Malaria Vaccines and Global Equity: A Scoping Review of Current Progress and Future Directions
Abstract
1. Introduction
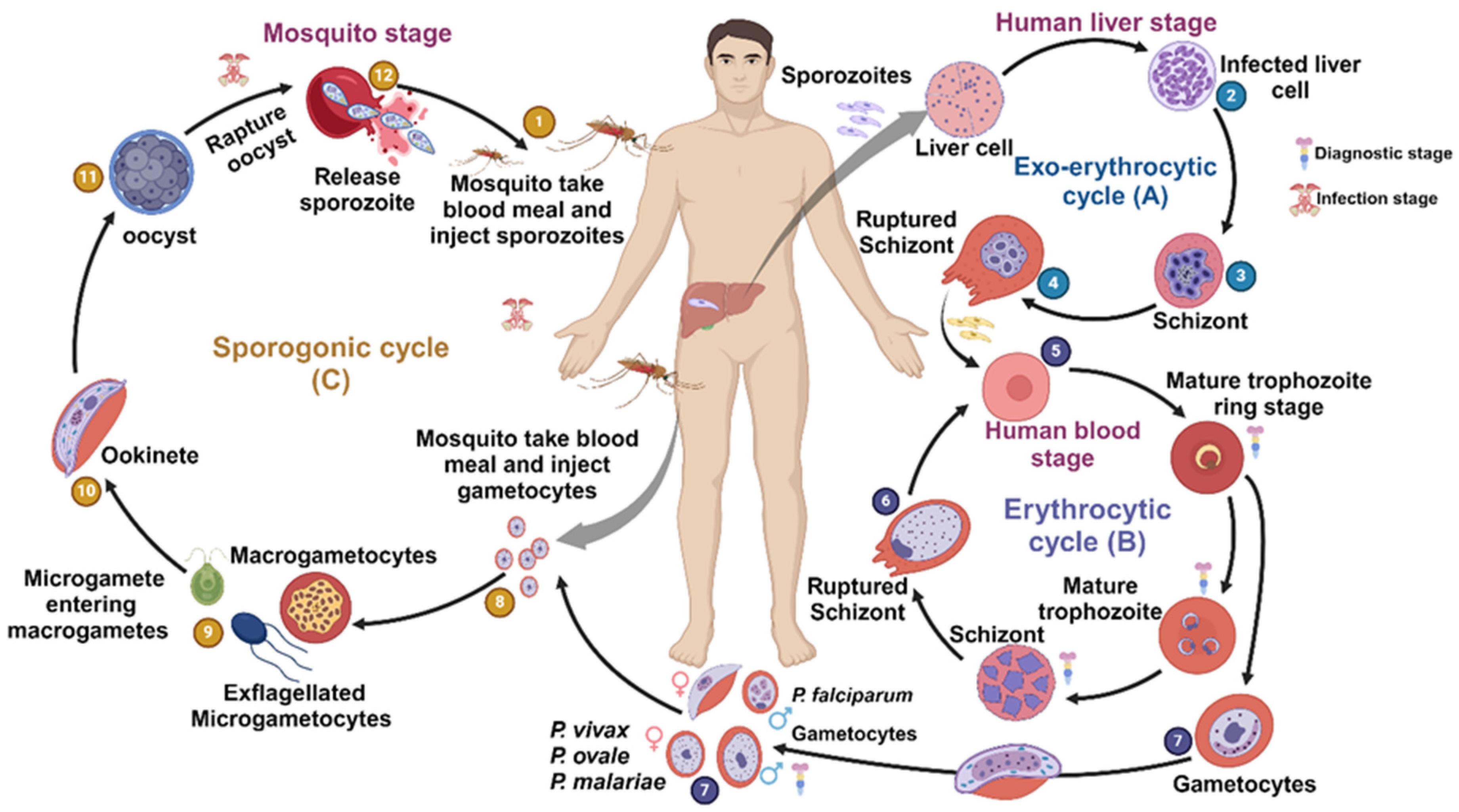
2. Types of Malaria Vaccines
2.1. Pre-Erythrocytic Vaccines
2.2. Erythrocytic Vaccines
2.3. Liver-Stage Subunit Vaccines
2.4. Transmission-Blocking Vaccine
2.5. Viral Vector Vaccines
2.6. DNA and mRNA Vaccines
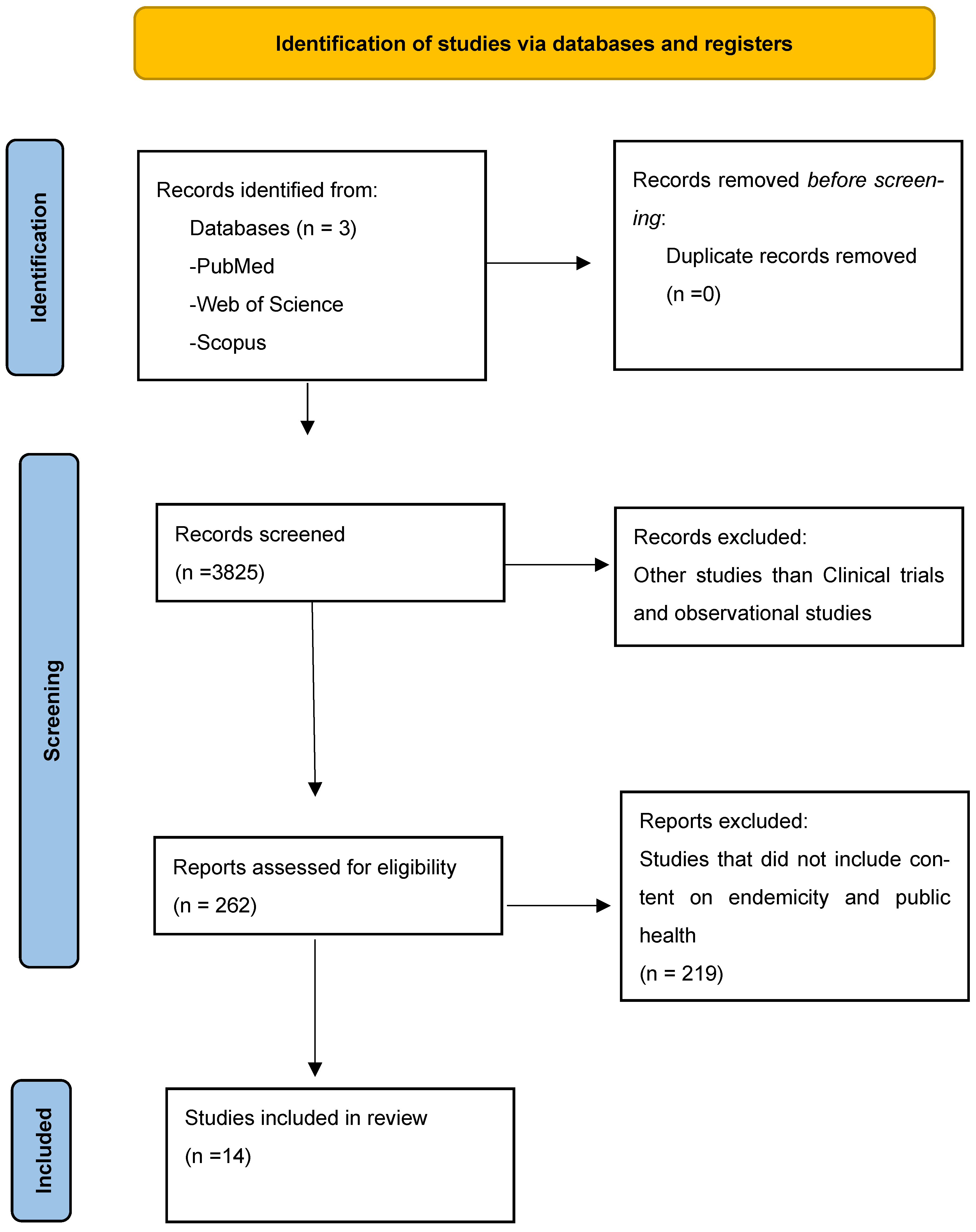
3. Methods
3.1. Inclusion Criteria
3.2. Exclusion Criteria
4. Discussion
5. Conclusions, Safety Concerns, and Limitations
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. World Malaria Report 2023; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Breman, J.G. The ears of the hippopotamus: Manifestations, determinants, and estimates of the malaria burden. Am. J. Trop. Med. Hyg. 2001, 64, 1–11. [Google Scholar] [CrossRef]
- Baden, L.; Catteruccia, F.; Diabaté, A.; Donini, C.; Nosten, F.; O’Neill, S.; Osier, F.; Phyo, A.P.; White, N. Malaria—Epidemiology, Treatment, and Prevention. N. Engl. J. Med. 2023, 388, e9. [Google Scholar] [CrossRef] [PubMed]
- O’Meara, W.P.; Mangeni, J.N.; Steketee, R.; Greenwood, B. Changes in the burden of malaria in sub-Saharan Africa. Lancet Infect. Dis. 2010, 10, 545–555. [Google Scholar] [CrossRef] [PubMed]
- Lengeler, C. Insecticide-treated bed nets and curtains for preventing malaria. Cochrane Database Syst. Rev. 2004, 2, CD000363. [Google Scholar] [CrossRef]
- Lim, S.S.; Fullman, N.; Stokes, A.; Ravishankar, N.; Masiye, F.; Murray, C.J.L.; Gakidou, E. Net benefits: A multicountry analysis of observational data examining associations between insecticide-treated mosquito nets and health outcomes. PLoS Med. 2011, 8, e1001091. [Google Scholar] [CrossRef] [PubMed]
- West, P.A.; Protopopoff, N.; Wright, A.; Kivaju, Z.; Tigererwa, R.; Mosha, F.W.; Kisinza, W.; Rowland, M.; Kleinschmidt, I. Enhanced protection against malaria by indoor residual spraying in addition to insecticide treated nets: Is it dependent on transmission intensity or net usage? PLoS ONE 2015, 10, e0115661. [Google Scholar] [CrossRef]
- Katureebe, A.; Zinszer, K.; Arinaitwe, E.; Rek, J.; Kakande, E.; Charland, K.; Kigozi, R.; Kilama, M.; Nankabirwa, J.; Yeka, A.; et al. Measures of Malaria Burden after Long-Lasting Insecticidal Net Distribution and Indoor Residual Spraying at Three Sites in Uganda: A Prospective Observational Study. PLoS Med. 2016, 13, e1002167. [Google Scholar] [CrossRef]
- Esu, E.B.; Oringanje, C.; Meremikwu, M.M. Intermittent preventive treatment for malaria in infants. Cochrane Database Syst. Rev. 2021, 7, CD011525. [Google Scholar] [CrossRef]
- de Sousa, A.; Rabarijaona, L.P.; Tenkorang, O.; Inkoom, E.; Ravelomanantena, H.V.; Njarasoa, S.; Whang, J.N.; Ndiaye, J.L.; Ndiaye, Y.; Ndiaye, M.; et al. Pharmacovigilance of malaria intermittent preventive treatment in infants coupled with routine immunizations in 6 African countries. J. Infect. Dis. 2012, 205 (Suppl. S1), S82–S90. [Google Scholar] [CrossRef]
- Adeleke, O.T.; Oyenuga, A.; Slusher, T.M.; Gbadero, D.A. Cluster-randomized controlled trial of intermittent preventive treatment in infancy using sulfadoxine–pyrimethamine (SP-IPTi): A pilot study in Nigeria. J. Trop. Pediatr. 2023, 69, fmad001. [Google Scholar] [CrossRef]
- Griffin, J.T.; Cairns, M.; Ghani, A.C.; Roper, C.; Schellenberg, D.; Carneiro, I.; Newman, R.D.; Grobusch, M.P.; Greenwood, B.; Chandramohan, D.; et al. Protective efficacy of intermittent preventive treatment of malaria in infants (IPTi) using sulfadoxine-pyrimethamine and parasite resistance. PLoS ONE 2010, 5, e12618. [Google Scholar] [CrossRef] [PubMed]
