Abstract
Background: In recent years, musculoskeletal ultrasonography (MSUS) has established itself as a reliable method for evaluating disease activity in combination with clinical examination and laboratory tests. Objectives: In this pilot study, we aimed to evaluate the ultrasound response to treatment with tofacitinib and upadacitinib on tendons and joints in comparison to clinical and laboratory results in patients with RA who have shown inadequate response to conventional synthetic and/or biologic disease-modifying antirheumatic drugs (cs/b DMARDs). Methods: This study presents the MSUS assessment of therapeutic response in RA patients treated with tofacitinib or upadacitinib over a 24-week period. In a prospective, single-center study, patients were treated with upadacitinib 15 mg/daily or tofacitinib 2 × 5 mg/daily or 11 mg/daily, in combination with or without methotrexate or another conventional DMARDs. Disease activity was assessed by DAS28-CRP, CDAI, and SDAI, as well as MSUS. Patients were evaluated at baseline for ultrasound measures and at weeks 2, 4, 8, 12, and 24 for the rest of the indicators. For each patient, we used two ultrasound (US) scores (gray-scale, GS, and power Doppler, PD scores) and the system of European Alliance of Associations for Rheumatology outcome measures in rheumatology (EULAR-OMERACT) US scoring (combined GS and PD). We also calculated the tenosynovitis score (GS and PD) according to OMERACT recommendations. Results: A total of 53 patients were recruited. A total of 25 patients with a mean age of 56 ± 11.6 SD were followed in the upadacitinib group, and 22 patients with a mean age of 56.9 ± 11.3 were followed in the tofacitinib group. At baseline, DAS28-CRP for the upadacitinib group was 5.57 ± 1.24, and for tofacitinib, it was 4.77 ± 1.47. The baseline visit (GS, PD, and combined—US) and tendon US scores (GS and PD) were, respectively, 23 ± 2.96, 15 ± 2.56, 24.08 ± 3.36, 11.04 ± 2.21, and 8.44 ± 1.65 for the upadacitinib group. USGS-J—23 ± 3.55, USPD-J—13.36 ± 2.44, OMERACT composite—23.4 ± 3.84, USGS-T—12.18 ± 2.23, and USPD-T—9.5 ± 1.92 were found in the patients treated with tofacitinib. In both groups of patients, a significant reduction was found in both DAS28-CRP and the described MSUS scores at weeks 8, 12, and 24 (p < 0.05). Conclusions: Upadacitinib managed to produce lower echography scores much faster than tofacitinib; however, the differences in effectiveness evened out at weeks 12 and 24, with all patients being adequately controlled.
1. Introduction
Rheumatoid arthritis (RA) is a chronic, progressive autoimmune disease characterized by synovial proliferation and the destruction of articular cartilage and bone [1,2]. The disability of the patients significantly impairs their quality of life [2,3]. The main goal of treatment is to achieve remission. Otherwise, if the first goal is not possible, the goal is to achieve minimal disease activity [4,5,6,7]. The advent of biologic therapy over the past 25 years has led to significant advances in the treatment of patients with rheumatoid arthritis [8,9]. In addition, the introduction of new small molecules—inhibitors of Janus kinases (JAKs)—offers a new alternative for the treatment of inflammatory joint diseases [10,11,12,13,14,15,16,17,18]. In recent years, the results of numerous clinical trials have proven their effectiveness [10,11,12,13,14,15,16,17,18]. However, there is still a lack of sufficient evidence from real clinical practice to confirm and validate the good therapeutic response and higher remission rate in patients with RA [19,20].
Disease states may be assessed by several scores. The disease activity score (DAS28) evaluates disease severity based on the assessment of 28 joints [21,22]. The clinical disease activity index (CDAI) and the simplified disease activity index (SDAI) are the other two scores frequently used in rheumatology practice [22,23]. Several publications have shown that treatment with tofacitinib and upadacitinib significantly reduces the disease activity assessed by DAS28–ESR and DAS28-CRP [15,18,24]. Moreover, recent evidence has demonstrated the superior effect of upadacitinib in combination with methotrexate (MTX) versus adalimumab + MTX in the inhibition of structural damage [20]. However, these simplified measures cannot compare to ultrasound evaluation, which can assess the reduction in disease activity in terms of radiographical progression. Therefore, full evaluation of the remission state remains questionable without the use of musculoskeletal ultrasound.
In 2017, the European Alliance of Associations for Rheumatology (EULAR) published recommendations for the use of musculoskeletal ultrasound in the management of RA [25]. The new ultrasound definitions (US) and quantifications of synovial hypertrophy (SH) and power Doppler (PD) signal, separately and in combination with the European League Against Rheumatism–Outcomes Measures in Rheumatology (EULAR-OMERACT) combined score for PD and SH, demonstrated moderate–good reliability of ultrasound in RA [25]. Images in gray-scale can provide additional information to regular joint assessment. PD signal is an extremely sensitive marker for joint and tendon inflammation, which can supplement the evaluation of therapeutic response [26]. As Di Matteo et al. pointed out, US is applicable from the prediction of the progression of RA to the confirmation of early diagnosis during the disease continuum [27].
Clinicians are still working on clarifying the principal US findings in RA, scoring systems for evaluation, and appropriate use of US in areas of RA diagnosis and disease prognostication [28,29]. Thus, musculoskeletal ultrasound is becoming a reliable method for the assessment of disease activity, not only in routine clinical practice but also in clinical trials [25,30].
In this pilot study, we aimed to evaluate the ultrasound response to treatment with tofacitinib and upadacitinib on tendons and joints in comparison to clinical and laboratory results in patients with RA who have shown inadequate response to conventional synthetic and/or biologic disease-modifying antirheumatic drugs (cs/b DMARDs). In addition, we performed a musculoskeletal ultrasound at each visit to assess early response and maintenance of the therapeutic outcome during the 24-week follow-up period.
2. Materials and Methods
2.1. Design of the Study
This was a prospective, observational, longitudinal study at the largest rheumatology clinic at the University Hospital “St. Ivan Rilski” in Sofa, Bulgaria, during the period 2018–2022. It was conducted in accordance with the 1989 Declaration of Helsinki and was approved by the Local Ethics Committee (Ethics Approval Protocol Number: 02/06.03.2018). All of the 53 patients provided written informed consent at the time of the first visit, out of which 47 remained at the end of the observation—week 24. The treatment with oral tofacitinib (TOF) or upadacitinib (UPA) was prescribed according to local guidelines and National Health Insurance Fund (NHIF) requirements for disease activity [31,32].
The rationale behind the selection of tofacitinib and upadacitinib was based on the study of Fleischmann RM et al. [20] (Figure 1). Janus kinase inhibitors (JAKi) are relatively new molecules in the armamentarium of RA therapy, and they are especially newly reimbursed in our country, which provoked our interest.
Figure 1.
Structural 2D formulas of tofacitinib and upadacitinib.
The inclusion criteria for this study were:
Age > 18 years < 85 years.
Clinically proven RA according to ACR (1987) and/or ACR/EULAR (2010) criteria.
Different disease duration.
Patients treated in combination with or without methotrexate or another csDMARD in a stable dose, and/or corticosteroid (CS) therapy up to 10 mg/daily, and/or non-steroidal anti-inflammatory drugs (NSAIDs).
Patients treated with bDMARD (up to 2 bDMARDs, discontinued 3 months before initiation of the treatment with JAK-inhibitor).
Patients in the individual treatment groups did not change the dosage regimen or discontinue csDMARD or CS therapy during the entire follow-up period.
NSAIDs should be at a stable dose throughout the study period.
The exclusion criteria were defined as presence of infectious diseases, cardiac insufficiency (NYHA III and IV grade), malignant hypertension, any neoplasms, or proliferative lymph diseases within the previous 5 years.
All patients were followed up for a period of 24 weeks through six visits—baseline, week 2, week 4, week 8, week 12, and week 24 after the initiation of tofacitinib or upadacitinib. Joint assessment was performed during each visit by the same rheumatologist. Blood samples were collected to measure the levels of C-reactive protein. Laboratory tests comprised hematology, liver and kidney function, lipid profile, HBV, HCV screening, interferon-gamma release assay for latent tuberculosis (LTB), rheumatoid factor (RF), and anti-cyclic citrullinated peptide antibody (ACPA), which were obtained at baseline. Radiographies of hands and the chest were performed within 6 months before enrollment. We evaluated the disease activity using DAS28-CRP [21]. In addition, we measured CDAI and SDAI to precisely define the severity of the disease in each patient and searched for correlation with ultrasound scores. The three parameters were calculated during each visit.
DAS28 was calculated as follows:
where
DAS28 (CRP) = 0.56*√(TJC28) + 0.28*√(SJC28) + 0.014*GH + 0.36*ln(CRP + 1) + 0.96
TJC = tender joint count, and SJC = swollen joint count.
CDAI was calculated as follows:
where
CDAI = SJC(28) + TJC(28) + PGA + EGA,
SJC(28) = swollen 28-joint count; TJC(28) = tender 28-joint count; PGA = patient global disease activity; and EGA = evaluator’s global disease activity.
SDAI was calculated as follows:
where SJC = swollen joint count; TJC = tender joint count; PGA = patient global assessment of disease activity; EGA = evaluator global assessment of disease activity; and CRP = CRP in mg/dL.
SDAI = SJC + TJC + PGA + EGA + CRP
2.2. Musculoskeletal Ultrasound
Ultrasound evaluation was performed using Esaote model ultrasound machines, MyLab Twice, with a 4–15 MHz frequency probe. Power Doppler parameters were adjusted with a pulse repetition rate ranging between 400 and 800 Hz. Two independent sonographers performed ultrasound examinations in a darkened room in accordance with standardized scans. Each patient was assessed for two joint scores—gray-scale (GS) and PD (USGS-J and USPD-J); the semi-quantitative scale was calculated from 0 to 3 for each joint, and a different score was calculated according to the OMERACT-EULAR composite US scale (OMERACT) from 0 to 3 for each joint. In addition, we assessed the tenosynovitis and calculated USGS-T and USPD-T according to the OMERACT-EULAR scoring system [25]. The targeted ultrasound initiative (TUI) synovitis evaluation form was used to record the results.
