A Promising Approach to Psoriasis Vulgaris Management with N-Acetylcysteine and Vitamin E: Targeting the Interplay of Inflammatory and Oxidative Stress
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Setting
2.3. Ethical Consideration
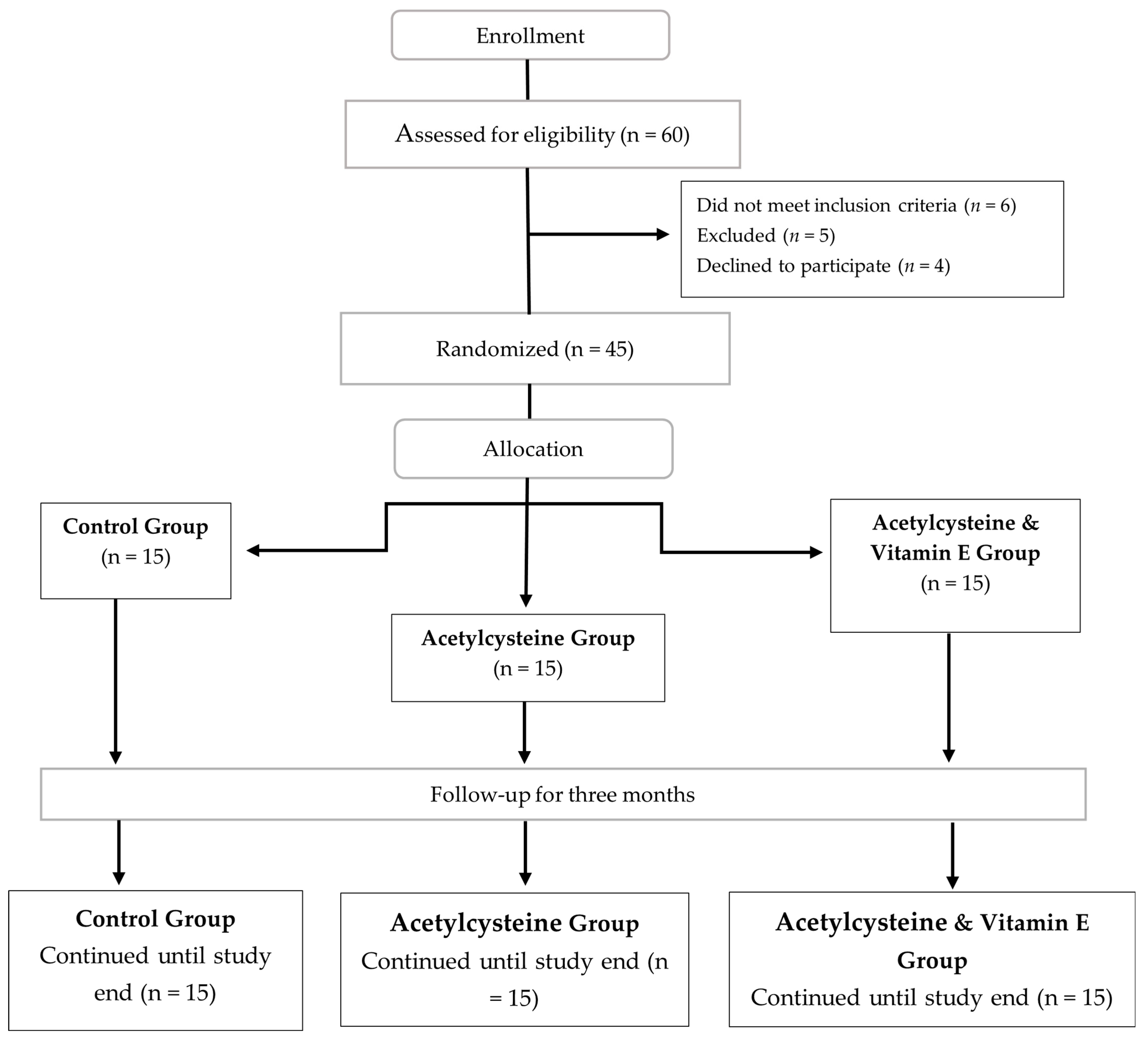
2.4. Study Subjects and Treatment
- Randomization Technique:
2.5. Baseline Assessment
2.6. Outcome Evaluation and Follow-Up
2.6.1. Psoriasis Area and Severity Index (PASI) [33]
2.6.2. Dermatology Life Quality Index (DLQI) [38]
2.6.3. Liver Function Test (ALT, AST, and Albumin)
2.6.4. Lipid Profile (Total Cholesterol, Triglycerides, LDL, and HDL)
2.6.5. Fasting Blood Glucose Test (FBG)
2.6.6. Complete Blood Count (CBC) (TLC Count, RBC Count, HB, HCT, Platelet Count, and RBC Indices)
2.6.7. Non-Specific Inflammatory Markers (CRP and ESR)
2.6.8. Oxidative Stress Marker Malondialdehyde (MDA)
2.6.9. Inflammatory Marker Interleukin-36 Gamma (IL-36γ)
2.7. Statistical Analysis
2.8. Sample Size Estimation
3. Results
3.1. Demographics and Clinical Characteristics of the Studied Groups
3.2. Baseline Laboratory Parameters Among the Studied Groups
3.3. Follow-Up Laboratory Parameters Among the Studied Groups
3.3.1. Liver Function Tests
- Lipid Profile
3.3.2. Blood Glucose Test
3.3.3. Complete Blood Count
3.3.4. Non-Specific Inflammatory Markers
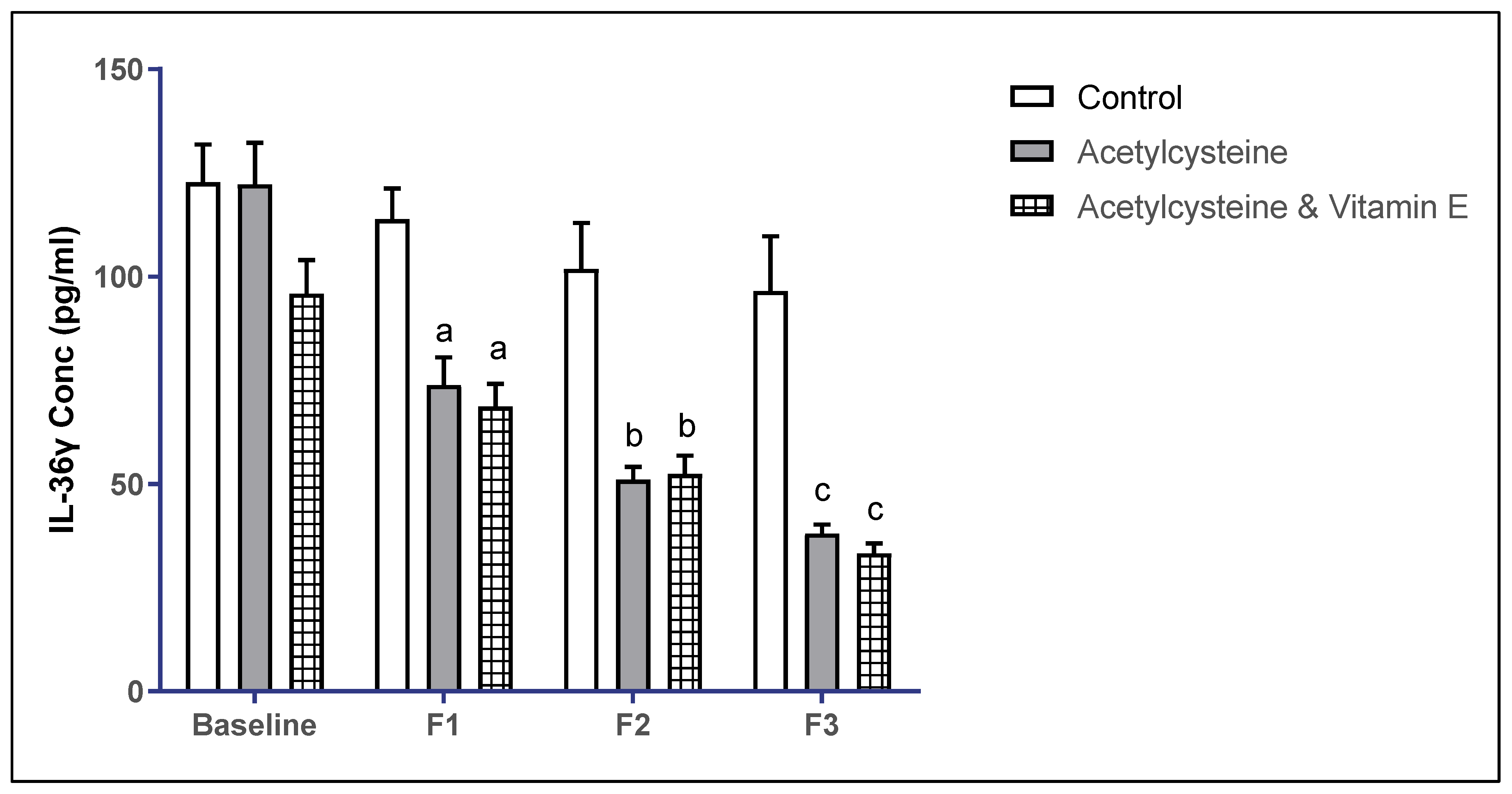
3.4. Inflammatory Marker Interleukin-36 Gamma (IL-36γ) Levels Among the Studied Groups
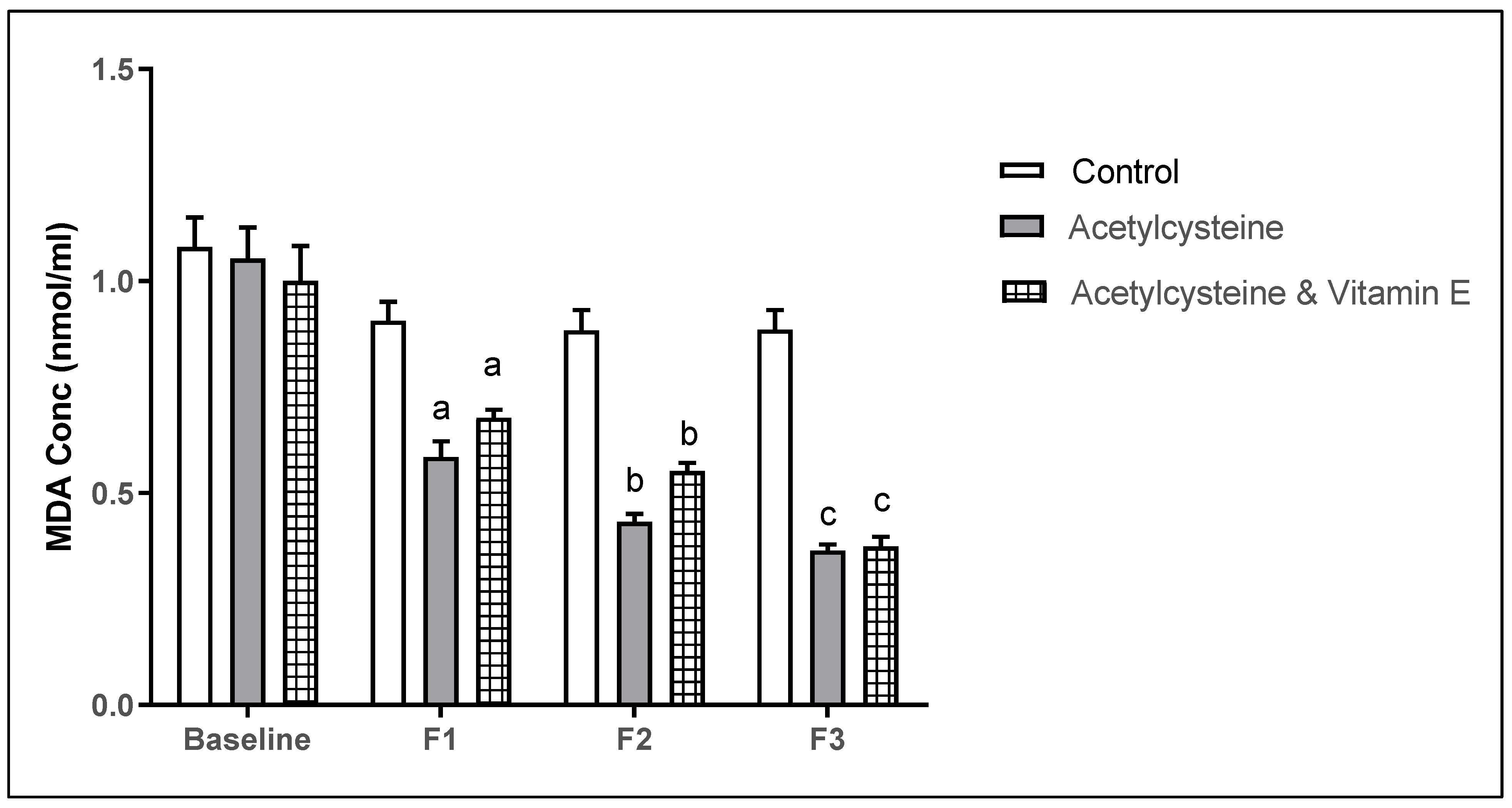
3.5. Oxidative Stress Marker Malondialdehyde (MDA) Levels Among the Studied Groups
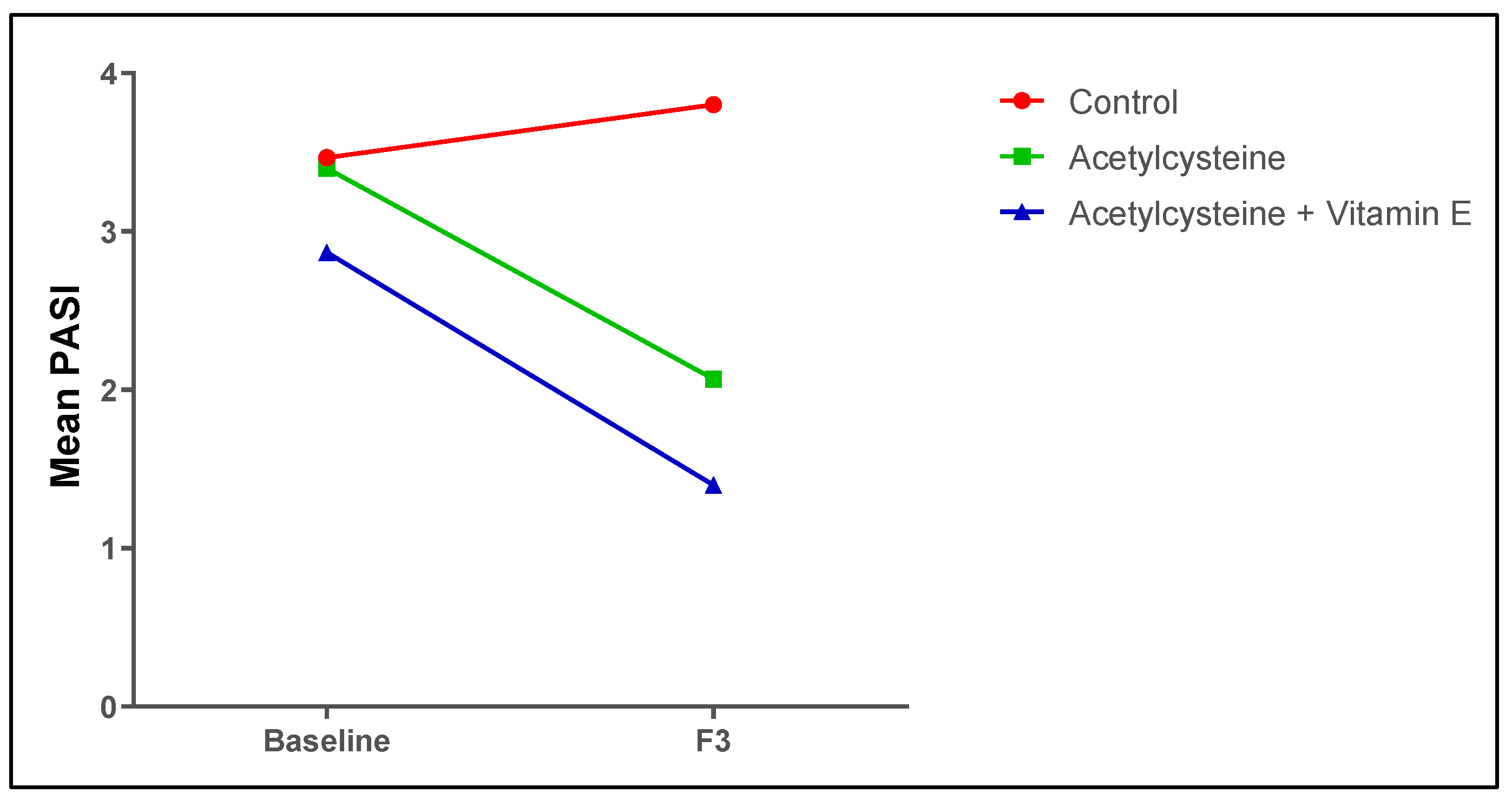
3.6. Follow-Up Assessment of the Psoriasis Indices (PASI Score and the DLQI) Among the Studied Groups
3.7. Correlations of IL-36γ and MDA with the PASI Score Among the Studied Groups
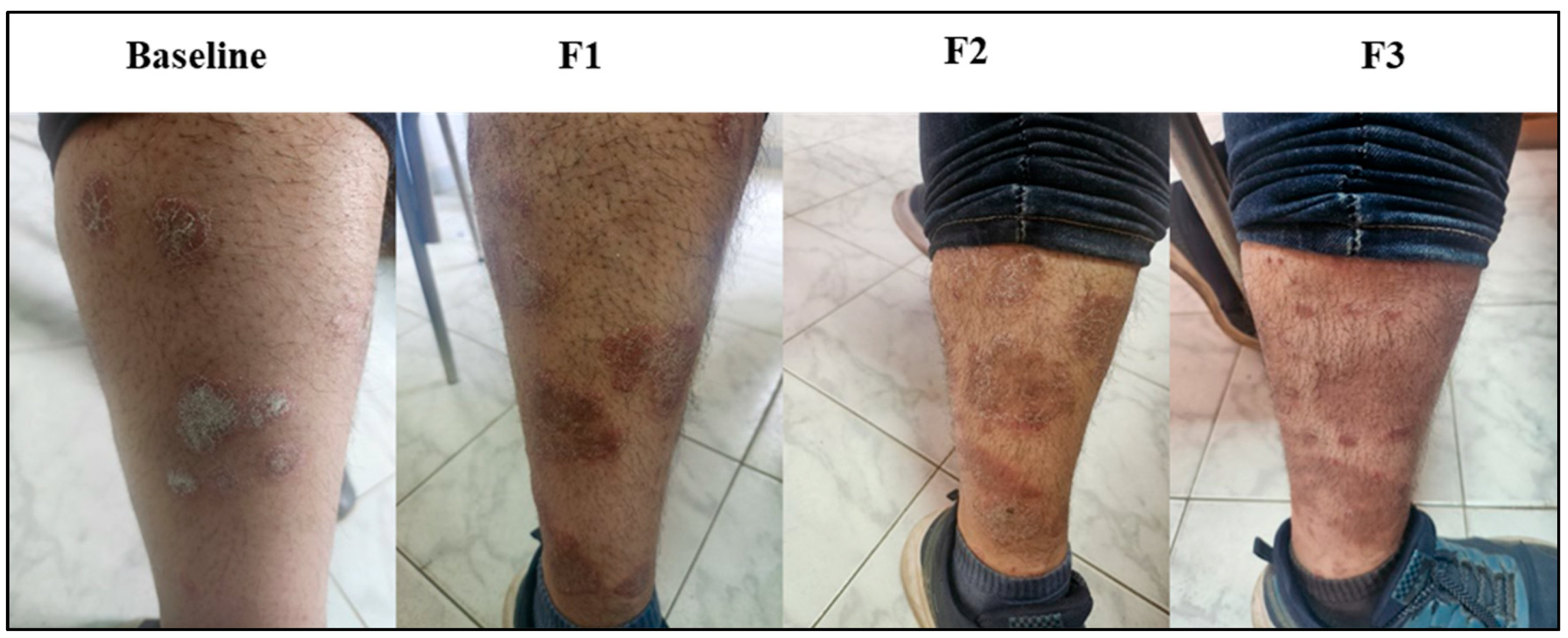
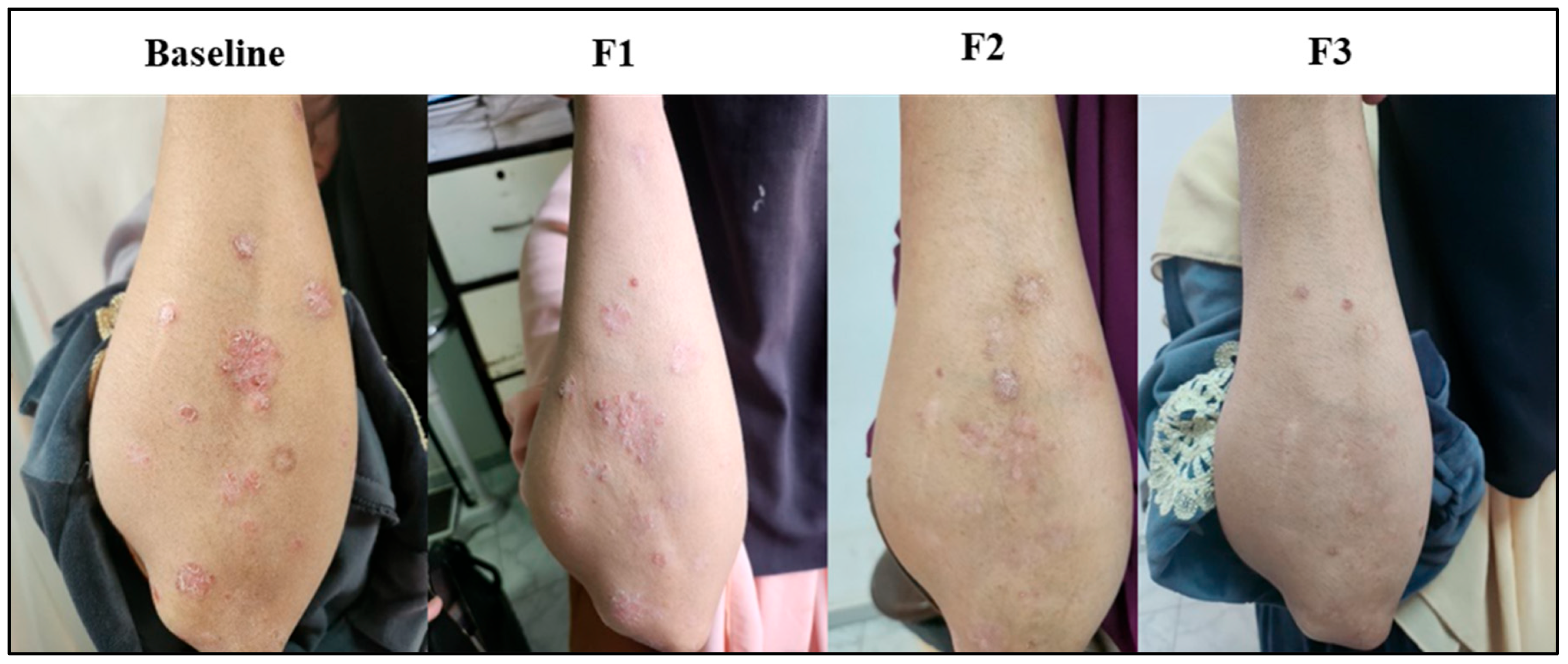
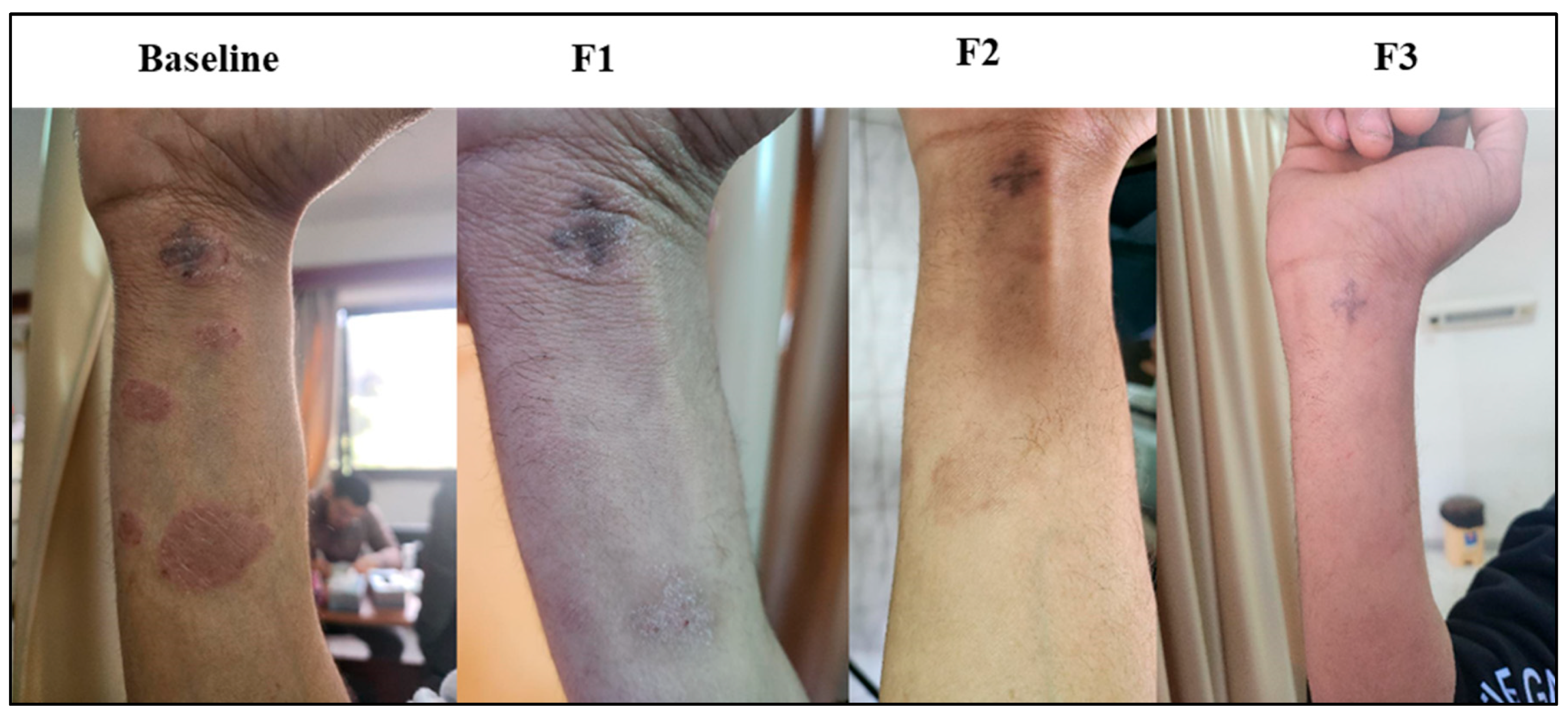
3.8. Subject Presentation
- Assessment of Safety of NAC and Vitamin E
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Hu, P.; Wang, M.; Gao, H.; Zheng, A.; Li, J.; Mu, D.; Tong, J. The Role of Helper T Cells in Psoriasis. Front. Immunol. 2021, 12, 788940. [Google Scholar] [CrossRef] [PubMed]
- Farci, F.; Mahabal, G.D. Hyperkeratosis. In Encyclopedia of Parasitology; Springer: Berlin/Heidelberg, Germany, 2023; p. 1311. [Google Scholar] [CrossRef]
- Maciel, T.T.; Merle, E.; Fricot, A.; Monteiro, R.; Moura, I.C.; Seleznik, G.; Seeger, H.; Papandile, A.; Fu, K.; Poreci, U.; et al. Pathology, Inflammation. Nephrol. Dial. Transplant. 2022, 29, iii25–iii26. [Google Scholar] [CrossRef]
- Duan, L.; Rao, X.; Sigdel, K.R. Regulation of Inflammation in Autoimmune Disease. J. Immunol. Res. 2019, 2019, 7403796. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Almeida, A.; Wróblewski, M.; Wróblewska, J.; Nuszkiewicz, J.; Pawłowska, M.; Wesołowski, R.; Wόzniak, A.W. The Role of Selected Trace Elements in Oxidoreductive Homeostasis in Patients with Thyroid Diseases. Int. J. Mol. Sci 2023, 24, 4840. [Google Scholar] [CrossRef]
- Mirończuk-Chodakowska, I.; Witkowska, A.M.; Zujko, M.E. Endogenous Non-Enzymatic Antioxidants in the Human Body. Adv. Med. Sci. 2018, 63, 68–78. [Google Scholar] [CrossRef]
- Kimmel, G.W.; Lebwohl, M. Psoriasis: Overview and Diagnosis. In Evidence-Based Psoriasis; Springer: Cham, Switzerland, 2018; p. 1. [Google Scholar] [CrossRef]
- Rodgers, M.; Epstein, D.; Bojke, L.; Yang, H.; Craig, D.; Fonseca, T.; Myers, L.; Bruce, I.; Chalmers, R.; Bujkiewicz, S. Etanercept, Infliximab and Adalimumab for the Treatment of Psoriatic Arthritis: A Systematic Review and Economic Evaluation. In NIHR Health Technology Assessment programme: Executive Summaries; NIHR: London, UK, 2011. [Google Scholar]
