Cartilage-Specific 18F-NaF Uptake in Rat Models: A Multimodal In Vitro and Ex Vitro Comparative Study with 99mTc-MDP
Abstract
1. Introduction
2. Methods
2.1. In Vitro Biodistribution Experiment
2.2. Time-Dependent Biodistribution Study
2.3. Age-Related Biodistribution Study
2.4. In Vivo Imaging Experiment
2.4.1. Unilateral Implantation Models
2.4.2. Bilateral Implantation Model
2.4.3. PET/CT Imaging Acquisition Method
2.5. Histopathological Verification
2.6. Autoradiography
2.7. Data Analysis
3. Results
3.1. Time-Dependent Biodistribution of 18F-NaF and 99mTc-MDP in Rat Tissues
3.2. Age-Related Biodistribution of 18F-NaF in Rat Musculoskeletal Tissues
3.3. Specific Uptake of 18F-NaF in Articular Cartilage
3.4. Reasons for Overlooking 18F-NaF Uptake Research
3.5. Investigation of Calcification Degree and 18F-NaF Uptake
3.6. Dynamic Imaging Changes of Implant Activity
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 18F-NaF | 18F-Sodium Fluoride |
| 99mTc-MDP | 99mTc-Methylene Diphosphonate |
| PET | Positron emission tomography |
| HA | Hydroxyapatite |
| ALP | Alkaline phosphatase |
| PPi | Pyrophosphate |
| Pi | Phosphate ions |
References
- Galasko, C.S. The pathological basis for skeletal scintigraphy. J. Bone Jt. Surgery. Br. Vol. 1975, 57, 353–359. [Google Scholar] [CrossRef]
- Eckstein, F.; Guermazi, A.; Gold, G.; Duryea, J.; Le Graverand, M.-P.H.; Wirth, W.; Miller, C. Comparison of the diagnostic value of 18F-NaF PET/CT and 99mTc-MDP SPECT for bone metastases: A systematic review and meta-analysis. Transl. Cancer Res. 2023, 12, 3166–3178. [Google Scholar]
- Eckstein, F.; Guermazi, A.; Gold, G.; Duryea, J.; Hellio Le Graverand, M.P.; Wirth, W.; Miller, C.G. Imaging of cartilage and bone: Promises and pitfalls in clinical trials of osteoarthritis. Osteoarthr. Cartil. 2014, 22, 1516–1532. [Google Scholar] [CrossRef]
- Mackie, E.J.; Ahmed, Y.A.; Tatarczuch, L.; Chen, K.-S.; Mirams, M. Endochondral ossification: How cartilage is converted into bone in the developing skeleton. Int. J. Biochem. Cell Biol. 2008, 40, 46–62. [Google Scholar] [CrossRef]
- Ghimire, S.; Miramini, S.; Edwards, G.; Rotne, R.; Xu, J.; Ebeling, P.; Zhang, L. The investigation of bone fracture healing under intramembranous and endochondral ossification. Bone Rep. 2021, 14, 100740. [Google Scholar] [CrossRef]
- Amizuka, N.; Hasegawa, T.; Oda, K.; Luiz de Freitas, P.H.; Hoshi, K.; Li, M.; Ozawa, H. Histology of epiphyseal cartilage calcification and endochondral ossification. Front. Biosci. (Elite Ed.) 2012, 4, 2085–2100. [Google Scholar] [CrossRef] [PubMed]
- Gheorghe, S.R.; Crăciun, A.M.; Ilyés, T.; Tisa, I.B.; Sur, L.; Lupan, I.; Samasca, G.; Silaghi, C.N. Converging Mechanisms of Vascular and Cartilaginous Calcification. Biology 2024, 13, 565. [Google Scholar] [CrossRef]