2.3. Statistical Analysis
Data were collected and analyzed in Microsoft Office’s Excel, with additional descriptive statistics performed by MedCalc 21.2 statistical software. Standard deviations of average scores are included in Table 1 and Table 2. The Pearson correlation coefficient was calculated for echographic variables vs. disease activity variables. A two-sample t-test was applied to mean values and obtained for all weeks of measurement, comparing upadacitinib vs. tofacitinib. Correlation matrixes were constructed through R studio, both separately for each drug and using a combined matrix to better follow changes. The main correlation coefficients are present on the graphs with the significance level in Tables 4 and 5. Baseline scores were available only for ultrasound measures and are presented additionally in Table 2 and Figure A1.

Table 1.
Demographic characteristics of the patients on upadacitinib and tofacitinib.

Table 2.
Baseline scores for ultrasonographic (US) measures for both medicines.
3. Results
3.1. Patient Population
A total of 53 patients were recruited, with 6 patients lost to follow-up due to adverse drug reactions (ADRs) or due to therapy changes. A total of 25 patients were on upadacitinib therapy and 22 on tofacitinib. The average age was 56 years old, with the majority of patients being women. The average durations of disease were 8.24 and 11.17 years, respectively (Table 1).
3.2. Disease Activity Scores
Baseline scores for all ultrasound measures and both groups of patients showed similarity (Table 2).
Both groups of patients experienced a steady decline in symptom severity and disease activity, as measured by SDAI, CDAI, and DAS-28. A steady linear downward trend was observed from week 2 to week 24. Patients on upadacitinib had average DAS-28 scores of 5.59 at week 2 and 2.93 at week 24 vs. 4.87 and 2.85 for tofacitinib (Table 3).

Table 3.
Average disease activity scores (DAS28-CRP) during the follow-up period for both medicines.
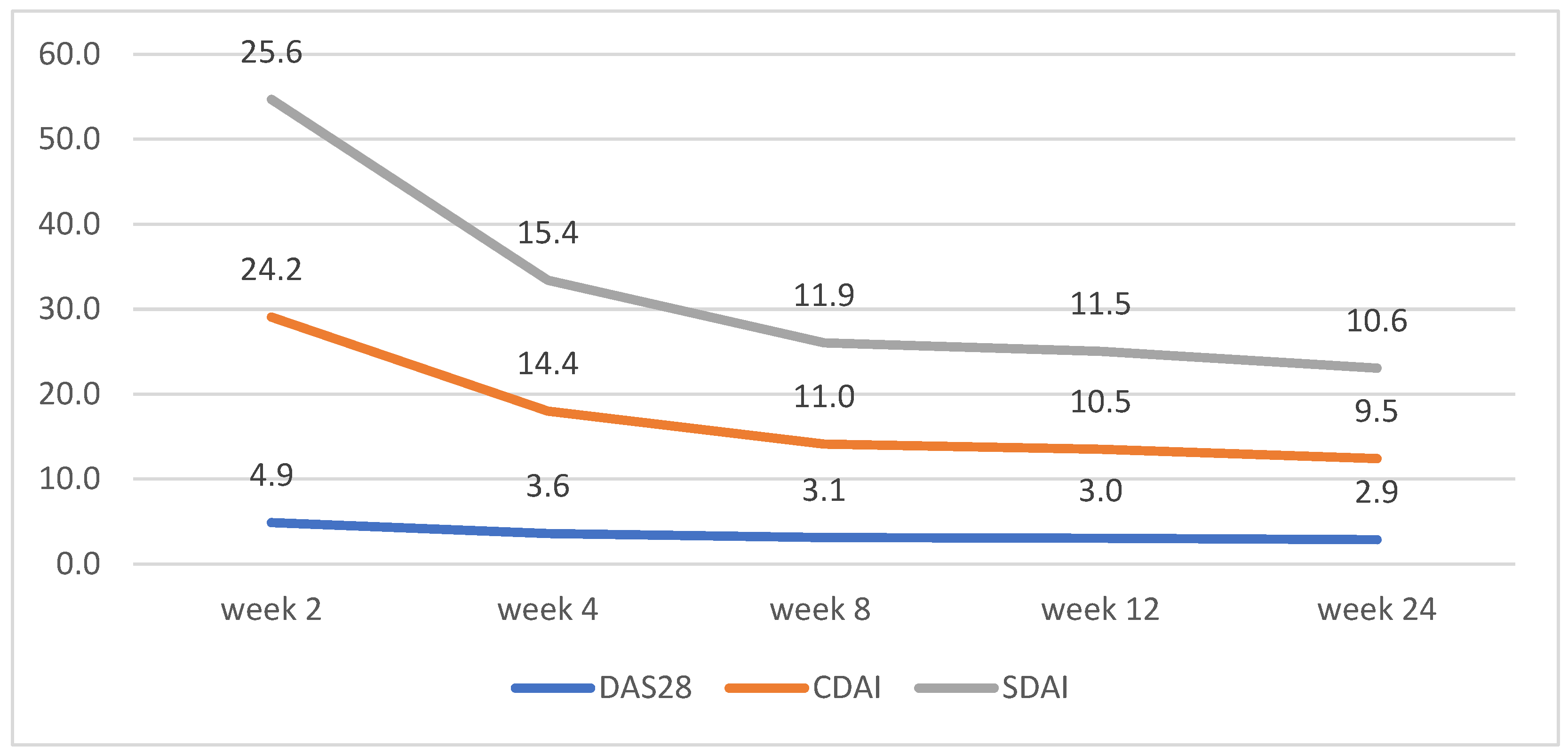
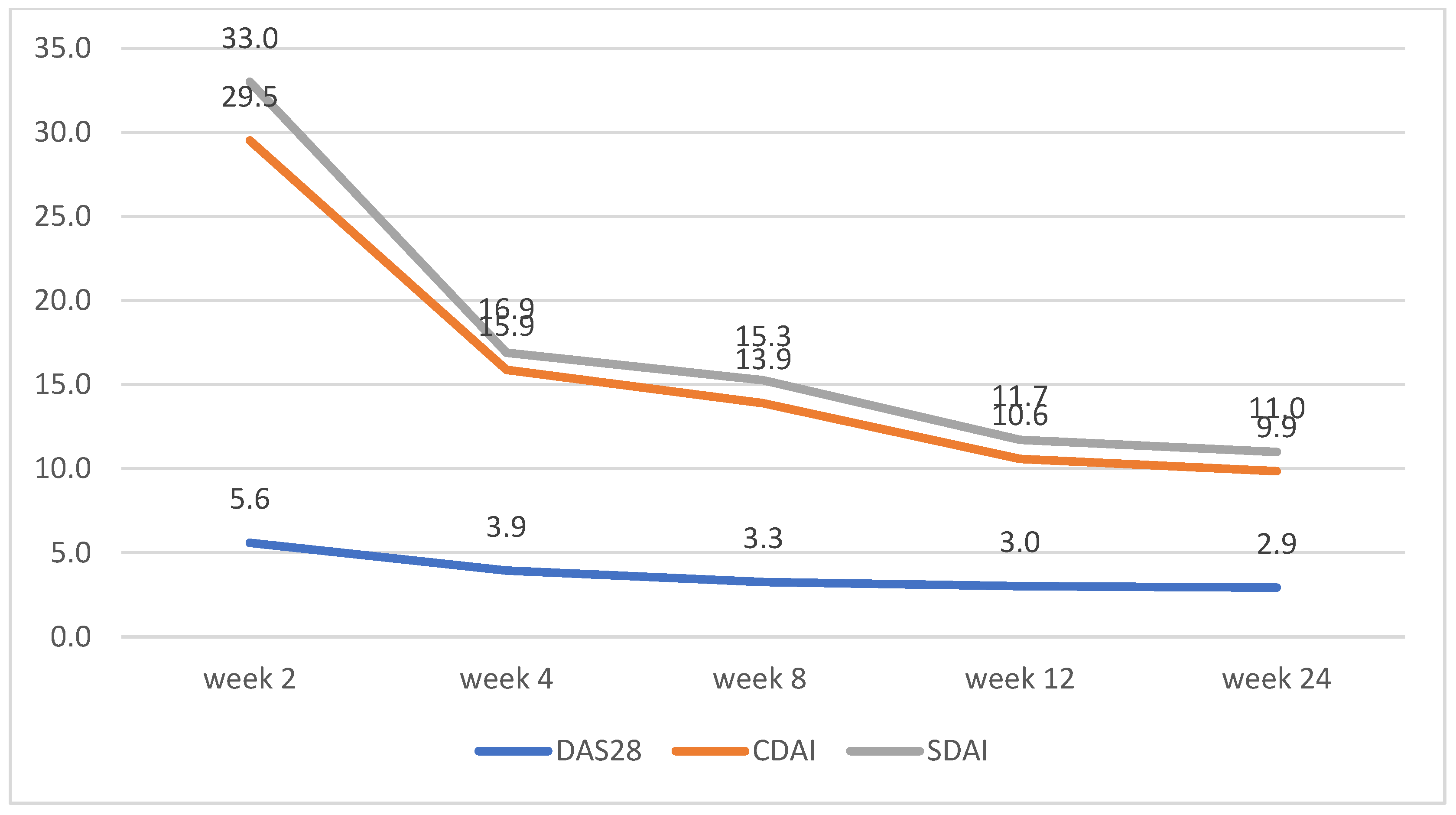
CDAI and SDAI scores decreased dramatically between weeks 2 and 4. No significant differences between the scores for both groups were observed, apart from week 2, where patients on tofacitinib had lower average scores for CDAI and SDAI (Figure 2 and Figure 3)
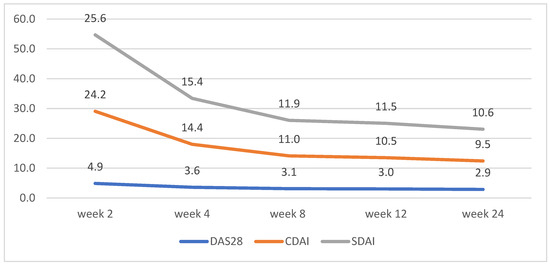
Figure 2.
Tofacitinib disease activity trends according to changes in DAS28, CDAI, and SDAI.
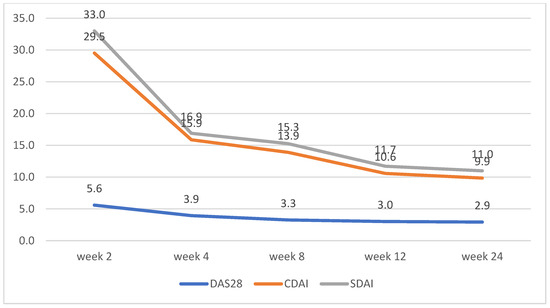
Figure 3.
