Combined Effects of Obstructive Sleep Apnea and Sleep Duration on Hypertension in Korean Adults: A Nationwide Study
Abstract
1. Introduction
2. Methods
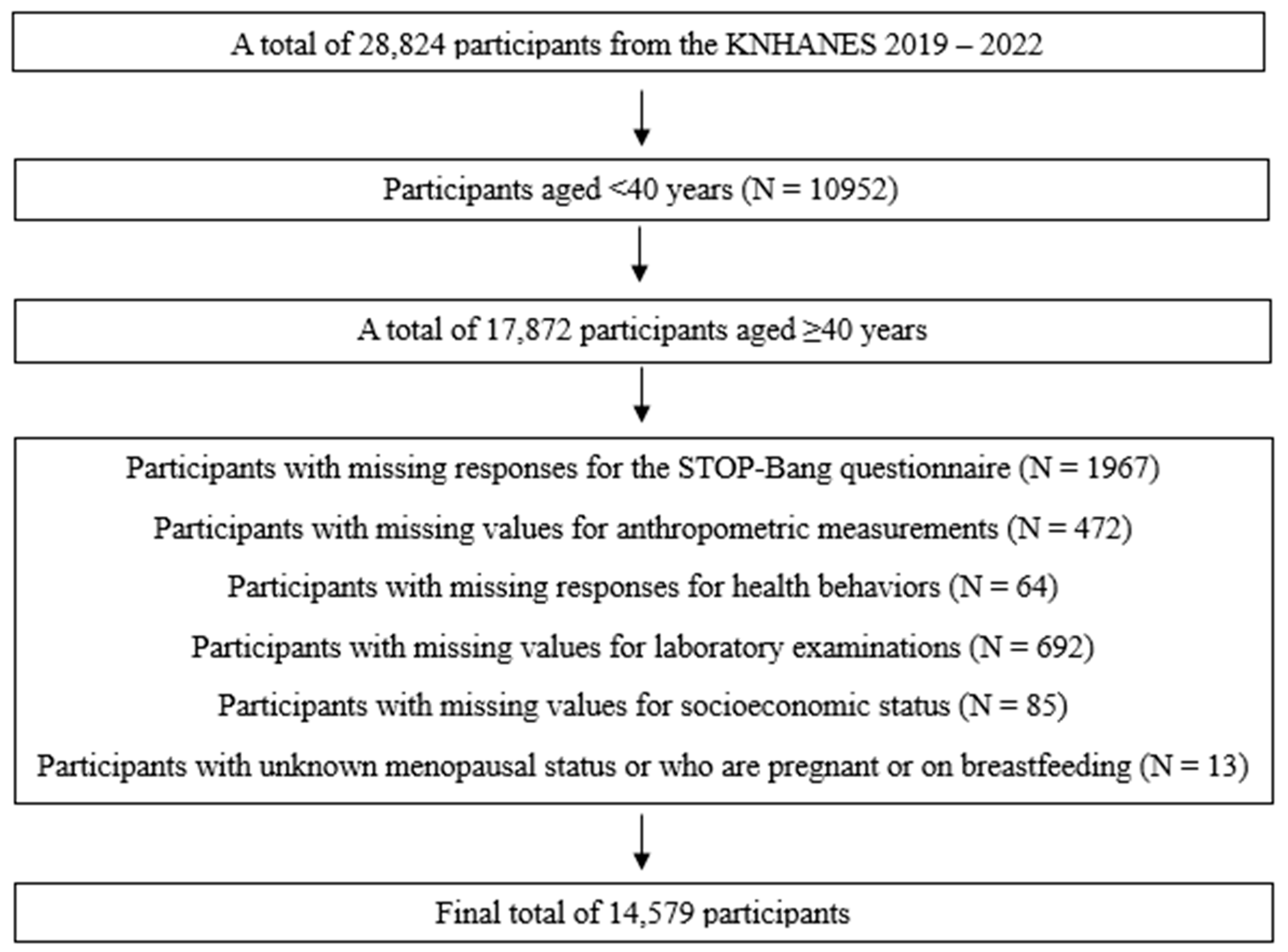
2.1. Study Participants
2.2. Measurements of OSA Risk and Sleep Duration
2.3. Measurements and Classifications of Blood Pressure
2.4. Covariates
2.5. Statistical Analysis
3. Results
3.1. Blood Pressure Status According to the Basic Characteristics of the Study Participants
3.2. Blood Pressure Status According to OSA Risk and Sleep Duration
3.3. Anthropometric and Laboratory Variables According to OSA Risk
3.4. Independent and Combined Effects of OSA Risk and Sleep Duration with Hypertension
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kario, K.; Okura, A.; Hoshide, S.; Mogi, M. The WHO Global report 2023 on hypertension warning the emerging hypertension burden in globe and its treatment strategy. Hypertens. Res. 2024, 47, 1099–1102. [Google Scholar] [CrossRef] [PubMed]
- The Korean Society of Hypertension. Korea Hypertension Fact Sheet 2023. Available online: https://www.koreanhypertension.org/reference/guide?mode=read&idno=10178 (accessed on 1 April 2025).