- Blumer, M.J.F. Bone tissue and histological and molecular events during development of the long bones. Ann. Anat. = Anat. Anz. Off. Organ Anat. Ges. 2021, 235, 151704. [Google Scholar] [CrossRef]
- Park, P.S.U.; Raynor, W.Y.; Sun, Y.; Werner, T.J.; Rajapakse, C.S.; Alavi, A. 18F-Sodium Fluoride PET as a Diagnostic Modality for Metabolic, Autoimmune, and Osteogenic Bone Disorders: Cellular Mechanisms and Clinical Applications. Int. J. Mol. Sci. 2021, 22, 6504. [Google Scholar] [CrossRef]
- Mathavan, N.; Koopman, J.; Raina, D.B.; Turkiewicz, A.; Tägil, M.; Isaksson, H. 18F-fluoride as a prognostic indicator of bone regeneration. Acta Biomater. 2019, 90, 403–411. [Google Scholar] [CrossRef]
- Aaltonen, L.; Koivuviita, N.; Seppänen, M.; Tong, X.; Kröger, H.; Löyttyniemi, E.; Metsärinne, K. Correlation between 18F-Sodium Fluoride positron emission tomography and bone histomorphometry in dialysis patients. Bone 2020, 134, 115267. [Google Scholar] [CrossRef] [PubMed]
- Raynor, W.Y.; Borja, A.J.; Hancin, E.C.; Werner, T.J.; Alavi, A.; Revheim, M.-E. Novel Musculoskeletal and Orthopedic Applications of 18F-Sodium Fluoride PET. PET Clin. 2021, 16, 295–311. [Google Scholar] [CrossRef] [PubMed]
- Dadgar, H.; Norouzbeigi, N.; Jokar, N.; Zareizadeh, J.; Gholamrezanezhad, A.; Ahmadzadehfar, H.; Abbaszadeh, M.; Assadi, M. Comparison of 18F-NaF Imaging, 99mTc-MDP Scintigraphy, and 18F-FDG for Detecting Bone Metastases. World J. Nucl. Med. 2022, 21, 001–008. [Google Scholar] [CrossRef]
- Watanabe, T.; Takase-Minegishi, K.; Ihata, A.; Kunishita, Y.; Kishimoto, D.; Kamiyama, R.; Hama, M.; Yoshimi, R.; Kirino, Y.; Asami, Y.; et al. (18)F-FDG and (18)F-NaF PET/CT demonstrate coupling of inflammation and accelerated bone turnover in rheumatoid arthritis. Mod. Rheumatol. 2016, 26, 180–187. [Google Scholar] [CrossRef]
- Irmler, I.M.; Gebhardt, P.; Hoffmann, B.; Opfermann, T.; Figge, M.-T.; Saluz, H.P.; Kamradt, T. 18 F-Fluoride positron emission tomography/computed tomography for noninvasive in vivo quantification of pathophysiological bone metabolism in experimental murine arthritis. Arthritis Res. Ther. 2014, 16, R155. [Google Scholar] [CrossRef]
- Moretti, R.; Meffe, G.; Annunziata, S.; Capotosti, A. Innovations in imaging modalities: A comparative review of MRI, long-axial field-of-view PET, and full-ring CZT-SPECT in detecting bone metastases. Q. J. Nucl. Med. Mol. Imaging 2023, 67, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Savisto, N.; Grönroos, T.J.; Oikonen, V.; Rajander, J.; Löyttyniemi, E.; Bergman, J.; Forsback, S.; Solin, O.; Haaparanta-Solin, M. [18F]Fluoride uptake in various bone types and soft tissues in rat. EJNMMI Res. 2023, 13, 21. [Google Scholar] [CrossRef]