Upadacitinib disease activity trends according to changes in DAS28, CDAI, and SDAI.
3.3. Echography Scores
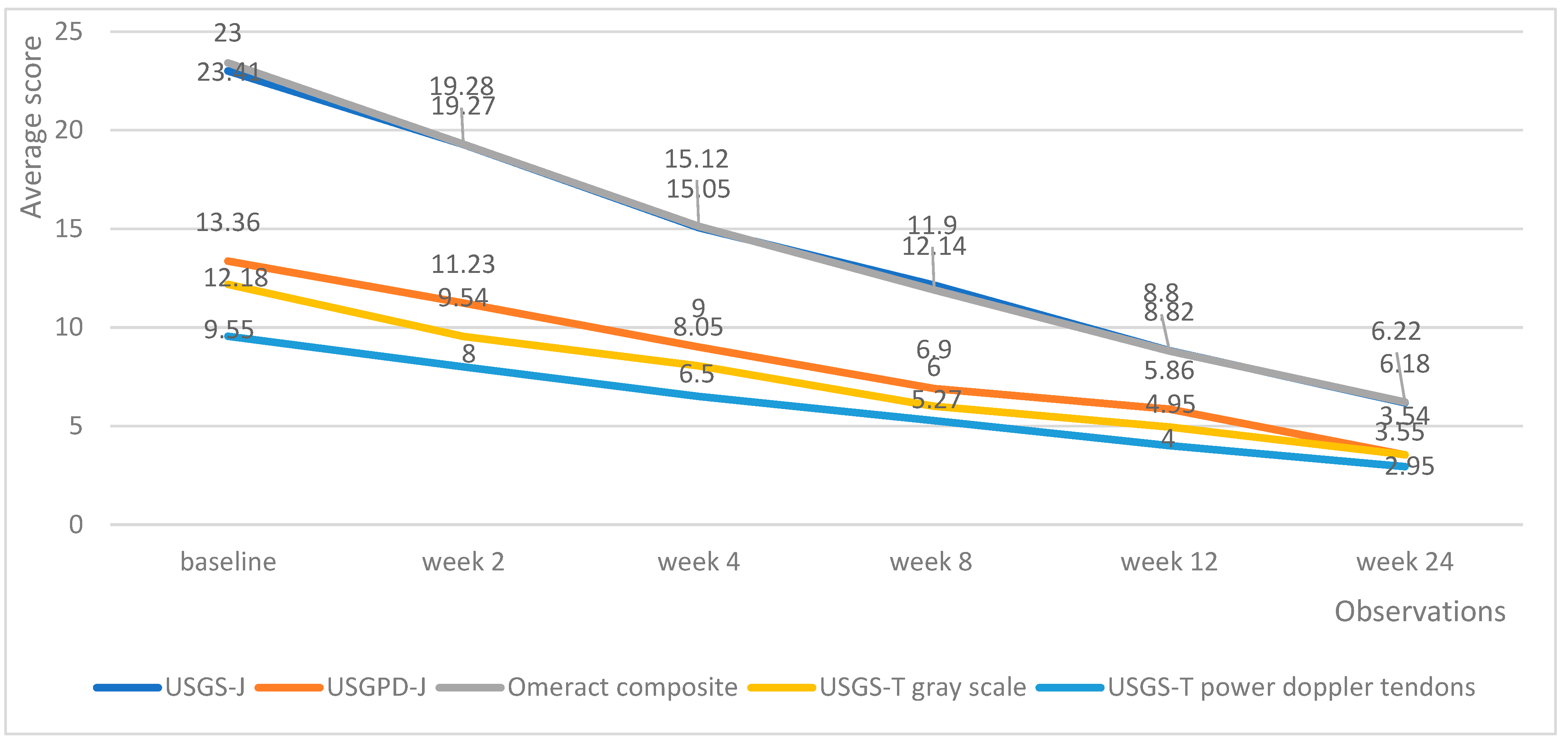
Both medicinal products managed to decrease the echography score in all domains. Tofacitinib managed to produce a linear reduction in echography scores, with the average lowering by roughly the same amount upon each successive measurement (Figure 4).
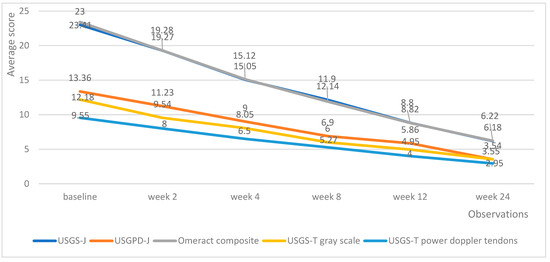
Figure 4.
Tofacitinib trends in echography average scores per week.
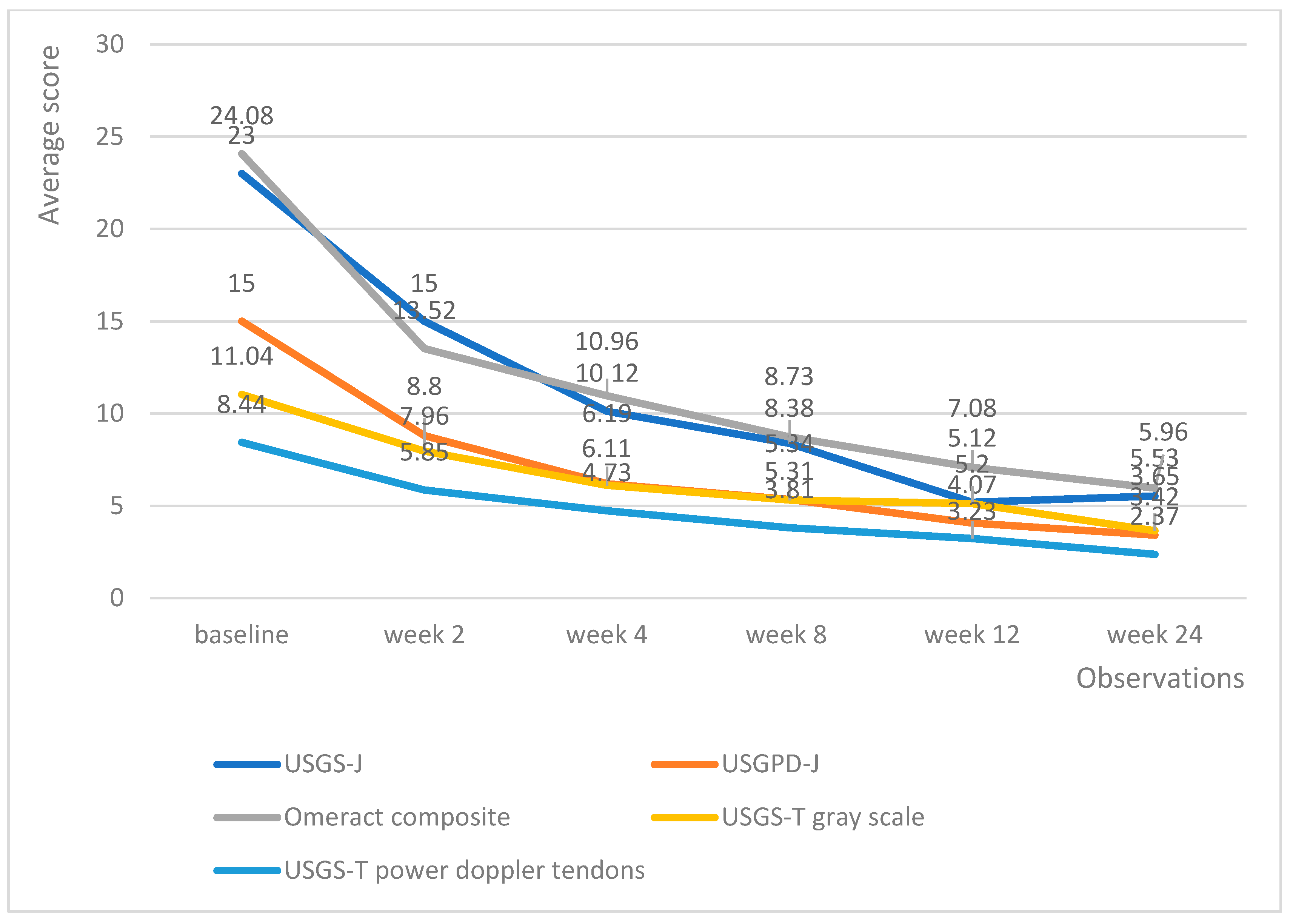
Upadacitinib managed to produce lower echography scores much faster than tofacitinib; however, the differences in effectiveness evened out at weeks 12 and 24, with all patients being adequately controlled (Figure 5). Of particular note is the fact that patients on upadacitinib showed a marked improvement beginning from week 2, with USGS-J experiencing a 10-point average reduction from baseline; USPD-J a 7.2-point reduction; OMERACT composite a 10.7-point reduction; USGS-T a 3.4-point reduction; and USPD-T a 2.6-point reduction.
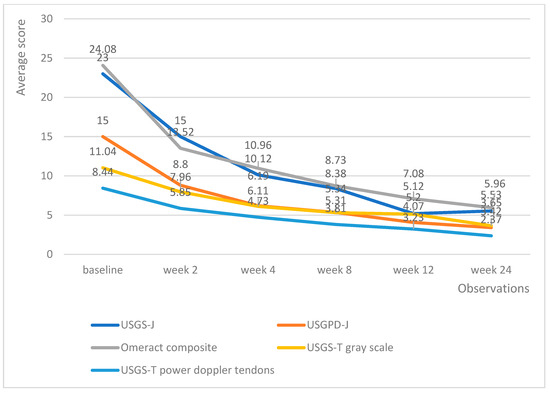
Figure 5.
Upadacitinib trends in echography average scores per week.
