Abstract
Hyaluronan, a key component of the extracellular matrix, plays a crucial role in joint development and maintenance. In order to determine the role of hyaluronan function in joint development and homeostasis, conditional loss-of-function experiments of Hyaluronan Synthase 2 (Has2) were carried out in mice. Has2 depletion in limb mesenchymal cells led to severely shortened limbs with appendicular joints that are deformed, decreased proteoglycan content as characterized by Safranin-O staining, and severely pitted epiphyseal ends of long bones and deformed joints as viewed by micro-CT reconstructions. The embryonic deletion of Has2 in mesoderm mesenchyme of limbs by Prx1-Cre confirmed its involvement in joint development, while in situ hybridization and hyaluronan staining confirmed Has2 expression and abundant accumulation of hyaluronan in the onset of joint formation, the joint interzone. These findings position Has2 as the main hyaluronan-making enzyme in articular cartilage and highlight its essential function in joint formation and retention of proteoglycans of the extracellular matrix of the cartilage.
1. Introduction
Synovial joints play a crucial role in movement and weight bearing. The epiphyseal ends of endochondral bones are covered by articular cartilage, a hyaline-based cartilage present within synovial joints [1,2]. The development of synovial joints is initiated by a specialized group of skeletal progenitors located in a distinct region of the developing endochondral bones known as the joint interzone [1,3]. Formation of the interzone occurs when differentiated chondrocytes lose their chondrocyte-like characteristics, alter their fate as growth plate chondrocytes, and acquire a new fate as progenitors of the synovial joint [1,2,3,4,5]. The embryonic development of synovial joints starts with interzone formation and cavitation, where condensed mesenchymal cells create a joint cavity. During postnatal maturation, cartilage undergoes structural transformation into three distinct zones known as superficial, intermediate, and deep zones, which exhibit unique cell and matrix characteristics necessary for smooth joint movement and mechanical stress resistance [6].
In articular cartilage, hyaluronan, a large glycosaminoglycan (GAG), represents an abundant component of the extracellular matrix [7,8,9]. Research has demonstrated the critical role of hyaluronan in synovial joint lubrication and overall joint health [9,10,11,12]. Conversely, clinical studies have shown that levels of hyaluronan are decreased in osteoarthritic cartilage [13,14,15]. Thus, an inverse correlation exists between hyaluronan abundance and joint health.
To better understand the function of hyaluronan, genetic mouse models for the Hyaluronan Synthase (Has) genes have been developed [16,17,18]. These genes (Has1, Has2, Has3) encode enzymes responsible for the production of hyaluronan. Has2 is the primary isoform expressed during endochondral bone formation and is responsible for hyaluronan production during skeletal development [8,19,20]. Prior studies on conditional or constitutive Has2 loss of function studies have highlighted its critical role in skeletal development [16,21]. Specifically, when Has2 was conditionally inactivated in using Prx1-Cre mice, defects were observed in joint formation, growth plate organization and gene expression of Ihh and Col10, and formation of the secondary ossification center [16]. While previous studies have demonstrated the critical role of Has2 in embryonic skeletal development, the long-term impact of its disruption on postnatal joint development and homeostasis remains unclear. This study explores the hypothesis that Hyaluronan Synthase 2 (Has2) is crucial for synovial joint development throughout the embryonic stage and into postnatal life. Specifically, we investigate that the conditional inactivation of Has2 in limb mesenchyme leads to abnormal joint development and long-term cartilage abnormalities.
In this report, we focus on the role of Has2 and hyaluronan in postnatal joint formation using the Prx1-Cre mice model.
Prx1 is a transcription factor essential for limb development, regulated by BMP and FGF pathways. Its limb-specific enhancer drives Cre recombinase in transgenic mice, enabling precise gene editing from embryonic day 9.5, with recombination complete by day 10.5. This tool allows spatially and temporally controlled studies of limb development. The Prx1-Cre model is used to conditionally inactivate Has2 in limb mesenchyme, aiding research on its developmental role in both forelimbs and hindlimbs [16,22,23,24].
Our results reveal that the conditional loss of Has2 at embryonic ages has long-term postnatal consequences, including diminished proteoglycan levels and progressive joint (postnatal maturation). The synthesis of hyaluronan via Has2 is essential for preserving extracellular matrix stability and protecting against joint degenerative conditions [25,26]. This work provides novel evidence for the essential role of hyaluronan through the long-term consequences of embryonic hyaluronan loss on joint health and postnatal joint integrity.