- Tibrewala, R.; Bahroos, E.; Mehrabian, H.; Foreman, S.C.; Link, T.M.; Pedoia, V.; Majumdar, S. [18F]-Sodium Fluoride PET/MR Imaging for Bone–Cartilage Interactions in Hip Osteoarthritis: A Feasibility Study. J. Orthop. Res. 2019, 37, 2671–2680. [Google Scholar] [CrossRef]
- Blake, G.M.; Puri, T.; Siddique, M.; Frost, M.L.; Moore, A.E.B.; Fogelman, I. Site specific measurements of bone formation using [18F] sodium fluoride PET/CT. Quant. Imaging Med. Surg. 2018, 8, 47–59. [Google Scholar] [CrossRef]
- Watkins, L.; MacKay, J.; Haddock, B.; Mazzoli, V.; Uhlrich, S.; Gold, G.; Kogan, F. Assessment of quantitative [18F]Sodium fluoride PET measures of knee subchondral bone perfusion and mineralization in osteoarthritic and healthy subjects. Osteoarthr. Cartil. 2021, 29, 849–858. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. Osteoarthr. Cartil. 2012, 20, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Bernhardsson, M.; Sandberg, O.; Ressner, M.; Koziorowski, J.; Malmquist, J.; Aspenberg, P. Shining dead bone-cause for cautious interpretation of [18F]NaF PET scans. Acta Orthop. 2018, 89, 124–127. [Google Scholar] [CrossRef]
- On behalf of the EANM Bone & Joint Committee and the Oncology Committee; Wyngaert, T.V.D.; Strobel, K.; Kampen, W.U.; Kuwert, T.; van der Bruggen, W.; Mohan, H.K.; Gnanasegaran, G.; Delgado-Bolton, R.; Weber, W.A.; et al. The EANM practice guidelines for bone scintigraphy. Eur. J. Nucl. Med. Mol. Imaging 2016, 43, 1723–1738. [Google Scholar] [CrossRef] [PubMed]
- Christensen, S.B.; Krogsgaard, O.W. Localization of Tc-99m MDP in epiphyseal growth plates of rats. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 1981, 22, 237–245. [Google Scholar]
- McKinstry, P.; Schnitzer, J.E.; Light, T.R.; Ogden, J.A.; Hoffer, P. Relationship of 99mTc-MDP uptake to regional osseous circulation in skeletally immature and mature dogs. Skelet. Radiol. 1982, 8, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Grant, F.D.; Fahey, F.H.; Packard, A.B.; Davis, R.T.; Alavi, A.; Treves, S.T. Skeletal PET with 18F-fluoride: Applying new technology to an old tracer. J. Nucl. Med. 2008, 49, 68–78. [Google Scholar] [CrossRef]
- Blau, M.; Nagler, W.; Bender, M.A. Fluorine-18: A new isotope for bone scanning. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 1962, 3, 332–334. [Google Scholar]
- Czernin, J.; Satyamurthy, N.; Schiepers, C. Molecular mechanisms of bone 18F-NaF deposition. J. Nucl. Med. 2010, 51, 1826–1829. [Google Scholar] [CrossRef]
- Beheshti, M.; Mottaghy, F.M.; Paycha, F.; Behrendt, F.F.F.; Van den Wyngaert, T.; Fogelman, I.; Strobel, K.; Celli, M.; Fanti, S.; Giammarile, F.; et al. (18)F-NaF PET/CT: EANM procedure guidelines for bone imaging. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1767–1777. [Google Scholar] [CrossRef]
