Salvage Pulmonary Resection After Immune Checkpoint or Tyrosine Kinase Inhibitor Therapy for Initially Unresectable Non-Small-Cell Lung Cancer: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
2.3. Information Sources and Search Strategy
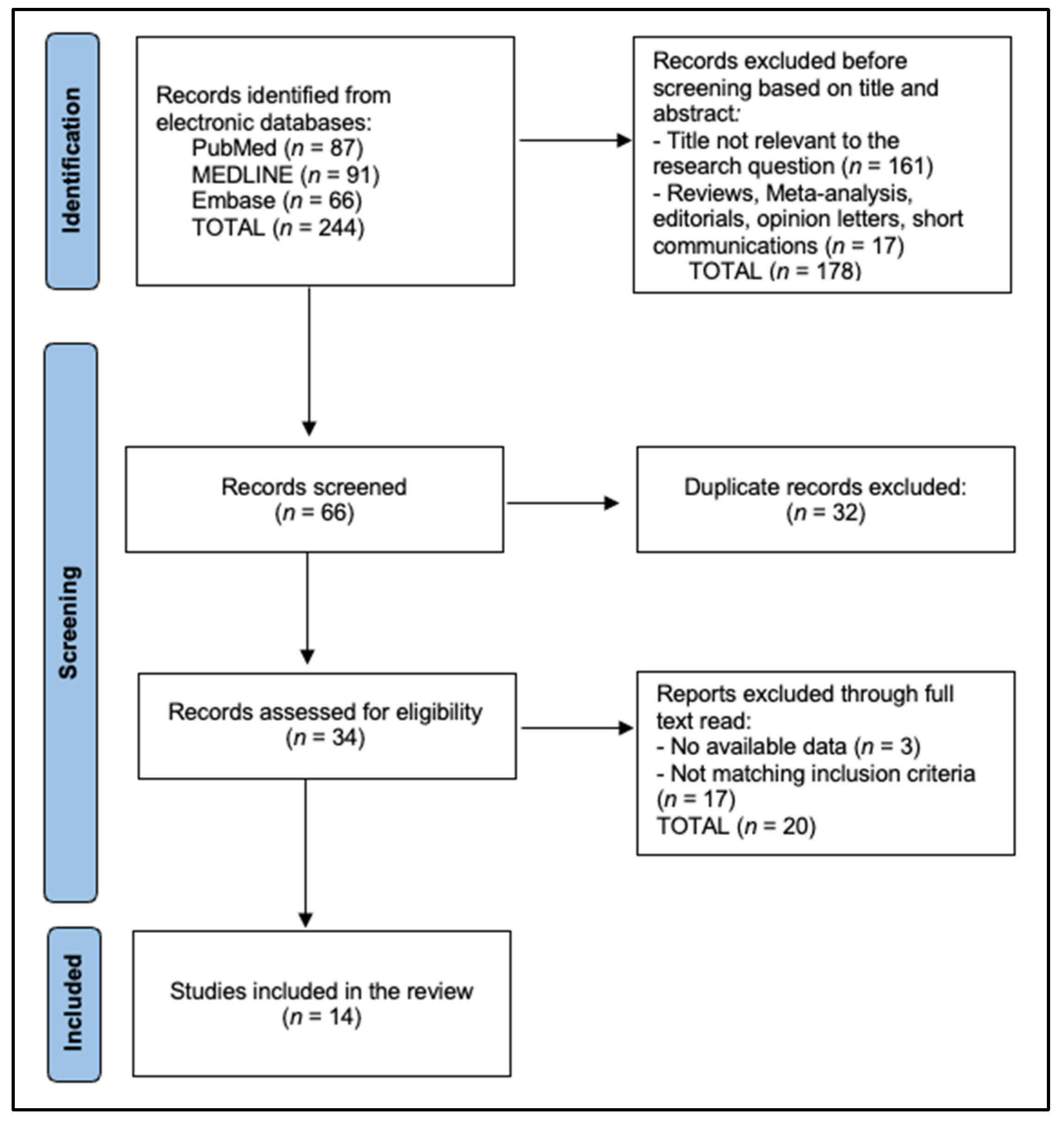
2.4. Study Selection, Data Extraction, and Risk-of-Bias
2.5. Statistical Synthesis and Heterogeneity
3. Results
4. Discussion
4.1. Summary of Evidence
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smith, A.; Wali, A.; Montes, A.; Hadaki, M.; Harrison-Phipps, K.; Karapanagiotou, E.M.; Bille, A. Salvage pulmonary resection in stages IIIb-IV lung cancer after treatment with immune checkpoint inhibitors case series and literature review. J. Surg. Oncol. 2022, 125, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Beattie, R.; Furrer, K.; Dolan, D.P.; Curioni-Fontecedro, A.; Lee, D.N.; Frauenfelder, T.; Hoeller, S.; Weder, W.; Bueno, R.; Opitz, I.; et al. Two centres experience of lung cancer resection in patients with advanced non-small cell lung cancer upon treatment with immune checkpoint inhibitors: Safety and clinical outcomes. Eur. J. Cardiothorac. Surg. 2021, 60, 1297–1305. [Google Scholar] [CrossRef] [PubMed]
- Ueno, T.; Yamashita, M.; Yamashita, N.; Uomoto, M.; Kawamata, O.; Sano, Y.; Inokawa, H.; Hirayama, S.; Okazaki, M.; Toyooka, S. Safety of salvage lung resection after immunotherapy for unresectable non-small cell lung cancer. Gen. Thorac. Cardiovasc. Surg. 2022, 70, 812–817. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, W.; Dudek, W.; Lettmaier, S.; Fietkau, R.; Sirbu, H. Should salvage surgery be considered for local recurrence after definitive chemoradiation in locally advanced non-small cell lung cancer? J. Cardiothorac. Surg. 2016, 11, 9. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Suzuki, S.; Asakura, K.; Okui, M.; Izawa, N.; Sawafuji, M.; Sakamaki, H.; Shigenobu, T.; Tajima, A.; Oka, N.; Masai, K.; et al. Prognostic factors affecting survival in patients with non-small cell lung cancer treated with salvage surgery after drug therapy: A multi-institutional retrospective study. World J. Surg. Oncol. 2023, 21, 290. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ohtaki, Y.; Shimizu, K.; Suzuki, H.; Suzuki, K.; Tsuboi, M.; Mitsudomi, T.; Takao, M.; Murakawa, T.; Ito, H.; Yoshimura, K.; et al. Salvage surgery for non-small cell lung cancer after tyrosine kinase inhibitor treatment. Lung Cancer 2021, 153, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, M.; Tanaka, F.; Kajiyama, K.; Manabe, T.; Yoshimatsu, K.; Mori, M.; Kanayama, M.; Taira, A.; Kuwata, T.; Nawata, A.; et al. Outcomes and pathologic response of primary lung cancer treated with tyrosine kinase inhibitor/immune checkpoint inhibitor before salvage surgery. Surg. Today 2024, 54, 1146–1153. [Google Scholar] [CrossRef] [PubMed]