2. Material and Methods
2.1. Mice
All animal experiments were performed under an approved protocol by the Institutional Animal Care and Use Committee at the University of Hartford (IACUC# UH-2023-01). We generated phenotypically normal wildtype Has2fl/fl mice with a functional conditional allele by targeting exon 2 (containing the start codon and transmembrane domains) using loxP-flanked Neo/DTA cassettes in 129SvJ-derived clones as described [16]. Prx1-Cre mice [22] were generously provided by Dr. Clifford Tabin. It was generated using a limb-specific enhancer to drive Cre recombinase in limb bud mesenchyme by 9.5 dpc, enabling targeted gene deletion for studying limb-patterning genes during limb development. All mice were housed in a room maintained on a 12:12 h light/dark cycle with controlled temperature and humidity and fed a standard rodent diet.
2.2. Mouse Crosses
Has2fl/fl homozygous females were intercrossed with Prx1-Cre male mice. All offspring showed normal phenotypes. Prx1-Cre; Has2fl/+ heterozygous males from the offspring were intercrossed with Has2fl/fl to generate mutant Prx1-Cre; Has2Δ/Δ mice. Age-matched littermates of Has2fl/fl served as wild-type embryo/mouse controls for mutant Prx1-Cre; Has2Δ/Δ mice. Logan et al. reported that Prx1-Cre can exhibit germline recombination in offspring of female Prx1-Cre carriers but not in offspring of male carriers, indicating sex-dependent leakiness [22]. Only male mice were used in the subsequent study due to potential Cre leaky in females, which could affect the interpretation of genotype effects.
2.3. Tissue Processing for Histology and Staining
Tissues were fixed with 4% paraformaldehyde for 2–4 h for embryonic day 14.5 (E14.5) and 2–3 days for post-natal tissues collected at neonatal day 3 (P3) and day 21 (P21). Postnatal tissues were decalcified with 14% EDTA for 3–5 days before paraffin embedding. Moreover, 8 μm paraffin sections were collected.
2.4. In Situ Hybridization
In situ hybridization was carried out on 8 μm paraffin tissue sections using a 33P labeled probe or digoxigenin-labeled probe for Has2 as previously described [16]. The Has2 probe was a 752 bp cDNA probe extended from 1461–2212 at the 3′ end of the cDNA derived from XmaI/EcoRI digestion of a pCI-Neo vector containing full-length mouse Has2. For spatial mapping, dark-field silver grains overlaid onto corresponding Hematoxylin-stained bright-field images in Photoshop version 26.6.1.
2.5. Histochemical Staining
Histochemical staining was used to detect the amount of hyaluronan accumulation in embryonic joints and adult joints by 2 μg/mL biotinylated hyaluronan-binding protein (HABP) overnight at 4 °C (Sigma-Aldrich, Saint Louis, MO, USA) as previously described [8,16]. Negative HABP staining control sections were pretreated for 2 h at 55 °C with 200 TRU/mL of Streptomyces hyalurolyticus hyaluronidase (Sigma-Aldrich, Saint Louis, MO, USA), which specifically degrades HA.
Histochemical staining with Safranin-O (Sigma-Aldrich, Saint Louis, MO, USA) with fast green (Sigma-Aldrich, Saint Louis, MO, USA) counterstaining was used to evaluate cartilage proteoglycan content and chondrocyte cellular morphology [16]. Paraffin sections were stained with Weigert’s Iron Hematoxylin and 0.02% aqueous Fast Green, followed by rinsing with 1% acetic acid and staining with 0.1% aqueous Safranin O. Alcian Blue and Alizarin Red staining were used for whole mount skeletal staining to visualize cartilage and mineralized bone, respectively [16]. Briefly, embryos and neonatal mice collected were fixed in 95% EtOH for 1 week, stained in 0.015% Alcian Blue (Sigma-Aldrich, Saint Louis, MO, USA) in 95% EtOH Glacial Acetic Acid for 3–4 days, and 75 µg/mL Alizarin Red (Sigma-Aldrich, Saint Louis, MO, USA) in 1% KOH for 1 day. Tissues were cleared in graded KOH and photography in 75% glycerol [16,27].
2.6. Microcomputed Tomography
The architecture of joints from both normal wildtype Has2fl/fl littermate and mutant Prx1-Cre; Has2Δ/Δ were compared using 3-dimensional (3D) microcomputed tomography (µCT) imaging (μCT40, ScanCo Medical AG, Bassersdorf, Switzerland) at UConn Health [28]. The limbs were scanned in water using high-resolution settings, with an energy level of 55 kVp and an intensity of 145 μA, at an integration time of 500 ms. The images were reconstructed and calibrated to an isotropic voxel size of 8 mm3.