- Pedrosa, R.P.; Drager, L.F.; Gonzaga, C.C.; Sousa, M.G.; de Paula, L.K.; Amaro, A.C.; Amodeo, C.; Bortolotto, L.A.; Krieger, E.M.; Bradley, T.D.; et al. Obstructive sleep apnea: The most common secondary cause of hypertension associated with resistant hypertension. Hypertension 2011, 58, 811–817. [Google Scholar] [CrossRef] [PubMed]
- Marin, J.M.; Agusti, A.; Villar, I.; Forner, M.; Nieto, D.; Carrizo, S.J.; Barbé, F.; Vicente, E.; Wei, Y.; Nieto, F.J.; et al. Association between treated and untreated obstructive sleep apnea and risk of hypertension. JAMA 2012, 307, 2169–2176. [Google Scholar] [CrossRef]
- Peppard, P.E.; Young, T.; Palta, M.; Skatrud, J. Prospective study of the association between sleep-disordered breathing and hypertension. N. Engl. J. Med. 2000, 342, 1378–1384. [Google Scholar] [CrossRef] [PubMed]
- Huh, G.; Han, K.D.; Park, Y.M.; Park, C.S.; Lee, K.N.; Lee, E.Y.; Cho, J.H. Comorbidities associated with high-risk obstructive sleep apnea based on the STOP-BANG questionnaire: A nationwide population-based study. Korean J. Intern. Med. 2023, 38, 80–92. [Google Scholar] [CrossRef]
- Kim, T.; Kang, J. Relationship between obstructive sleep apnea, insulin resistance, and metabolic syndrome: A nationwide population-based survey. Endocr. J. 2023, 70, 107–119. [Google Scholar] [CrossRef]
- Cano-Pumarega, I.; Durán-Cantolla, J.; Aizpuru, F.; Miranda-Serrano, E.; Rubio, R.; Martínez-Null, C.; de Miguel, J.; Egea, C.; Cancelo, L.; Alvarez, A.; et al. Obstructive sleep apnea and systemic hypertension: Longitudinal study in the general population: The Vitoria Sleep Cohort. Am. J. Respir. Crit. Care Med. 2011, 184, 1299–1304. [Google Scholar] [CrossRef]
- Ren, R.; Covassin, N.; Yang, L.; Li, Y.; Zhang, Y.; Zhou, J.; Tan, L.; Li, T.; Li, X.; Wang, Y.; et al. Objective but Not Subjective Short Sleep Duration Is Associated With Hypertension in Obstructive Sleep Apnea. Hypertension 2018, 72, 610–617. [Google Scholar] [CrossRef]
- Willoughby, A.R.; Alikhani, I.; Karsikas, M.; Chua, X.Y.; Chee, M.W.L. Country differences in nocturnal sleep variability: Observations from a large-scale, long-term sleep wearable study. Sleep. Med. 2023, 110, 155–165. [Google Scholar] [CrossRef]
- Lusardi, P.; Zoppi, A.; Preti, P.; Pesce, R.M.; Piazza, E.; Fogari, R. Effects of insufficient sleep on blood pressure in hypertensive patients: A 24-h study. Am. J. Hypertens. 1999, 12 Pt 1, 63–68. [Google Scholar] [CrossRef]
- Guo, X.; Zheng, L.; Wang, J.; Zhang, X.; Zhang, X.; Li, J.; Sun, Y. Epidemiological evidence for the link between sleep duration and high blood pressure: A systematic review and meta-analysis. Sleep. Med. 2013, 14, 324–332. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Lee, S.K.; Kim, S.H.; Yun, C.H.; Kim, J.H.; Thomas, R.J.; Shin, C. Genetic association of short sleep duration with hypertension incidence--a 6-year follow-up in the Korean genome and epidemiology study. Circ. J. 2012, 76, 907–913. [Google Scholar] [CrossRef]
