol. 1998, 80, 2699–2709. [Google Scholar] [CrossRef]
- Bottini, G.; Sterzi, R.; Paulesu, E.; Vallar, G.; Cappa, S.F.; Erminio, F.; Passingham, R.E.; Frith, C.D. Frackowiak RS. Identification of the central vestibular projections in man: A positron emission tomography activation study. Exp. Brain Res. 1994, 99, 164–169. [Google Scholar] [CrossRef]
- zu Eulenburg, P.; Baumgärtner, U.; Treede, R.-D.; Dieterich, M. Interoceptive and multimodal functions of the operculo-insular cortex: Tactile, nociceptive and vestibular representations. NeuroImage 2013, 83, 75–86. [Google Scholar] [CrossRef]
- Frank, S.M.; Greenlee, M.W. The parieto-insular vestibular cortex in humans: More than a single area? J. Neurophysiol. 2018, 120, 1438–1450. [Google Scholar] [CrossRef]
- Moro, V.; Pacella, V.; Scandola, M.; Besharati, S.; Rossato, E.; Jenkinson, P.M.; Fotopoulou, A. A fronto-insular-parietal network for the sense of body ownership. Cereb. Cortex 2023, 33, 512–522. [Google Scholar] [CrossRef]
- Deen, B.; Pitskel, N.B.; Pelphrey, K.A. Three systems of insular functional connectivity identified with cluster analysis. Cereb. Cortex 2011, 21, 1498–1506. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Lee, Y.; Cheng, C. Anterior insular cortex activity to emotional salience of voices in a passive oddball paradigm. Front. Human. Neurosci. 2014, 8, 743. [Google Scholar] [CrossRef]
- Zhou, K.; Zhu, L.; Hou, G.; Chen, X.; Chen, B.; Yang, C.; Zhu, Y. The Contribution of Thalamic Nuclei in Salience Processing. Front. Behav. Neurosci. 2021, 15, 634618. [Google Scholar] [CrossRef] [PubMed]
- Bamiou, D.E.; Musiek, F.E.; Luxon, L.M. The insula (Island of Reil) and its role in auditory processing: Literature review. Brain Res. Rev. 2003, 42, 143–154. [Google Scholar] [CrossRef]
- Chen, T.; Michels, L.; Supekar, K.; Kochalka, J.; Ryali, S.; Menon, V. Role of the anterior insular cortex in integrative causal signaling during multisensory auditory–visual attention. Eur. J. Neurosci. 2015, 41, 264–274. [Google Scholar] [CrossRef]
- Maddison, R.; Nazar, H.; Obara, I.; Vuong, Q.C. The efficacy of sensory neural entrainment on acute and chronic pain: A systematic review and meta-analysis. Br. J. Pain. 2023, 17, 126–141. [Google Scholar] [CrossRef] [PubMed]
- Colloca, L. The Placebo Effect in Pain Therapies. Rev. Pharmacol. Toxicol. 2019, 59, 191–211. [Google Scholar] [CrossRef]
- Oken, B.S. Placebo effects: Clinical aspects and neurobiology. Brain 2008, 131, 2812–2823. [Google Scholar] [CrossRef]
- Freeman, S.; Yu, R.; Egorova, N.; Chen, X.; Kirsch, I.; Claggett, B.; Kaptchuk, T.J.; Gollub, R.L.; Kong, J. Distinct neural representations of placebo and nocebo effects. NeuroImage 2015, 112, 197–207. [Google Scholar] [CrossRef]
- Vickers, A.J.; Cronin, A.M.; Maschino, A.C.; Lewith, G.; MacPherson, H.; Foster, N.E.; Sherman, K.J.; Witt, C.M.; Linde, K.; for the Acupuncture Trialists’ Collaboration. Acupuncture for Chronic Pain: Individual Patient Data Meta-analysis. Arch. Intern. Med. 2012, 172, 1444–1453. [Google Scholar] [CrossRef]