3. Results
3.1. Genetic Loss of Has2 in the Limb Bud Mesenchyme Causes Joint Defects
To investigate the effect of Has2 on synovial joint formation, we examined Prx1-Cre; Has2Δ/Δ mice, in which Has2 was conditionally inactivated by Prx1-Cre. In these mice, Has2 was removed in early limb bud mesenchyme prior to the onset of mesenchymal condensations and the formation of joint interzones. Similar to previous reports [16], the appendicular skeleton of mutant Prx1-Cre; Has2Δ/Δ mice was severely compromised at neonatal day 3 (P3). The forelimbs and hindlimbs of mutant Prx1-Cre; Has2Δ/Δ mice were dramatically shorter than their wildtype Has2fl/fl littermates. The distal limb region and paws were also shortened (Figure 1A,B). In all sixteen P3 mutant Prx1-Cre; Has2Δ/Δ mice examined, the limbs displayed consistent phenotypic changes, with shortening of the skeletal elements. The skeletal elements of P3 mutant Prx1-Cre; Has2Δ/Δ from proximal to distal—stylopod, zeugopod, and autopod—were shortened by 58%, 60%, and 35%, respectively, in the forelimbs and by 52%, 58%, and 32%, respectively, in the hindlimbs comparing to the wildtype Has2fl/fl mice.
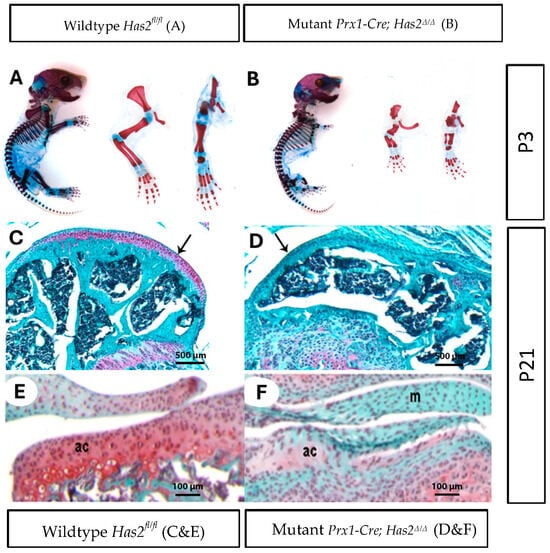
Figure 1.
Limb bud mesenchyme-specific knockout of Has2 induced severe joint defects and impaired the differentiation and formation of articular cartilage. (A,B) Whole mount staining of cartilage and mineralized bone with Alcian Blue and Alizarin Red, respectively, in neonatal day 3 (P3) (A) wildtype Has2fl/fl and (B) mutant Prx1-Cre; Has2Δ/Δ mice. Left to right: whole body, forelimb, hindlimb (0.63×). (C–F) Paraffin sections of the joint surface of 3-week-old (C) wildtype Has2fl/fl and (D) mutant Prx1-Cre; Has2Δ/Δ femurs stained with Safranin-O (red) for proteoglycan accumulation (counterstained with fast green). The wildtype Has2fl/fl femur is capped by a thick layer of articular cartilage, which stains intensely with Safranin-O, while the hyaluronan-deficient femur is capped by a thin layer of tissue that does not stain with Safranin-O, indicating normal articular cartilage is not present (4×) (pointed by black arrow). (E,F) Sections of the tibia joint surface of 3-week-old (P21) (E) wildtype Has2fl/fl and (F) mutant Prx1-Cre; Has2Δ/Δ mice stained with Safranin-O/fast green. In the wildtype Has2fl/fl tibia, a layer of articular cartilage (ac) with round chondrocytes and abundant Safranin-O matrix is present, but the joint surface in the hyaluronan-deficient tibia is covered by a highly cellular tissue with flattened cells and little or no Safranin-O staining, indicating initial articular cartilage differentiation is impaired. Menisci (M) in the hyaluronan-deficient joint also showed no or very little Safranin-O staining. This underscores the importance of Has2 in joint development for different types of chondrocytes (20×).
Next, we determined the long-term consequences of Prx1-Cre-mediated inactivation of Has2 in 3-week-old animals. Safranin-O staining on joint tissue sections revealed remarkable differences in proteoglycan content within the cartilage matrix. Whereas the wildtype Has2fl/fl femurs were capped by a thick layer of Safranin-O-positive articular cartilage, Has2 deficient femurs were capped by a thin layer of Safranin-O-negative tissue (Figure 1C,D), black arrows demarcate articular surface). Not surprisingly, significant reductions in cartilage proteoglycan content were also observed in mutant Prx1-Cre; Has2Δ/Δ tibia (Figure 1E,F). Moreover, Has2 conditional mutants appeared to have less cartilage matrix production and denser intercellular distances among chondrocytes within the matrix.