- Min, H.; Um, Y.J.; Jang, B.S.; Shin, D.; Choi, E.; Park, S.M.; Lee, K. Association between Sleep Duration and Measurable Cardiometabolic Risk Factors in Healthy Korean Women: The Fourth and Fifth Korean National Health and Nutrition Examination Surveys (KNHANES IV and V). Int. J. Endocrinol. 2016, 2016, 3784210. [Google Scholar] [CrossRef]
- Xu, H.; Liang, C.; Zou, J.; Yi, H.; Guan, J.; Gu, M.; Feng, Y.; Yin, S. Interaction between obstructive sleep apnea and short sleep duration on insulin resistance: A large-scale study: OSA, short sleep duration and insulin resistance. Respir. Res. 2020, 21, 151. [Google Scholar] [CrossRef]
- Priou, P.; Le Vaillant, M.; Meslier, N.; Paris, A.; Pigeanne, T.; Nguyen, X.L.; Alizon, C.; Bizieux-Thaminy, A.; Leclair-Visonneau, L.; Humeau, M.P.; et al. Cumulative association of obstructive sleep apnea severity and short sleep duration with the risk for hypertension. PLoS ONE 2014, 9, e115666. [Google Scholar] [CrossRef] [PubMed]
- Drager, L.F.; Santos, R.B.; Silva, W.A.; Parise, B.K.; Giatti, S.; Aielo, A.N.; Souza, S.P.; Furlan, S.F.; Lorenzi-Filho, G.; Lotufo, P.A.; et al. OSA, Short Sleep Duration, and Their Interactions With Sleepiness and Cardiometabolic Risk Factors in Adults: The ELSA-Brasil Study. Chest 2019, 155, 1190–1198. [Google Scholar] [CrossRef]
- Kweon, S.; Kim, Y.; Jang, M.J.; Kim, Y.; Kim, K.; Choi, S.; Chun, C.; Khang, Y.H.; Oh, K. Data resource profile: The Korea National Health and Nutrition Examination Survey (KNHANES). Int. J. Epidemiol. 2014, 43, 69–77. [Google Scholar] [CrossRef]
- Jeon, H.J.; Bang, Y.R.; Yoon, I.Y. A validation study on three screening questionnaires for obstructive sleep apnea in a Korean community sample. Sleep Breath. 2019, 23, 969–977. [Google Scholar] [CrossRef] [PubMed]
- Henríquez-Beltrán, M.; Dreyse, J.; Jorquera, J.; Jorquera-Diaz, J.; Salas, C.; Fernandez-Bussy, I.; Labarca, G. The U-Shaped Association between Sleep Duration, All-Cause Mortality and Cardiovascular Risk in a Hispanic/Latino Clinically Based Cohort. J. Clin. Med. 2023, 12, 4961. [Google Scholar] [CrossRef]
- The Korean Society of Hypertension. 2022 Hypertension Guideline. Available online: https://drive.google.com/file/d/1EhSQ-zqGIBsBRTjXgJO4Xym_9viky1jy/view (accessed on 1 April 2025).
- Korean Standard Statistical Classification Portal. The Korean Standard Classification of Occupations (KSCO). Available online: https://kssc.kostat.go.kr (accessed on 1 April 2025).