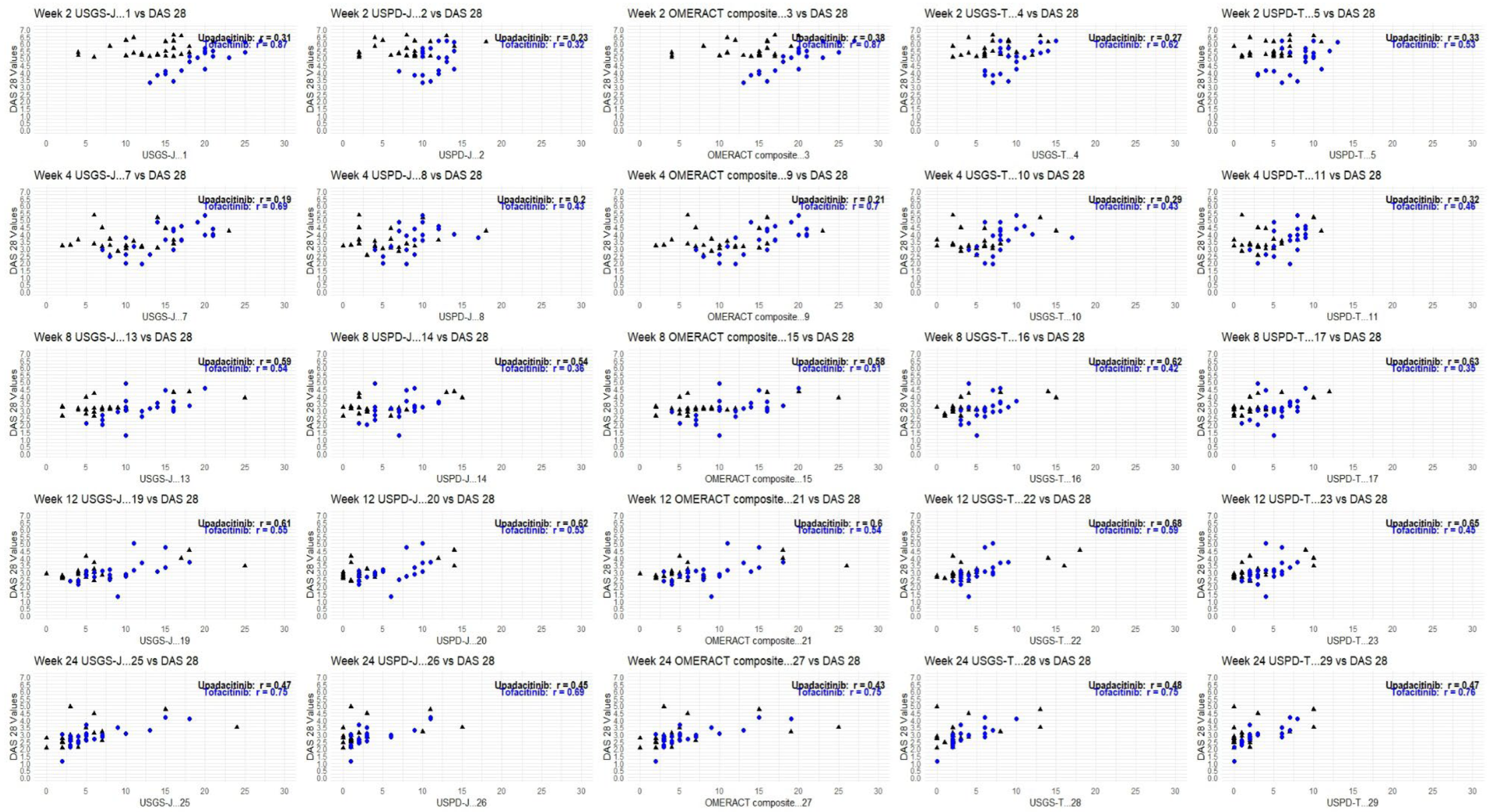
3.4. Correlation Analysis
Table 4 and Table 5 show where the correlation measured reached statistical significance in relation to echography scores. Overall, there was a correlation between echography scores and disease activity measurements. Patients with low DAS 28, CDAI, and SDAI also showed lower joint and tendon inflammation. DAS 28 was the most sensitive to this correlation.

Table 4.
Statistically significant correlations between echography and disease activity scores of upadacitinib.

Table 5.
Statistically significant correlations between echography and disease activity scores of tofacitinib.
Despite the average echography being low from week 2 for upadacitinib, this was not reflected as much in the correlation analysis. A significant correlation began to be observed from week 8 onward.
Contrary to that, the linear reduction for tofacitinib resulted in higher correlation coefficients, as well as statistically significant correlations beginning from week 2. Here, both DAS 28-CRP and CDAI were sensitive enough to confirm this correlation.
Overall, results showed that both products led to a measurable clinical response while at the same time reducing inflammation in the joints and tendons. Weeks 12 and 24 showed the highest correlation coefficients, which was to be expected, since both products achieved disease control at a similar rate from week 8 onward.
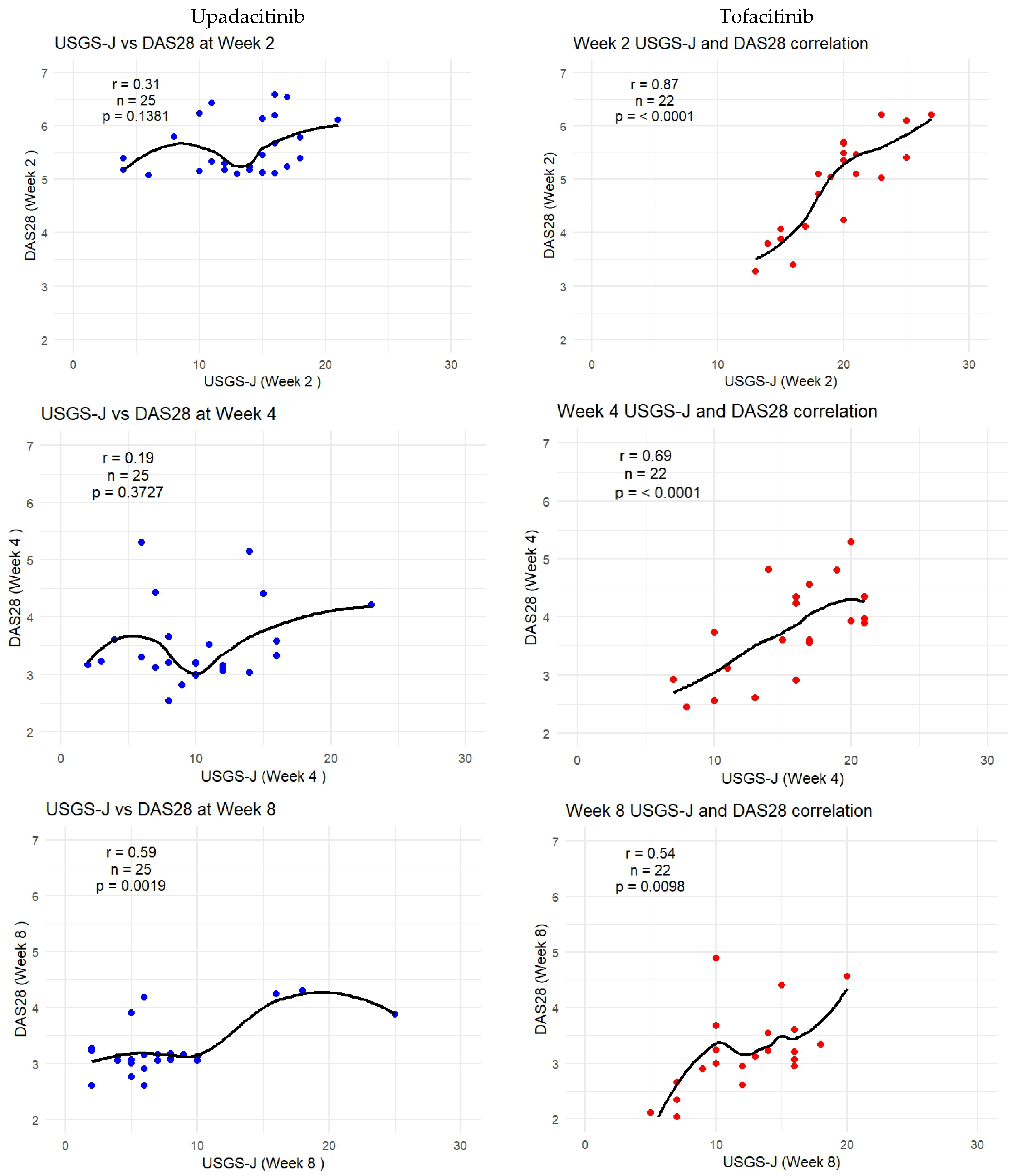
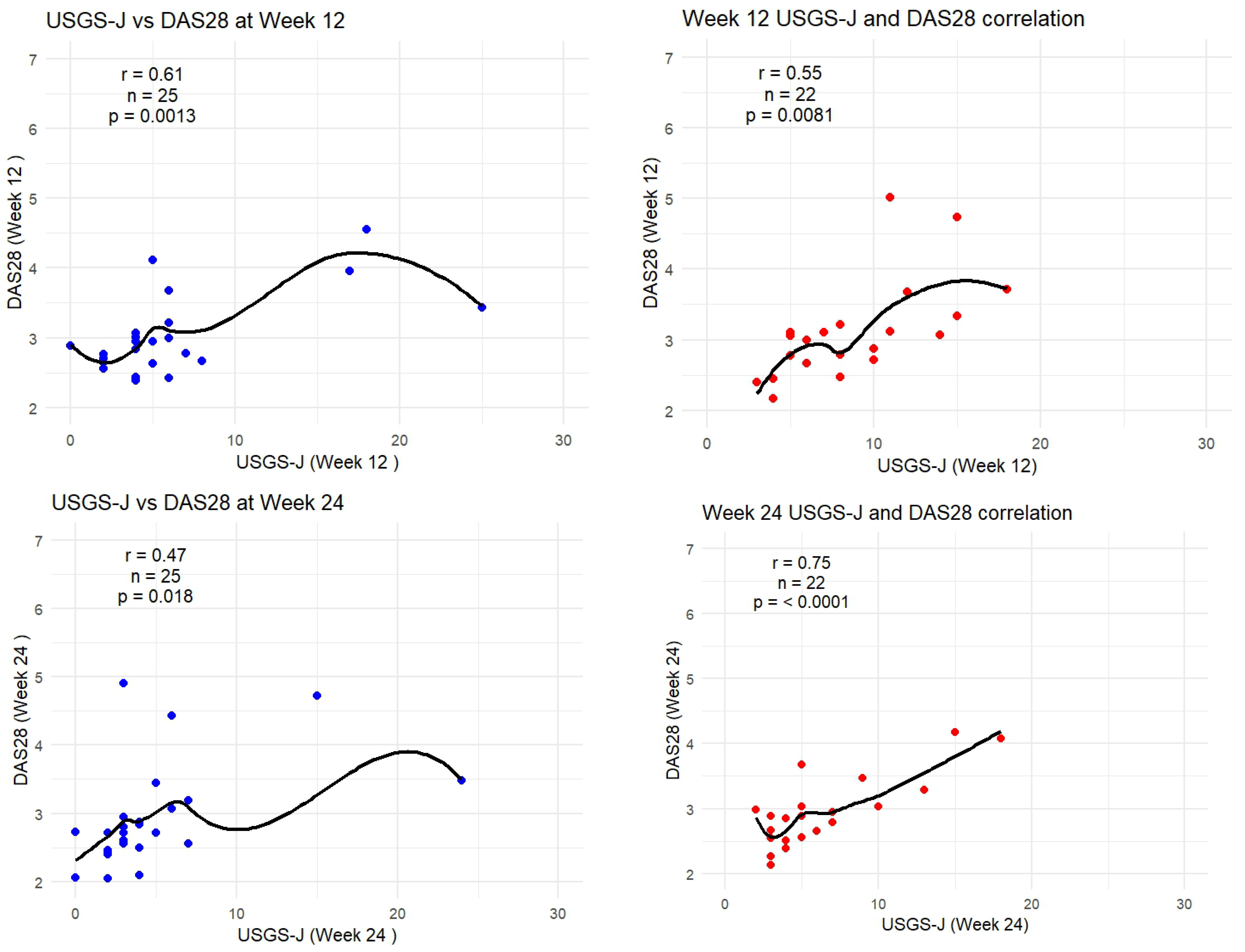
As DAS-28-CRP was the most sensitive to measuring correlation, the following graphs show how the trendlines shifted for both products. Tofacitinib results were more in line with expected correlation coefficients; however, there seemed to be a mismatch for upadacitinib. That is to say, despite echography scores being low from week 2 onward, it seemed clinical disease activity measures were able to capture this beginning from week 8 onward. Figure 6 captures this gradual progression for USGS-J as a point illustration.