- Ahuja, K.; Sotoudeh, H.; Galgano, S.J.; Singh, R.; Gupta, N.; Gaddamanugu, S.; Choudhary, G. 18F-Sodium Fluoride PET: History, Technical Feasibility, Mechanism of Action, Normal Biodistribution, and Diagnostic Performance in Bone Metastasis Detection Compared with Other Imaging Modalities. J. Nucl. Med. Technol. 2020, 48, 9–16. [Google Scholar] [CrossRef]
- Iagaru, A.; Young, P.; Mittra, E.; Dick, D.W.; Herfkens, R.; Gambhir, S.S. Pilot prospective evaluation of 99mTc-MDP scintigraphy, 18F NaF PET/CT, 18F FDG PET/CT and whole-body MRI for detection of skeletal metastases. Clin. Nucl. Med. 2013, 38, e290–e296. [Google Scholar] [CrossRef] [PubMed]
- Jambor, I.; Kuisma, A.; Ramadan, S.; Huovinen, R.; Sandell, M.; Kajander, S.; Kemppainen, J.; Kauppila, E.; Auren, J.; Merisaari, H.; et al. Prospective evaluation of planar bone scintigraphy, SPECT, SPECT/CT, 18F-NaF PET/CT and whole body 1.5T MRI, including DWI, for the detection of bone metastases in high risk breast and prostate cancer patients: SKELETA clinical trial. Acta Oncol. 2016, 55, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Sheikhbahaei, S.; Jones, K.M.; Werner, R.A.; Salas-Fragomeni, R.A.; Marcus, C.V.; Higuchi, T.; Rowe, S.P.; Solnes, L.B.; Javadi, M.S. 18F-NaF-PET/CT for the detection of bone metastasis in prostate cancer: A meta-analysis of diagnostic accuracy studies. Ann. Nucl. Med. 2019, 33, 351–361. [Google Scholar] [CrossRef]
- Bernhard, J.C.; Marolt Presen, D.; Li, M.; Monforte, X.; Ferguson, J.; Leinfellner, G.; Heimel, P.; Betti, S.L.; Shu, S.; Teuschl-Woller, A.H.; et al. Effects of Endochondral and Intramembranous Ossification Pathways on Bone Tissue Formation and Vascularization in Human Tissue-Engineered Grafts. Cells 2022, 11, 3070. [Google Scholar] [CrossRef]
- Hall, A.C. The Role of Chondrocyte Morphology and Volume in Controlling Phenotype—Implications for Osteoarthritis, Cartilage Repair, and Cartilage Engineering. Curr. Rheumatol. Rep. 2019, 21, 38. [Google Scholar] [CrossRef]
- Funck-Brentano, T.; Cohen-Solal, M. Crosstalk between cartilage and bone: When bone cytokines matter. Cytokine Amp; Growth Factor Rev. 2011, 22, 91–97. [Google Scholar] [CrossRef]
- Golub, E.E. Biomineralization and matrix vesicles in biology and pathology. Semin. Immunopathol. 2011, 33, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Micheletti, C.; Hurley, A.; Gourrier, A.; Palmquist, A.; Tang, T.; Shah, F.A.; Grandfield, K. Bone mineral organization at the mesoscale: A review of mineral ellipsoids in bone and at bone interfaces. Acta Biomater. 2022, 142, 1–13. [Google Scholar] [CrossRef]