- Hwang, S. Estimation of High-Risk Drinkers and Drinking Behavior in Korea. J. Environ. Health Sci. 2020, 46, 65–77. [Google Scholar]
- Chung, F.; Subramanyam, R.; Liao, P.; Sasaki, E.; Shapiro, C.; Sun, Y. High STOP-Bang score indicates a high probability of obstructive sleep apnoea. Br. J. Anaesth. 2012, 108, 768–775. [Google Scholar] [CrossRef] [PubMed]
- Chung, F.; Yegneswaran, B.; Liao, P.; Chung, S.A.; Vairavanathan, S.; Islam, S.; Khajehdehi, A.; Shapiro, C.M. STOP questionnaire: A tool to screen patients for obstructive sleep apnea. Anesthesiology 2008, 108, 812–821. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Pivetta, B.; Nagappa, M.; Saripella, A.; Islam, S.; Englesakis, M.; Chung, F. Validation of the STOP-Bang questionnaire for screening of obstructive sleep apnea in the general population and commercial drivers: A systematic review and meta-analysis. Sleep Breath. 2021, 25, 1741–1751. [Google Scholar] [CrossRef] [PubMed]
- Lam, B.; Ip, M.S.; Tench, E.; Ryan, C.F. Craniofacial profile in Asian and white subjects with obstructive sleep apnoea. Thorax 2005, 60, 504–510. [Google Scholar] [CrossRef]
- Lee, R.W.; Vasudavan, S.; Hui, D.S.; Prvan, T.; Petocz, P.; Darendeliler, M.A.; Cistulli, P.A. Differences in craniofacial structures and obesity in Caucasian and Chinese patients with obstructive sleep apnea. Sleep 2010, 33, 1075–1080. [Google Scholar] [CrossRef]
- Hou, H.; Zhao, Y.; Yu, W.; Dong, H.; Xue, X.; Ding, J.; Xing, W.; Wang, W. Association of obstructive sleep apnea with hypertension: A systematic review and meta-analysis. J. Glob. Health 2018, 8, 010405. [Google Scholar] [CrossRef]
- Shiina, K. Obstructive sleep apnea -related hypertension: A review of the literature and clinical management strategy. Hypertens. Res. 2024, 47, 3085–3098. [Google Scholar] [CrossRef]
- Maniaci, A.; Lavalle, S.; Parisi, F.M.; Barbanti, M.; Cocuzza, S.; Iannella, G.; Magliulo, G.; Pace, A.; Lentini, M.; Masiello, E.; et al. Impact of Obstructive Sleep Apnea and Sympathetic Nervous System on Cardiac Health: A Comprehensive Review. J. Cardiovasc. Dev. Dis. 2024, 11, 204. [Google Scholar] [CrossRef]
- Patel, A.R.; Patel, A.R.; Singh, S.; Singh, S.; Khawaja, I. The Association of Obstructive Sleep Apnea and Hypertension. Cureus 2019, 11, e4858. [Google Scholar] [CrossRef]
- Muxfeldt, E.S.; Margallo, V.S.; Guimarães, G.M.; Salles, G.F. Prevalence and associated factors of obstructive sleep apnea in patients with resistant hypertension. Am. J. Hypertens. 2014, 27, 1069–1078. [Google Scholar] [CrossRef]