- Naidoo, I.; Roper, C. Drug resistance maps to guide intermittent preventive treatment of malaria in African infants. Parasitology 2011, 138, 1469–1479. [Google Scholar] [CrossRef] [PubMed]
- Jones, T.R.; Hoffman, S.L. Malaria vaccine development. Clin. Microbiol. Rev. 1994, 7, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Good, M.F.; Kumar, S.; Miller, L.H. The real difficulties for malaria sporozoite vaccine development: Nonresponsiveness and antigenic variation. Immunol. Today 1988, 9, 351–355. [Google Scholar] [CrossRef]
- Khusmith, S.; Charoenvit, Y.; Kumar, S.; Sedegah, M.; Beaudoin, R.L.; Hoffman, S.L. Protection against malaria by vaccination with sporozoite surface protein 2 plus CS protein. Science 1991, 252, 715–718. [Google Scholar] [CrossRef]
- Smythe, J.A.; Coppel, R.L.; Brown, G.V.; Ramasamy, R.; Kemp, D.J.; Anders, R.F. Identification of two integral membrane proteins of Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 1988, 85, 5195–5199. [Google Scholar] [CrossRef]
- Williams, B.G.; King, L.D.W.; Pulido, D.; Quinkert, D.; Lias, A.M.; Silk, S.E.; Ragotte, R.J.; Davies, H.; Barrett, J.R.; McHugh, K.; et al. Development of an improved blood-stage malaria vaccine targeting the essential RH5-CyRPA-RIPR invasion complex. Nat. Commun. 2024, 15, 4857. [Google Scholar] [CrossRef]
- Doolan, D.L.; Hoffman, S.L. DNA-based vaccines against malaria: Status and promise of the Multi-Stage Malaria DNA Vaccine Operation. Int. J. Parasitol. 2001, 31, 753–762. [Google Scholar] [CrossRef]
- Sklar, M.J.; Maiolatesi, S.; Patterson, N.; Sedegah, M.; Limbach, K.; Teneza-Mora, N.; Chuang, I.; Hollis-Perry, K.M.; Banania, J.G.; Guzman, I.; et al. A three-antigen Plasmodium falciparum DNA prime-Adenovirus boost malaria vaccine regimen is superior to a two-antigen regimen and protects against controlled human malaria infection in healthy malaria-naïve adults. PLoS ONE 2021, 16, e0256980. [Google Scholar] [CrossRef]
- Draper, S.J.; Sack, B.K.; King, C.R.; Nielsen, C.M.; Rayner, J.C.; Higgins, M.K.; Long, C.A.; Seder, R.A. Malaria Vaccines: Recent Advances and New Horizons. Cell Host Microbe 2018, 24, 43–56. [Google Scholar] [CrossRef]
- Zheng, J.; Pan, H.; Gu, Y.; Zuo, X.; Ran, N.; Yuan, Y.; Zhang, C.; Wang, F. Prospects for Malaria Vaccines: Pre-Erythrocytic Stages, Blood Stages, and Transmission-Blocking Stages. Biomed Res. Int. 2019, 2019, 9751471. [Google Scholar] [CrossRef] [PubMed]
- Duffy, P.E. Current approaches to malaria vaccines. Curr. Opin. Microbiol. 2022, 70, 102227. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, S.L.; Vekemans, J.; Richie, T.L.; Duffy, P.E. The march toward malaria vaccines. Vaccine 2015, 33 (Suppl. S4), D13–D23. [Google Scholar] [CrossRef]
- Mariano, R.M.d.S.; Gonçalves, A.A.M.; de Oliveira, D.S.; Ribeiro, H.S.; Pereira, D.F.S.; Santos, I.S.; Lair, D.F.; da Silva, A.V.; Galdino, A.S.; Chávez-Fumagalli, M.A.; et al. A Review of Major Patents on Potential Malaria Vaccine Targets. Pathogens 2023, 12, 247. [Google Scholar] [CrossRef] [PubMed]
- Tajudeen, Y.A.; Oladipo, H.J.; Yusuff, S.I.; Abimbola, S.O.; Abdulkadir, M.; Oladunjoye, I.O.; Omotosho, A.O.; Egbewande, O.M.; Shittu, H.D.; Yusuf, R.O.; et al. A landscape review of malaria vaccine candidates in the pipeline. Trop. Dis. Travel Med. Vaccines 2024, 10, 19. [Google Scholar] [CrossRef]
- Graves, P.; Gelband, H. Vaccines for preventing malaria (pre-erythrocytic). Cochrane Database Syst. Rev. 2006, 2006, CD006198. [Google Scholar] [CrossRef]