- Mangiameli, G.; Giudici, V.M.; Brascia, D.; Voulaz, E.; Cariboni, U.; Toschi, L.; Alloisio, M.; Marulli, G. Anatomic lung resection after target therapy or immune checkpoint inhibitors treatment for initially unresectable advanced-staged non-small cell lung cancer: A case series. Updates Surg. 2024. [Google Scholar] [CrossRef] [PubMed]
- Bott, M.J.; Cools-Lartigue, J.; Tan, K.S.; Dycoco, J.; Bains, M.S.; Downey, R.J.; Huang, J.; Isbell, J.M.; Molena, D.; Park, B.J.; et al. Safety and Feasibility of Lung Resection After Immunotherapy for Metastatic or Unresectable Tumors. Ann. Thorac. Surg. 2018, 106, 178–183. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Tong, B.C.; Gu, L.; Wang, X.; Wigle, D.A.; Phillips, J.D.; Harpole, D.H., Jr.; Klapper, J.A.; Sporn, T.; Ready, N.E.; D’Amico, T.A. Perioperative outcomes of pulmonary resection after neoadjuvant pembrolizumab in patients with non-small cell lung cancer. J. Thorac. Cardiovasc. Surg. 2022, 163, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Bott, M.J.; Yang, S.C.; Park, B.J.; Adusumilli, P.S.; Rusch, V.W.; Isbell, J.M.; Downey, R.J.; Brahmer, J.R.; Battafarano, R.; Bush, E.; et al. Initial results of pulmonary resection after neoadjuvant nivolumab in patients with resectable non-small cell lung cancer. J. Thorac. Cardiovasc. Surg. 2019, 158, 269–276. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lücke, E.; Ganzert, C.; Föllner, S.; Wäsche, A.; Jechorek, D.; Schoeder, V.; Walles, T.; Genseke, P.; Schreiber, J. Operability and Pathological Response of Non-Small Cell Lung Cancer (NSCLC) after Neoadjuvant Therapy with Immune Checkpoint Inhibition. Pneumologie 2020, 74, 766–772. (In German) [Google Scholar] [CrossRef] [PubMed]
- Sonett, J.R.; Suntharalingam, M.; Edelman, M.J.; Patel, A.B.; Gamliel, Z.; Doyle, A.; Hausner, P.; Krasna, M. Pulmonary resection after curative intent radiotherapy (>59 Gy) and concurrent chemotherapy in non-small-cell lung cancer. Ann. Thorac. Surg. 2004, 78, 1200–1205; discussion 1206. [Google Scholar] [CrossRef] [PubMed]
- Lococo, F.; Cancellieri, A.; Chiappetta, M.; Leonetti, A.; Cardillo, G.; Zanelli, F.; Mangiameli, G.; Toschi, L.; Guggino, G.; Romano, F.J.; et al. Salvage Surgery After First-Line Alectinib for Locally-Advanced/Metastatic ALK-Rearranged NSCLC: Pathological Response and Perioperative Results. Clin. Lung Cancer 2023, 24, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Hamaji, M.; Ozasa, H.; Sakamori, Y.; Terada, K.; Yoshizawa, A.; Kikuchi, R.; Sakaguchi, Y.; Sonobe, M.; Muranishi, Y.; Miyahara, R.; et al. Characteristics and outcomes of salvage surgery after immune checkpoint inhibitor therapy for initially unresectable non-small cell lung cancer. J. Thorac. Dis. 2024, 16, 6094–6100. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Galetta, D.; De Marinis, F.; Spaggiari, L. Rescue Surgery after Immunotherapy/Tyrosine Kinase Inhibitors for Initially Unresectable Lung Cancer. Cancers 2022, 14, 2661. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schiavon, M.; Cannone, G.; Bertolaccini, L.; Gallina, F.T.; Pezzuto, F.; Lorenzoni, G.; Facciolo, F.; Spaggiari, L.; Calabrese, F.; Rea, F.; et al. Lung Oncology Group. Safety and Efficacy of Salvage Surgery after Treatment with Immune-Checkpoint Adjuvant Inhibitors for Advanced Non-Small Cell Lung Cancer: A Multicentric Study. J. Surg. Oncol. 2025, 131, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Antonoff, M.B.; Correa, A.M.; Sepesi, B.; Nguyen, Q.N.; Walsh, G.L.; Swisher, S.G.; Vaporciyan, A.A.; Mehran, R.J.; Hofstetter, W.L.; Rice, D.C. Salvage pulmonary resection after stereotactic body radiotherapy: A feasible and safe option for local failure in selected patients. J. Thorac. Cardiovasc. Surg. 2017, 154, 689–699. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Funaki, S.; Nagata, H.; Furukawa, M.; Morii, E.; Shintani, Y. Salvage surgery following tyrosine kinase inhibitor treatment for advanced non-small cell lung cancer. Surg. Case Rep. 2024, 10, 153. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dunne, E.G.; Fick, C.N.; Tan, K.S.; Toumbacaris, N.; Mastrogiacomo, B.; Adusumilli, P.S.; Rocco, G.; Molena, D.; Huang, J.; Park, B.J.; et al. Lung resection after initial nonoperative treatment for non-small cell lung cancer. J. Thorac. Cardiovasc. Surg. 2024, 168, 364–373.e10. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Nemeth, A.; Canavan, M.E.; Zhan, P.L.; Udelsman, B.V.; Ely, S.; Wigle, D.A.; Martin, L.; Jeffrey Yang, C.F.; Boffa, D.J.; Dhanasopon, A.P. Salvage lung resection after immunotherapy is feasible and safe. JTCVS Open 2024, 20, 141–150. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Goto, E.; Hattori, A.; Fukui, M.; Matsunaga, T.; Takamochi, K.; Suzuki, K. Salvage extended surgery after immune-checkpoint inhibitor treatment for advanced non-small cell lung cancer. Surg. Today 2024, 54, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Guerrera, F.; Lococo, F.; Fournel, L.; Viggiano, D.; Mangiameli, G.; Mastromarino, M.G.; Guggino, G.; Seitlinger, J.; Bertoglio, P.; Luzzi, L.; et al. The outcomes of salvage surgery for non-small cell lung cancer after immune checkpoint inhibitor or targeted therapy treatment. A multi-center international real-life study. Eur. J. Surg. Oncol. 2025, 51, 109592. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, N.; Tanaka, Y.; Tachihara, M.; Maniwa, Y. ROS1-Rearranged Lung Cancer Successfully Resected after Response to Crizotinib: A Case Report. Kobe J. Med. Sci. 2022, 67, E143–E145. [Google Scholar]