- Doonan, R.J.; Scheffler, P.; Lalli, M.; Kimoff, R.J.; Petridou, E.T.; Daskalopoulos, M.E.; Daskalopoulou, S.S. Increased arterial stiffness in obstructive sleep apnea: A systematic review. Hypertens. Res. 2011, 34, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Heizhati, M.; Li, N.; Gan, L.; Lin, M.; Yang, W.; Li, M.; Yao, L.; Liu, M.; Maitituersun, A.; et al. Association of objective and subjective parameters of obstructive sleep apnea with plasma aldosterone concentration in 2066 hypertensive and 25,368 general population. Front. Endocrinol. 2023, 13, 1016804. [Google Scholar] [CrossRef] [PubMed]
- Tao, M.; Dong, X.; Tu, J.; Fang, Q.; Shao, C. Symptom and comorbidity burden in hypertensive patients with obstructive sleep apnea. Front. Endocrinol. 2024, 15, 1361466. [Google Scholar] [CrossRef]
- Worku, A.; Ayele, E.; Alemu, S.; Legese, G.L.; Yimam, S.M.; Kassaw, G.; Diress, M.; Asres, M.S. Obstructive sleep apnea risk and determinant factors among type 2 diabetes mellitus patients at the chronic illness clinic of the University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia. Front. Endocrinol. 2023, 14, 1151124. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.J.; Park, E. The Association of Sleep Duration and Hypertension in Adults in Korea. Korean J. Health Promot. 2013, 13, 141–147. [Google Scholar]
- Ong, J.L.; Tandi, J.; Patanaik, A.; Lo, J.C.; Chee, M.W.L. Large-scale data from wearables reveal regional disparities in sleep patterns that persist across age and sex. Sci. Rep. 2019, 9, 3415. [Google Scholar] [CrossRef]
- Han, B.; Chen, W.Z.; Li, Y.C.; Chen, J.; Zeng, Z.Q. Sleep hypertension. Sleep Breath. 2020, 24, 351–356. [Google Scholar] [CrossRef]
- Gao, C.; Guo, J.; Gong, T.T.; Lv, J.L.; Li, X.Y.; Liu, F.H.; Zhang, M.; Shan, Y.T.; Zhao, Y.H.; Wu, Q.J. Sleep Duration/Quality With Health Outcomes: An Umbrella Review of Meta-Analyses of Prospective Studies. Front. Med. 2021, 8, 813943. [Google Scholar] [CrossRef]
- Bock, J.M.; Vungarala, S.; Covassin, N.; Somers, V.K. Sleep Duration and Hypertension: Epidemiological Evidence and Underlying Mechanisms. Am. J. Hypertens. 2022, 35, 3–11. [Google Scholar] [CrossRef]
- Liu, D.; Yu, C.; Huang, K.; Thomas, S.; Yang, W.; Liu, S.; Kuang, J. The Association between Hypertension and Insomnia: A Bidirectional Meta-Analysis of Prospective Cohort Studies. Int. J. Hypertens. 2022, 2022, 4476905. [Google Scholar] [CrossRef]
- Kapur, V.K.; Auckley, D.H.; Chowdhuri, S.; Kuhlmann, D.C.; Mehra, R.; Ramar, K.; Harrod, C.G. Clinical Practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea: An American Academy of Sleep Medicine Clinical Practice Guideline. J. Clin. Sleep. Med. 2017, 13, 479–504. [Google Scholar] [CrossRef] [PubMed]
- Nagappa, M.; Liao, P.; Wong, J.; Auckley, D.; Ramachandran, S.K.; Memtsoudis, S.; Mokhlesi, B.; Chung, F. Validation of the STOP-Bang Questionnaire as a Screening Tool for Obstructive Sleep Apnea among Different Populations: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0143697. [Google Scholar] [CrossRef] [PubMed]