- Bejon, P.; Cook, J.; Bergmann-Leitner, E.; Olotu, A.; Lusingu, J.; Mwacharo, J.; Vekemans, J.; Njuguna, P.; Leach, A.; Lievens, M.; et al. Effect of the pre-erythrocytic candidate malaria vaccine RTS,S/AS01E on blood stage immunity in young children. J. Infect. Dis. 2011, 204, 9–18. [Google Scholar] [CrossRef]
- Olotu, A.; Lusingu, J.; Leach, A.; Lievens, M.; Vekemans, J.; Msham, S.; Lang, T.; Gould, J.; Dubois, M.-C.; Jongert, E.; et al. Efficacy of RTS,S/AS01E malaria vaccine and exploratory analysis on anti-circumsporozoite antibody titres and protection in children aged 5-17 months in Kenya and Tanzania: A randomised controlled trial. Lancet Infect. Dis. 2011, 11, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Otieno, L.; Guerra Mendoza, Y.; Adjei, S.; Agbenyega, T.; Agnandji, S.T.; Aide, P.; Akoo, P.; Ansong, D.; Asante, K.P.; Berkley, J.A.; et al. Safety and immunogenicity of the RTS,S/AS01 malaria vaccine in infants and children identified as HIV-infected during a randomized trial in sub-Saharan Africa. Vaccine 2020, 38, 897–906. [Google Scholar] [CrossRef]
- RTS SCTP; Agnandji, S.T.; Lell, B.; Soulanoudjingar, S.S.; Fernandes, J.F.; Abossolo, B.P.; Conzelmann, C.; Methogo, B.G.N.O.; Doucka, Y.; Flamen, A.; et al. First results of phase 3 trial of RTS,S/AS01 malaria vaccine in African children. N. Engl. J. Med. 2011, 365, 1863–1875. [Google Scholar]
- RTS SCTP; Agnandji, S.T.; Lell, B.; Fernandes, J.F.; Abossolo, B.P.; Kabwende, A.L.; Adegnika, A.A.; Mordmüller, B.; Issifou, S.; Kremsner, P.G.; et al. Efficacy and Safety of the RTS,S/AS01 Malaria Vaccine during 18 Months after Vaccination: A Phase 3 Randomized, Controlled Trial in Children and Young Infants at 11 African Sites. PLoS Med. 2014, 11, e1001685. [Google Scholar]
- Guerra Mendoza, Y.; Garric, E.; Leach, A.; Lievens, M.; Ofori-Anyinam, O.; Pirçon, J.-Y.; Stegmann, J.-U.; Vandoolaeghe, P.; Otieno, L.; Otieno, W.; et al. Safety profile of the RTS,S/AS01 malaria vaccine in infants and children: Additional data from a phase III randomized controlled trial in sub-Saharan Africa. Hum. Vaccin. Immunother. 2019, 15, 2386–2398. [Google Scholar] [CrossRef]
- Verma, A.; Anand, A.; Patel, V.A.; Nazar, M.W.; Mukherjee, A.; Karim, K.A.; Oduoye, M.O.; Satapathy, P.; Rustagi, S. Breaking the malaria barrier: The WHO-approved R21/Matrix-M vaccine and its global impact—An editorial. Ann. Med. Surg. 2024, 86, 1824–1827. [Google Scholar] [CrossRef] [PubMed]
- Sirima, S.B.; Ouédraogo, A.; Tiono, A.B.; Kaboré, J.M.; Bougouma, E.C.; Ouattara, M.S.; Kargougou, D.; Diarra, A.; Henry, N.; Ouédraogo, I.N.; et al. A randomized controlled trial showing safety and efficacy of a whole sporozoite vaccine against endemic malaria. Sci. Transl. Med. 2022, 14, eabj3776. [Google Scholar] [CrossRef]
- Jongo, S.A.; Preston Church, L.W.; Mtoro, A.T.; Schindler, T.; Chakravarty, S.; Ruben, A.J.; Swanson, P.A.; Kassim, K.R.; Mpina, M.; Tumbo, A.-M.; et al. Increase of dose associated with decrease in protection against controlled human malaria infection by PfSPZ vaccine in Tanzanian Adults. Clin. Infect. Dis. 2020, 71, 2849–2857. [Google Scholar] [CrossRef]
- Ntege, E.H.; Takashima, E.; Morita, M.; Nagaoka, H.; Ishino, T.; Tsuboi, T. Blood-stage malaria vaccines: Post-genome strategies for the identification of novel vaccine candidates. Expert Rev. Vaccines 2017, 16, 769–779. [Google Scholar] [CrossRef]
- Cai, J.; Chen, S.; Zhu, F.; Lu, X.; Liu, T.; Xu, W. Whole-Killed Blood-Stage Vaccine: Is It Worthwhile to Further Develop It to Control Malaria? Front. Microbiol. 2021, 12, 670775. [Google Scholar] [CrossRef] [PubMed]
- Good, M.F. Towards a blood-stage vaccine for malaria: Are we following all the leads? Nat. Rev. Immunol. 2001, 1, 117–125. [Google Scholar] [CrossRef]