- Binder, A.; Koroschetz, J.; Baron, R. Disease mechanisms in neuropathic itch. Nat. Rev. Neurol. 2008, 4, 329–337. [Google Scholar] [CrossRef] [PubMed]
- van Laarhoven, A.M.; Vogelaar, M.L.; Wilder, S.O.H.; van Riel, P.L.; van de Kerkhof, P.; Kraaimaat, F.W.; Evers, A.W.M. Induction of nocebo and placebo effects on itch and pain by verbal suggestions. Pain 2011, 152, 1486–1494. [Google Scholar] [CrossRef]
- Kooijman, C.M.; Dijkstra, P.U.; Geertzen, J.H.; Elzinga, A.; van der Schans, C.P. Phantom pain and phantom sensations in upper limb amputees: An epidemiological study. Pain 2000, 87, 3341. [Google Scholar] [CrossRef]
- Hunter, J.P.; Katz, J.; Davis, K.D. Dissociation of phantom limb phenomena from stump tactile spatial acuity and sensory thresholds. Brain 2005, 128, 308. [Google Scholar] [CrossRef] [PubMed]
- Ladds, E.; Redgrave, N.; Hotton, M.; Lamyman, M. Systematic review: Predicting adverse psychological outcomes after hand trauma. J. Hand Ther. 2017, 30, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Opsteegh, L.; Reinders-Messelink, H.A.; Schollier, D.; Groothoff, J.W.; Postema, K.; Dijkstra, P.U.; van der Sluis, C.K. Determinants of Return to Work in Patients with Hand Disorders and Hand Injuries. J. Occup. Rehab. 2009, 19, 245–255. [Google Scholar] [CrossRef]
- Gustafsson, M.; Windahl, J.; Blomberg, K. Ten years follow-up of trauma-related psychological distress in a cohort of patients with acute traumatic hand injury. Int. J. Orthop. Trauma. Nurs. 2012, 16, 128–135. [Google Scholar] [CrossRef]
| Variable | All | First Stimulus Subgroups | ||
|---|---|---|---|---|
| (n = 125) | Caloric (n = 42) | Centrifuge (n = 41) | TOAE (n = 42) | |
| Mean ± S.D. | Mean ± S.D. | Mean ± S.D. | Mean ± S.D. | |
| Years of age | 38.2 ± 8.1 | 40.1 ± 9.0 | 37.3 ± 7.6 | 37.3 ± 7.4 |
| Body mass index | 27.7 ± 4.1 | 27.3 ± 3.7 | 27.7 ± 4.2 | 28.0 ± 4.5 |
| Visual analog scale of pain | 5.4 ± 1.7 | 5.3 ± 1.6 | 5.4 ± 1.5 | 5.8 ± 1.9 |
| PAIN CHARACTERISTICS (DN4) | Percentage (95% C.I.) | Percentage (95% C.I.) | Percentage (95% C.I.) | Percentage (95% C.I.) |
| Electric shocks | 44.8 (35.9–53.6) | 38.0 (22.7–53.4) | 60.9 (45.3–76.5) | 35.7 (20.6–50.8) |
| Painful cold | 55.2 (46.3–64.0) | 50.0 (34.2–65.7) | 65.8 (50.7–81.0) | 50.0 (34.7–65.7) |
| Burning | 57.6 (48.5–66.6) | 59.5 (44.0–75.0) | 48.7 (31.3–66.2) | 34.2 (49.1–79.3) |
| Associated symptoms | ||||
| Tingling | 59.2 (50.4–67.9) | 61.9 (46.5–77.2) | 65.8 (50.7–81.0) | 50 3 (4.2–65.7) |
| Pins and needles | 56.0 (47.1–64.8) | 66.6 (51.7–81.5) | 48.7 (32.8–64.7) | 52.3 (36.6–68.1) |
| Numbness | 60.8 (52.1–69.4) | 57.1 (41.5–72.7) | 58.5 (42.7–74.2) | 66.6 (51.7–81.5) |
| Itching | 45.6 (36.7–54.4) | 45.2 (29.5–60.9) | 43.9 (28.0–59.7) | 47.6 (31.8–63.3) |