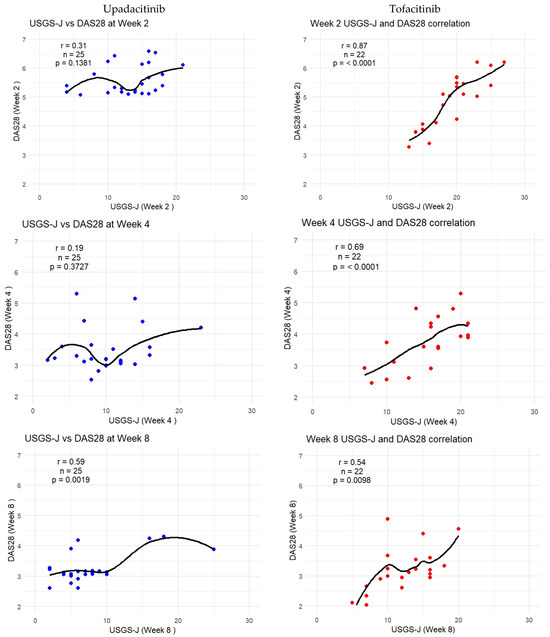
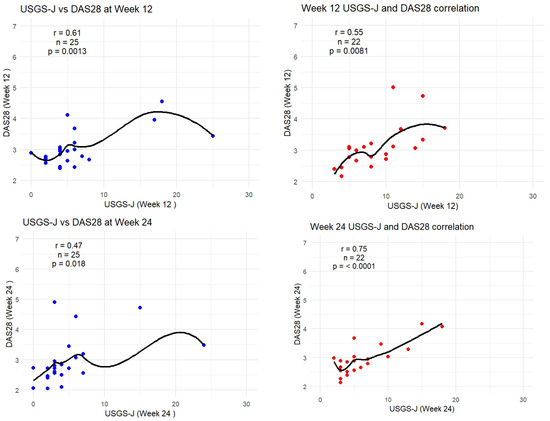
Figure 6.
USGS-J score correlations with DAS28-CRP for weeks 2, 4, 8, 12, and 24.
Although tofacitinib trends appeared to be more predictable, both sets of measurements showed a clear trend—lower echography scores corresponded to lower disease state scores, which appeared to be significant. Correlation matrices were constructed through R studio, both separately for each drug and as a combined matrix to better follow the changes (Appendix B).
4. Discussion
Imaging techniques have long been used in rheumatoid arthritis and is one of the main sources of understanding the pathology of the disease, helping physicians assess both synovial and bone damage. Better disease understanding, treat-to-target approaches, advent of TNF-alpha inhibitors, and better csDMARD usage have allowed patients to experience significant improvement in disease course [33]. This includes better outcomes, higher remission rates, less radiographical damage, and better quality of life [34]. Despite these improvements, many patients still fail to respond to therapy. JAK inhibitors, small-sized oral molecules, have provided a targeted approach to treat these patients, with similar efficacy demonstrated by clinical trials [35]. However, these molecules are still accumulating evidence in real-world settings, and our study adds to the body of evidence, showing results consistent with randomized clinical trials (RCTs) [36,37,38,39].
Progression of joint damage is linked with disease activity, with studies showing that TNF-alpha inhibitors disrupt this link [40]. Assessing disease activity and extensive radiography evaluation is not commonplace, with studies of tofacitinib assessing only the van der Heijde Total Sharp Score [41], while upadacitinib studies have also assessed erosion scores and joint space narrowing as measurements [42]. In 2008, Hameed et al. found that composite US markers of synovial disease relate significantly to DAS28 [43]. The study compared RA patients with normal controls only in terms of imaging and disease severity, without investigating the impact of therapy. Our findings corroborate the results of that study, showing that DAS28 is extremely sensitive to ultrasound improvement while additionally providing point estimates.
Our study showed that upadacitinib achieved rapid effect in the first 2–4 weeks, which gradually leveled out over the 6-month observation period, leading to stability in disease management. Similarly, Baldi et al. followed upadacitinib patients for 6 months in three Italian rheumatology centers, observing DAS28-CRP, SDAI, CDAI, USGS, and USPD improvements in the first month [44]. However, in that study, patients were examined at months 1, 3, and 6, showing gradual improvement. The additional measurement of patients at week 2 showed that the effect was more rapid than previously suspected. This has implications for clinical decision making when patients with severe RA require quick, clinically meaningful disease control. The other study for upadacitinib reported that after 12 weeks and 24 weeks, 40% and 63.6% of patients, respectively, achieved US plus clinical remission [45].
Our results confirmed the data from the aforementioned studies, providing additional information about the change in disease activity in patients between weeks 2, 4, 8, and 12.
The improvement with tofacitinib was non-inferior to that of upadacitinib, with patients also experiencing improvement as early as week 2 of treatment, continuing up to month 6. However, our findings showed that this decrease was more linear and gradual, with a high correlation to all disease activity measurement scales, observable from week 2 as well. The findings of our ultrasound measurements are in line with what Germano et al. discovered in a real-world study with an identical study design [19], where joint and tendon scores were also significantly reduced at week 2. Curiously, as the authors themselves noted, they did not find any correlation between the variations of DAS28-CRP and any US scores at any visits. Our findings dispute this claim, showing that the gradual control offered by TOF is linked with improvement in DAS28 scores and seem to be more in line with other studies in the field, such as Razmjou et al. [26], whose study showed a correlation between US gray-scale and power Doppler parameters from baseline to week 2 and a persistent correlation with changes in CDAI and DAS28. Similarly, Ceccarelli et al. [46] found significant correlation between changes in DAS28-CRP and changes in the mean synovial hypertrophy score for both joints with and without Doppler activity. These results revealed that the improvement of the joints is directly related to the reduction of disease activity and the overall improvement of the condition of the patients.
The implementation of the EULAR/OMERACT scoring system in our study assessed joint inflammation using the combination of gray-scale and color power Doppler. The addition of the OMERACT composite score in combination with all of the other routine ultrasound scores offered a more precise evaluation of the regression of the joint inflammatory process. Our results are very similar to the very small number of studies from real-life clinical practice that evaluate the correlations between clinical parameters and US scores in patients with RA treated with tofacitinib [19]. However, one of the strengths of our study is the direct head-to-head comparison of disease activity assessed by ultrasound and DAS28-CRP, CDAI, and SDAI in patients treated with tofacitinib and upadacitinib in a real-life clinical setting. In addition, it presents the important role of ultrasound in the evaluation of remission and low disease activity and reveals discrepancies between the results reported by DAS28-CRP, CDAI, and SDAI. Another strength of our study is the frequent MSUS assessment of the patients (baseline and weeks 2, 4, 8, 12,16, and 24) and the results that highlight the early effectiveness of the treatment with both tsDMARDs.
The limitations of our study are the single-center participation, sample size of 47 patients, and open-label design, as well as the initial evaluation of disease activity. Some of these limitations are overcome by the fact that this is the reference rheumatology clinic for Bulgaria and admits a variety of complicated cases, so our sample includes difficult-to-treat patients. Furthermore, the collection of ultrasound measures for all 47 patients is a very intensive resource undertaking, so we can consider this study as the pilot for our hospital. Further enlargement of samples needs to be performed when the JAKi starts to be more frequently prescribed, and we can recruit more patients.
5. Conclusions
In conclusion, the results obtained provide evidence that treatment with both tofacitinib and upadacitinib leads to an early clinical response to treatment (week 2) and a long-lasting reduction in US signs of inflammation. However, further investigations are needed to make general conclusions about which JAK inhibitor has an early effect and whether the selectivity of the mechanism of action has a crucial role in determining the better effect on the signs of inflammation. Our study highlights the individual nature of patient responses to therapy.
Author Contributions
Conceptualization, V.B., N.S., G.P., and R.S.; methodology, V.B., N.S. and K.T.; software, K.T.; validation, S.E. and Z.A.; formal analysis, V.B. and K.T.; investigation, V.B., N.S., S.E. and Z.A.; resources, V.B. and N.S.; data curation, V.B. and K.T.; writing—original draft preparation, V.B. and K.T.; writing—review and editing, G.P. and R.S.; visualization, K.T. and N.S.; supervision, G.P. and R.S.; project administration, V.B. and N.S.; funding acquisition, All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the European Union Next-Generation EU through the National Recovery and Resilience Plan of the Republic of Bulgaria, project N BG-RRP 2.004-0004-C01.