- Liluashvili, S.; Kituashvili, T. Dermatology Life Quality Index and Disease Coping Strategies in Psoriasis Patients. Adv. Dermatol. Allergol./Postȩpy Dermatol. I Alergologii 2019, 36, 419. [Google Scholar] [CrossRef]
- Svoboda, S.A.; Ghamrawi, R.I.; Owusu, D.A.; Feldman, S.R. Treatment Goals in Psoriasis: Which Outcomes Matter Most? Am. J. Clin. Dermatol. 2020, 21, 505–511. [Google Scholar] [CrossRef]
- Gisondi, P.; Talamonti, M.; Chiricozzi, A.; Piaserico, S.; Amerio, P.; Balato, A.; Bardazzi, F.; Calzavara Pinton, P.; Campanati, A.; Cattaneo, A.; et al. Treat-to-Target Approach for the Management of Patients with Moderate-to-Severe Plaque Psoriasis: Consensus Recommendations. Dermatol. Ther. 2021, 11, 235. [Google Scholar] [CrossRef]
- Bowcock, A.M.; Cookson, W.O.C.M. The Genetics of Psoriasis, Psoriatic Arthritis and Atopic Dermatitis. Hum. Mol. Genet. 2004, 13, R43–R55. [Google Scholar] [CrossRef]
- Kim, N.; Thrash, B.; Menter, A. Comorbidities in Psoriasis Patients. In Proceedings of the Seminars in Cutaneous Medicine and Surgery; WB Saunders: Philadelphia, PA, USA, 2010; Volume 29, pp. 10–15. [Google Scholar]
- Afifi, T.; de Gannes, G.; Huang, C.; Zhou, Y. Topical Therapies for Psoriasis: Evidence-Based Review. Can. Fam. Physician 2005, 51, 519. [Google Scholar] [PubMed]
- Trémezaygues, L.; Reichrath, J. Vitamin D Analogs in the Treatment of Psoriasis: Where Are We Standing and Where Will We Be Going? Dermato-Endocrinology 2011, 3, 180. [Google Scholar] [CrossRef] [PubMed]
- Malecic, N.; Young, H. Tacrolimus for the Management of Psoriasis: Clinical Utility and Place in Therapy. Psoriasis 2016, 6, 153. [Google Scholar] [CrossRef] [PubMed]
- Amiri, D.; Willy Schwarz, C.; Gether, L.; Skov, L. Safety and Efficacy of Topical Calcineurin Inhibitors in the Treatment of Facial and Genital Psoriasis: A Systematic Review. Acta Derm. Venereol. 2023, 103, adv00890. [Google Scholar] [CrossRef]
- Wolff, K. Side-effects of Psoralen Photochemotherapy (PUVA). Br. J. Dermatol. 1990, 122, 117–125. [Google Scholar] [CrossRef]
- Wong, T.; Hsu, L.; Liao, W. Phototherapy in Psoriasis: A Review of Mechanisms of Action. J. Cutan. Med. Surg. 2013, 17, 6. [Google Scholar] [CrossRef]
- Balighi, K.; Zahra, G.; Azadeh, G.; Pardis, H.; Ehsan, S.; Arghavan, A. Assessment of the Therapeutic Aspect of Systemic Non-Biologic Anti-Psoriatic Treatment Modalities Used in Combination with Methotrexate. Indian J. Dermatol. 2016, 61, 118. [Google Scholar] [CrossRef]
- Zito, P.M.; Mazzoni, T. Acitretin. Handbook of Systemic Drug Treatment in Dermatology, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2022; pp. 1–7. [Google Scholar] [CrossRef]
- Maverakis, E.; Bowen, M.; Raychaudhuri, S.; Sivamani, R.; Correa, G.; Ono, Y. Biological Therapy of Psoriasis. Indian J. Dermatol. 2010, 55, 161. [Google Scholar] [CrossRef]
- Millsop, J.W.; Bhatia, B.K.; Debbaneh, M.; Koo, J.; Liao, W. Diet and Psoriasis: Part 3. Role of Nutritional Supplements. J. Am. Acad. Dermatol. 2014, 71, 561. [Google Scholar] [CrossRef]
- Stanescu, A.M.A.; Simionescu, A.A.; Diaconu, C.C. Oral Vitamin D Therapy in Patients with Psoriasis. Nutrients 2021, 13, 163. [Google Scholar] [CrossRef]
- Cao, H.; Han, M.; Li, X.; Dong, S.; Shang, Y.; Wang, Q.; Xu, S.; Liu, J. Clinical Research Evidence of Cupping Therapy in China: A Systematic Literature Review. BMC Complement. Altern. Med. 2010, 10, 70. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, A.; Shah, A.; Quraishi, H.A.; Rather, S.A.; Raheem, A. Effect of Leech Therapy in the Management of Psoriasis. J. Res. Tradit. Med. 2018, 4, 16. [Google Scholar] [CrossRef]
- Mokhtari, V.; Afsharian, P.; Shahhoseini, M.; Kalantar, S.M.; Moini, A. A Review on Various Uses of N-Acetyl Cysteine. Cell J. 2017, 19, 11. [Google Scholar] [CrossRef] [PubMed]