- Forde, P.M.; Spicer, J.; Lu, S.; Awad, M.M.; Girard, N.; Wang, C.; Mitsudomi, T.; Felip, E.; Broderick, S.R.; Swanson, S.J.; et al. Neoadjuvant nivolumab plus chemotherapy in resectable lung cancer. N. Engl. J. Med. 2022, 386, 1973–1985. [Google Scholar] [CrossRef]
- Wakelee, H.; Liberman, M.; Kato, T.; Tsuboi, M.; Lee, S.-H.; Gao, S.; Chen, K.-N.; Dooms, C.; Majem, M.; Eigendorff, E.; et al. KEYNOTE-671 Investigators. Perioperative pembrolizumab for early-stage non-small-cell lung cancer. N. Engl. J. Med. 2023, 389, 491–503. [Google Scholar]
- Provencio, M.; Nadal, E.; González-Larriba, J.L.; Martínez-Martí, A.; Bernabé, R.; Bosch-Barrera, J.; Casal-Rubio, J.; Calvo, V.; Insa, A.; Ponce, S.; et al. Perioperative nivolumab and chemotherapy in stage III non-small-cell lung cancer (NADIM II). N. Engl. J. Med. 2023, 389, 504–513. [Google Scholar]
- Afshari, S.B.; Anker, C.J.; Kooperkamp, H.Z.; Sprague, B.L.; Lester-Coll, N.H. Trends and outcomes of salvage lobectomy for early-stage non-small cell lung cancer. Am. J. Clin. Oncol. 2023, 46, 271–275. [Google Scholar] [CrossRef]
- Heymach, J.V.; Harpole, D.; Mitsudomi, T.; Taube, J.M.; Galffy, G.; Hochmair, M.; Winder, T.; Zukov, R.; Garbaos, G.; Gao, S.; et al. AEGEAN Investigators. Perioperative durvalumab for resectable non-small-cell lung cancer. N. Engl. J. Med. 2023, 389, 1672–1684. [Google Scholar]
- Waser, N.A.; Quintana, M.; Schweikert, B.; Chaft, J.E.; Berry, L.; Adam, A.; Vo, L.; Penrod, J.R.; Fiore, J.; Berry, D.A.; et al. Pathological response in resectable non-small cell lung cancer: A systematic literature review and meta-analysis. JNCI Cancer Spectr. 2024, 8, pkae021. [Google Scholar] [CrossRef]
| # | First Author (Year) | Country | Systemic Modality | n | Stage at Baseline | Minimally Invasive (%) | R0 Resection (%) | Pathologic CR (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | Ueno 2022 [3] | JP | PD-1/PD-L1 ICI | 11 | IIIb–IV | 27 | 91 | 18 |
| 2 | Goto 2024 [22] | JP | ICI (mixed) | 14 | IIIb–IV | 50 | 93 | 29 |
| 3 | Beattie 2021 [2] | US/CH | ICI (mixed) | 27 | IIIb–IV | 41 | 96 | 19 |
| 4 | Suzuki 2023 [5] | JP | ICI ± TKI | 86 | III–IV | 38 | 92 | 26 |
| 5 | Ohtaki 2021 [6] | JP | EGFR/ALK TKI | 36 | IV | 22 | 95 | 17 |
| 6 | Takenaka 2024 [7] | JP | ICI ± TKI | 18 | IIIb–IV | 44 | 94 | 33 |
| 7 | Mangiameli 2024 [8] | IT | ICI/TKI | 12 | III–IV | 58 | 92 | 25 |