- Salinas, N.D.; Tang, W.K.; Tolia, N.H. Blood-Stage Malaria Parasite Antigens: Structure, Function, and Vaccine Potential. J. Mol. Biol. 2019, 431, 4259–4280. [Google Scholar] [CrossRef]
- Graves, P.; Gelband, H. Vaccines for preventing malaria (blood-stage). Cochrane Database Syst. Rev. 2006, 2006, CD006199. [Google Scholar] [CrossRef]
- Remarque, E.J.; Faber, B.W.; Kocken, C.H.M.; Thomas, A.W. Apical membrane antigen 1: A malaria vaccine candidate in review. Trends Parasitol. 2008, 24, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, G.H.; Thomas, A.W.; Margos, G.; Dluzewski, A.R.; Bannister, L.H. Apical membrane antigen 1, a major malaria vaccine candidate, mediates the close attachment of invasive merozoites to host red blood cells. Infect. Immun. 2004, 72, 154–158. [Google Scholar] [CrossRef] [PubMed]
- Pattnaik, P.; Shakri, A.R.; Singh, S.; Goel, S.; Mukherjee, P.; Chitnis, C.E. Immunogenicity of a recombinant malaria vaccine based on receptor binding domain of Plasmodium falciparum EBA-175. Vaccine 2007, 25, 806–813. [Google Scholar] [CrossRef]
- Hodgson, S.H.; Ewer, K.J.; Bliss, C.M.; Edwards, N.J.; Rampling, T.; Anagnostou, N.A.; de Barra, E.; Havelock, T.; Bowyer, G.; Poulton, I.D.; et al. Evaluation of the efficacy of ChAd63-MVA vectored vaccines expressing circumsporozoite protein and ME-TRAP against controlled human malaria infection in malaria-naive individuals. J. Infect. Dis. 2015, 211, 1076–1086. [Google Scholar] [CrossRef]
- Afolabi, M.O.; Tiono, A.B.; Adetifa, U.J.; Yaro, J.B.; Drammeh, A.; Nébié, I.; Bliss, C.; Hodgson, S.H.; A Anagnostou, N.; Sanou, G.S.; et al. Safety and Immunogenicity of ChAd63 and MVA ME-TRAP in West African Children and Infants. Mol. Ther. 2016, 24, 1470–1477. [Google Scholar] [CrossRef] [PubMed]
- Sagara, I.; Healy, S.A.; Assadou, M.H.; Kone, M.; Swihart, B.J.; Kwan, J.L.; Fintzi, J.; Sissoko, K.; Kamate, B.; Samake, Y.; et al. Malaria transmission-blocking vaccines Pfs230D1-EPA and Pfs25-EPA in Alhydrogel in healthy Malian adults; a phase 1, randomised, controlled trial. Lancet Infect. Dis. 2023, 23, 1266–1279. [Google Scholar] [CrossRef]
- Healy, S.A.; Anderson, C.; Swihart, B.J.; Mwakingwe, A.; Gabriel, E.E.; Decederfelt, H.; Hobbs, C.V.; Rausch, K.M.; Zhu, D.; Muratova, O.; et al. Pfs230 yields higher malaria transmission-blocking vaccine activity than Pfs25 in humans but not mice. J. Clin. Investig. 2021, 131, e146221. [Google Scholar] [CrossRef]
- Mulamba, C.; Williams, C.; Kreppel, K.; Ouedraogo, J.B.; Olotu, A.I. Evaluation of the Pfs25-IMX313/Matrix-M malaria transmission-blocking candidate vaccine in endemic settings. Malar. J. 2022, 21, 159. [Google Scholar] [CrossRef]
- Sheehy, S.H.; Duncan, C.J.A.; Elias, S.C.; Choudhary, P.; Biswas, S.; Halstead, F.D.; Collins, K.A.; Edwards, N.J.; Douglas, A.D.; Anagnostou, N.A.; et al. ChAd63-MVA-vectored blood-stage malaria vaccines targeting MSP1 and AMA1: Assessment of efficacy against mosquito bite challenge in humans. Mol. Ther. 2012, 20, 2355–2368. [Google Scholar] [CrossRef]
- Ishola, D. Asymptomatic Malaria Infection and the Immune Response to the 2-Dose Ad26.ZEBOV, MVA-BN-Filo Ebola Vaccine Regimen in Adults and Children. Clin. Infect. Dis. 2022, 75, 1585–1593. [Google Scholar] [CrossRef]
- Emmanuel, B.N.; Ishaq, A.N.; Akunne, O.Z.; Saidu, U.F. Evaluating the knowledge, attitude, perception, and readiness of caregivers of under 5-year-old children to accept malaria vaccine in Nigeria. Clin. Exp. Vaccine Res. 2024, 13, 121–131. [Google Scholar] [CrossRef] [PubMed]
- RTS SCTP. Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: Final results of a phase 3, individually randomised, controlled trial. Lancet 2015, 386, 31–45. [Google Scholar] [CrossRef]