Institutional Review Board Statement
The study was conducted in accordance with the 1989 Declaration of Helsinki and was approved by the Local Ethics Committee (Ethics Approval Protocol Number: 02/06.03.2018).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are unavailable due to privacy or ethical restrictions.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| ADR | adverse drug reaction |
| ACPA | anti-cyclic citrullinated peptide antibody |
| CDAI | clinical disease activity index |
| cs/b DMARDs | synthetic and/or biologic disease-modifying antirheumatic drugs |
| DAS28 | disease activity score |
| DAS28-CRP | disease activity score with C-reactive protein |
| DAS28-ESR | disease activity score with erythrocyte sedimentation rate |
| DMARD | disease-modifying antirheumatic drugs |
| EGA | evaluator global assessment of disease activity |
| EULAR | European Alliance of Associations for Rheumatology |
| HBV | hepatitis B virus |
| HCV | hepatitis C virus |
| GS | gray-scale |
| JAKi | Janus kinases inhibitors |
| LTB | interferon-gamma release assay for latent tuberculosis |
| MSUS | musculoskeletal ultrasonography |
| MTX | methotrexate |
| NHIF | National Health Insurance Fund |
| NYHA | New York Heart Association |
| OMERACT | Outcome Measures in Rheumatology |
| PD | power Doppler |
| PGA | patient global assessment of disease activity |
| RA | rheumatoid arthritis |
| RCT | randomized clinical trials |
| RF | rheumatoid factor |
| SDAI | simplified disease activity index |
| SH | synovial hypertrophy |
| SJC | swollen joint count |
| TJC | tender joint count |
| TOF | tofacitinib |
| USPD | ultrasound power Doppler |
| UPA | upadacitinib |
| US | ultrasound |
| USGS | ultrasound gray-scale |
Appendix A
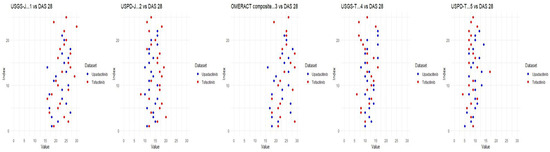
Figure A1.
Baseline ultrasonographic characteristics for DAS28 for both INNs.
Figure A1.
Baseline ultrasonographic characteristics for DAS28 for both INNs.
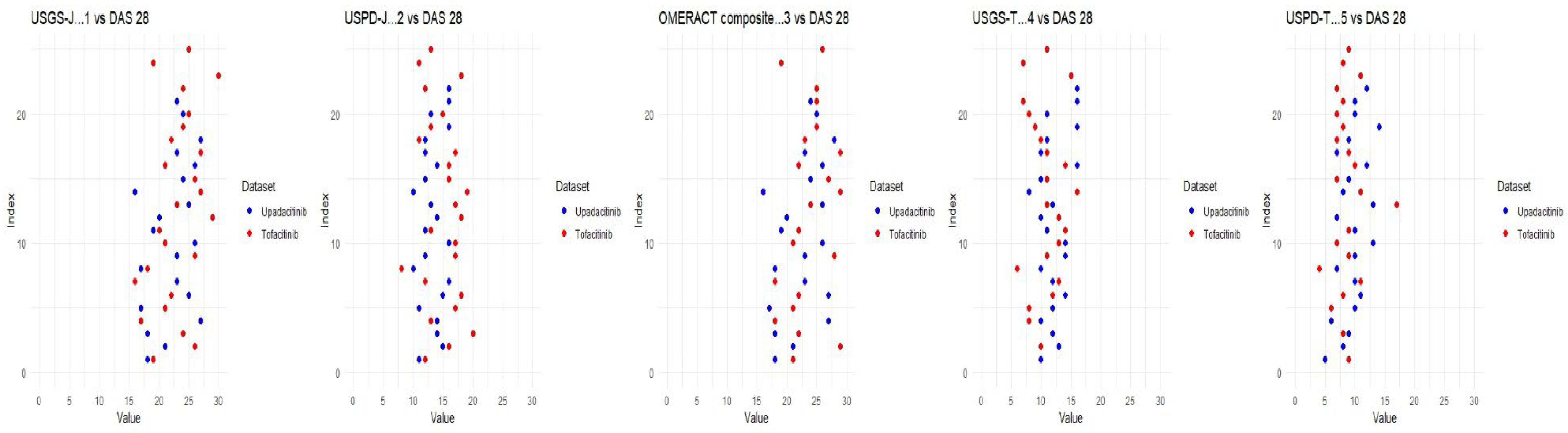
Appendix B
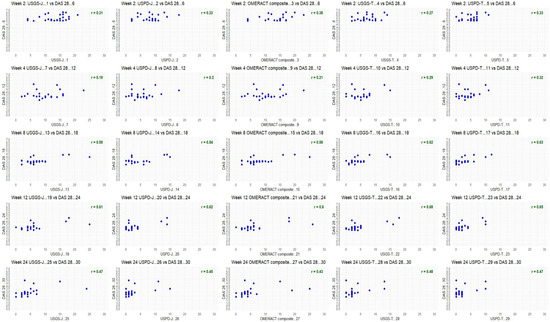
Figure A2.
DAS28 for upadacitinib; week by week comparison.
Figure A2.
DAS28 for upadacitinib; week by week comparison.
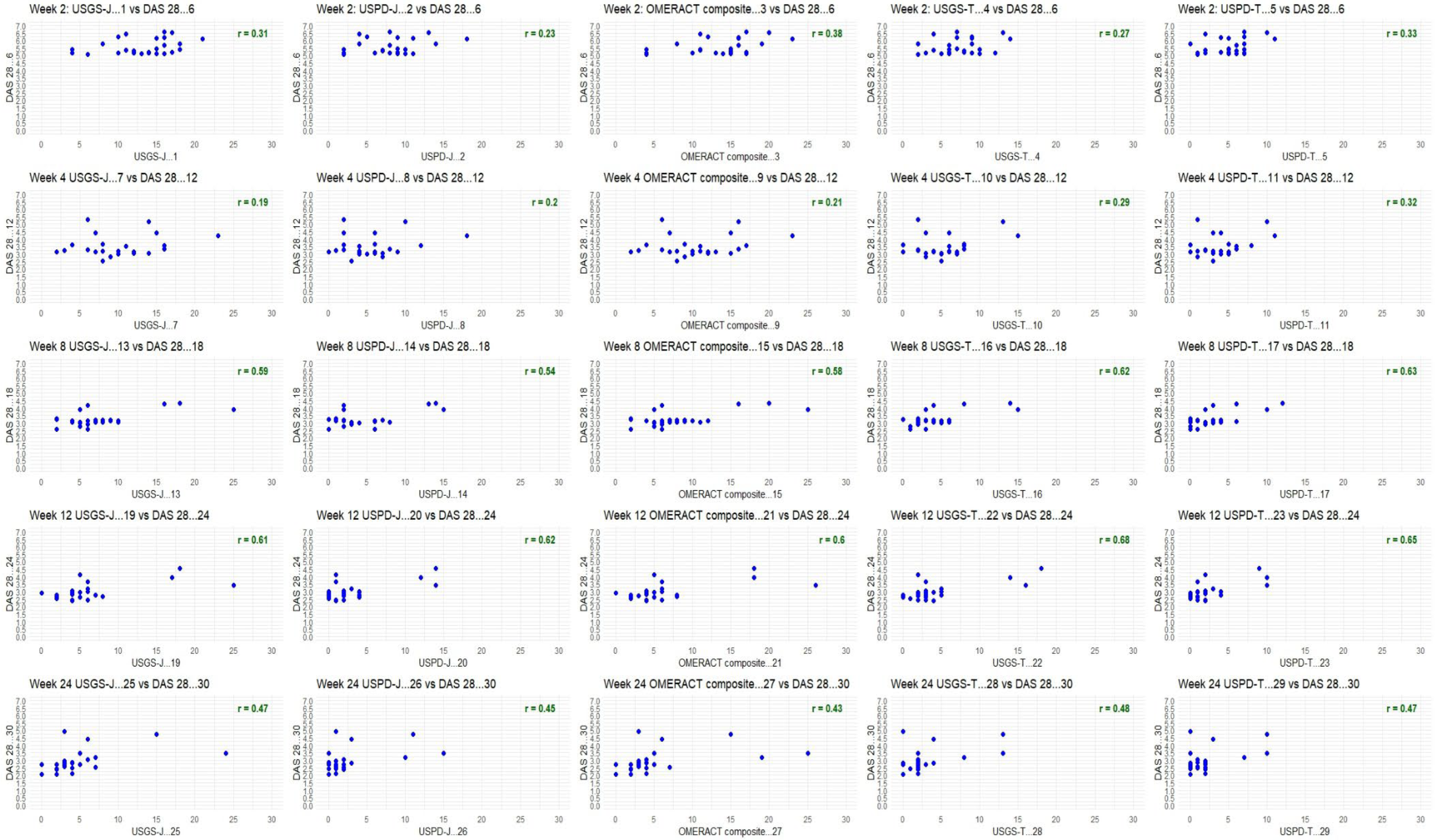
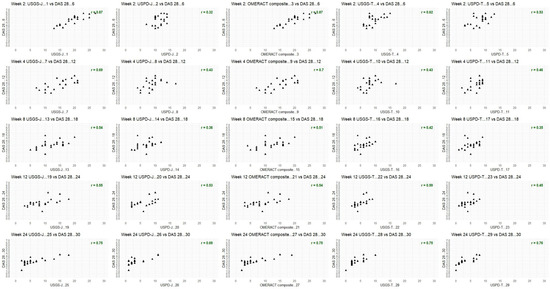
Figure A3.
DAS28 for tofacitinib; week by week comparison.
Figure A3.
DAS28 for tofacitinib; week by week comparison.