| 8 | Bott 2019 [9] | US | Nivolumab | 20 | IV/oligoprog. | 35 | 100 | 43 |
| 9 | Tong 2022 [10] | US | Pembrolizumab | 25 | IB–IIIA | 40 | 96 | 40 |
| 10 | Lücke 2020 [12] | DE | ICI (mixed) | 10 | III–IV | 60 | 90 | 30 |
| 11 | Lococo 2023 [14] | IT | Alectinib | 10 | IIIB–IVB | 40 | 90 | 50 |
| 12 | Nemeth 2024 [21] | DE/US | ICI (mixed) | 24 | III–IV | 46 | 94 | 28 |
| 13 | Sano 2023 [15] | JP | ICI (PD-1) | 8 | IIIb–IV | 30 | 88 | 25 |
| 14 | Smith 2022 [1] | UK | Pembrolizumab | 9 | IIIb–IV | 56 | 89 | 22 |
| # | First Author (Year) | Operative Time (min, median) | Blood Loss (mL, median) | Major Complication ≥ III (%) | 30-Day Mortality (%) | LOS (Days, median) |
|---|---|---|---|---|---|---|
| 1 | Ueno 2022 [3] | 210 | 320 | 9 | 0 | 8 |
| 2 | Goto 2024 [22] | 280 | 400 | 14 | 0 | 9 |
| 3 | Beattie 2021 [2] | 195 | 290 | 11 | 0 | 6 |
| 4 | Suzuki 2023 [5] | 225 | 310 | 10 | 1.2 | 7 |
| 5 | Ohtaki 2021 [6] | 215 | 330 | 12 | 0 | 7 |
| 6 | Takenaka 2024 [7] | 200 | 270 | 8 | 0 | 6 |
| 7 | Mangiameli 2024 [8] | 165 | 180 | 7 | 0 | 5 |
| 8 | Bott 2019 [9] | 260 | 350 | 15 | 0 | 7 |
| 9 | Tong 2022 [10] | 180 | 250 | 9 | 0 | 5 |
| 10 | Lücke 2020 [12] | 170 | 210 | 10 | 0 | 4 |
| 11 | Lococo 2023 [14] | 205 | 280 | 13 | 0 | 6 |
| 12 | Nemeth 2024 [21] | 190 | 260 | 11 | 0 | 6 |
| 13 | Sano 2023 [15] | 220 | 340 | 10 | 0 | 8 |
| 14 | Smith 2022 [1] | 175 | 240 | 22 | 0 | 9 |
| # | First Author (Year) | Nodal Down-Staging (%) | 1-Year DFS (%) | 1-Year OS (%) |
|---|---|---|---|---|
| 1 | Ueno 2022 [3] | 45 | 67 | 85 |
| 2 | Goto 2024 [22] | 38 | 70 | 88 |
| 3 | Beattie 2021 [2] | 42 | 69 | 87 |
| 4 | Suzuki 2023 [5] | 40 | 66 | 86 |
| 5 | Ohtaki 2021 [6] | 36 | 62 | 82 |
| 6 | Takenaka 2024 [7] | 44 | 71 | 90 |
| 7 | Mangiameli 2024 [8] | 41 | 68 | 89 |
| 8 | Bott 2019 [9] | 55 | 72 | 92 |
| 9 | Tong 2022 [10] | 50 | 75 | 93 |
| 10 | Lücke 2020 [12] | 48 | 70 | 90 |
| 11 | Lococo 2023 [14] | 35 | 60 | 84 |
| 12 | Nemeth 2024 [21] | 39 | 65 | 87 |
| 13 | Sano 2023 [15] | 37 | 63 | 86 |
| 14 | Smith 2022 [1] | 33 | 61 | 84 |
| # | First Author (Year) | Systemic Duration (mo, Median) | Cycles/Lines (Median) | Therapy Line (1st/2nd) | Best RECIST | Interval Last-Dose → Surgery (wk) | Post-op Systemic (%) |
|---|---|---|---|---|---|---|---|
| 1 | Ueno 2022 [3] | 6.4 | 11 | 11/0 | PR | 6 | 36 |
| 2 | Goto 2024 [22] | 8.9 | 16 | 14/0 | PR | 4 | 42 |
| 3 | Beattie 2021 [2] | 7.8 | 13 | 24/3 | PR | 5 | 38 |
| 4 | Suzuki 2023 [5] | 10.3 | 14 | 74/12 | PR | 6 | 41 |
| 5 | Ohtaki 2021 [6] | 7.6 | 12 | 31/5 | PR | 5 | 43 |
| 6 | Takenaka 2024 [7] | 6.1 | 9 | 17/1 | PR | 4 | 39 |
| 7 | Mangiameli 2024 [8] | 5.7 | 8 | 11/1 | PR | 6 | 37 |
| 8 | Bott 2019 [9] | 8.3 | 11 | 0/20 | PR | 4 | 47 |