- Keating, C. The history of the RTS,S/AS01 malaria vaccine trial. Lancet 2020, 395, 1336–1337. [Google Scholar] [CrossRef]
- Olotu, A.; Fegan, G.; Wambua, J.; Nyangweso, G.; Leach, A.; Lievens, M.; Kaslow, D.C.; Njuguna, P.; Marsh, K.; Bejon, P. Seven-Year Efficacy of RTS,S/AS01 Malaria Vaccine among Young African Children. N. Engl. J. Med. 2016, 374, 2519–2529. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, A.Y.; Shehzad, A.; Islam, S.U.; Al-Suhaimi, E.A.; Lee, Y.S. MosquirixTM RTS, S/AS01 Vaccine Development, Immunogenicity, and Efficacy. Vaccines 2022, 10, 713. [Google Scholar] [CrossRef]
- Mumtaz, H.; Nadeem, A.; Bilal, W.; Ansar, F.; Saleem, S.; Khan, Q.A.; Tango, T.; Farkouh, C.; Belay, N.F.; Verma, R.; et al. Acceptance, availability, and feasibility of RTS, S/AS01 malaria vaccine: A review. Immun. Inflamm. Dis. 2023, 11, e899. [Google Scholar] [CrossRef] [PubMed]
- Locke, E.; Flores-Garcia, Y.; Mayer, B.T.; MacGill, R.S.; Borate, B.; Salgado-Jimenez, B.; Gerber, M.W.; Mathis-Torres, S.; Shapiro, S.; King, C.R.; et al. Establishing RTS,S/AS01 as a benchmark for comparison to next-generation malaria vaccines in a mouse model. NPJ Vaccines 2024, 9, 29. [Google Scholar] [CrossRef]
- Björkman, A.; Benn, C.S.; Aaby, P.; Schapira, A. RTS,S/AS01 malaria vaccine-proven safe and effective? Lancet Infect. Dis. 2023, 23, e318–e322. [Google Scholar] [CrossRef]
- Greenwood, B.; Doumbo, O.K. Implementation of the malaria candidate vaccine RTS,S/AS01. Lancet 2016, 387, 318–319. [Google Scholar] [CrossRef]
- Moorthy, V.S.; Newman, R.D.; Duclos, P.; Okwo-Bele, J.M.; Smith, P.G. Assessment of the RTS,S/AS01 malaria vaccine. Lancet Infect. Dis. 2013, 13, 280–282. [Google Scholar] [CrossRef]
- Rosenthal, P.J. The RTS,S/AS01 vaccine continues to show modest protection against malaria in African infants and children. Evid. Based Med. 2015, 20, 179. [Google Scholar] [CrossRef] [PubMed]
- White, M.T.; Verity, R.; Griffin, J.T.; Asante, K.P.; Owusu-Agyei, S.; Greenwood, B.; Drakeley, C.; Gesase, S.; Lusingu, J.; Ansong, D.; et al. Immunogenicity of the RTS,S/AS01 malaria vaccine and implications for duration of vaccine efficacy: Secondary analysis of data from a phase 3 randomised controlled trial. Lancet Infect. Dis. 2015, 15, 1450–1458. [Google Scholar] [CrossRef] [PubMed]
- Tsoumani, M.E.; Voyiatzaki, C.; Efstathiou, A. Malaria Vaccines: From the Past towards the mRNA Vaccine Era. Vaccines 2023, 11, 1452. [Google Scholar] [CrossRef] [PubMed]
- Tizifa, T.A.; Kabaghe, A.N.; McCann, R.S.; van den Berg, H.; Van Vugt, M.; Phiri, K.S. Prevention Efforts for Malaria. Curr. Trop. Med. Rep. 2018, 5, 41–50. [Google Scholar] [CrossRef]
- Furnival-Adams, J.; Olanga, E.A.; Napier, M.; Garner, P. House modifications for preventing malaria. Cochrane Database Syst. Rev. 2020, 10, CD013398. [Google Scholar]
- Messenger, L.A.; Furnival-Adams, J.; Chan, K.; Pelloquin, B.; Paris, L.; Rowland, M. Vector control for malaria prevention during humanitarian emergencies: A systematic review and meta-analysis. Lancet Glob. Health 2023, 11, e534–e545. [Google Scholar] [CrossRef]
- Gabaldón Figueira, J.C.; Wagah, M.G.; Adipo, L.B.; Wanjiku, C.; Maia, M.F. Topical repellents for malaria prevention. Cochrane Database Syst. Rev. 2023, 8, CD015422. [Google Scholar]

| S. No | Reference | Year of Publication | Purpose of Research | Number of Patients Initially Assigned | Result |
|---|---|---|---|---|---|