| Median (Quartiles 1–3) | Median (Quartiles 1–3) | Median (Quartiles 1–3) | Median (Quartiles 1–3) | |
| Months elapsed since amputation | 4 (4–6) | 5 (3–6) | 4 (4–5) | 4 (3–5) |
| LATTINEN INDEX total score | 7 (5–8) | 7 (5–10) | 7 (6–8) | 7 (6–8) |
| Pain intensity | 2 (1–3) | 2 (1–2) | 2 (1–2) | 2 (1–3) |
| Pain frequency | 2 (2–3) | 2 (2–3) | 2 (1–3) | 2 (2–3) |
| Need for medication | 1 (0–1) | 1 (0–1) | 1 (0–1) | 1 (0–1) |
| Handicap | 1 (0–2) | 1 (0–2) | 1 (0–2) | 1 (0–2) |
| Sleep time | 1 (0–2) | 1 (0–2) | 1 (0–2) | 1 (0–2) |
| MENTAL SYMPTOMS | ||||
| Anxiety and depression | 14 (10–17) | 14.5 (10–18) | 14 (10–17) | 13.5 (10–18) |
| Anxiety | 7 (5–10) | 8 (4–10) | 7 (5–9) | 8.5 (5–11) |
| Depression | 6 (4–8) | 6 (4–8) | 7 (4–9) | 5.5 (3–8) |
| Dissociative experiences | 10.0 (6.4–16.7) | 11.0 (5.3–17.8) | 10.3 (7.5–16.4) | 9.1 (6.4–16.7) |
| Depersonalization/derealization | 7 (3–17) | 8 (5–21) | 5 (2–12) | 7 (3–16) |
| Variable | Mean ± S.D. | Mean ± S.D. | p (F, d.f.) | T.H.S.D. p |
|---|---|---|---|---|
| Pain Intensity | Before | After | <0.00001 (17.6, 5) | |
| Caloric | 4.7 ± 2.0 | 3.8 ± 2.3 | 0.00002 | |
| Centrifuge | 4.8 ± 2.1 | 3.9 ± 2.3 | 0.00002 | |
| Transient Otoacoustic Emissions | 5.0 ± 2.1 | 4.7 ± 2.3 | 0.30 | |
| Depersonalization/Derealization Score | Before | After | <0.00001 (17.9, 5) | |
| Caloric | 9.5 ± 10.9 | 13.2 ± 9.8 | 0.00002 | |
| Centrifuge | 9.2 ± 10.2 | 13.2 ± 8.8 | 0.00002 | |
| Transient Otoacoustic Emissions | 9.1 ± 9.8 | 8.2 ± 7.7 | 0.84 |
| Caloric | Centrifuge | TOAE | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Right 30 °C (n = 32) | Right 44 °C (n = 32) | Left 30 °C (n = 31) | Left 44 °C (n = 30) | ANOVA | Right (n = 60) | Left (n = 65) | t test | Right (n = 64) | Left (n = 61) | t test | |
| Pain intensity | Mean ± S.D. | Mean ± S.D. | Mean ± S.D. | Mean ± S.D. | p (F, d.f.) | Mean ± S.D. | Mean ± S.D. | p (t, d.f.) | Mean ± S.D. | Mean ± S.D. | p (t, d.f.) |
| Before | 4.8 ± 1.9 | 4.8 ± 1.6 | 4.6 ± 2.0 | 4.5 ± 2.4 | 0.95 (0.10; 3, 121) | 4.7 ± 2.2 | 4.8 ± 2.0 | 0.71 (0.36, 123) | 4.9 ± 2.1 | 5.2 ± 2.2 | 0.3 2 (0.99, 123) |
| After | 4.0 ± 2.6 | 3.7 ± 1.8 | 3.6 ± 2.4 | 4.0 ± 2.7 | 0.84 (0.27; 3, 121) | 3.9 ± 2.6 | 4.0 ± 2.0 | 0.75 (0.31, 123) | 4.6 ± 2.4 | 4.8 ± 2.2 | 0.51 (0.65, 123) |
| Difference | −0.7 ± 1.7 | −1.0 ± 1.6 | −1.0 ± 1.1 | −0.5 ± 1.3 | 0.40 (0.98; 3, 121) | −0.8 ± 1.3 | −0.8 ± 1.1 | 0.96 (0.39, 123) | −0.3 ± 0.9 | −0.36 ± 1.1 | 0.85 (0.18, 123) |
| DD symptoms | Mean ± S.D. | Mean ± S.D. | Mean ± S.D. | Mean ± S.D. | p (F, d.f.) | Mean ± S.D. | Mean ± S.D. | p (t, d.f.) | Mean ± S.D. | Mean ± S.D. | p (t, d.f.) |
| Before | 8.9 ± 10.6 | 10.6 ± 12.0 | 8.8 ± 10.7 | 9.8 ± 10.9 | 0.90 (0.19; 3, 121) | 10.0 ± 11.2 | 8.5 ± 9.0 | 0.41 (0.81, 123) | 9.1 ± 8.2 | 9.0 ± 11.3 | 0.92 (0.10, 121) |
| After | 12.5 ± 7.4 | 13.5 ± 11.9 | 13.7 ± 9.0 | 13.2 ± 10.8 | 0.96 (0.09; 3, 121) | 13.7 ± 8.7 | 12.7 ± 9.0 | 0.51 (0.65, 123) | 7.7 ± 6.2 | 8.6 ± 9.0 | 0.50 (0.67, 121) |