- Cui, L.; Houston, D.; Farquharson, C.; MacRae, V. Characterisation of matrix vesicles in skeletal and soft tissue mineralisation. Bone 2016, 87, 147–158. [Google Scholar] [CrossRef]
- Wu, L.N.; Yoshimori, T.; Genge, B.R.; Sauer, G.R.; Kirsch, T.; Ishikawa, Y.; E Wuthier, R. Characterization of the nucleational core complex responsible for mineral induction by growth plate cartilage matrix vesicles. J. Biol. Chem. 1993, 268, 25084–25094. [Google Scholar] [CrossRef]
- Rosenthal, A.K. Articular cartilage vesicles and calcium crystal deposition diseases. Curr. Opin. Rheumatol. 2016, 28, 127–132. [Google Scholar] [CrossRef]
- Iwayama, T.; Bhongsatiern, P.; Takedachi, M.; Murakami, S. Matrix Vesicle–Mediated Mineralization and Potential Applications. J. Dent. Res. 2022, 101, 1554–1562. [Google Scholar] [CrossRef]
- Lin, T.S.; Hsieh, C.H.; Kuo, C.; Juang, Y.P.; Hsieh, Y.S.Y.; Chiang, H.; Hung, S.C.; Jiang, C.C.; Liang, P.H. Sulfation pattern of chondroitin sulfate in human osteoarthritis cartilages reveals a lower level of chondroitin-4-sulfate. Carbohydr. Polym. 2020, 229, 115496. [Google Scholar] [CrossRef]
- Jung, Y.K.; Park, H.R.; Cho, H.J.; Jang, J.A.; Lee, E.J.; Han, M.S.; Kim, G.W.; Han, S. Degrading products of chondroitin sulfate can induce hypertrophy-like changes and MMP-13/ADAMTS5 production in chondrocytes. Sci. Rep. 2019, 9, 15846. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Khong, M.L.; Lui, E.L.; Mebarek, S.; Magne, D.; Buchet, R.; Tanner, J.A. Long-chain polyphosphate in osteoblast matrix vesicles: Enrichment and inhibition of mineralization. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2019, 1863, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Millán, J.L. The role of phosphatases in the initiation of skeletal mineralization. Calcif. Tissue Int. 2013, 93, 299–306. [Google Scholar] [CrossRef] [PubMed]
- McLean, F.M.; Keller, P.J.; Genge, B.R.; Walters, S.A.; Wuthier, R.E. Disposition of preformed mineral in matrix vesicles. Internal localization and association with alkaline phosphatase. J. Biol. Chem. 1987, 262, 10481–10488. [Google Scholar] [CrossRef]
- Frost, H.M. A 2003 update of bone physiology and Wolff’s Law for clinicians. Angle Orthod. 2004, 74, 3–15. [Google Scholar]
- Mitsuyama, H.; Healey, R.; Terkeltaub, R.; Coutts, R.; Amiel, D. Calcification of human articular knee cartilage is primarily an effect of aging rather than osteoarthritis. Osteoarthr. Cartil. 2007, 15, 559–565. [Google Scholar] [CrossRef]
- Hubert, J.; Beil, F.; Rolvien, T.; Butscheidt, S.; Hischke, S.; Püschel, K.; Frosch, S.; Mussawy, H.; Ries, C.; Hawellek, T. Cartilage calcification is associated with histological degeneration of the knee joint: A highly prevalent, age-independent systemic process. Osteoarthr. Cartil. 2020, 28, 1351–1361. [Google Scholar] [CrossRef]