3.2. Has2 Is Essential for Proper Bone Formation at Synovial Joints
We further examined the forelimb and wrist joints of wildtype Has2fl/fl and mutant Prx1-Cre; Has2Δ/Δ mice at 3 weeks of age using 3-D µCT (Figure 2A–D). Remarkably, Has2 conditional mutants showed severe erosion at the epiphyseal ends of bones as evidenced by extensive pitting (Figure 2B,D). Arch-like deformations of the radius and ulna were also observed in Has2 mutants (Figure 2B,D).
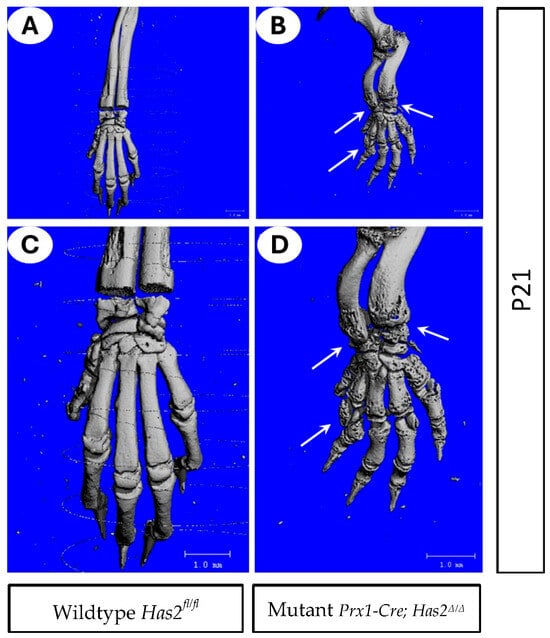
Figure 2.
Mutant Prx1-Cre; Has2Δ/Δ mice exhibited improper bone formation (A–D) 3-D µCT images of forelimb and wrist joints of 3-week-old (P21) (A,C) wildtype Has2fl/fl and (B,D) mutant Prx1-Cre; Has2Δ/Δ mice. (C,D) are magnified images of (A,B), respectively. Joint spaces were intact in wild-type littermate (A,C) but showed erosion of bone and heavily eroded wrist (arrows in (B,D)). Arch-like deformation of radius and ulna was also observed in the mutant.
3.3. Has2 and Hyaluronan Are Localized at the Joint Interzone
To provide additional evidence for the early role of Has2 and hyaluronan in joint formation, Has2 expression and hyaluronan production were examined in E14 embryos in phalangeal joints. In situ hybridization of Has2 mRNA revealed selective expression at the joint interzone (Figure 3A, white arrows). We further observed the abundant accumulation of hyaluronan in the interzone, as well as in the surrounding chondrocytes (Figure 3B; asterisk) by immunostaining of hyaluronan with biotinylated hyaluronan-binding protein (HABP) (Figure 3B, black arrow).
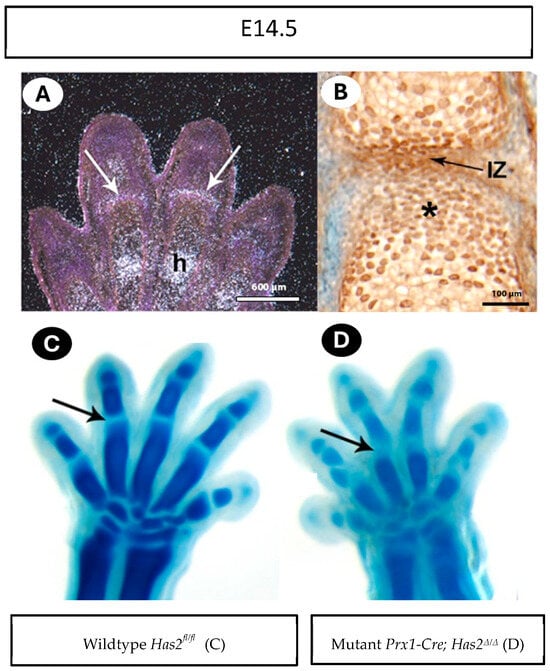
Figure 3.
Has2 and hyaluronan are localized at the joint interzone. (A) At embryonic day 14.5 (E14.5), Has2 mRNA is present in joint interzones (arrows) and chondrocytes undergoing hypotrophy (h). (B) HABP staining shows abundant hyaluronan in the interzone (IZ) and abundant in the cartilage cells adjacent to IZ (asterisk). (C,D) E14.5 wildtype Has2fl/fl and mutant Prx1-Cre; Has2Δ/Δ paws stained with Alcian blue. The wildtype Has2fl/fl joint interzones are narrow (arrow in (A)), while Has2-deficient interzones are wide (arrow in (B)).
The presence of Has2 and hyaluronan in the joint interzone suggests a direct and early role for hyaluronan in joint formation (Figure 3A). Indeed, Alcian blue staining of the paws of E14.5 embryos revealed that the interzone regions of mutant Prx1-Cre; Has2Δ/Δ mutants were wider and less defined than their wildtype Has2fl/fl littermates (Figure 3C,D, arrows).