- Tenório, M.C.D.S.; Graciliano, N.G.; Moura, F.A.; de Oliveira, A.C.M.; Goulart, M.O.F. N-Acetylcysteine (NAC): Impacts on Human Health. Antioxidants 2021, 10, 967. [Google Scholar] [CrossRef]
- Sadowska, A.M.; Verbraecken, J.; Darquennes, K.; De Backer, W.A. Role of N-Acetylcysteine in the Management of COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2006, 1, 425. [Google Scholar] [CrossRef]
- Traber, M.G.; Atkinson, J. Vitamin E, Antioxidant and Nothing More. Free Radic. Biol. Med. 2007, 43, 4. [Google Scholar] [CrossRef]
- Rizvi, S.; Raza, S.T.; Ahmed, F.; Ahmad, A.; Abbas, S.; Mahdi, F. The Role of Vitamin E in Human Health and Some Diseases. Sultan Qaboos Univ. Med. J. 2014, 14, e157. [Google Scholar] [CrossRef]
- Salgado-Boquete, L.; Carrascosa, J.M.; Llamas-Velasco, M.; Ruiz-Villaverde, R.; de la Cueva, P.; Belinchón, I. A New Classification of the Severity of Psoriasis: What’s Moderate Psoriasis? Life 2021, 11, 627. [Google Scholar] [CrossRef]
- Timis, T.-L.; Mitrea, D.-R.; Florian, I.-A.; Timis, T.-L.; Mitrea, D.-R.; Florian, I.-A. Medical Management of Chronic Plaque Psoriasis in the Modern Age. In Healthcare Access—Regional Overviews; Intechopen: London, UK, 2019. [Google Scholar] [CrossRef]
- Calverley, P.; Rogliani, P.; Papi, A. Safety of N-Acetylcysteine at High Doses in Chronic Respiratory Diseases: A Review. Drug Saf. 2021, 44, 273–290. [Google Scholar] [CrossRef]
- Pizzorno, J. IMCJ_13_1.Indd. Integr. Med. 2014, 13, 8. [Google Scholar]
- El Hadi, H.; Vettor, R.; Rossato, M. Vitamin E as a Treatment for Nonalcoholic Fatty Liver Disease: Reality or Myth? Antioxidants 2018, 7, 12. [Google Scholar] [CrossRef] [PubMed]
- FINLAY, A.Y.; KHAN, G.K. Dermatology Life Quality Index (DLQI)—A Simple Practical Measure for Routine Clinical Use. Clin. Exp. Dermatol. 1994, 19, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Kassoff, A.; Kassoff, J.; Buehler, J.; Eglow, M.; Kaufman, F.; Mehu, M.; Kieval, S.; Mairs, M.; Graig, B.; Quattrocchi, A.; et al. A Randomized, Placebo-Controlled, Clinical Trial of High-Dose Supplementation with Vitamins C and E, Beta Carotene, and Zinc for Age-Related Macular Degeneration and Vision Loss: AREDS Report No. 8. Arch. Ophthalmol. 2001, 119, 1417–1436. [Google Scholar] [CrossRef]
- Miller, E.R.; Pastor-Barriuso, R.; Dalal, D.; Riemersma, R.A.; Appel, L.J.; Guallar, E. Meta-Analysis: High-Dosage Vitamin E Supplementation May Increase All-Cause Mortality. Ann. Intern. Med. 2005, 142, 37–46. [Google Scholar] [CrossRef]
- Brien, S.B.; Bishop, F.L.; Riggs, K.; Stevenson, D.; Freire, V.; Lewith, G. Integrated Medicine in the Management of Chronic Illness: A Qualitative Study. Br. J. Gen. Pract. 2011, 61, e89. [Google Scholar] [CrossRef]
- Kowalewska, B.; Cybulski, M.; Jankowiak, B.; Krajewska-Kułak, E. Acceptance of Illness, Satisfaction with Life, Sense of Stigmatization, and Quality of Life among People with Psoriasis: A Cross-Sectional Study. Dermatol. Ther. 2020, 10, 413–430. [Google Scholar] [CrossRef]
- Pleńkowska, J.; Gabig-Cimińska, M.; Mozolewski, P. Oxidative Stress as an Important Contributor to the Pathogenesis of Psoriasis. Int. J. Mol. Sci. 2020, 21, 6206. [Google Scholar] [CrossRef]
- Uraz, S.; Tahan, G.; Aytekin, H.; Tahan, V. N-Acetylcysteine Expresses Powerful Anti-Inflammatory and Antioxidant Activities Resulting in Complete Improvement of Acetic Acid-Induced Colitis in Rats. Scand J. Clin. Lab. Investig. 2013, 73, 61–66. [Google Scholar] [CrossRef]
- Prussick, R.; Prussick, L.; Gutman, J. Psoriasis Improvement in Patients Using Glutathione-Enhancing, Nondenatured Whey Protein Isolate: A Pilot Study. J. Clin. Aesthet. Dermatol. 2013, 6, 23. [Google Scholar]