| Difference | 3.6 ± 6.6 | 2.9 ± 9.4 | 4.8 ± 5.7 | 3.6 ± 6.9 | 0.72 (0.43; 3, 121) | 3.7 ± 8.1 | 4.2 ± 5.6 | 0.72 (0.35, 123) | −1.4 ± 4.5 | −0.3 ± 6.2 | 0.25 (1.1, 121) |
| Symptom | Percentage (95% C.I.) | n |
|---|---|---|
| 1. Surroundings seem strange and unreal | 39.2% (30.6–47.7) | 49 |
| 2. Time seems to pass very slowly | 36.0% (27.5–44.4) | 45 |
| 3. Body feels strange or different in some way | 50.4% (41.6–59.1) | 63 |
| 4. Feel like you’ve been here before (déjà vu) | 38.4% (29.8–46.9) | 48 |
| 5. Feel as though in a dream | 35.2% (26.8–43.5) | 44 |
| 6. Body feels numb | 47.2% (38.4–55.9) | 59 |
| 7. Feeling of detachment or separation from surroundings | 28.0% (20.1–35.8) | 35 |
| 8. Numbing of emotions | 52.0% (43.2–60.7) | 65 |
| 9. People and objects seem far away | 23.2% (15.8–30.6) | 29 |
| 10. Feeling detached or separated from your body | 22.4% (15.0–29.7) | 28 |
| 11. Thoughts seem blurred | 38.4% (29.8–46.9) | 48 |
| 12. Events seem to happen in slow motion | 23.2% (15.8–30.6) | 29 |
| 13. Your emotions seem disconnected from yourself | 31.2% (23.0–39.3) | 39 |
| 14. Feeling of not being in control of self | 30.4% (22.3–38.4) | 38 |
| 15. People appear strange or unreal | 25.6% (17.9–33.2) | 32 |
| 16. Dizziness | 36.8% (28.3–45.2) | 46 |
| 17. Surroundings appear covered with a haze | 14.4% (8.2–20.5) | 18 |
| 18. Vision is dulled | 32.8% (24.5–41.0) | 41 |
| 19. Feel as if walking on shifting ground | 17.6% (10.9–24.2) | 22 |
| 20. Difficulty understanding what others say to you | 29.6% (21.6–37.6) | 37 |
| 21. Difficulty focusing attention | 48.0% (39.2–56.7) | 60 |
| 22. Feel as though in a trance | 26.4% (18.6–34.1) | 33 |
| 23. The distinction between close and distant is blurred | 30.4% (22.3–38.4) | 38 |
| 24. Difficulty concentrating | 42.4% (33.7–51.0) | 53 |
| 25. Feel as though your personality is different | 34.4% (26.0–42.7) | 43 |
| 26. Feel confused or bewildered | 44.0% (35.3–52.7) | 55 |
| 27. Feel isolated from the world | 26.4% (18.6–34.1) | 33 |
| 28. Feel “spacy” or “spaced out” | 8.0% (3.2–12.7) | 10 |
| Repeated Measures | Caloric | Centrifuge | TOAE | |
|---|---|---|---|---|
| Variables | Adjusted R2 (F Value, p) 0.15 (2.59, 0.002) | Adjusted R2 (F Value, p) 0.15 (2.61, 0.002) | Adjusted R2 (F Value, p) 0.17 (2.93, 0.0008) | |
| p (F Value, d.f.) | p (F Value, d.f.) | p (F Value, d.f.) | p (F Value, d.f.) | |
| Intercept | 0.028 (4.92, 1) * | 0.044 (4.12) * | 0.39 (0.73) | 0.60 (0.26) |
| Anxiety/depression HADS total score | 0.019 (5.63, 1) * | 0.68 (0.16) | 0.012 (6.49) * | 0.024 (5.23) * |
| First DD_3: “Body feels strange or different in some way” | 0.026 (5.08, 1) * | 0.005 (7.86) * | 0.11 (2.46) | 0.17 (1.84) |
| First DD_23: “The distinction between close and distant is blurred” | 0.007 (7.29, 1) * | 0.71 (0.13) | 0.018 (5.69) * | 0.002 (9.45) * |