- Findlay, D.M.; Kuliwaba, J.S. Bone–cartilage crosstalk: A conversation for understanding osteoarthritis. Bone Res. 2016, 4, 16028. [Google Scholar] [CrossRef] [PubMed]
- Høilund-Carlsen, P.F.; Sturek, M.; Alavi, A.; Gerke, O. Atherosclerosis imaging with 18F-sodium fluoride PET: State-of-the-art review. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1538–1551. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, A.J.; Theng, E.H.; Paravastu, S.S.; Wojnowski, N.M.; Farhadi, F.; Morris, M.A.; Hartley, I.R.; Gafni, R.I.; Roszko, K.L.; Collins, M.T.; et al. Spatial Atlas for Mapping Vascular Microcalcification Using 18F-NaF PET/CT: Application in Hyperphosphatemic Familial Tumoral Calcinosis. Arterioscler. Thromb. Vasc. Biol. 2024, 44, 1432–1446. [Google Scholar] [CrossRef]
- Vidavsky, N.; Kunitake, J.A.M.R.; Estroff, L.A. Multiple Pathways for Pathological Calcification in the Human Body. Adv. Healthc. Mater. 2021, 10, 2001271. [Google Scholar] [CrossRef] [PubMed]
- Collins, M.T.; Marcucci, G.; Anders, H.-J.; Beltrami, G.; Cauley, J.A.; Ebeling, P.R.; Kumar, R.; Linglart, A.; Sangiorgi, L.; Towler, D.A.; et al. Skeletal and extraskeletal disorders of biomineralization. Nat. Rev. Endocrinol. 2022, 18, 473–489. [Google Scholar] [CrossRef]
- Lee, S.J.; Lee, I.K.; Jeon, J.H. Vascular Calcification—New Insights into Its Mechanism. Int. J. Mol. Sci. 2020, 21, 2685. [Google Scholar] [CrossRef]
- Kapustin, A.; Shanahan, C.M. Targeting vascular calcification: Softening-up a hard target. Curr. Opin. Pharmacol. 2009, 9, 84–89. [Google Scholar] [CrossRef]
- Viegas, C.; Araújo, N.; Marreiros, C.; Simes, D. The interplay between mineral metabolism, vascular calcification and inflammation in Chronic Kidney Disease (CKD): Challenging old concepts with new facts. Aging 2019, 11, 4274–4299. [Google Scholar] [CrossRef]
- Persy, V.; D’Haese, P. Vascular calcification and bone disease: The calcification paradox. Trends Mol. Med. 2009, 15, 405–416. [Google Scholar] [CrossRef]
- Whitfield, J.F. Osteogenic PTHs and vascular ossification—Is there a danger for osteoporotics? J. Cell. Biochem. 2005, 95, 437–444. [Google Scholar] [CrossRef]
- Evenepoel, P.; Opdebeeck, B.; David, K.; D’HAese, P.C. Bone-Vascular Axis in Chronic Kidney Disease. Adv. Chronic Kidney Dis. 2019, 26, 472–483. [Google Scholar] [CrossRef] [PubMed]
- Lodge, M.A.; Chaudhry, M.A.; Wahl, R.L. Noise Considerations for PET Quantification Using Maximum and Peak Standardized Uptake Value. J. Nucl. Med. 2012, 53, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Akamatsu, G.; Ikari, Y.; Nishida, H.; Nishio, T.; Ohnishi, A.; Maebatake, A.; Sasaki, M.; Senda, M. Influence of Statistical Fluctuation on Reproducibility and Accuracy of SUVmax and SUVpeak: A Phantom Study. J. Nucl. Med. Technol. 2015, 43, 222–226. [Google Scholar] [CrossRef] [PubMed]