| 1 | Agnandji et al. [31] | 17 November 2011 | To test the efficacy of the phase III malaria vaccine RTS,S/AS01 in seven African countries | N = 15,460 children | In the study, the first 600 children were from the 5 to 17 months category. There were 2 groups where one received the RTS,S/AS01 vaccine and the other got the non-malaria comparator (control) vaccine. The study had looked at vaccine episode to person-year ratios of RTS,S/AS01 and control, which showed values of 0.32 and 0.55, respectively. The study revealed that the efficacy RTS,S/AS01 was 50.4% (95% confidence interval [CI], 45.8 to 54.6) in comparison to the control. |
| 2 | Agnandji et al. [32] | 29 July 2014 | Observing the effects of phase 3 RTS,S/AS01 after 18 months in children at 11 African sites | N = 6537 infants (6–12 weeks), N = 8923 children (15 to 17 months) | The study was looking at the vaccine efficacy in both groups with either the RTS,S/AS01 or the comparator (control vaccine). The vaccine efficacy rate (VE) for the children group for the first 18 months was 46% (95% CI 42% to 50%) (range 40% to 77%; VE, p = 0.01 across all sites). The VE for the infant group was 27% (95% CI 20% to 32%, per protocol; 27% [95% CI 21% to 33%]). The children group had better protection against severe malaria, malaria hospitalization, and all cause hospitalization than the infant group. One big adverse condition with this vaccine was meningitis. In the RTS,S/AS01 vaccine groups, there were 16/5949 and 9/4358 adverse events in the children and infant groups, respectively. |
| 3 | Tsoumani et al. [63] | 4 September 2023 | Testing the use of mRNA-based vaccines to treat malaria | Previously, mRNA vaccines have been helpful in treating various infections such as COVID-19. In terms of malaria, mRNA amplifying vaccines have been showing clinical efficacy. The vaccine targets Plasmodium macrophage migration inhibitory factor (PMIF) released from the parasites. The study has also shown that vaccines with antigens containing Pfs25 and PfCSP had generated a stronger immune response. These mRNA-lipid nanoparticles (LNP) vaccines prevent the parasite from moving into red blood cells. | |
| 4 | Sirima et al. [35] | 7 December 2022 | Understand vaccine efficacy of whole sporozoite vaccine in the area of Balonghin, Burkina Faso | N = 80 patients, n = 39 Plasmodium falciparum sporozoite vaccine (PfSPZ vaccine), n = 41 normal saline (control) | The adults had been given three doses of either vaccine. For the PfSPZ vaccine, the vaccine efficacy (1 − risk ratio; primary VE endpoint) was 38% at 6 months (p = 0.017) and 15% at 18 months (0.078). After a 2-week period from the vaccine administration, patients who took PfSPZ had shown more antibodies to P. falciparum circumsporozoite proteins than the control group. |
| 5 | Olotu et al. [29] | 11 February 2011 | Studying the efficacy of RTS,S/AS01 in children 5–17 months in Kenya and Tanzania | N = 894, n = 447 (RTS,S/AS01), n = 447 (rabies vaccinated) | The study focused on determining the estimated duration that the anti circumsporozoite antibodies lasted with the RTS,S/AS01 vaccine. The vaccine efficacy rates (VEs) at 12 and 18 months were 39.2% (95% CI 19.5–54.1, p = 0.0005) and 45.8% (24.1–61.3, p = 0.0004), respectively. Some adverse effects included gastroenteritis, pneumonia, and convulsions. |
| 6 | Mendoza et al. [33] | 23 April 2019 | Understanding the possible safety outcomes with the use of RTS,S/AS01in infants and children of sub-Saharan Africa | N = 8922 children (5–17 months), n = 6537 infants (6–12 weeks) | The study was trying to compare RTS,S/AS01(R3R) to the non-malaria control vaccine (C3C). During the 2–3-day period after the vaccine, there was an increase in febrile convulsions in the RTS,S/AS01 group compared with the control. The study also showed there were increased meningitis cases in children receiving RTS,S/AS01 (R3R: 11, R3C: 10, C3C: 1) but not infants. There was increased all-cause mortality in girls receiving RTS,S/AS01 (2.4% vs. 1.3%, all ages) compared with the C3C group. |