| Total (N = 14,579) | Normotension (N = 4914) | Prehypertension (N = 3606) | Hypertension (N = 6059) | p-Value | |
|---|---|---|---|---|---|
| Age (yrs) | |||||
| 40–49 | 3481 (23.9) | 1906 (54.8) | 930 (26.7) | 645 (18.5) | <0.001 |
| 50–59 | 3723 (25.5) | 1506 (40.5) | 1017 (27.3) | 1200 (32.2) | |
| 60–69 | 3928 (26.9) | 1001 (25.5) | 979 (24.9) | 1948 (49.6) | |
| 70–80 | 3447 (23.6) | 501 (14.5) | 680 (19.7) | 2266 (65.7) | |
| Sex | |||||
| Men | 6326 (43.4) | 1672 (26.4) | 1763 (27.9) | 2891 (45.7) | <0.001 |
| Women | 8253 (56.6) | 3242 (39.3) | 1843 (22.3) | 3168 (38.4) | |
| Residence | |||||
| City | 11,219 (77.0) | 4049 (36.1) | 2759 (24.6) | 4411 (39.3) | <0.001 |
| Rural area | 3360 (23.0) | 865 (25.7) | 847 (25.2) | 1648 (49.0) | |
| Marrital status | |||||
| Married/cohabitated | 11,287 (77.4) | 3956 (35.0) | 2895 (25.6) | 4436 (39.3) | <0.001 |
| Unmarried/separated/divorced/widowed | 3292 (22.6) | 958 (29.1) | 711 (21.6) | 1623 (49.3) | |
| Education | |||||
| ≤Middle school graduate | 5170 (35.5) | 992 (19.2) | 1122 (21.7) | 3056 (59.1) | <0.001 |
| High school graduate | 4717 (32.4) | 1774 (37.6) | 1232 (26.1) | 1711 (36.3) | |
| Community college graduate | 1428 (9.8) | 677 (47.4) | 354 (24.8) | 397 (27.8) | |
| ≥College graduate | 3264 (22.4) | 1471 (45.1) | 898 (27.5) | 895 (27.4) | |
| Household income | |||||
| First quartile | 3137 (21.5) | 667 (21.3) | 667 (21.3) | 1803 (57.5) | <0.001 |
| Second quartile | 3644 (25.0) | 1112 (30.5) | 889 (24.4) | 1643 (45.1) | |
| Third quartile | 3745 (25.7) | 1433 (38.3) | 961 (25.7) | 1351 (36.1) | |
| Fourth quartile | 4053 (27.8) | 1702 (42.0) | 1089 (26.9) | 1262 (31.1) | |
| Occupation | |||||
| No occupation | 5887 (40.4) | 1777 (30.2) | 1292 (21.9) | 2818 (47.9) | <0.001 |
| Manual workers | 3932 (27.0) | 1068 (27.2) | 1027 (26.1) | 1837 (46.7) | |
| Non-manual workers | 4760 (32.6) | 2069 (43.5) | 1287 (27.0) | 1404 (29.5) | |
| Frequnecy of high-risk drinking | |||||
| None | 9015 (61.8) | 3103 (34.4) | 2077 (23.0) | 3835 (42.5) | <0.001 |
| <1/month | 1718 (11.8) | 721 (42.0) | 489 (28.5) | 508 (29.6) | |
| ≥1/month | 3846 (26.4) | 1090 (28.3) | 1040 (27.0) | 1716 (44.6) | |
| Smoking status | |||||
| Never smoker | 8716 (59.8) | 3202 (36.7) | 2039 (23.4) | 3475 (39.9) | <0.001 |
| Former smoker | 3701 (25.4) | 1016 (27.5) | 968 (26.2) | 1717 (46.4) | |
| Current smoker | 2162 (14.8) | 696 (32.2) | 599 (27.7) | 867 (40.1) | |
| Aerobic exercise | |||||
| No | 8861 (60.8) | 2814 (31.8) | 2111 (23.8) | 3936 (44.4) | <0.001 |
| Yes | 5718 (39.2) | 2100 (36.7) | 1495 (26.1) | 2123 (37.1) | |
| Muscle strengthening exercise | |||||
| No | 11,501 (78.9) | 3831 (33.3) | 2786 (24.2) | 4884 (42.5) | <0.001 |
| Yes | 3078 (21.1) | 1083 (35.2) | 820 (26.6) | 1175 (38.2) |
| Total | Normotension | Prehypertension | Hypertension | p-Value | |
