Circulating Tumor Cells in Early Breast Cancer: A 10-Year Follow-Up Update
Abstract
1. Introduction
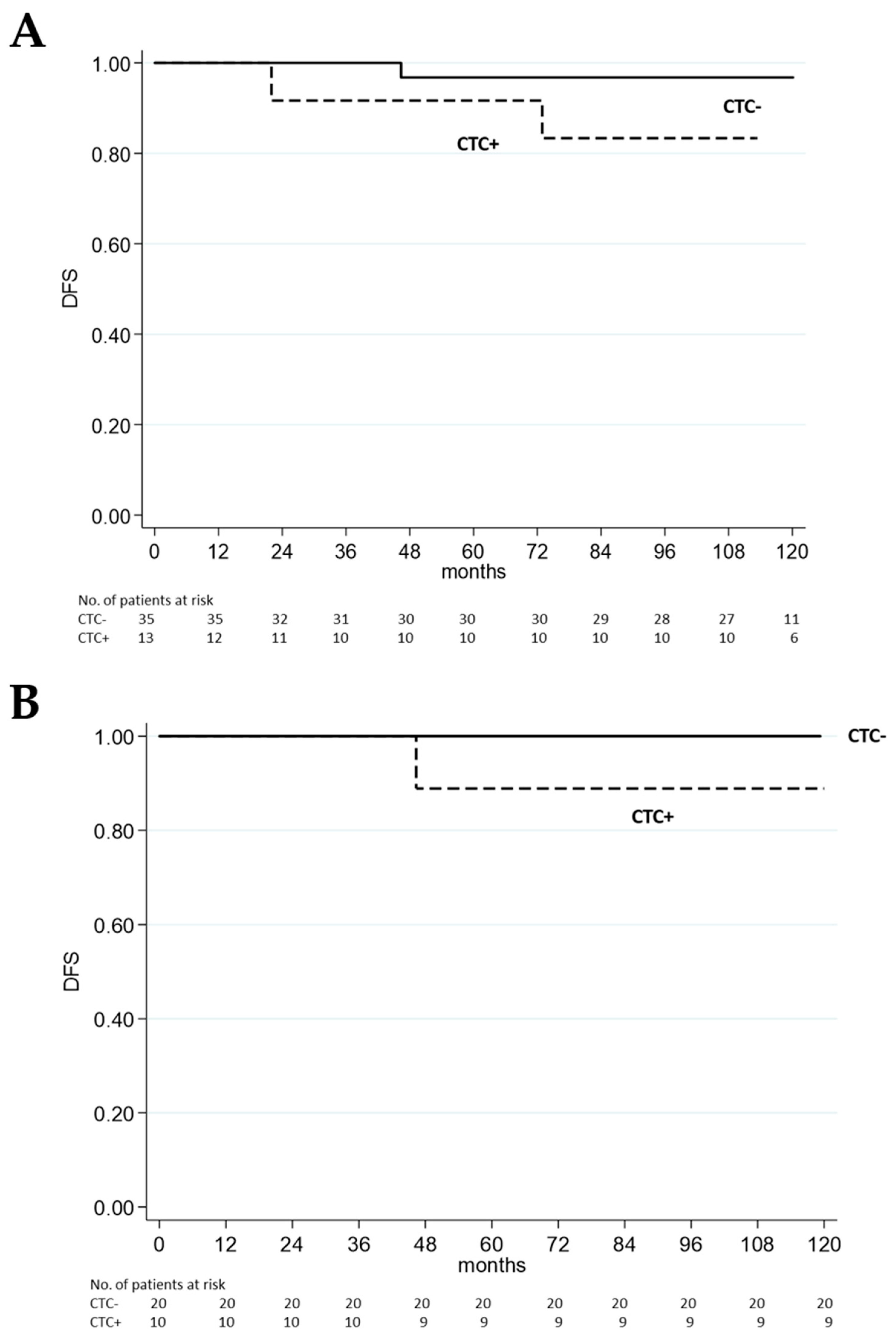
2. Patients After 10-Year Follow-Up
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Reduzzi, C.; Nicolo’, E.; Singhal, S.; Venetis, K.; Ortega-Franco, A.; de Miguel-Perez, D.; Dipasquale, A.; Gouda, M.A.; Saldanha, E.F.; Kasi, P.M.; et al. Unveiling the Impact of Circulating Tumor Cells: Two Decades of Discovery and Clinical Advancements in Solid Tumors. Crit. Rev. Oncol./Hematol. 2024, 203, 104483. [Google Scholar] [CrossRef] [PubMed]
- Cristofanilli, M.; Budd, G.T.; Ellis, M.J.; Stopeck, A.; Matera, J.; Miller, M.C.; Reuben, J.M.; Doyle, G.V.; Allard, W.J.; Terstappen, L.W.M.M.; et al. Circulating Tumor Cells, Disease Progression, and Survival in Metastatic Breast Cancer. N. Engl. J. Med. 2004, 351, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Magbanua, M.J.M.; Hendrix, L.H.; Hyslop, T.; Barry, W.T.; Winer, E.P.; Hudis, C.; Toppmeyer, D.; Carey, L.A.; Partridge, A.H.; Pierga, J.; et al. Serial Analysis of Circulating Tumor Cells in Metastatic Breast Cancer Receiving First-Line Chemotherapy. JNCI J. Natl. Cancer Inst. 2021, 113, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Maltoni, R.; Fici, P.; Amadori, D.; Gallerani, G.; Cocchi, C.; Zoli, M.; Rocca, A.; Cecconetto, L.; Folli, S.; Scarpi, E.; et al. Circulating Tumor Cells in Early Breast Cancer: A Connection with Vascular Invasion. Cancer Lett. 2015, 367, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Alix-Panabières, C.; Pantel, K. Advances in Liquid Biopsy: From Exploration to Practical Application. Cancer Cell 2024, 43, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Lucci, A.; Hall, C.S.; Lodhi, A.K.; Bhattacharyya, A.; Anderson, A.E.; Xiao, L.; Bedrosian, I.; Kuerer, H.M.; Krishnamurthy, S. Circulating Tumour Cells in Non-Metastatic Breast Cancer: A Prospective Study. Lancet Oncol. 2012, 13, 688–695. [Google Scholar] [CrossRef] [PubMed]
- Rack, B.; Schindlbeck, C.; Fehm, T.; Schneeweiss, A.; Lichtenegger, W.; Beckmann, M.W.; Friese, K.; Pantel, K.; Janni, W.; Jückstock, J.; et al. Circulating Tumor Cells Predict Survival in Early Average-to-High Risk Breast Cancer Patients. JNCI J. Natl. Cancer Inst. 2014, 106, dju066. [Google Scholar] [CrossRef] [PubMed]