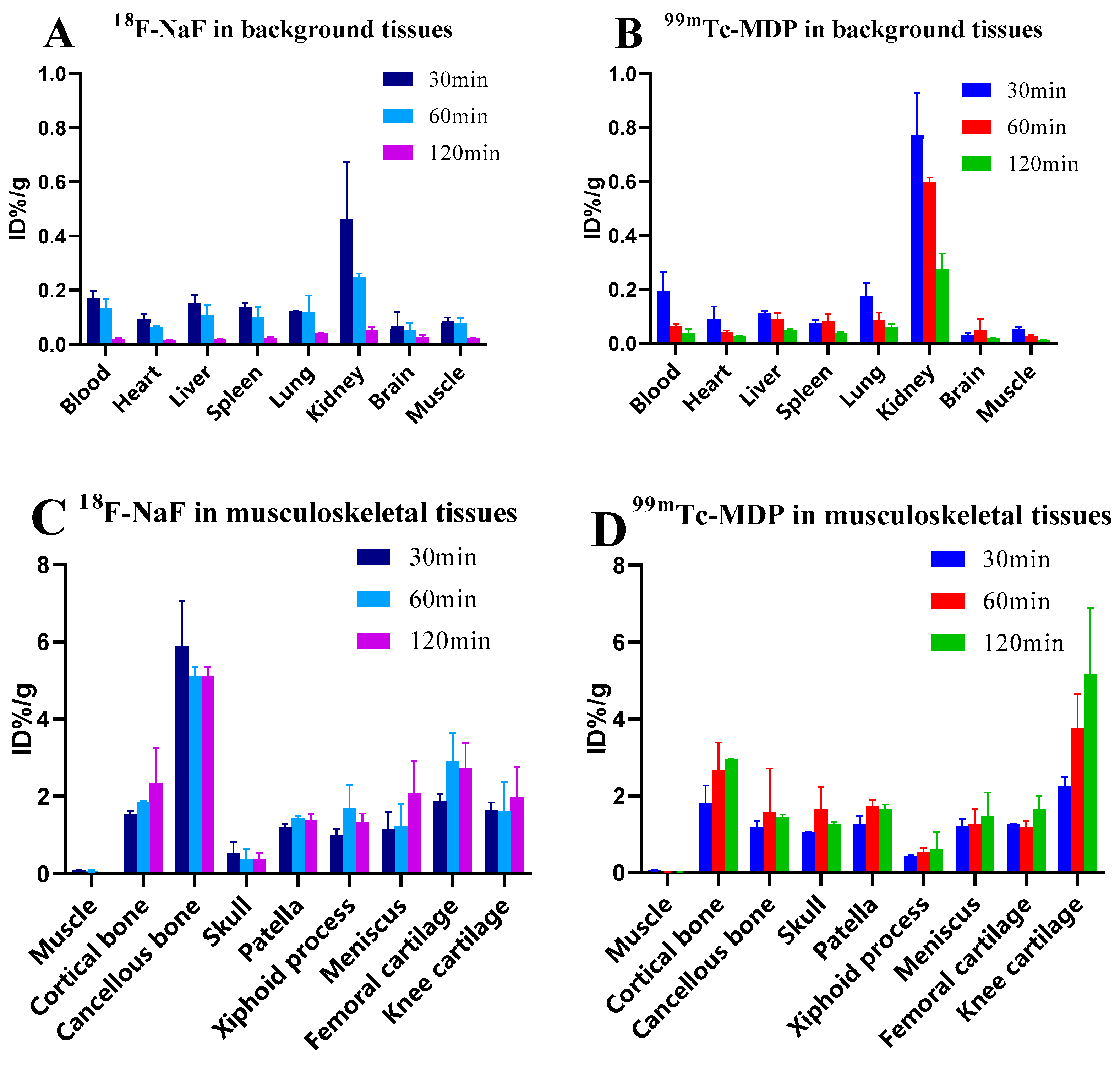

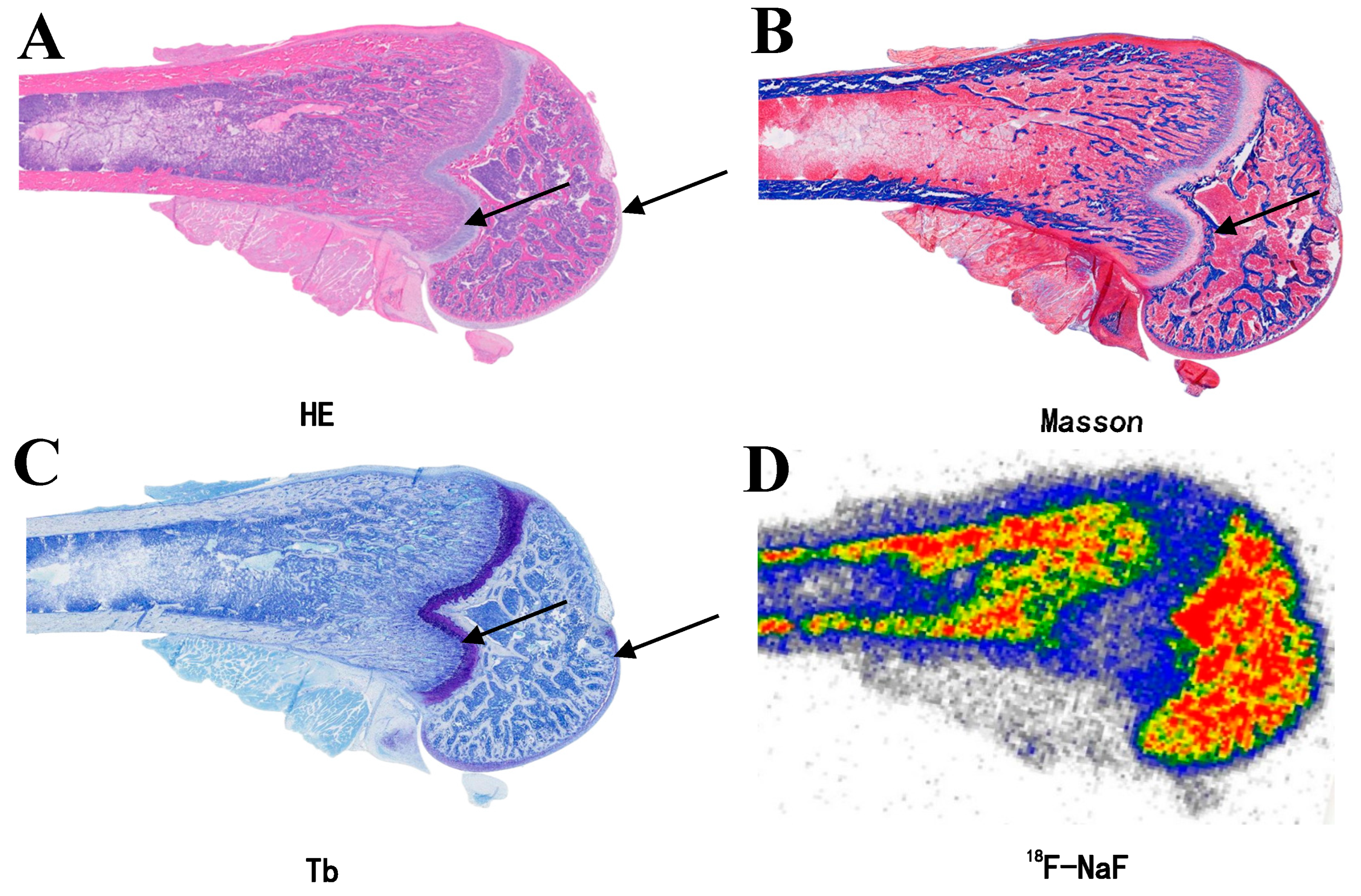


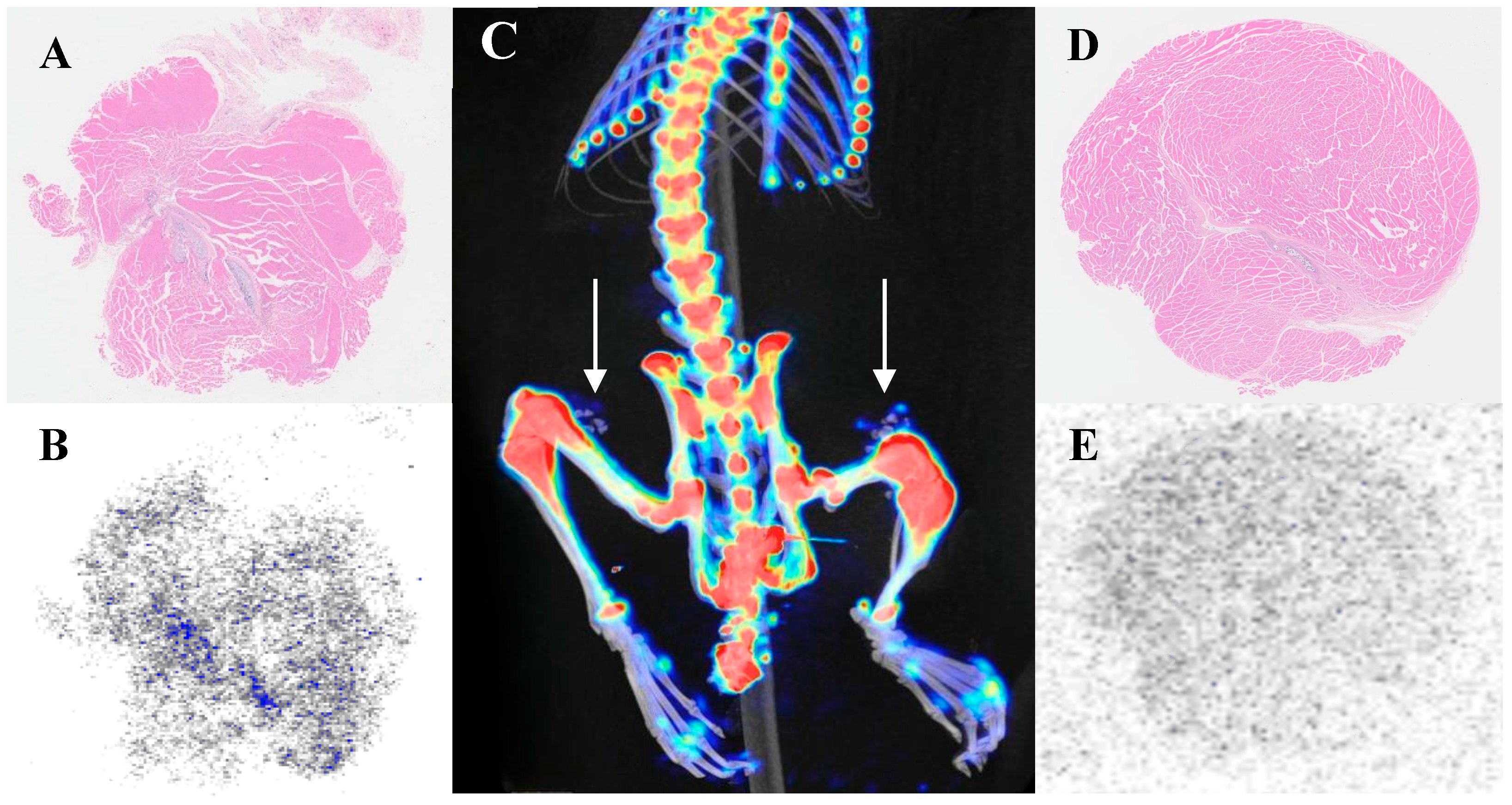
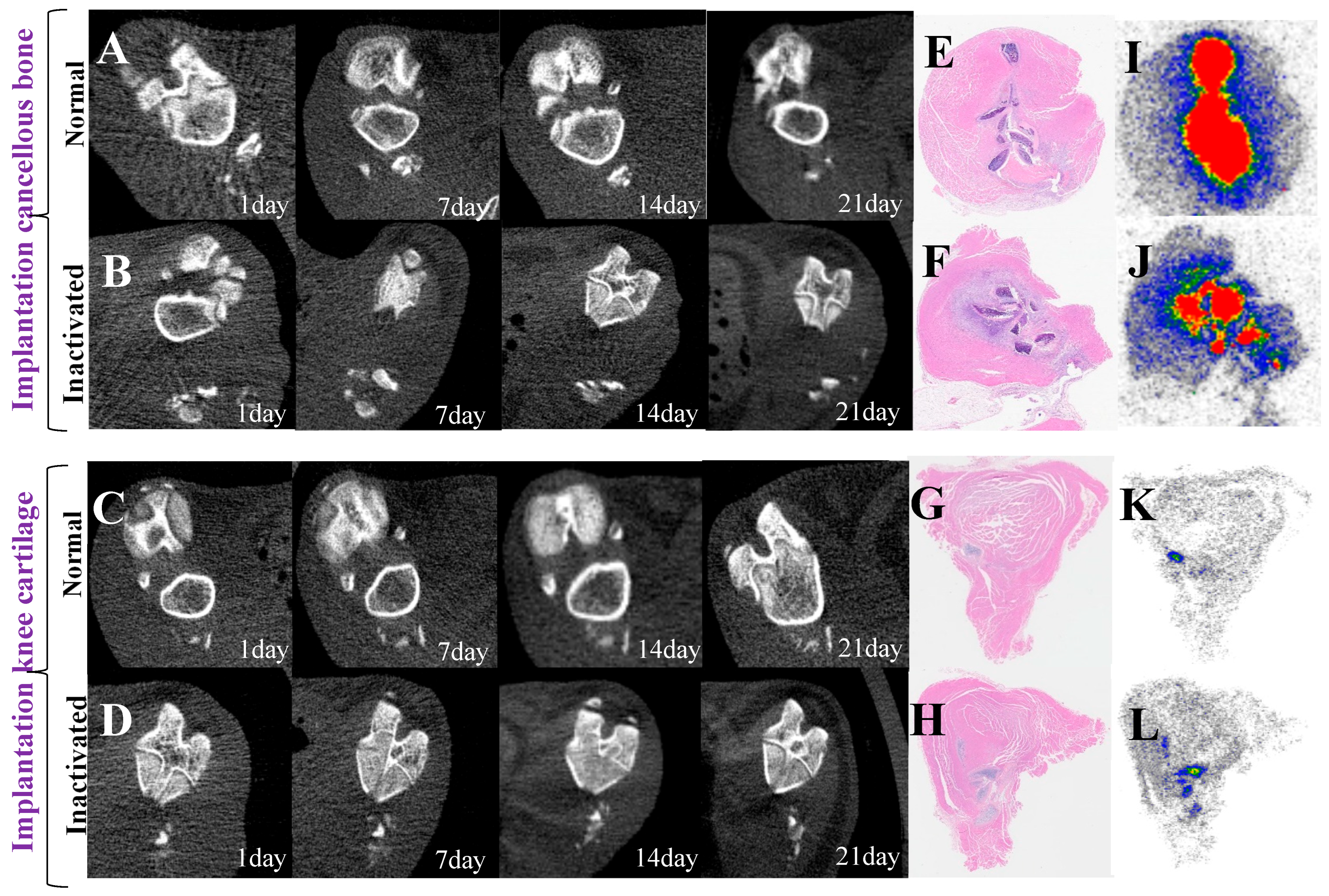

| Tissue Classification | 18F-NaF | 99mTc-MDP | t-Value | p-Value |
|---|---|---|---|---|
| Bone Tissue | ||||
| femoral condyle (Cancellous) | 5.11 ± 0.23 | 1.45 ± 0.07 | 31.48 | <0.001 |
| femoral cortex (Cortical) | 2.36 ± 0.90 | 2.95 ± 0.01 | −1.31 | 0.24 |
| skull (Flat bone) | 0.38 ± 0.16 | 1.27 ± 0.06 | −10.93 | <0.001 |
| patella (Sesamoid bone) | 1.38 ± 0.18 | 1.65 ± 0.12 | −2.83 | 0.028 |
| Cartilage Tissue | ||||
| Femoral cartilage (weight-bearing) | 2.75 ± 0.62 | 1.66 ± 0.35 | 2.93 | 0.023 |
| Knee cartilage (weight-bearing) | 1.99 ± 0.78 | 5.18 ± 1.71 | −3.39 | <0.001 |
| Meniscus (load-bearing area) | 2.08 ± 0.83 | 1.48 ± 0.61 | 1.34 | 0.230 |
| Xiphoid process (hyaline cartilage) | 1.33 ± 0.23 | 0.60 ± 0.46 | 3.71 | 0.010 |
| Background Tissue | ||||
| Blood (blood pool) | 0.020 ± 0.005 | 0.039 ± 0.014 | −2.70 | 0.035 |
| Liver (metabolism) | 0.019 ± 0.001 | 0.049 ± 0.004 | −15.81 | <0.001 |
| Spleen (reticuloendothelial) | 0.023 ± 0.005 | 0.039 ± 0.002 | −6.32 | <0.001 |
| Lung (capillaries) | 0.042 ± 0.001 | 0.061 ± 0.010 | −3.71 | 0.010 |
| Tissue Classification | Juvenile (4 Weeks) | Adult (4 Months) | Aged (14 Months) | F-Value | p-Value |
|---|---|---|---|---|---|
| Cortical bone | 20.07 ± 3.58 **** | 1.94 ± 0.77 | 1.98 ± 0.35 | 152.73 | <0.001 |
| Cancellous bone | 15.95 ± 2.67 *** | 0.91 ± 0.06 | 2.19 ± 0.99 ** | 98.56 | 0.009 |
| Knee cartilage | 2.84 ± 0.23 | 2.86 ± 0.56 | 3.87 ± 0.79 *** | 4.28 | 0.033 |
| Femoral cartilage | 0.82 ± 0.19 | 1.32 ± 0.37 ** | 1.25 ± 0.19 ** | 5.92 | 0.016 |
| Meniscus | 0.47 ± 0.20 | 0.63 ± 0.09 | 0.84 ± 0.44 | 3.1 | 0.075 |
| Patella | 0.14 ± 0.04 | 1.44 ± 0.60 ** | 1.46 ± 0.32 ** | 21.5 | <0.001 |
| Xiphoid cartilage | 0.10 ± 0.04 | 0.19 ± 0.05 | 0.15 ± 0.07 | 1.2 | 0.335 |
| Muscle | 0.043 ± 0.004 | 0.030 ± 0.0167 | 0.020 ± 0.010 | 4.1 | 0.055 |
| Tissue Type | Mean ± SD (%ID/g) | Target-to- Muscle Ratio | F-Value | p-Value | Post Hoc Comparisons |
|---|---|---|---|---|---|
| Cancellous Bone (Bone Tissue) | 5.11 ± 0.23 | 232.96:1 | 152.63 | < 0.0001 | Cancellous Bone vs. Knee Cartilage: p < 0.0001 |
| Knee Cartilage (Cartilage Tissue) | 1.99 ± 0.78 | 90.72:1 | Cancellous Bone vs. Muscle: p < 0.0001 | ||
| Quadriceps Muscle (Background Tissue) | 0.022 ± 0.002 | Knee Cartilage vs. Muscle: p < 0.0001 |
| Time | Group Comparison | Mean ± SD (CT Mean) | t-Value | p-Value |
|---|---|---|---|---|
| 1 day | Inactivated vs. Normal | 607.34 ± 35.55 vs. 613.97 ± 22.85 | −0.31 | 0.762 |