|---|---|---|---|---|---|
| OSA risk | |||||
| Low | 7529 (51.6) | 3697 (49.1) | 2128 (28.3) | 1704 (22.6) | <0.001 |
| Moderate | 5403 (37.1) | 1072 (19.8) | 1260 (23.3) | 3071 (56.8) | |
| High | 1647 (11.3) | 145 (8.8) | 218 (13.2) | 1284 (78.0) | |
| Sleep duration (hrs) | |||||
| < 6 | 2917 (20.0) | 831 (28.5) | 719 (24.6) | 1367 (46.9) | <0.001 |
| ≥6 and <7 | 3933 (27.0) | 1395 (35.5) | 971 (24.7) | 1567 (39.8) | |
| ≥7 and <8 | 4439 (30.4) | 1607 (36.2) | 1159 (26.1) | 1673 (37.7) | |
| ≥8 and <9 | 2607 (17.9) | 892 (34.2) | 615 (23.6) | 1100 (42.2) | |
| ≥9 | 683 (4.7) | 189 (27.7) | 142 (20.8) | 352 (51.5) | |
| OSA risk and sleep duration (hrs) | |||||
| Low and <6 | 1403 (9.6) | 596 (42.5) | 435 (31.0) | 372 (26.5) | <0.001 |
| Low and ≥6 and <7 | 2037 (14.0) | 1032 (50.7) | 569 (27.9) | 436 (21.4) | |
| Low and ≥7 and <8 | 2411 (16.5) | 1228 (50.9) | 681 (28.2) | 502 (20.8) | |
| Low and ≥8 and <9 | 1358 (9.3) | 702 (51.7) | 364 (26.8) | 292 (21.5) | |
| Low and ≥9 | 320 (2.2) | 139 (43.4) | 79 (24.7) | 102 (31.9) | |
| Moderate and <6 | 1165 (8.0) | 212 (18.2) | 239 (20.5) | 714 (61.3) | |
| Moderate and ≥6 and <7 | 1458 (10.0) | 318 (21.8) | 347 (23.8) | 793 (54.4) | |
| Moderate and ≥7 and <8 | 1548 (10.6) | 330 (21.3) | 413 (26.7) | 805 (52.0) | |
| Moderate and ≥8 and <9 | 950 (6.5) | 164 (17.3) | 206 (21.7) | 580 (61.1) | |
| Moderate and ≥9 | 282 (1.9) | 48 (17.0) | 55 (19.5) | 179 (63.5) | |
| High and <6 | 349 (2.4) | 23 (6.6) | 45 (12.9) | 281 (80.5) | |
| High and ≥6 and <7 | 438 (3.0) | 45 (10.3) | 55 (12.6) | 338 (77.2) | |
| High and ≥7 and <8 | 480 (3.3) | 49 (10.2) | 65 (13.5) | 366 (76.3) | |
| High and ≥8 and <9 | 299 (2.1) | 26 (8.7) | 45 (15.1) | 228 (76.3) | |
| High and ≥9 | 81 (0.6) | 2 (2.5) | 8 (9.9) | 71 (87.7) |
| OSA Risk | |||||
|---|---|---|---|---|---|
| Low a | Moderate b | High c | |||
| Mean ± SE | Mean ± SE | Mean ± SE | p-Value | Post Hoc | |
| BMI (kg/m2) | 23.13 ± 0.04 | 25.01 ± 0.05 | 27.02 ± 0.10 | <0.001 | a < b < c |
| WC (cm) | 80.62 ± 0.13 | 88.83 ± 0.14 | 94.71 ± 0.27 | <0.001 | a < b < c |
| NC (cm) | 33.10 ± 0.04 | 36.93 ± 0.05 | 39.17 ± 0.07 | <0.001 | a < b < c |
| SBP (mmHg) | 117.04 ± 0.24 | 125.22 ± 0.28 | 127.94 ± 0.43 | <0.001 | a < b < c |
| DBP (mmHg) | 73.97 ± 0.14 | 78.23 ± 0.17 | 81.52 ± 0.32 | <0.001 | a < b < c |
| Fasting glucose (mg/dL) | 99.61 ± 0.28 | 108.23 ± 0.43 | 111.29 ± 0.79 | <0.001 | a < b < c |
| HbA1c (%) | 5.72 ± 0.01 | 5.98 ± 0.02 | 6.12 ± 0.03 | <0.001 | a < b < c |
| Total cholesterol (mg/dL) | 198.11 ± 0.55 | 188.80 ± 0.69 | 187.41 ± 1.29 | <0.001 | a > b, c |
| Triglyceride (mg/dL) | 117.24 ± 1.15 | 155.07 ± 2.15 | 178.52 ± 4.21 | <0.001 | a < b < c |
| HDL cholesterol (mg/dL) | 56.56 ± 0.20 | 49.39 ± 0.22 | 46.86 ± 0.31 | <0.001 | a > b > c |
| LDL cholesterol (mg/dL) | |||||