4. Discussion
Synovial joints are essential for our mobility and diseases that impact joint function are closely tied to quality of life and our overall health. Diseases of the joint include congenital conditions that can result in joint deformity resulting in premature malfunction, sometimes affecting one specific joint type over another. The genetic causes of synovial joint birth defects and the signaling molecules required for joint homeostasis after birth remain poorly understood.
Hyaluronan is a large glycosaminoglycan molecule abundant in the cartilage matrix. Traditionally, hyaluronan function in cartilage matrix has been believed as a space-filling molecule, where it serves a structural role through binding to other cartilage matrix molecules by forming large proteoglycan aggregates during limb development. However, emerging evidence suggests that hyaluronan also plays a signaling role during joint formation. For instance, overexpression of hyaluronan in embryonic chicken limb mesenchymal cells results in missing or malformed joints [8], whereas loss of hyaluronan leads to severely shortened limbs with defective synovial joint cavities [16]. These findings indicate that hyaluronan is not only a passive ECM component but also a key regulator of joint morphogenesis. Our previous study found that Has2 is expressed in the distal posterior subridge mesoderm and AER during early limb formation, as well as in digit tips but not precartilage or interdigital mesoderm. While HA downregulation enables precartilage condensation and cartilage differentiation, later Has2 and HA accumulate in joint-forming interzones and in hypertrophic chondrocytes, emphasizing the importance of HA in interzone formation and cartilage-to-bone transition. Studies using fate-mapping have shown all of the structures of the adult synovial joint, including the articular cartilage, as well as non-chondrogenic tissue such as ligament and synovium, arise from these joint progenitor cells [1,29]. Thus, the joint interzones contain a mixed population of progenitors with distinct chondrogenic or non-chondrogenic fates. In mutants lacking hyaluronan, interzone regions were wider and less defined because hyaluronan loss disrupts the balance between chondrogenic and non-chondrogenic fates. Without hyaluronan, joint progenitor cells may favor non-chondrogenic differentiation (e.g., ligaments) over articular cartilage formation. This shift leads to less distinct interzones with mixed cell fates and impaired tissue boundaries. The absence of Alcian blue staining in the expanded interzone, suggesting no cartilage formation, further confirms the altered fate specification. The absence of hyaluronan leads to an increase in interzone space because hyaluronan normally provides hydration and swelling pressure that helps maintain tissue cohesion and structure; without it, the matrix becomes disorganized and less compact [30,31]. Hyaluronan is a major component of the cartilage matrix, along with aggrecan and other proteoglycans. It forms a hydrated network in cartilage tissues. Other molecules, such as collagens that can contribute to interzone structure and are often crosslinked with proteoglycans for proper extracellular matrix (ECM) organization, do not possess the hydrophilic and space-filling qualities of hyaluronan. Additionally, hyaluronan plays a key role in establishing proteoglycan aggregates, which are essential components of the cartilage extracellular matrix. The primary proteoglycan aggregate in cartilage consists of aggrecan, hyaluronan, and link protein. Due to these functions, hyaluronan’s unique contribution cannot be replaced in preserving proper tissue structure and spacing for normal joint formation [32].
While hyaluronan contributes to the extracellular matrix of articular cartilage, we demonstrate that Has2 plays a critical role in both embryonic and postnatal joint development. Our data show that loss of Has2 results in important long-term impairments of joint health. These observations indicate that Has2 and hyaluronan are dynamic mediators of cartilage repair and degeneration. This work reveals novel information on the central function of Has2 and hyaluronan in embryonic and postnatal joint development. These data provide a novel insight into how genetic loss of Has2 has far-reaching, long-term impacts on cartilage health and joint function. The correlation between articular cartilage loss of Has2 and diminished levels of proteoglycan and the erosion of bone on the epiphyseal edges underscores the role of Has2-mediated hyaluronan synthesis in maintaining the ECM of articular cartilage. It provides a new phenotype that underscores the critical role of Has2 in the dynamic maintenance and repair of synovial joints.
Using the Prx1-Cre genetic mouse model, we show that deletion of Has2 from embryonic limb mesenchymal cells results in rapid cartilage degradation, suggesting that hyaluronan synthesis is essential for continuous cartilage maintenance. Consistent with what has been previously reported [15], the loss of Has2 in limb bud mesenchyme with Prx1-Cre resulted in defective joint formation. Interestingly, while changes in joint formation were histologically apparent at the earliest stages of joint formation, the long-term consequences on articular cartilage formation and joint health have not been reported. Examination of 3-week-old femurs and tibias from mutant Prx1-Cre; Has2Δ/Δ mice, when all types of chondrocytes are already differentiated, indicated a considerable drop in cartilage proteoglycan content as determined by Safranin-O staining. Moreover, examination of forelimbs by 3D-µCT at 3 weeks of age not only revealed bone deformities but extensive pitting, suggesting extensive erosion has occurred in focal areas at the epiphyseal ends of long bones.