- Ayala-Fontánez, N.; Soler, D.C.; McCormick, T.S. Current Knowledge on Psoriasis and Autoimmune Diseases. Psoriasis 2016, 6, 7. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of Its Protective Roles, Measurement, and Biosynthesis. Mol. Asp. Med. 2008, 30, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Tualeka, A.R.; Martiana, T.; Ahsan, A.; Russeng, S.S.; Meidikayanti, W. Association between Malondialdehyde and Glutathione (L-Gamma-Glutamyl-Cysteinyl-Glycine/GSH) Levels on Workers Exposed to Benzene in Indonesia. Open Access Maced. J. Med. Sci. 2019, 7, 1198. [Google Scholar] [CrossRef] [PubMed]
- Farag, A.; Abass, W.; Qassem, H. Evaluation of the Antioxidant and Anti-Inflammatory Effect of Sublingual Glutathione on COPD Patients. J. Med. Life 2023, 16, 1796. [Google Scholar] [CrossRef] [PubMed]
- Adeoye, O.; Olawumi, J.; Opeyemi, A.; Christiania, O. Review on the Role of Glutathione on Oxidative Stress and Infertility. JBRA Assist. Reprod. 2018, 22, 61. [Google Scholar] [CrossRef]
- Matuz-Mares, D.; Riveros-Rosas, H.; Vázquez-Meza, H.; Vilchis-Landeros, M.M. Glutathione Participation in the Prevention of Cardiovascular Diseases. Antioxidants 2021, 10, 1220. [Google Scholar] [CrossRef]
- Kurd, S.K.; Smith, N.; VanVoorhees, A.; Troxel, A.B.; Badmaev, V.; Seykora, J.T.; Gelfand, J.M. Oral Curcumin in the Treatment of Moderate to Severe Psoriasis Vulgaris: A Prospective Clinical Trial. J. Am. Acad. Dermatol. 2008, 58, 625–631. [Google Scholar] [CrossRef]
- Thiele, J.J.; Ekanayake-Mudiyanselage, S. Vitamin E in Human Skin: Organ-Specific Physiology and Considerations for Its Use in Dermatology. Mol. Asp. Med. 2007, 28, 646–667. [Google Scholar] [CrossRef]
- Keen, M.A.; Hassan, I. Vitamin E in Dermatology. Indian Dermatol Online J 2016, 7, 311. [Google Scholar] [CrossRef]
- Zheng, Y. High Serum IgE Concentration in Patients with Psoriasis. J. Clin. Res. Dermatol. 2017, 4, 1–4. [Google Scholar] [CrossRef]
- Lee, S.; Ahn, K.; Paik, H.Y.; Chung, S.J. Serum Immunoglobulin E (IgE) Levels and Dietary Intake of Korean Infants and Young Children with Atopic Dermatitis. Nutr. Res. Pract. 2012, 6, 429. [Google Scholar] [CrossRef]
- Aryaeian, N.; Djalali, M.; Shahram, F.; Djazayery, A.; Eshragian, M.R. Effect of Conjugated Linoleic Acid, Vitamin E, Alone or Combined on Immunity and Inflammatory Parameters in Adults with Active Rheumatoid Arthritis: A Randomized Controlled Trial. Int. J. Prev. Med. 2014, 5, 1567. [Google Scholar] [PubMed]
- Borel, P.; Preveraud, D.; Desmarchelier, C. Bioavailability of Vitamin E in Humans: An Update. Nutr. Rev. 2013, 71, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Reboul, E. Vitamin E Bioavailability: Mechanisms of Intestinal Absorption in the Spotlight. Antioxidants 2017, 6, 95. [Google Scholar] [CrossRef]
- Ahmad, F.; Alam, M.A.; Ansari, A.W.; Jochebeth, A.; Leo, R.; Al-Abdulla, M.N.; Al-Khawaga, S.; AlHammadi, A.; Al-Malki, A.; Al Naama, K.; et al. Emerging Role of the IL-36/IL-36R Axis in Multiple Inflammatory Skin Diseases. J. Investig. Dermatol. 2024, 144, 206–224. [Google Scholar] [CrossRef]
- Sachen, K.L.; Arnold Greving, C.N.; Towne, J.E. Role of IL-36 Cytokines in Psoriasis and Other Inflammatory Skin Conditions. Cytokine 2022, 156, 155897. [Google Scholar] [CrossRef]
- Calder, P.C. Omega-3 Fatty Acids and Inflammatory Processes. Nutrients 2010, 2, 355–374. [Google Scholar] [CrossRef]
- Farshchian, M.; Sobhan, M.; Ansar, A.; Hoseinpoor, V. C-Reactive Protein Serum Level in Patients with Psoriasis before and after Treatment with Narrow-Band Ultraviolet B. An. Bras. Dermatol. 2016, 91, 355–374. [Google Scholar] [CrossRef]