| 7 | Verma et al. [34] | 4 March 2024 | Looking at the effectiveness of the R21/Matrix-M malaria vaccine and RTS,S/AS01 (RTS,S) in children across 4 African countries | N = 4800 children | The study wanted to test if the R21/M-Matrix had a faster effect than the standard RTS,S/AS01. The study showed vaccine efficacy values for seasonal areas of 75% and 74% at 12 and 18 months, respectively. There was also a higher seasonal effectiveness in children within the 5-to-17-month range. After the one-year point, the anti-NANP antibody titers had doubled after administration of the third dose. |
| 8 | Ishola et al. [51] | 29 October 2022 | Study focuses on whether the asymptomatic malaria affects the immune response to the 2-dose Ad26.ZEBOV and MVA-BN-Filo Ebola vaccine regimen in malaria endemic countries like Sierra Leone | N = 587 patients, n = 188 adults, n = 399 children | The study was looking at the geometric mean concentrations (GMCs) after the first and second doses of the vaccine using ELISA. The geometric mean ratios using malaria-positive and malaria-negative GMCs were also done. After the first dose, malaria-positive children around 1 to 3 years old (age group-specific GMR, 0.56; 95% CI, 0.39–0.81) had lower GMCs compared to the malaria-negative children. After the second dose, there was a decreasing GMC trend in all groups with no age specific data (GMR, 0.82; 95% CI, 0.67–1.02). |
| 9 | Katureebe et al. [8] | 8 November 2016 | Researchers studies the malaria burden in 3 locations around Uganda after using insecticides and residual spray | Walukuba (n) = 42,833, Kihihi (n) = 28,790, and Nagongera (n) = 38,690 | The study was looking at the test positivity rate (TPR), incidence of malaria (episodes per person per year (PPY)), and human biting rates (HBR) for the three regions. The two control methods used were (LLIN) and indoor residual spraying (IRS) of insecticides. After the 28-month period in Walukuba using LLIN, no changes were found in TPR (26.5% pre-intervention versus 26.2% post-intervention; aRR = 0.70, 95% CI 0.46–1.06, p = 0.09) or incidence (0.39 episodes PPY pre-intervention versus 0.20 post-intervention; adjusted rate ratio [aRR] = 1.02, 95% CI 0.36–2.91, p = 0.97). Following the 21-month period of LLIN in Kihihi, there were no changes in TPR (49.3% pre-intervention versus 45.9% post-intervention; aRR = 0.83, 95% 0.58–1.18, p = 0.30), but a reduction in malaria incidence (1.77 pre-intervention versus 1.89 post-intervention; aRR = 0.65, 95% CI 0.43–0.98, p = 0.04) was observed. After the 12-month LLIN period in Nagongera, there was no change in the malaria incidence (2.82 pre-intervention versus 3.28 post-intervention; aRR = 1.10, 95% 0.76–1.59, p = 0.60), but there was a decrease in TPR (45.3% pre-intervention versus 36.5% post-intervention; aRR = 0.82, 95% CI 0.76–0.88, p < 0.001) |
| 10 | Jongo et al. [36] | 31 December 2020 | The study observed the immune response for different dosages of the PfSPZ vaccine (Plasmodium falciparum [Pf] sporozoites [SPZ]) in Tanzania | N = 9 (age 18–45 years, assigned to 3 doses of 9 × 105 PfSPZ or NS), N = 9 (age 18–45 years, assigned to 3 doses of 1.8 × 106 PfSPZ or NS for Group 1b), N = 12 (age 18–45 years, assigned as infectivity controls) | The vaccine efficacy for the 9 × 105 PfSPZ group during the 3rd or 11th week was 100% (p < 0.000l, Barnard test, 2-tailed). For the 1.8 × 106 PfSPZ group, the vaccine efficacy at 7.5 weeks was around 33% (p = 0.028). The researchers also concluded that the time to first time parasitemia after the vaccine was longer in the PfSPZ groups compared with the control. |