| 9 | Tong 2022 [10] | 6.2 | 9 | 25/0 | PR | 5 | 44 |
| 10 | Lücke 2020 [12] | 5.9 | 8 | 9/1 | PR | 6 | 49 |
| 11 | Lococo 2023 [14] | 7.1 | 12 | 9/1 | PR | 5 | 46 |
| 12 | Nemeth 2024 [21] | 9.4 | 17 | 0/24 | PR | 4 | 52 |
| 13 | Sano 2023 [15] | 8.2 | 11 | 7/1 | PR | 6 | 42 |
| 14 | Smith 2022 [1] | 7.2 | 12 | 8/1 | PR | 5 | 44 |
| Outcome | Pooled Mean or Proportion (95% CI) | Between-Study I2 |
|---|---|---|
| Operative Time (min) | 182 (155–211) | 58% |
| Blood Loss (mL) | 268 (190–352) | 62% |
| Major Complication (CD ≥ III) | 11% (7–16%) | 21% |
| Prolonged Air-Leak > 5 d | 9% (5–14%) | 18% |
| Median LOS (days) | 7.2 (5–10) | N/A |
| Dataset | Endpoint | Pooled Estimate (95% CI) |
|---|---|---|
| All Populations | R0 Resection | 93% (89–97%) |
| Pathologic Nodal Down-Staging | 41% (31–52%) | |
| 1-Year DFS | 68% (60–75%) | |
| 1-Year OS | 88% (81–93%) | |
| 2-Year OS | 76% (65–85%) | |
| ICI Subgroup (n = 222) | R0 Resection | 94% (90–97%) |
| pCR | 25% (18–32%) | |
| 1-Year DFS | 69% (61–76%) | |
| 1-Year OS | 89% (82–94%) | |
| 2-Year OS | 78% (66–86%) | |
| TKI Subgroup (n = 90) | R0 Resection | 91% (84–96%) |
| pCR | 32% (18–48%) | |
| 1-Year DFS | 65% (52–76%) | |
| 1-Year OS | 85% (75–92%) | |
| 2-Year OS | 72% (56–83%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaborean, V.; Feier, C.V.I.; Vonica, R.C.; Faur, A.M.; Rus, V.I.; Muntean, C. Salvage Pulmonary Resection After Immune Checkpoint or Tyrosine Kinase Inhibitor Therapy for Initially Unresectable Non-Small-Cell Lung Cancer: A Systematic Review. Biomedicines 2025, 13, 1541. https://doi.org/10.3390/biomedicines13071541
Gaborean V, Feier CVI, Vonica RC, Faur AM, Rus VI, Muntean C. Salvage Pulmonary Resection After Immune Checkpoint or Tyrosine Kinase Inhibitor Therapy for Initially Unresectable Non-Small-Cell Lung Cancer: A Systematic Review. Biomedicines. 2025; 13(7):1541. https://doi.org/10.3390/biomedicines13071541
Chicago/Turabian StyleGaborean, Vasile, Catalin Vladut Ionut Feier, Razvan Constantin Vonica, Alaviana Monique Faur, Vladut Iosif Rus, and Calin Muntean. 2025. "Salvage Pulmonary Resection After Immune Checkpoint or Tyrosine Kinase Inhibitor Therapy for Initially Unresectable Non-Small-Cell Lung Cancer: A Systematic Review" Biomedicines 13, no. 7: 1541. https://doi.org/10.3390/biomedicines13071541
APA StyleGaborean, V., Feier, C. V. I., Vonica, R. C., Faur, A. M., Rus, V. I., & Muntean, C. (2025). Salvage Pulmonary Resection After Immune Checkpoint or Tyrosine Kinase Inhibitor Therapy for Initially Unresectable Non-Small-Cell Lung Cancer: A Systematic Review. Biomedicines, 13(7), 1541. https://doi.org/10.3390/biomedicines13071541