- Solak, B.; Kara, R.Ö. Assessing Systemic Inflammatory Markers in Psoriasis: A Retrospective Study. Trop. Med. Int. Health 2024, 29, 580–583. [Google Scholar] [CrossRef] [PubMed]
- Şener, G.; İnan Yuksel, E.; Gökdeniz, O.; Karaman, K.; Canat, H.D. The Relationship of Hematological Parameters and C-Reactive Protein (CRP) With Disease Presence, Severity, and Response to Systemic Therapy in Patients With Psoriasis. Cureus 2023, 15, e43790. [Google Scholar] [CrossRef]
- Tan, M.; Luo, Y.; Hu, J.; Hu, K.; Li, X.; Yang, J.; Chen, J.; Zhu, W.; Kuang, Y. Elevated C-Reactive Protein and Erythrocyte Sedimentation Rate Correlates with Depression in Psoriasis: A Chinese Cross-Sectional Study. Clin. Cosmet. Investig. Dermatol. 2023, 16, 397–405. [Google Scholar] [CrossRef]
- Houttekiet, C.; De Vlam, K.; Neerinckx, B.; Lories, R. Systematic Review of the Use of CRP in Clinical Trials for Psoriatic Arthritis: A Concern for Clinical Practice? RMD Open 2022, 8, e001756. [Google Scholar] [CrossRef] [PubMed]
- Grechin, C.; Solovăstru, L.; Vâță, D.; Pătrașcu, A.; Grăjdeanu, A.; Porumb-Andrese, E. Inflammatory Marker Alteration in Response to Systemic Therapies in Psoriasis. Exp. Ther. Med. 2020, 20, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, H.; Zhao, X.; Huang, J.; Zhang, J.; Liu, Z.; Wen, J.; Qin, S. The Role of C-Reactive Protein and Genetic Predisposition in the Risk of Psoriasis: Results from a National Prospective Cohort. BMC Rheumatol. 2024, 8, 72. [Google Scholar] [CrossRef] [PubMed]
- Santus, P.; Signorello, J.C.; Danzo, F.; Lazzaroni, G.; Saad, M.; Radovanovic, D. Anti-Inflammatory and Anti-Oxidant Properties of N-Acetylcysteine: A Fresh Perspective. J. Clin. Med. 2024, 13, 4127. [Google Scholar] [CrossRef]
| Variables | Control (n = 15) | Acetylcysteine (n = 15) | Acetylcysteine and Vitamin E (n = 15) | P1-Value | P2-Value | P3-Value | ||
|---|---|---|---|---|---|---|---|---|
| Demographics | ||||||||
| Age (years) | Mean ± SD | 43.10 ± 8.3 | 40.00 ± 12.65 | 41.93 ± 9.30 | 0.99 # | 0.94 # | 0.94 # | |
| Sex (n) | Male | 6 (40%) | 8 (43.33%) | 13 (86.67%) | 0.027 $* | |||
| Female | 9 (60%) | 7 (46.67%) | 2 (13.33%) | |||||
| Smoking status (n) | Smoker | 3 (6.67%) | 2 (4.44%) | 3 (6.67%) | 0.86 $ | |||
| Non-smoker | 12 (26.67%) | 13 (28.89%) | 12 (26.67%) | |||||
| Family History(n) | Yes | 3 | 2 | 0 | 0.21 $ | |||
| No | 12 | 13 | 15 | |||||
| PASI score | Mean ± SD | 3.47 ± 0.64 | 3.40 ± 0.63 | 2.87 ± 0.92 | >0.999 # | 0.697 # | 0.791 # | |
| DLQI | Mean ± SD | 4.25 ± 2.87 | 5.64 ± 3.59 | 5.91 ± 2.28 | 0.959 # | 0.913 # | >0.999 # | |
| Variables | Control (n = 15) | Acetylcysteine (n = 15) | Acetylcysteine and Vitamin E (n = 15) | P1-Value | P2-Value | P3-Value |
|---|---|---|---|---|---|---|
| Routine Lab Analysis | ||||||
| AST (U/L) Mean ± SD | 26.43 ± 8.31 | 28.47 ± 5.44 | 22.60 ± 6.25 | 0.0646 $$ | ||
| ALT (U/L) Mean ± SD | 23.20 ± 5.69 | 26.87 ± 7.98 | 21.93 ± 5.74 | 0.117 $$ | ||
| Albumin (g/dL) Mean ± SD | 4.14 ± 0.44 | 4.19 ± 0.45 | 4.16 ± 0.47 | 0.966 $$ | ||
| Total cholesterol (mg/dL) Mean ± SD | 149.00 ± 30.80 | 154.00 ± 32.00 | 129.00 ± 28.20 | 0.0716 $$ | ||
| TG (mg/dL) Mean ± SD | 99.80 ± 25.4 | 114.90 ± 18.72 | 94.14 ± 24.59 | 0.0610 $$ | ||
| LDL (mg/dL) Mean ± SD | 86.31 ± 30.51 | 93.04 ± 31.59 | 78.64 ± 31.55 | 0.510 $$ | ||
| HDL (mg/dL) Mean ± SD | 40.80 ± 3.56 | 40.30 ± 5.87 | 41.90 ± 3.71 | 0.689 $$ | ||
| FBG (mg/dL) Mean ± SD | 86.14 ± 19.23 | 67.53 ± 14.91 | 85.75 ± 19.51 | 0.106 $$ | >0.99 $$ | 0.104 $$ |
| Complete Blood Count | ||||||
| Platelets (×103/µL) Median (range) | 300.50 (35.4–414) | 272.40 (227–385.2) | 345.00 (194–433) | 0.410 ## | ||
| TLC count (×103/µL) Median (range) | 8.10 (5.1–10.1) | 8.50 (4.9–9.7) | 7.80 (3.7–10.3) | 0.822 ## | ||
| RBC count (×103/µL) Median (range) | 6.88 (4–8.18) | 5.50 (3.5–7.62) | 7.24 (4.6–8.94) | 0.0614 ## | ||