| 11 | Emmanuel et al. [54] | 13 April 2024 | The study sought to understand the willingness of the caregivers under the age of 5 years to give malaria vaccines to their children in Nigeria | N = 347 | The study showed a higher percentage of people that accepted the vaccine, with around 78.4% and around 21.6% rejecting it. Researchers had also found that people with post-secondary or higher certification were more willing to accept the vaccine, with values around 55.6%. |
| 12 | Adeleke et al. [11] | 5 December 2022 | The study wanted to explore sulfadoxine-pyrimethamine intermittent preventive treatment in infants (SP-IPTi) in Nigeria to prevent malaria. | N = 1379 patients | The study showed that SP-IPT was safe in infants. However, there was no difference in the 9-month risks of hospitalizations and fever compared with the control. |
| 13 | Draper et al. [21] | 11 July 2018 | The study observed different vaccines for malaria that have recently been used in the clinical setting. | The study found that higher levels of monoclonal antibodies against the NANP repeat region were strongly correlated with better vaccine efficacy, particularly in vaccines like RTS,S/AS01. The researchers have also concluded that IV administration of the PfSPZ vaccine had a stronger CD8+ response than the subcutaneous method. | |
| 14 | Otieno et al. [30] | 22 January 2020 | Testing the safety and immune response of the RTS,S/AS01 malaria vaccine in HIV-infected children in sub-Saharan Africa | N = 15,459 | The study consisted of 6537 infants (6–12 weeks) and 8922 children (5–17 months). The study reported the anti-circumsporozoite (CS) antibodies of the RTS,S/AS01 vaccine and comparator vaccine group. Each group had received 4 doses. The antibody levels for RTS,S/AS01 and comparator groups were 193.3 EU/mL and 491.5 EU/mL, respectively (p = 0.0001). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaithamanakallam, R.P.; Patel, T.; Balachandran, B.; Fernandez, N.; Jillwin, J.; Kashyap, D.; Shivaprasad, A.; Udayan, U.; Kalyandrug, P.; Aakanksha, A.; et al. Malaria Vaccines and Global Equity: A Scoping Review of Current Progress and Future Directions. Biomedicines 2025, 13, 1270. https://doi.org/10.3390/biomedicines13061270
Kaithamanakallam RP, Patel T, Balachandran B, Fernandez N, Jillwin J, Kashyap D, Shivaprasad A, Udayan U, Kalyandrug P, Aakanksha A, et al. Malaria Vaccines and Global Equity: A Scoping Review of Current Progress and Future Directions. Biomedicines. 2025; 13(6):1270. https://doi.org/10.3390/biomedicines13061270
Chicago/Turabian StyleKaithamanakallam, Rajesh Perumbilavil, Tirath Patel, Bharati Balachandran, Neville Fernandez, Joseph Jillwin, Dharambir Kashyap, Aparna Shivaprasad, Uttam Udayan, Pragnesh Kalyandrug, Aakanksha Aakanksha, and et al. 2025. "Malaria Vaccines and Global Equity: A Scoping Review of Current Progress and Future Directions" Biomedicines 13, no. 6: 1270. https://doi.org/10.3390/biomedicines13061270
APA StyleKaithamanakallam, R. P., Patel, T., Balachandran, B., Fernandez, N., Jillwin, J., Kashyap, D., Shivaprasad, A., Udayan, U., Kalyandrug, P., Aakanksha, A., & Honnavar, P. (2025). Malaria Vaccines and Global Equity: A Scoping Review of Current Progress and Future Directions. Biomedicines, 13(6), 1270. https://doi.org/10.3390/biomedicines13061270