| Model 1 | Model 2 | |||
|---|---|---|---|---|
| OR (95% CI) | p-Value | OR (95% CI) | p-Value | |
| Independent effects | ||||
| OSA risk | ||||
| Low | 1.00 | 1.00 | ||
| Moderate | 11.63 (10.13–13.36) | <0.001 | 9.69 (8.37–11.23) | <0.001 |
| High | 51.55 (41.92–63.38) | <0.001 | 36.58 (29.35–45.59) | <0.001 |
| Sleep duration (hrs) | ||||
| <6 | 1.07 (0.93–1.23) | 0.339 | 0.96 (0.83–1.11) | 0.586 |
| ≥6 and <7 | 1.07 (0.94–1.22) | 0.284 | 1.04 (0.91–1.18) | 0.583 |
| ≥7 and <8 | 1.00 | 1.00 | ||
| ≥8 and <9 | 1.08 (0.93–1.26) | 0.289 | 1.06 (0.91–1.23) | 0.488 |
| ≥9 | 1.13 (0.90–1.41) | 0.295 | 1.00 (0.80–1.26) | 1.000 |
| Combined effects | ||||
| OSA risk and sleep duration (hrs) | ||||
| Low and <6 | 0.87 (0.71–1.06) | 0.172 | 0.76 (0.61–0.93) | 0.008 |
| Low and ≥6 and <7 | 0.95 (0.79–1.14) | 0.553 | 0.93 (0.77–1.12) | 0.441 |
| Low and ≥7 and <8 | 1.00 | 1.00 | ||
| Low and ≥8 and <9 | 0.90 (0.74–1.10) | 0.311 | 0.89 (0.72–1.09) | 0.253 |
| Low and ≥9 | 1.20 (0.88–1.65) | 0.251 | 1.02 (0.74–1.41) | 0.891 |
| Moderate and <6 | 11.97 (9.49–15.10) | <0.001 | 9.17 (7.20–11.67) | <0.001 |
| Moderate and ≥6 and <7 | 11.29 (9.02–14.12) | <0.001 | 8.95 (7.10–11.28) | <0.001 |
| Moderate and ≥7 and <8 | 9.58 (7.79–11.77) | <0.001 | 7.95 (6.43–9.83) | <0.001 |
| Moderate and ≥8 and <9 | 13.01 (10.20–16.60) | <0.001 | 10.30 (8.04–13.21) | <0.001 |
| Moderate and ≥9 | 9.16 (6.61–12.71) | <0.001 | 7.05 (5.01–9.91) | <0.001 |
| High and <6 | 55.34 (37.60–81.46) | <0.001 | 35.09 (23.70–51.96) | <0.001 |
| High and ≥6 and <7 | 52.38 (37.70–72.77) | <0.001 | 35.38 (25.17–49.74) | <0.001 |
| High and ≥7 and <8 | 45.17 (32.84–62.12) | <0.001 | 31.98 (23.02–44.43) | <0.001 |
| High and ≥8 and <9 | 40.73 (27.45–60.44) | <0.001 | 28.74 (19.48–42.41) | <0.001 |
| High and ≥9 | 79.27 (32.79–191.68) | <0.001 | 48.49 (19.68–119.50) | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, S.Y.; Kim, Y. Combined Effects of Obstructive Sleep Apnea and Sleep Duration on Hypertension in Korean Adults: A Nationwide Study. Biomedicines 2025, 13, 1475. https://doi.org/10.3390/biomedicines13061475
Kang SY, Kim Y. Combined Effects of Obstructive Sleep Apnea and Sleep Duration on Hypertension in Korean Adults: A Nationwide Study. Biomedicines. 2025; 13(6):1475. https://doi.org/10.3390/biomedicines13061475
Chicago/Turabian StyleKang, Seo Young, and Yunmi Kim. 2025. "Combined Effects of Obstructive Sleep Apnea and Sleep Duration on Hypertension in Korean Adults: A Nationwide Study" Biomedicines 13, no. 6: 1475. https://doi.org/10.3390/biomedicines13061475
APA StyleKang, S. Y., & Kim, Y. (2025). Combined Effects of Obstructive Sleep Apnea and Sleep Duration on Hypertension in Korean Adults: A Nationwide Study. Biomedicines, 13(6), 1475. https://doi.org/10.3390/biomedicines13061475