| Hemoglobin (g/dL) Mean ± SD | 13.16 ± 1.39 | 13.47 ± 1.58 | 14.08 ± 1.70 | 0.281 $$ | ||
| HCT (%) Median (range) | 39.80 (31.6–46.2) | 39.30 (31.3–54.4) | 41.70 (30.1–4.9) | 0.158 ## | ||
| MCV (fl) Median (range) | 49.25 (42.0–58.0) | 50.00 (42.7–58.0) | 52.70 (43.0–61) | 0.064 ## | ||
| MCH (pg) Median (range) | 15.75 (11.5–21.5) | 14.10 (12.0–25.5) | 18.70 (12.5–28.1) | 0.824 ## | 0.072 ## | 0.02 ##* |
| MCHC (g/dL) Median (range) | 34.05 (28.0–37.5) | 36.10 (30.0–38.6) | 35.20 (32.0–36.0) | 0.0699 ## | ||
| Inflammatory Markers | ||||||
| CRP (g/dL) Median (range) | 0.255 (0.0410–0.428) | 0.400 (0.062–0.520) | 0.211 (0.060–0.50) | 0.0816 ## | ||
| ESR (mm/h) Median (range) | 12 (3–35) | 5 (3–30) | 15 (5–50) | 0.116 ## | ||
| IL-36 (pg/mL) Mean ± SD | 122.70 ± 34.40 | 122.20 ± 39.06 | 95.89 ± 31.17 | >0.999 ^ | 0.351 ^ | 0.381 ^ |
| Oxidative Stress Marker | ||||||
| MDA (nmol/mL) Mean ± SD | 1.08 ± 0.27 | 1.05 ± 0.28 | 1.00 ± 0.32 | >0.999 ^ | 0.989 ^ | 0.999 ^ |
| Variables | Control (n = 15) | Acetylcysteine (n = 15) | Acetylcysteine and Vitamin E (n = 15) | P1-Value | P2-Value | P3-Value | |
|---|---|---|---|---|---|---|---|
| Liver Function tests | |||||||
| AST (U/L) Mean ± SD | Baseline | 26.43 ± 8.31 | 28.47 ± 5.44 | 22.60 ± 6.25 | 0.0646 $$ | ||
| F1 | 25.07 ± 6.22 | 28.20 ± 6.67 | 25.33 ± 5.14 | 0.305 $$ | |||
| F2 | 26.33 ± 4.52 | 25.33 ± 4.68 | 25.87 ± 4.63 | 0.869 $$ | |||
| F3 | 28.33 ± 5.20 | 27.45 ± 4.55 | 25.50 ± 6.11 | 0.465 $$ | |||
| ALT (U/L) Mean ± SD | Baseline | 23.20 ± 5.69 | 26.87 ± 7.98 | 21.93 ± 5.74 | 0.117 $$ | ||
| F1 | 27.86 ± 6.18 | 28.07 ± 6.82 | 28.87 ± 4.98 | 0.892 $$ | |||
| F2 | 27.17 ± 5.46 | 30.08 ± 6.50 | 25.07 ± 6.34 | 0.122 $$ | |||
| F3 | 28.78 ± 6.82 | 26.64 ± 5.84 | 25.42 ± 5.16 | 0.440 $$ | |||
| Albumin (g/dL) Mean ± SD | Baseline | 4.14 ± 0.44 | 4.19 ± 0.45 | 4.16 ± 0.47 | 0.966 $$ | ||
| F1 | 4.21 ± 0.51 | 4.09 ± 0.53 | 4.18 ± 0.46 | 0.796 $$ | |||
| F2 | 4.27 ± 0.53 | 4.28 ± 0.52 | 4.13 ± 0.46 | 0.670 $$ | |||
| F3 | 4.13 ± 0.42 | 4.01 ± 0.56 | 4.21 ± 0.34 | 0.566 $$ | |||
| Lipid Profile | |||||||
| Total cholesterol (mg/dL) Mean ±SD | Baseline | 149.00 ± 30.80 | 154.00 ± 32.00 | 129.00 ± 28.20 | 0.0716 $$ | ||
| F1 | 142.80 ± 27.92 | 125.50 ± 23.19 | 131.90 ± 30.57 | 0.241 $$ | |||
| F2 | 141.00 ± 29.90 | 162.70 ± 25.45 | 145.00 ± 24.29 | 0.113 $$ | |||
| F3 | 140.00 ± 38.45 | 155.00 ± 13.04 | 157.50 ± 16.75 | 0.233 $$ | |||
| TG (mg/dL) Mean ± SD | Baseline | 99.80 ± 25.4 | 114.90 ± 18.72 | 94.14 ± 24.59 | 0.0610 $$ | ||
| F1 | 130.40 ± 24.36 | 129.50 ± 20.92 | 138.70 ± 26.16 | 0.367 $$ | |||
| F2 | 128.20 ± 33.64 | 123.90 ± 24.31 | 132.50 ± 28.28 | 0.748 $$ | |||
| F3 | 123.80 ± 21.00 | 125.40 ± 36.12 | 113.20 ± 26.22 | 0.555 $$ | |||
| LDL (mg/dL) Mean ± SD | Baseline | 86.31 ± 30.51 | 93.04 ± 31.59 | 78.64 ± 31.55 | 0.510 $$ | ||
| F1 | 76.64 ± 29.18 | 61.97 ± 20.78 | 63.45 ± 31.65 | 0.312 $$ | |||
| F2 | 75.57 ± 29.69 | 97.30 ± 23.29 | 77.31 ± 26.35 | 0.0917 $$ | |||
| F3 | 76.24 ± 36.14 | 90.67 ± 13.90 | 93.54 ± 14.32 | 0.205 $$ | |||
| Variables | Control (n = 15) | Acetylcysteine (n = 15) | Acetylcysteine and Vitamin E (n = 15) | P1-Value | P2-Value | P3-Value | |
|---|---|---|---|---|---|---|---|
| Blood Glucose test | |||||||
| FBG (mg/dL) Mean ± SD | Baseline | 86.14 ± 19.23 | 67.53 ± 14.91 | 85.75 ± 19.51 | 0.106 $$ | >0.99 $$ | 0.104 $$ |
| F1 | 87.50 ± 22.03 | 74.47 ± 14.26 | 70.09 ± 7.006 | 0.497 $$ | 0.189 $$ | 0.99 $$ | |
| F2 | 76.75 ± 14.51 | 71.08 ± 16.37 | 67.20 ± 5.92 | 0.999 $$ | 0.906 $$ | >0.99 $$ | |
| F3 | 77.00 ± 10.64 | 77.91 ± 11.14 | 78.58 ± 17.87 | >0.99 $$ | >0.99 $$ | >0.99 $$ | |
| Complete Blood Count | |||||||