Hyaluronidases and matrix metalloproteases (MMP) are known to degrade hyaluronan [33,34,35], but the continued synthesis of hyaluronan by Has2 has limited our ability to determine which protease(s) are primarily responsible. Certainly, developing a deeper understanding of the dynamic turnover of cartilage extracellular matrix molecules has important implications for diseases like osteoarthritis. Observing the marked changes that have occurred in the cartilage extracellular matrix due to the loss of Has2 and hyaluronan will clearly need to be further investigated to gain a better understanding of its mechanistic role in cartilage. Further studies investigating the involvement of MMPs and inflammatory cytokines in Has2-deficient models may provide mechanistic insights into how hyaluronan depletion contributes to cartilage breakdown and joint pathology.
The results of our study provide essential insights into the development mechanisms of osteoarthritis (OA). OA develops through articular cartilage degradation, which occurs alongside diminished hyaluronan concentrations in the extracellular matrix. The joint pathology displayed in Has2-deficient mice, which includes eroded cartilage and diminished proteoglycan levels, mirrors the typical OA disease characteristics. The results demonstrate that Has2 activity and hyaluronan production help maintain cartilage health by preventing its degradation. Our findings demonstrate that the impairment in Has2 function could increase OA risk while identifying Has2 as a potential early-stage joint disease biomarker. The development of treatments that target Has2 signaling pathways offers a potential new therapeutic strategy for OA by both restoring hyaluronan synthesis and maintaining cartilage integrity. Researchers need to investigate drug treatments that can boost Has2 activity or hyaluronan production to stop OA from advancing.
In conclusion, the central role of Has2 and hyaluronan in joint health makes them prime candidates for novel clinical interventions. Such research not only broadens our knowledge of joint biology but also opens a better way to treat and prevent chondrocyte diseases.
Author Contributions
Conceptualization, Y.L.; methodology, Y.L.; validation, Y.L., A.T., K.E., A.F.; formal analysis, Y.L., A.T., K.E., A.F. and K.W.-H.L.; investigation, Y.L., A.T., K.E., A.F. and K.W.-H.L.; resources, P.M. and D.W.R.; data curation, Y.L., K.W.-H.L., P.M. and S.S.K.C.; writing—original draft preparation, Y.L.; writing—review and editing, Y.L., K.W.-H.L., G.L.-C., S.S.K.C., Y.Y. and N.A.D.; visualization, Y.L., K.W.-H.L., S.S.K.C. and P.M.; supervision, Y.L., K.W.-H.L. and D.W.R.; project administration, Y.L. and K.W.-H.L.; funding acquisition, Y.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Arthritis National Research Foundation (ANRF), Woman’s Advancement Initiative (WAI) Faculty Fellowship, Greenburg Junior Faculty Research Fund, Bell Ribicoff Junior Faculty Prize, Dean’s Faculty Development Fund of College of Arts and Sciences, University of Hartford to Y.L. We thank Clifford Tabin for the Prx1-Cre mice. We thank Robert Kosher and Caroline Dealy Labs at UConn Health for their support.
Institutional Review Board Statement
The animal study protocol was approved by the Institutional Animal Care and Use Committee at the University of Hartford (IACUC# UH-2023-01, 2023-09-28).
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Chijimatsu, R.; Saito, T. Mechanisms of synovial joint and articular cartilage development. Cell. Mol. Life Sci. 2019, 76, 3939–3952. [Google Scholar] [CrossRef] [PubMed]
- Breeland, G.; Sinkler, M.A.; Menezes, R.G. Embryology, Bone Ossification. [Updated 2023 May 1]. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2025. Available online: https://www.ncbi.nlm.nih.gov/books/NBK539718/ (accessed on 25 May 2025).