| 7 days | Inactivated vs. Normal | 578.15 ± 30.92 vs. 580.67 ± 27.38 | −0.15 | 0.886 |
| 14 days | Inactivated vs. Normal | 265.45 ± 45.11 vs. 408.01 ± 37.23 | −5.12 | <0.001 |
| 21 days | Inactivated vs. Normal | 238.26 ± 35.62 vs. 325.87 ± 32.71 | −4.89 | <0.001 |
| Time | Group Comparison | Mean ± SD (SUVmax) | t-Value | p-Value |
|---|---|---|---|---|
| 1 day | Inactivated vs. Normal | 3.43 ± 1.55 vs. 7.90 ± 0.71 | −5.72 | <0.001 |
| 7 days | Inactivated vs. Normal | 6.34 ± 1.89 vs. 8.07 ± 1.61 | −1.84 | 0.107 |
| 14 days | Inactivated vs. Normal | 4.77 ± 1.03 vs. 5.56 ± 0.65 | −1.65 | 0.142 |
| 21 days | Inactivated vs. Normal | 2.46 ± 1.13 vs. 3.09 ± 0.42 | −1.34 | 0.225 |
| Time | Group Comparison | Mean ± SD (CT Mean) | t-Value | p-Value |
|---|---|---|---|---|
| 1day | Inactivated vs. Normal | 167.82 ± 24.19 vs. 160.86 ± 19.37 | 0.54 | 0.606 |
| 7days | Inactivated vs. Normal | 182.67 ± 26.13 vs. 171.78 ± 25.12 | 0.79 | 0.457 |
| 14days | Inactivated vs. Normal | 232.41 ± 14.74 vs. 202.82 ± 16.38 | 3.41 | 0.014 |
| 21days | Inactivated vs. Normal | 239.34 ± 21.15 vs. 211.49 ± 13.80 | 2.64 | 0.032 |
| Time | Group Comparison | Mean ± SD (SUVmax) | t-Value | p-Value |
|---|---|---|---|---|
| 1 day | Inactivated vs. Normal | 2.22 ± 0.49 vs. 3.22 ± 0.41 | −3.52 | 0.008 |
| 7 days | Inactivated vs. Normal | 3.21 ± 0.49 vs. 4.62 ± 0.50 | −4.89 | 0.001 |
| 14 days | Inactivated vs. Normal | 2.55 ± 0.55 vs. 5.39 ± 1.04 | −5.67 | <0.001 |
| 21 days | Inactivated vs. Normal | 2.49 ± 0.44 vs. 3.72 ± 1.34 | −2.56 | 0.032 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Gao, J.; Wang, Y.; Song, Y.; Liu, L.; Zhang, C.; Li, M.; Dang, H.; Tian, J. Cartilage-Specific 18F-NaF Uptake in Rat Models: A Multimodal In Vitro and Ex Vitro Comparative Study with 99mTc-MDP. Biomedicines 2025, 13, 1540. https://doi.org/10.3390/biomedicines13071540
Li Q, Gao J, Wang Y, Song Y, Liu L, Zhang C, Li M, Dang H, Tian J. Cartilage-Specific 18F-NaF Uptake in Rat Models: A Multimodal In Vitro and Ex Vitro Comparative Study with 99mTc-MDP. Biomedicines. 2025; 13(7):1540. https://doi.org/10.3390/biomedicines13071540
Chicago/Turabian StyleLi, Qingxiao, Jianpeng Gao, Yiqun Wang, Yaoyao Song, Liwei Liu, Cong Zhang, Ming Li, Haodan Dang, and Jiahe Tian. 2025. "Cartilage-Specific 18F-NaF Uptake in Rat Models: A Multimodal In Vitro and Ex Vitro Comparative Study with 99mTc-MDP" Biomedicines 13, no. 7: 1540. https://doi.org/10.3390/biomedicines13071540
APA StyleLi, Q., Gao, J., Wang, Y., Song, Y., Liu, L., Zhang, C., Li, M., Dang, H., & Tian, J. (2025). Cartilage-Specific 18F-NaF Uptake in Rat Models: A Multimodal In Vitro and Ex Vitro Comparative Study with 99mTc-MDP. Biomedicines, 13(7), 1540. https://doi.org/10.3390/biomedicines13071540