| Platelets (×103/µL) Median (range) | Baseline | 300.50 (35.4–414) | 272.41 (227–385.2) | 345.00 (194–433) | 0.410 ## | ||
| F1 | 291.50 (233.5–410) | 307.00 (246–410) | 273.60 (205–417) | 0.579 ## | |||
| F2 | 332.00 (205–417) | 312.00 (234–410) | 328.00 (251–417) | 0.711 ## | |||
| F3 | 294.00 (35.2–410) | 297.00 (34.6–433) | 355.50 (36.5–414) | 0.0816 ## | |||
| TLC count (×103/µL) Median (range) | Baseline | 8.10 (5.1–10.1) | 8.50 (4.9–9.7) | 7.80 (3.7–10.3) | 0.822 ## | ||
| F1 | 6.50 (4.1–9.4) | 6.20 (4.5–9.7) | 5.90 (4.1–8.3) | 0.455 ## | |||
| F2 | 6.35 (5.6–9.7) | 5.66 (4.15–10.1) | 6.40 (4–10.3) | 0.656 ## | |||
| F3 | 6.30 (5.1–9.7) | 8.10 (4.15–9.7) | 6.00 (4.2–9.4) | 0.326 ## | |||
| RBC count (×103/µL) Median (range) | Baseline | 6.88 (4–8.18) | 5.5 (3.5–7.62) | 7.24 (4.6–8.94) | 0.0614 ## | ||
| F1 | 4.40 (3.5–7.3) | 5.10 (3.9–9.6) | 4.30 (3.6–8.7) | 0.686 ## | |||
| F2 | 6.35 (5.6–8.9) | 5.69 (3.7–8.4) | 5.50 (4.15–8.4) | 0.050 ## | |||
| F3 | 4.50 (3.8–7.86) | 5.90 (5.1–8.8) | 5.90 (4.5–7.2) | 0.255 ## | |||
| Hemoglobin (g/dL) Mean ± SD | Baseline | 13.16 ± 1.39 | 13.47 ± 1.58 | 14.08 ± 1.70 | 0.281 $$ | ||
| F1 | 13.16 ± 1.04 | 12.91 ± 1.50 | 13.55 ± 1.14 | 0.385 $$ | |||
| F2 | 13.47 ± 1.36 | 13.33 ± 1.36 | 13.25 ±0.91 | 0.900 $$ | |||
| F3 | 13.40 ± 1.79 | 13.62 ± 1.57 | 13.07 ± 0.95 | 0.655 $$ | |||
| HCT (%) Median (range) | Baseline | 39.80 (31.6–46.2) | 39.30 (31.3–54.4) | 41.70 (30.1–4.9) | 0.158 ## | ||
| F1 | 35.85 (28.4–43.3) | 34.30 (30.4–50.0) | 36.20 (30.3–49.5) | 0.970 ## | |||
| F2 | 35.30 (31.6–54.4) | 38.40 (33.8–48.0) | 40.00 (36.0–48.0) | 0.051 ## | |||
| F3 | 36.40 (31.3–54.4) | 37.20 (33.6–47.9) | 41.10 (31.8–46.2) | 0.471 ## | |||
| MCV (fl) Median (range) | Baseline | 49.25 (42.0–58.0) | 50.00 (42.7–58.0) | 52.70 (43.0–61) | 0.064 ## | ||
| F1 | 50.85 (43.2–56.2) | 50.00 (43.2–55.0) | 49.50 (43.7–57.0) | 0.839 ## | |||
| F2 | 51.1 (43.2–55.0) | 52.0 (49.0–56.0) | 52.0 (41.0–59.0) | 0.418 ## | |||
| F3 | 52.5 (43.7–58.0) | 51.2 (44.0–58.0) | 49.9 (41.8–56.3) | 0.833 ## | |||
| MCH (pg) Median (range) | Baseline | 15.75 (11.5–21.5) | 14.10 (12.0–25.5) | 18.70 (12.5–28.1) | 0.824 ## | 0.072 ## | 0.02 ##* |
| F1 | 14.45 (11.6–19.3) | 18.40 (12.1–21.3) | 16.50 (12.0–19.3) | 0.029 ##* | >0.99 ## | 0.196 ## | |
| F2 | 17.90 (13.3–21.7) | 14.50 (11.7–18.3) | 14.90 (13.2–18.3) | 0.018 ##* | 0.017 ##* | >0.99 ## | |
| F3 | 16.50 (12.6–21.7) | 15.60 (11.0–18.7) | 17.20 (14.4–21.4) | 0.19 ## | |||
| MCHC (g/dL) Median (range) | Baseline | 34.05 (28.0–37.5) | 36.10 (30.0–38.6) | 35.20 (32.0–36.0) | 0.0699 ## | ||
| F1 | 30.00 (25.0–37.0) | 32.00 (26.0–38.5) | 30.00 (25.0–36.0) | 0.172 ## | |||
| F2 | 31.50 (26.0–37.0) | 32.40 (29.0–39.0) | 33.00 (30.0–39.0) | 0.411 ## | |||
| F3 | 33.80 (26.0–36.2) | 33.50 (30.0–39.0) | 35.30 (30.0–39.2) | 0.337 ## | |||
| Variables | Control (n = 15) | Acetylcysteine (n = 15) | Acetylcysteine and Vitamin E (n = 15) | P1-Value | P2-Value | P3-Value | |
|---|---|---|---|---|---|---|---|
| Non-specific Inflammatory Markers | |||||||
| CRP (g/dL) Median (range) | Baseline | 0.255 (0.0410–0.428) | 0.400 (0.062–0.520) | 0.211 (0.060–0.50) | 0.0816 ## | ||
| F1 | 0.425 (0.344–0.538) | 0.418 (0.242–0.538) | 0.362 (0.161–0.638) | 0.263 ## | |||
| F2 | 0.376 (0.066–0.662) | 0.341 (0.271–0.638) | 0.329 (0.131–0.583) | 0.844 ## | |||
| F3 | 0.390 (0.037–0528) | 0.331 (0.162–0.53) | 0.157 (0.057–0.400) | >0.99 ## | 0.123 ## | 0.0145 *## | |
| P4-value | 0.8351 ^ | >0.999 ^ | 0.708 ^ | ||||
| ESR (mm/h) Median (range) | Baseline | 12 (3–35) | 5 (3–30) | 15 (5–50) | 0.116 ## | ||
| F1 | 17.5 (2–35) | 10 (3–30) | 10 (5–15) | 0.303 ## | |||
| F2 | 16 (6–35) | 10 (3–20) | 5 (3–45) | 0.05 ## | 0.010 *## | >0.99 ## | |
| F3 | 10 (3–40) | 10 (3–20) | 5 (3–33) | 0.209 ## | |||