- Pacifici, M.; Koyama, E.; Shibukawa, Y.; Wu, C.; Tamamura, Y.; Enomoto-Iwamoto, M.; Iwamoto, M. Cellular and molecular mechanisms of synovial joint and articular cartilage formation. Ann. N. Y. Acad. Sci. 2006, 1068, 74–86. [Google Scholar] [CrossRef]
- Roelofs, A.J.; Zupan, J.; Riemen, A.H.; Kania, K.; Ansboro, S.; White, N.; Clark, S.M.; De Bari, C. Joint morphogenetic cells in the adult mammalian synovium. Nat. Commun. 2017, 8, 15040. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Koyama, E.; Saunders, C.M.; Querido, W.; Pleshko, N.; Pacifici, M. Synovial joint cavitation initiates with microcavities in interzone and is coupled to skeletal flexion and elongation in developing mouse embryo limbs. Biol. Open 2022, 11, bio059381. [Google Scholar] [CrossRef]
- Decker, R.S.; Koyama, E.; Pacifici, M. Articular cartilage: Structural and developmental intricacies and questions. Curr. Osteoporos. Rep. 2015, 13, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Asari, A.; Miyauchi, S.; Miyazaki, K.; Hamai, A.; Horie, K.; Takahashi, T.; Sekiguchi, T.; Machida, A.; Kohno, K.; Uchiyama, Y. Intra-and extracellular localization of hyaluronic acid and proteoglycan constituents (chondroitin sulfate, keratan sulfate, and protein core) in articular cartilage of rabbit tibia. J. Histochem. Cytochem. 1992, 40, 1693–1704. [Google Scholar] [CrossRef]
- Li, Y.; Toole, B.P.; Dealy, C.N.; Kosher, R.A. Hyaluronan in limb morphogenesis. Dev. Biol. 2007, 305, 411–420. [Google Scholar] [CrossRef]
- Ma, S.K.Y.; Chan, A.S.F.; Rubab, A.; Chan, W.C.W.; Chan, D. Extracellular matrix and cellular plasticity in musculoskeletal development. Front. Cell Dev. Biol. 2020, 8, 781. [Google Scholar] [CrossRef]
- Bonnevie, E.D.; Galesso, D.; Secchieri, C.; Cohen, I.; Bonassar, L.J. Elastoviscous transitions of articular cartilage reveal a mechanism of synergy between lubricin and hyaluronic acid. PLoS ONE 2015, 10, e0143415. [Google Scholar] [CrossRef]
- Nečas, D.; Vrbka, M.; Galandáková, A.; Křupka, I.; Hartl, M. On the observation of lubrication mechanisms within hip joint replacements. Part I: Hard-on-soft bearing pairs. J. Mech. Behav. Biomed. Mater. 2019, 89, 237–248. [Google Scholar] [CrossRef]
- Rebenda, D.; Vrbka, M.; Čípek, P.; Toropitsyn, E.; Nečas, D.; Pravda, M.; Hartl, M. On the dependence of rheology of hyaluronic acid solutions and frictional behavior of articular cartilage. Materials 2020, 13, 2659. [Google Scholar] [CrossRef] [PubMed]
- Dahl, L.; Dahl, I.; Engström-Laurent, A.; Granath, K. Concentration and molecular weight of sodium hyaluronate in synovial fluid from patients with rheumatoid arthritis and other arthropathies. Ann. Rheum. Dis. 1985, 44, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Buckwalter, J.A.; Mankin, H.J.; Grodzinsky, A.J. Articular cartilage and osteoarthritis. Instr. Course Lect.-Am. Acad. Orthop. Surg. 2005, 54, 465. [Google Scholar]
- Kosinska, M.K.; Ludwig, T.E.; Liebisch, G.; Zhang, R.; Siebert, H.-C.; Wilhelm, J.; Kaesser, U.; Dettmeyer, R.B.; Klein, H.; Ishaque, B. Articular joint lubricants during osteoarthritis and rheumatoid arthritis display altered levels and molecular species. PLoS ONE 2015, 10, e0125192. [Google Scholar] [CrossRef]
- Matsumoto, K.; Li, Y.; Jakuba, C.; Sugiyama, Y.; Sayo, T.; Okuno, M.; Dealy, C.N.; Toole, B.P.; Takeda, J.; Yamaguchi, Y.; et al. Conditional inactivation of Has2 reveals a crucial role for hyaluronan in skeletal growth, patterning, chondrocyte maturation and joint formation in the developing limb. Development 2009, 136, 2825–2835. [Google Scholar] [CrossRef]
- Mack, J.A.; Feldman, R.J.; Itano, N.; Kimata, K.; Lauer, M.; Hascall, V.C.; Maytin, E.V. Enhanced inflammation and accelerated wound closure following tetraphorbol ester application or full-thickness wounding in mice lacking hyaluronan synthases Has1 and Has3. J. Investig. Dermatol. 2012, 132, 198–207. [Google Scholar] [CrossRef]