| Sequence of the stimuli | 0.001 (4.10, 5) * | 0.016 (2.91) * | 0.029 (2.59) * | 0.06 (2.11) |
| First DN4_7: Itching | 0.0002 (14.63, 1) * | 0.07 (3.33) | 0.11 (2.56) | 0.002 (9.72) * |
| Sequence of stimuli * and DN4_7: Itching | 0.42 (1.25, 5) | 0.33 (1.15) | 0.47 (0.91) | 0.46 (0.93) |
| R1 Repeated measures (R1) | 0.36 (1.01, 2) | - | - | - |
| R1 and HADS score | 0.10 (2.32, 2) | - | - | - |
| R1 and DD1_3 | 0.01 (4.67, 2) * | - | - | - |
| R1 and DD1_23 | 0.07 (2.00, 2) | - | - | - |
| R1 and sequence of stimuli | 0.03 (2.59, 10) * | - | - | - |
| R1 and DN1_7 | 0.78 (0.24, 2) | - | - | - |
| Sequence of stimuli and DN4_7 | 0.40 (1.04, 10) | - | - | - |
| Repeated Measures | Caloric | Centrifuge | TOAE | |
|---|---|---|---|---|
| Variables | Adjusted R2 (F Value, p) 0.13 (3.48, 0.001) | Adjusted R2 (F Value, p) 0.13 (3.52, 0.001)) | Adjusted R2 (F Value, p) 0.15 (3.78, 0.0005)) | |
| p (F Value, d.f.) | p (F Value, d.f.) | p (F Value, d.f.) | p (F Value, d.f.) | |
| Intercept | 0.0000001 (31.24, 1) * | 0.00001 (19.78) | 0.00002 (19.30) | 0.021 (5.43) |
| Age | 0.006 (7.62, 1) * | 0.015 (6.01) * | 0.07 (3.21) | 0.19 (1.73) |
| Anxiety/depression HADS Score | 0.0003 (13.32, 1) * | 0.015 (6.07) * | 0.004 (8.25) * | 0.039 (4.33) * |
| Dissociative Experiences Score | 0.0001 (14.97, 5) * | 0.21 (1.57) | 0.004 (8.46) * | 0.00006 (17.01) * |
| Sequence of stimuli | 0.054 (2.24, 2) | 0.022 (2.73) * | 0.15 (1.64) | 0.41 (1.01) |
| Repeated measures (R1) | ||||
| R1 * Age | 0.038 (3.29, 2) * | - | - | - |
| R1 * HADS Score | 0.48 (0.72, 2) | - | - | - |
| R1 * Dissociative Experiences Score | 0.54 (0.61, 2) | - | - | - |
| R1 * Sequence of stimuli | 0.20 (1.60, 10) | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-López, J.J.; Miguel-Puga, J.A.; Jaime-Esquivias, M.I.; Peña-Chávez, M.; Jáuregui-Renaud, K. Vestibular Versus Cochlear Stimulation on the Relief of Phantom Pain After Traumatic Finger Amputation. Biomedicines 2025, 13, 1601. https://doi.org/10.3390/biomedicines13071601
Díaz-López JJ, Miguel-Puga JA, Jaime-Esquivias MI, Peña-Chávez M, Jáuregui-Renaud K. Vestibular Versus Cochlear Stimulation on the Relief of Phantom Pain After Traumatic Finger Amputation. Biomedicines. 2025; 13(7):1601. https://doi.org/10.3390/biomedicines13071601
Chicago/Turabian StyleDíaz-López, José Joaquín, José Adán Miguel-Puga, María Isabel Jaime-Esquivias, Maricela Peña-Chávez, and Kathrine Jáuregui-Renaud. 2025. "Vestibular Versus Cochlear Stimulation on the Relief of Phantom Pain After Traumatic Finger Amputation" Biomedicines 13, no. 7: 1601. https://doi.org/10.3390/biomedicines13071601
APA StyleDíaz-López, J. J., Miguel-Puga, J. A., Jaime-Esquivias, M. I., Peña-Chávez, M., & Jáuregui-Renaud, K. (2025). Vestibular Versus Cochlear Stimulation on the Relief of Phantom Pain After Traumatic Finger Amputation. Biomedicines, 13(7), 1601. https://doi.org/10.3390/biomedicines13071601