- Sparano, J.; O’Neill, A.; Alpaugh, K.; Wolff, A.C.; Northfelt, D.W.; Dang, C.T.; Sledge, G.W.; Miller, K.D. Association of Circulating Tumor Cells with Late Recurrence of Estrogen Receptor–Positive Breast Cancer. JAMA Oncol. 2018, 4, 1700. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, R.; Watters, M.; Davies, C.R.; Pantel, K.; Lu, Y. Circulating Tumour Cells for Early Detection of Clinically Relevant Cancer. Nat. Rev. Clin. Oncol. 2023, 20, 487–500. [Google Scholar] [CrossRef] [PubMed]
- Thomas-Bonafos, T.; Pierga, J.Y.; Bidard, F.; Cabel, L.; Kiavue, N. Circulating Tumor Cells in Breast Cancer: Clinical Validity and Utility. Npj Breast Cancer 2024, 10, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Nicolazzo, C.; Gradilone, A.; Loreni, F.; Raimondi, C.; Gazzaniga, P. Circulating Tumor Cells: Gold in the Waste. Dis. Markers 2019, 2019, 1718920. [Google Scholar] [CrossRef] [PubMed]
- Gallerani, G.; Rossi, T.; Ferracin, M.; Bonafè, M. Settling the Uncertainty about Unconventional Circulating Tumor Cells: Epithelial-to-Mesenchymal Transition, Cell Fusion and Trogocytosis. Int. Rev. Cell Mol. Biol. 2023, 381, 99–111. [Google Scholar] [PubMed]
| ID | ER (%) | PgR (%) | HER2 | Histology | Tumor Grade | Type of Surgery | Adjuvant Therapy | Recurrence Site | Time to Relapse (Months) | CTC pre Surgery | CTC Post Surgery |
|---|---|---|---|---|---|---|---|---|---|---|---|
| #11 | 100 | 5 | Not amplified | Ductal | 2 | Quadrantectomy | TC + H | Mediastinal Node | 46.3 | − | + |
| #35 | 0 | 0 | Not amplified | Ductal | 3 | Quadrantectomy | AC + T | Bone | 22.0 | + | − |
| #39 | 95 | 75 | Not amplified | Ductal | 3 | Mastectomy | TC + H | Node | 72.9 | + | NA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossi, T.; Scarpi, E.; Maltoni, R. Circulating Tumor Cells in Early Breast Cancer: A 10-Year Follow-Up Update. Biomedicines 2025, 13, 1278. https://doi.org/10.3390/biomedicines13061278
Rossi T, Scarpi E, Maltoni R. Circulating Tumor Cells in Early Breast Cancer: A 10-Year Follow-Up Update. Biomedicines. 2025; 13(6):1278. https://doi.org/10.3390/biomedicines13061278
Chicago/Turabian StyleRossi, Tania, Emanuela Scarpi, and Roberta Maltoni. 2025. "Circulating Tumor Cells in Early Breast Cancer: A 10-Year Follow-Up Update" Biomedicines 13, no. 6: 1278. https://doi.org/10.3390/biomedicines13061278
APA StyleRossi, T., Scarpi, E., & Maltoni, R. (2025). Circulating Tumor Cells in Early Breast Cancer: A 10-Year Follow-Up Update. Biomedicines, 13(6), 1278. https://doi.org/10.3390/biomedicines13061278