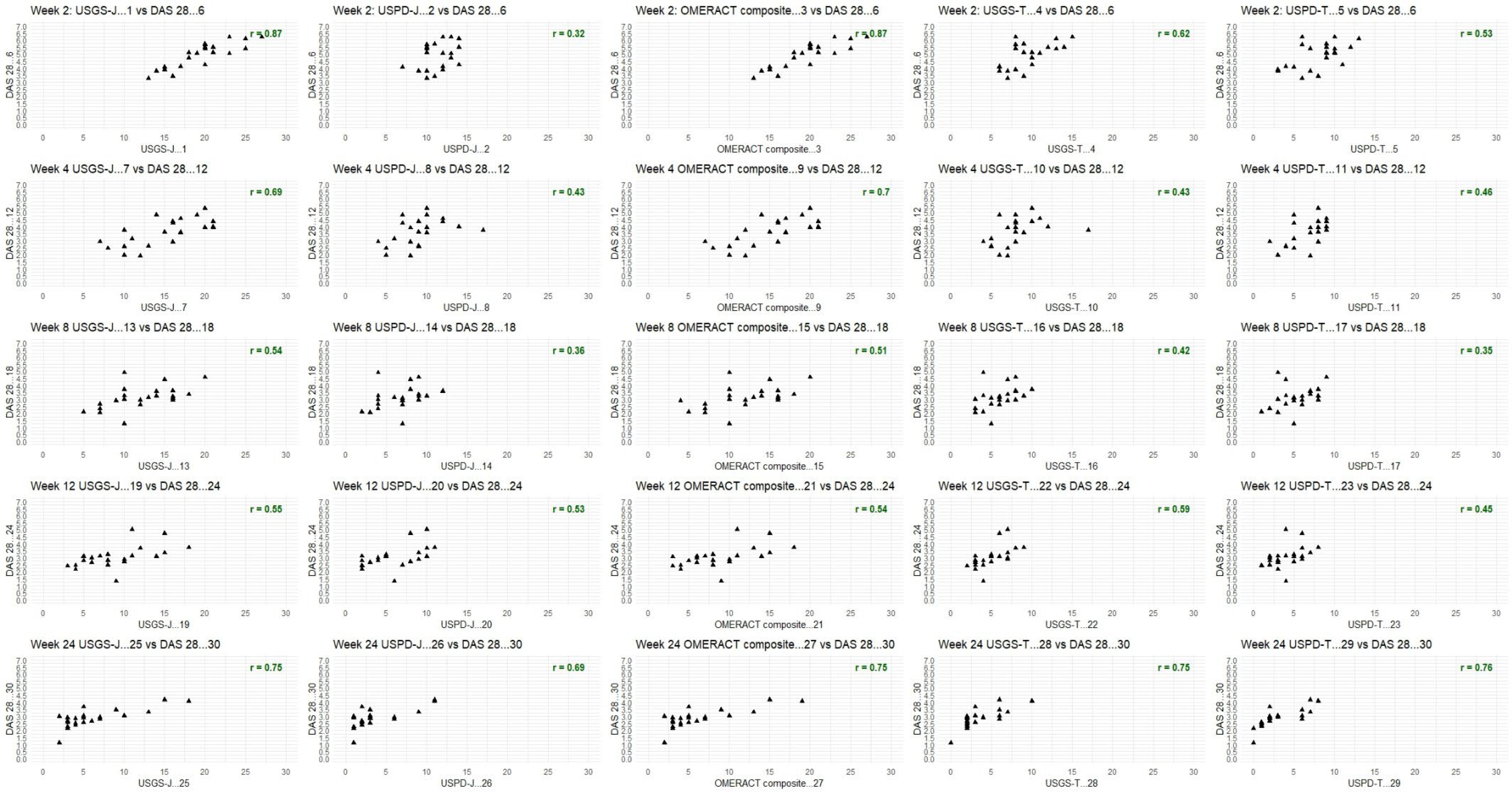
Figure A4.
DAS28 combined analysis for upadacitinib and tofacitinib; week by week comparison.
Figure A4.
DAS28 combined analysis for upadacitinib and tofacitinib; week by week comparison.
References
- Boyadzhieva, V.; Stoilov, N.; Ivanova, M.; Stoilov, R. Treatment of rheumatoid arthritis: csDMARDS versus bDMARDS. Prospective study to evaluate disease activity. Rheumatology 2019, 27, 3–15. [Google Scholar] [CrossRef]
- Boyadzhieva, V.; Stoilov, N.; Stoilov, R. Assessment of health-related quality of life in patients with rheumatoid arthritis and analysis of its change during csDMARDS and bDMARDS therapy after one year of follow-up. Rheumatology 2019, 27, 29–43. [Google Scholar] [CrossRef]
- Tachkov, K.; Boyadzhieva, V.; Stoilov, N.; Mitov, K.; Petrova, G. Is There a Symmetry in Disease Control and Quality of Life of Patients with Rheumatoid Arthritis Treated with Biological Therapy? Symmetry 2021, 13, 538. [Google Scholar] [CrossRef]
- Smolen, J.S.; Aletaha, D.; Bijlsma, J.W.J.; Breedveld, F.; Boumpas, D.; Burmester, G.; Combe, B.; Cutolo, M.; De Wit, M.; Dougados, M.; et al. Treating rheumatoid arthritis to target: Recommendations of an international task force. Ann. Rheum. Dis. 2010, 69, 631–637. [Google Scholar] [CrossRef]
- Singh, J.A.; Saag, K.G.; Bridges, S.L., Jr.; Akl, E.A.; Bannuru, R.R.; Sullivan, M.C.; Vaysbrot, E.; McNaughton, C.; Oscani, M.; Shmerling, R.H.; et al. 2015 American College of Rheumatology Guideline for the treatment of rheumatoid arthritis. Arthritis Rheumatol. 2016, 68, 1–26. [Google Scholar] [CrossRef]
- National Institute for Health and Clinical Excellence (NICE). 2 Rheumatoid Arthritis: The Management of Rheumatoid Arthritis in Adults; Royal College of Physicians: London, UK; National Collaborating Centre for Chronic Conditions: London, UK, 2009. [Google Scholar]
- van Vollenhoven, R. Treat-to-target in rheumatoid arthritis—Are we there yet? Nat. Rev. Rheumatol. 2019, 15, 180–186. [Google Scholar] [CrossRef]
- Wells, A.F.; Curtis, J.R.; Betts, K.A.; Douglas, K.; Du Xiaoyan, E.; Ganguli, A. Systematic literature review and meta-analysis of tumor necrosis factor-alpha experienced rheumatoid arthritis. Clin. Ther. 2017, 39, 1680–1694.e2. [Google Scholar] [CrossRef]
- Tracey, D.; Klareskog, L.; Sasso, E.H.; Salfeld, J.G.; Tak, P.P. Tumor necrosis factor antagonist mechanisms of action: A comprehensive review. Pharmacol. Ther. 2008, 117, 244–279. [Google Scholar] [CrossRef]
- Fleischmann, R.; Mysler, E.; Hall, S.; Kivitz, A.J.; Moots, R.J.; Luo, Z.; De Masi, R.; Soma, K.; Zhang, R.; Takiya, L.; et al. Efficacy and safety of tofacitinib monotherapy, tofacitinib with methotrexate, and adalimumab with methotrexate in patients with rheumatoid arthritis (ORAL Strategy): A phase 3b/4, double- blind, head-to-head, randomised controlled trial. Lancet 2017, 390, 457–468. [Google Scholar] [CrossRef]
- van der Heijde, D.; Strand, V.; Tanaka, Y.; Keystone, E.; Kremer, J.; Zerbini, C.A.F.; Cardiel, M.H.; Cohen, S.; Nash, P.; Song, Y.-W.; et al. Tofacitinib in combination with methotrexate in patients with rheumatoid arthritis: Clinical efficacy, radiographic, and safety outcomes from a twenty-four-month, phase III study. Arthritis Rheumatol. 2019, 71, 878–891. [Google Scholar] [CrossRef]
- Strand, V.; de Vlam, K.; Covarrubias-Cobos, J.A.; Mease, P.J.; Gladman, D.D.; Graham, D.; Wang, C.; Cappelleri, J.C.; Hendrikx, T.; Hsu, M.-A. Tofacitinib or adalimumab versus placebo: Patient-reported outcomes from OPAL Broaden-a phase III study of active psoriatic arthritis in patients with an inadequate response to conventional synthetic disease-modifying antirheumatic drugs. RMD Open 2019, 5, e000806. [Google Scholar] [CrossRef] [PubMed]
- Kavanaugh, A.; Kremer, J.; Ponce, L.; Cseuz, R.; Reshetko, O.V.; Stanislavchuk, M.; Greenwald, M.; Van der, A.A.; Vanhoutte, F.; Tasset, C.; et al. Filgotinib (GLPG0634/GS-6034), an oral selective JAK1 inhibitor, is effective as monotherapy in patients with active rheumatoid arthritis: Results from a randomised, dose-finding study (DARWIN 2). Ann. Rheum. Dis. 2017, 76, 1009–1019. [Google Scholar] [CrossRef] [PubMed]
- Genovese, M.C.; Kremer, J.; Zamani, O.; Ludivico, C.; Krogulec, M.; Xie, L.; Beattie, S.D.; Koch, A.E.; Cardillo, T.E.; Rooney, T.P.; et al. Baricitinib in patients with refractory rheumatoid arthritis. N. Engl. J. Med. 2016, 374, 1243–1252. [Google Scholar] [CrossRef] [PubMed]
- Genovese, M.C.; Fleischmann, R.; Combe, B.; Hall, S.; Rubbert-Roth, A.; Zhang, Y.; Zhao, Y.; Mohamed, M.-E.F.; Meerwein, S.; Pangan, A.L. Safety and efficacy of upadacitinib in patients with active rheumatoid arthritis refractory to biologic disease-modifying antirheumatic drugs (SELECT-BEYOND): A double-blind, randomised controlled phase 3 trial. Lancet 2018, 391, P2513–P2524. [Google Scholar] [CrossRef]
- Burmester, G.R.; Kremer, J.M.; Van den Bosch, F.; Kivitz, A.; Bessette, L.; Li, Y.; Zhou, Y.; Othman, A.A.; Pangan, A.L.; Camp, H.S. Safety and efficacy of upadacitinib in patients with rheumatoid arthritis and inadequate response to conventional synthetic disease-modifying anti-rheumatic drugs (SELECT-NEXT): A randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2018, 391, 2503–2512. [Google Scholar] [CrossRef]
- Märker-Hermann, E.; Nitschmann, S. Upadacitinib bei Patienten mit rheumatoider Arthritis: SELECT-NEXT und SELECT-BEYOND. (Upadacitinib in patients with rheumatoid arthritis: SELECT-NEXT and SELECT-BEYOND). Internist 2019, 60, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.S.; Pangan, A.L.; Emery, P.; Rigby, W.; Tanaka, Y.; Vargas, J.I.; Zhang, Y.; Damjanov, N.; Friedman, A.; Othman, A.A.; et al. Upadacitinib as monotherapy in patients with active rheumatoid arthritis and inadequate response to methotrexate (SELECT-MONOTHERAPY): A randomised, placebo-controlled, double-blind phase 3 study. Lancet 2019, 393, 2303–2311, Erratum in Lancet 2019, 393, 2590. [Google Scholar] [CrossRef]
- Germanò, G.; Macchioni, P.; Maranini, B.; Ciancio, G.; Bonazza, S.; Govoni, M.; Salvarani, C. Ultrasound response to tofacitinib in patients with rheumatoid arthritis: Data from a multicenter 24 weeks prospective study. Front. Med. 2022, 9, 990317. [Google Scholar] [CrossRef]
- Fleischmann, R.M.; Genovese, M.C.; Enejosa, J.V.; Mysler, E.; Bessette, L.; Peterfy, C.; Durez, P.; Ostor, A.; Li, Y.; Song, I.-H. Safety and effectiveness of upadacitinib or adalimumab plus methotrexate in patients with rheumatoid arthritis over 48 weeks with switch to alternate therapy in patients with insufficient response. Ann. Rheum. Dis. 2019, 78, 1454–1462. [Google Scholar] [CrossRef]
- The DAS28 Score. Available online: https://nras.org.uk/resource/the-das28-score/ (accessed on 15 January 2021).