| P4-value | 0.993 ^ | >0.999 ^ | 0.159 ^ | ||||
| Variables | Control (n = 15) | Acetylcysteine (n = 15) | Acetylcysteine and Vitamin E (n = 15) | P1-Value | P2-Value | P3-Value | |
|---|---|---|---|---|---|---|---|
| Inflammatory Marker IL-36γ | |||||||
| IL-36γ (pg/mL) Mean ± SD | Baseline | 122.70 ± 34.40 | 122.20 ± 39.06 | 95.89 ± 31.17 | >0.999 ^ | 0.351 | 0.381 |
| F1 | 113.80 ± 27.99 | 73.79 ± 25.99 | 68.66 ± 21.20 | 0.014 *^ | 0.0025 *^ | >0.999 ^ | |
| F2 | 101.90 ± 41.25 | 51.07 ± 10.57 | 52.45 ± 17.02 | 0.0004 *^ | 0.0005 *^ | >0.999 ^ | |
| F3 | 96.54 ± 49.27 | 38.06 ± 7.17 | 33.24 ± 9.49 | 0.0001 *^ | <0.0001 *^ | >0.999 ^ | |
| P4-value | 0.154 | <0.0001 *^ | <0.0001 *^ | ||||
| Variables | Control (n = 15) | Acetylcysteine (n = 15) | Acetylcysteine and Vitamin E (n = 15) | P1-Value | P2-Value | P3-Value | |
|---|---|---|---|---|---|---|---|
| Oxidative Stress Marker (MDA) | |||||||
| MDA (nmol/mL) Mean ± SD | Baseline | 1.08 ± 0.27 | 1.05 ± 0.28 | 1.00 ± 0.32 | >0.999 ^ | 0.989 ^ | 0.999 ^ |
| F1 | 0.89 ± 0.19 | 0.58 ± 0.14 | 0.68 ± 0.073 | 0.0003 *^ | 0.039 *^ | 0.966 ^ | |
| F2 | 0.88 ± 0.18 | 0.43 ± 0.072 | 0.55 ± 0.077 | 0.0001 *^ | 0.0001 *^ | 0.845 ^ | |
| F3 | 0.86 ± 0.19 | 0.36 ± 0.052 | 0.37 ± 0.089 | <0.0001 *^ | <0.0001 *^ | >0.999 ^ | |
| P4-value | 0.154 | <0.0001 *^ | <0.0001 *^ | ||||
| PASI Score (Mean ± SD) | DLQI (Mean ± SD) | ||||||
|---|---|---|---|---|---|---|---|
| Baseline | F3 | % Improvement | P4-Value | Baseline | F3 | P4-Value | |
| Control | 3.47 ± 0.64 | 3.80 ± 2.21 | −6% | 0.966 | 5.53 ± 3.40 | 6.67 ± 4.50 | 0.833 |
| Acetylcysteine | 3.40 ± 0.63 | 2.07 ± 0.80 | 42% | 0.0217 * | 5.93 ± 3.31 | 2.93 ± 2.90 | 0.025 * |
| Acetylcysteine and Vitamin E | 2.87 ± 0.92 | 1.40 ± 0.74 | 52% | 0.0083 * | 6.20 ± 2.62 | 2.60 ± 1.50 | 0.0037 * |
| P1-value | >0.999 | 0.0010 * | 0.959 | 0.0182 * | |||
| P2-value | 0.697 | <0.0001 * | 0.913 | 0.0278 * | |||
| P3-value | 0.791 | 0.594 | >0.999 | >0.999 | |||
| IL-36γ | MDA | ||||
|---|---|---|---|---|---|
| r | p-Value | r | p-Value | ||
| Control | Baseline | 0.562 | 0.037 * | 0.751 | 0.0013 * |
| F3 | 0.655 | 0.011 * | 0.791 | 0.0004 * | |
| Acetylcysteine | Baseline | 0.674 | 0.0059 * | 0.756 | 0.0011 * |
| F3 | 0.640 | 0.034 * | 0.817 | 0.0012 * | |
| Acetylcysteine and Vitamin E | Baseline | 0.755 | 0.011 * | 0.753 | 0.0012 * |
| F3 | 0.715 | 0.0040 * | 0.519 | 0.040 * | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elkalla, N.; Elhamammsy, M.H.; Bedair, N.I.; Elazazy, O.; El Kholy, A.A. A Promising Approach to Psoriasis Vulgaris Management with N-Acetylcysteine and Vitamin E: Targeting the Interplay of Inflammatory and Oxidative Stress. Biomedicines 2025, 13, 1275. https://doi.org/10.3390/biomedicines13061275
Elkalla N, Elhamammsy MH, Bedair NI, Elazazy O, El Kholy AA. A Promising Approach to Psoriasis Vulgaris Management with N-Acetylcysteine and Vitamin E: Targeting the Interplay of Inflammatory and Oxidative Stress. Biomedicines. 2025; 13(6):1275. https://doi.org/10.3390/biomedicines13061275
Chicago/Turabian StyleElkalla, Nira, Manal H. Elhamammsy, Nermeen Ibrahim Bedair, Ola Elazazy, and Amal A. El Kholy. 2025. "A Promising Approach to Psoriasis Vulgaris Management with N-Acetylcysteine and Vitamin E: Targeting the Interplay of Inflammatory and Oxidative Stress" Biomedicines 13, no. 6: 1275. https://doi.org/10.3390/biomedicines13061275
APA StyleElkalla, N., Elhamammsy, M. H., Bedair, N. I., Elazazy, O., & El Kholy, A. A. (2025). A Promising Approach to Psoriasis Vulgaris Management with N-Acetylcysteine and Vitamin E: Targeting the Interplay of Inflammatory and Oxidative Stress. Biomedicines, 13(6), 1275. https://doi.org/10.3390/biomedicines13061275