- Chan, D.D.; Xiao, W.; Li, J.; de la Motte, C.A.; Sandy, J.D.; Plaas, A. Deficiency of hyaluronan synthase 1 (Has1) results in chronic joint inflammation and widespread intra-articular fibrosis in a murine model of knee joint cartilage damage. Osteoarthr. Cartil. 2015, 23, 1879–1889. [Google Scholar] [CrossRef]
- Spicer, A.P.; McDonald, J.A. Characterization and molecular evolution of a vertebrate hyaluronan synthase gene family. J. Biol. Chem. 1998, 273, 1923–1932. [Google Scholar] [CrossRef] [PubMed]
- Knudson, W.; Ishizuka, S.; Terabe, K.; Askew, E.B.; Knudson, C.B. The pericellular hyaluronan of articular chondrocytes. Matrix Biol. 2019, 78, 32–46. [Google Scholar] [CrossRef]
- Roughley, P.J.; Lamplugh, L.; Lee, E.R.; Matsumoto, K.; Yamaguchi, Y. The role of hyaluronan produced by Has2 gene expression in development of the spine. Spine 2011, 36, E914–E920. [Google Scholar] [CrossRef]
- Logan, M.; Martin, J.F.; Nagy, A.; Lobe, C.; Olson, E.N.; Tabin, C.J. Expression of Cre Recombinase in the developing mouse limb bud driven by a Prxl enhancer. Genesis 2002, 33, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.F.; Olson, E.N. Identification of a prx1 limb enhancer. Genesis 2000, 26, 225–229. [Google Scholar] [CrossRef]
- Martin, J.F.; Bradley, A.; Olson, E.N. The paired-like homeo box gene MHox is required for early events of skeletogenesis in multiple lineages. Genes Dev. 1995, 9, 1237–1249. [Google Scholar] [CrossRef] [PubMed]
- Saalbach, A.; Stein, M.; Lee, S.; Krügel, U.; Haffner-Luntzer, M.; Krohn, K.; Franz, S.; Simon, J.; Tuckermann, J.; Anderegg, U. Bone quality relies on hyaluronan synthesis–Insights from mice with complete knockout of hyaluronan synthase expression. Matrix Biol. Plus 2024, 24, 100163. [Google Scholar] [CrossRef]
- Weigel, P.H.; DeAngelis, P.L. Hyaluronan synthases: A decade-plus of novel glycosyltransferases. J. Biol. Chem. 2007, 282, 36777–36781. [Google Scholar] [CrossRef]
- McLeod, M.J. Differential staining of cartilage and bone in whole mouse fetuses by alcian blue and alizarin red S. Teratology 1980, 22, 299–301. [Google Scholar] [CrossRef]
- Meo Burt, P.; Xiao, L.; Dealy, C.; Fisher, M.C.; Hurley, M.M. FGF2 high molecular weight isoforms contribute to osteoarthropathy in male mice. Endocrinology 2016, 157, 4602–4614. [Google Scholar] [CrossRef]
- Koyama, E.; Shibukawa, Y.; Nagayama, M.; Sugito, H.; Young, B.; Yuasa, T.; Okabe, T.; Ochiai, T.; Kamiya, N.; Rountree, R.B. A distinct cohort of progenitor cells participates in synovial joint and articular cartilage formation during mouse limb skeletogenesis. Dev. Biol. 2008, 316, 62–73. [Google Scholar] [CrossRef]
- Garantziotis, S.; Savani, R.C. Hyaluronan biology: A complex balancing act of structure, function, location and context. Matrix Biol. 2019, 78, 1–10. [Google Scholar] [CrossRef]
- Dicker, K.T.; Gurski, L.A.; Pradhan-Bhatt, S.; Witt, R.L.; Farach-Carson, M.C.; Jia, X. Hyaluronan: A simple polysaccharide with diverse biological functions. Acta Biomater. 2014, 10, 1558–1570. [Google Scholar] [CrossRef]
- Xu, Q.; Torres, J.E.; Hakim, M.; Babiak, P.M.; Pal, P.; Battistoni, C.M.; Nguyen, M.; Panitch, A.; Solorio, L.; Liu, J.C. Collagen-and hyaluronic acid-based hydrogels and their biomedical applications. Mater. Sci. Eng. R Rep. 2021, 146, 100641. [Google Scholar] [CrossRef] [PubMed]
- Fink, S.P.; Triggs-Raine, B. Genetic deficiencies of hyaluronan degradation. Cells 2024, 13, 1203. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, Y.; Nishida, Y.; Kozawa, E.; Zhuo, L.; Arai, E.; Hamada, S.; Morita, D.; Ikuta, K.; Kimata, K.; Ushida, T. Conditional knockdown of hyaluronidase 2 in articular cartilage stimulates osteoarthritic progression in a mice model. Sci. Rep. 2017, 7, 7028. [Google Scholar] [CrossRef]
- Little, C.B.; Barai, A.; Burkhardt, D.; Smith, S.; Fosang, A.; Werb, Z.; Shah, M.; Thompson, E. Matrix metalloproteinase 13–deficient mice are resistant to osteoarthritic cartilage erosion but not chondrocyte hypertrophy or osteophyte development. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 2009, 60, 3723–3733. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).