- Prevoo, M.L.; van’t Hof, M.A.; Kuper, H.H.; van Leeuwen, M.A.; van de Putte, L.B.; van Riel, P.L. Modified disease activity scores that include twenty-eight–joint counts: Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995, 38, 44–48. [Google Scholar] [CrossRef]
- Smolen, J.S.; Breedveld, F.C.; Schiff, M.H.; Kalden, J.R.; Emery, P.; Eberl, G.; van Riel, P.L.; Tugwell, P. A simplified disease activity index for rheumatoid arthritis for use in clinical practice. Rheumatology 2003, 42, 244–257. [Google Scholar] [CrossRef] [PubMed]
- Kremer, J.; Li, Z.G.; Hall, S.; Fleischmann, R.; Genovese, M.; Martin-Mola, E.; Isaacs, J.D.; Gruben, D.; Wallenstein, G.; Krishnaswami, S.; et al. Tofacitinib in combination with nonbiologic disease-modifying antirheumatic drugs in patients with active rheumatoid arthritis: A randomized trial. Ann. Intern. Med. 2013, 159, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Terslev, L.; Naredo, E.; Aegerter, P.; Wakefield, R.J.; Backhaus, M.; Balint, P.; Bruyn, G.A.; Filippucci, E.; Grassi, W.; Lagnocco, A.; et al. Scoring ultrasound synovitis in rheumatoid arthritis: A EULAR OMERACT ultrasound taskforce-Part 2: Reliability and application to multiple joints of a standardised consensus-based scoring system. RMD Open 2017, 3, e000427. [Google Scholar] [CrossRef]
- Razmjou, A.A.; Brook, J.; Elashoff, D.; Kaeley, G.; Choi, S.; Kermani, T.; Ranganath, V.K. Ultrasound and multi-biomarker disease activity score for assessing and predicting clinical response to tofacitinib treatment in patients with rheumatoid arthritis. BMC Rheumatol. 2020, 4, 55. [Google Scholar] [CrossRef]
- Di Matteo, A.; Mankia, K.; Azukizawa, M.; Wakefield, R.J. The Role of Musculoskeletal Ultrasound in the Rheumatoid Arthritis Continuum. Curr. Rheumatol. Rep. 2020, 22, 41. [Google Scholar] [CrossRef] [PubMed]
- Elangovan, S.; Kiat Tan, Y. The Role of Musculoskeletal Ultrasound Imaging in Rheumatoid Arthritis. Ultrasound Med. Biol. 2020, 46, 1841–1853. [Google Scholar] [CrossRef]
- Felson, D.T.; Smolen, J.S.; Wells, G.; Zhang, B.; van Tuyl, L.H.; Funovits, J.; Aletaha, D.; Allaart, C.F.; Bathon, J.; Bombardieri, S.; et al. American college of rheumatology/European league against rheumatism provisional definition of remission in rheumatoid arthritis for clinical trials. Ann. Rheum. Dis. 2011, 70, 404–413. [Google Scholar] [CrossRef] [PubMed]
- Kgoebane, K.; Ally, M.M.T.M.; Duim-Beytell, M.C.; Suleman, F.E. The role of imaging in rheumatoid arthritis. SA J. Radiol. 2018, 22, 1316. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- National Council of Prices and Reimbursement of Medicines. Pharmacotherapeutic Guideline on Rheumatology. Available online: https://www.ncpr.bg/images/farmako-terapevtichni/2019/01.04.2019/ftr_po_revmatologiia.pdf (accessed on 14 May 2025).
- National Health Insurance Fund. Requirements for Rheumatology. Available online: https://www.nhif.bg/upload/22768/18.%D0%98%D0%B7%D0%B8%D1%81%D0%BA%D0%B2%D0%B0%D0%BD%D0%B8%D1%8F%20%D0%BD%D0%B0%20%D0%9D%D0%97%D0%9E%D0%9A%20%20%D1%80%D0%B5%D0%B2%D0%BC%D0%B0%D1%82%D0%BE%D0%BB%D0%BE%D0%B3%D0%B8%D1%8F.pdf (accessed on 14 May 2025).
- Aletaha, D.; Smolen, J.S. Diagnosis and management of rheumatoid arthritis: A review. JAMA 2018, 320, 1360–1372. [Google Scholar] [CrossRef]
- Sanmartí, R.; Corominas, H. Upadacitinib for Patients with Rheumatoid Arthritis: A Comprehensive Review. J. Clin. Med. 2023, 12, 1734. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Harrington, R.; Al Nokhatha, S.A.; Conway, R. JAK Inhibitors in Rheumatoid Arthritis: An Evidence-Based Review on the Emerging Clinical Data. J. Inflamm. Res. 2020, 13, 519–531. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- van Vollenhoven, R.; Takeuchi, T.; Pangan, A.L.; Friedman, A.; Mohamed, M.F.; Chen, S.; Rischmueller, M.; Blanco, R.; Xavier, R.M.; Strand, V. Efficacy and safety of upadacitinib monotherapy in methotrexate-naïve patients with moderately to severely active rheumatoid arthritis (SELECT-EARLY): A randomized, double-blind, active-comparator, multi-center, multi-country trial. Arthritis Rheumatol. 2020, 72, 1607–1620. [Google Scholar] [CrossRef]
- Rubbert-Roth, A.; Enejosa, J.; Pangan, A.L.; Haraoui, B.; Rischmueller, M.; Khan, N.; Zhang, Y.; Martin, N.; Xavier, R.M. Trial of Upadacitinib or Abatacept in Rheumatoid Arthritis. N. Engl. J. Med. 2020, 383, 1511–1521. [Google Scholar] [CrossRef]
- Rubbert-Roth, A.; Enejosa, J.; Pangan, A.; Xavier, R.; Haraoui, B.; Rischmueller, M.; Khan, N.; Zhang, Y.; Martin, N.; Genovese, M.C. Efficacy and safety of upadacitinib versus abatacept in patients with active rheumatoid arthritis and prior inadequate response or intolerance to biologic disease-modifying anti-rheumatic drugs (SELECT-CHOICE): A double-blind, rnadomized controlled Phase 3 trial. Ann. Rheum. Dis. 2020, 79, 1011. [Google Scholar]
- Fleischmann, R.; Mease, P.J.; Schwartzman, S.; Hwang, L.J.; Soma, K.; Connell, C.A.; Takiya, L.; Bananis, E. Efficacy of tofacitinib in patients with rheumatoid arthritis stratified by background methotrexate dose group. Clin. Rheum. 2017, 36, 15–24. [Google Scholar] [CrossRef]
- Aletaha, D.; Alasti, F.; Smolen, J.S. Rituximab dissociates the tight link between disease activity and joint damage in rheumatoid arthritis patients. Ann. Rheum. Dis. 2013, 72, 7–12. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Fleischmann, R.; Hall, S.; Wilkinson, B.; Bradley, J.D.; Gruben, D.; Koncz, T.; Krishaswami, S.; Wallenstein, G.V.; Zang, C.; et al. Tofacitinib versus methotrexate in rheumatoid arthritis. N. Engl. J. Med. 2014, 370, 2377–2386. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, R.; Pangan, A.L.; Mysler, E.; Bessette, L.; Peterfy, C.; Durez, P.; Ostor, A.J.; Li, Y.; Zhao, Y.; Othman, A.; et al. A phase 3, randomized, double-blind study comparing upadacitinib to placebo and to adalimumab, in patients with active rheumatoid arthritis with inadequate response to methotrexate. Arthritis Rheumatol. 2018, 70 (Suppl. S10), 1788–1800. [Google Scholar]
- Hameed, B.; Pilcher, J.; Heron, C.; Kiely, P.D. The relation between composite ultrasound measures and the DAS28 score, its components and acute phase markers in adult RA. Rheumatology 2008, 47, 476–480. [Google Scholar] [CrossRef] [PubMed]
- Baldi, C.; Parisi, S.; Falsetti, P.; Sota, J.; Ditto, M.C.; Capassoni, M.; D’alessandro, M.; Conticini, E.; Nacci, F.; Peroni, C.L.; et al. Efficacy and Safety of Upadacitinib in Rheumatoid Arthritis: Real-Life Experience from a Prospective Longitudinal Multicentric Study. J. Clin. Med. 2024, 13, 401. [Google Scholar] [CrossRef]
- Picchianti Diamanti, A.; Cattaruzza, M.S.; Salemi, S.; De Rosa, R.; Sesti, G.; De Lorenco, C.; Felice, G.M.; Fediani, B.; Baldi, C.; Chimenti, M.S.; et al. Clinical and Ultrasonographic Remission in Bio-naïve and Bio-failure Patients with Rheumatoid Arthritis at 24 Weeks of Upadacitinib Treatment: The UPARAREMUS Real-Life Study. Rheumatol. Ther. 2024, 11, 1347–1361. [Google Scholar] [CrossRef] [PubMed]
- Ceccarelli, F.; Spinelli, F.R.; Garufi, C.; Mancuso, S.; Alessandri, C.; Di Franco, M.; Orefice, V.; Pacucci, V.A.; Pirone, C.; Priori, R.; et al. The role of musculoskeletal ultrasound in predicting the response to JAK inhibitors: Results from a monocentric cohort. Clin. Exp. Rheumatol. 2022, 40, 921–927. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).