1. Introduction
Preeclampsia (PE) is a hypertensive disorder unique to pregnancy and is characterized by new-onset hypertension (≥140/90 mmHg) and proteinuria (≥300 mg/24 h) or other organ dysfunction after 20 weeks of gestation [
1]. It remains one of the three causes of maternal and fetal morbidity and mortality worldwide. In addition to hypertension and renal dysfunction, PE can result in severe complications, including endothelial dysfunction, cardiovascular abnormalities, and multiorgan failure, posing significant risks to both mothers and fetuses [
2]. The pathogenesis of PE is multifactorial, with placental insufficiency, hypoxia, excessive oxidative stress, and trophoblast dysfunction playing central roles. Aberrant trophoblast invasion and impaired migration contribute to defective placentation, ultimately leading to the clinical manifestations of PE [
3]. However, the molecular mechanisms governing trophoblast dysfunction remain incompletely understood.
RNA-binding proteins (RBPs) are key regulators of posttranscriptional gene expression and influence mRNA splicing, stability, transport, and translation [
4]. Dysregulated RBP activity is implicated in various pathological conditions, including cancer and cardiovascular diseases [
5]. Alternative splicing (AS) is a post-transcriptional mechanism by which a single gene can generate multiple mRNA isoforms through the selective inclusion or exclusion of specific exons or introns. This process significantly expands the proteomic diversity without increasing the number of genes, allowing cells to fine-tune gene expression in response to developmental cues and environmental stimuli [
6]. Increasing evidence suggests that alternative splicing (AS) plays a critical role in trophoblast differentiation and placental development [
7]. Aberrant RNA processing, including altered AS, has been associated with PE, but the underlying regulatory mechanisms remain elusive. Identifying key RBPs involved in PE-related AS events may provide novel insights into disease pathogenesis and therapeutic targets.
Insulin-like growth factor 2 mRNA-binding protein 3 (IGF2BP3), an RBP known for its role in RNA metabolism, which was first demonstrated in neuronal development of mouse embryos [
8], has been extensively studied in cancer, where it promotes proliferation, adhesion, and invasion [
9]. IGF2BP3 functions as an m6A reader, stabilizing oncogenic transcripts such as CCND1 and VEGF to increase tumor progression [
10]. Additionally, IGF2BP3 modulates AS events, including those of PKM, contributing to metabolic reprogramming in cancer [
11,
12]. While its role in malignancies is well established, the function of IGF2BP3 in trophoblast biology and PE remains poorly defined. Notably, IGF2BP3 expression is significantly downregulated in PE placentas but is elevated in early pregnancy trophoblasts, suggesting a potential role in placental development [
13]. Knockdown of IGF2BP3 in trophoblast cells impairs migration and invasion; however, its involvement in AS regulation in PE has not been fully explored.
In this study, we investigated the regulatory role of IGF2BP3 in PE, focusing on its function in AS and mRNA stability. Using RNA sequencing (RNA-seq) and RNA immunoprecipitation sequencing (RIP-seq), we identified PDE3A as a key IGF2BP3 target. IGF2BP3 directly stabilizes PDE3A mRNA, modulating its splicing patterns and expression in trophoblast cells. Functional assays demonstrated that IGF2BP3 overexpression promoted trophoblast proliferation, migration, and invasion, whereas PDE3A knockdown partially reversed these effects. Moreover, we identified miR-196a-5p as a negative regulator of IGF2BP3, further modulating the IGF2BP3-PDE3A axis. Collectively, our findings reveal a novel miR-196a-5p/IGF2BP3/PDE3A regulatory pathway involved in trophoblast function, providing mechanistic insights into PE pathogenesis and identifying potential therapeutic targets.
2. Materials and Methods
2.1. Tissue Collection
A total of 38 fresh-frozen placental samples, including 13 from patients with preeclampsia (PE) and 25 from normotensive controls, were collected at Renmin Hospital of Wuhan University between 1 February and 30 July 2022. The gestational age at delivery ranged from 29+3 to 37+1 weeks in the PE group and from 37 to 39+2 weeks in the control group. Among the 13 PE cases, 6 were just diagnosed with preeclampsia and 7 with severe preeclampsia (sPE). All the tissue samples were immediately snap-frozen and stored at −80 °C until further analysis. This study was approved by the Ethics Committee of Renmin Hospital of Wuhan University (Approval No. WDRY2022-K103), and written informed consent was obtained from all participants.
2.2. RNA Extraction, Sequencing, and Data Analysis
To comprehensively characterize AS events in preeclampsia and their underlying regulation, we performed transcriptomic profiling on two sample types. Placental tissues from 7 sPE patients and 7 normotensive controls were used to identify clinically relevant AS events. In parallel, the HTR-8/SVneo trophoblast cell line was used to conduct RNA-seq and functional experiments under defined experimental conditions, enabling mechanistic validation of candidate regulators and splicing events.
Total RNA was extracted from 14 fresh-frozen placental tissues, including 7 from sPE patients and 7 from normotensive controls, using TRIzol Reagent (Invitrogen, Carlsbad, CA, USA). RNA quality and concentration were evaluated using NanoDrop™ One C and Qubit™ 3.0 fluorometer (Thermo Fisher Scientific, Waltham, MA, USA), and integrity was assessed via 1.5% agarose gel electrophoresis.
Total RNA was extracted from HTR-8/SVneo cells via TRIzol Reagent (Invitrogen, Cat. No. 15596026) and subsequently treated with DNase I to remove genomic DNA contamination. RNA purity was confirmed by measuring the A260/A280 ratio (NanoDrop™ One C, Thermo Fisher Scientific), and RNA integrity was verified via 1.5% agarose gel electrophoresis. The RNA concentrations were quantified via a Qubit™ RNA Broad Range Assay Kit (Life Technologies, Carlsbad, CA, USA, Q10210) on a Qubit™ 3.0 fluorometer. The quality and integrity of total RNA were assessed using an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). Samples with RIN values greater than 7.0 were selected for RNA-seq library construction and downstream analyses.
For library preparation, 2 μg of total RNA was used to construct stranded mRNA libraries with the KCTM Stranded mRNA Library Prep Kit for Illumina (Cat. No. DR08402, Wuhan Seqhealth Co., Ltd., Wuhan, China) following the manufacturer’s protocol. PCR products in the range of 200–500 bp were enriched and quantified before sequencing on an Illumina NovaSeq 6000 platform (PE150) (San Diego, CA, USA).
Raw sequencing reads were initially filtered by removing those containing >2 ambiguous bases (N) and trimming adaptor sequences and low-quality bases via the FASTX-Toolkit (version 0.0.13). Reads shorter than 16 nt were discarded [
14]. Clean reads were aligned to the GRCh38 reference genome via HISAT2, allowing up to four mismatches [
15]. Only uniquely mapped reads were retained for subsequent analyses.
Gene expression levels were quantified as fragments per kilobase of transcript per million mapped reads (FPKM) [
16]. For differentially expressed gene analysis, the R Bioconductor package DESeq214 was used to screen out the DEGs. Adjusted
p value < 0.05 (Benjamini–Hochberg) and |log2 fold change| > 1 were used as cutoffs [
17].
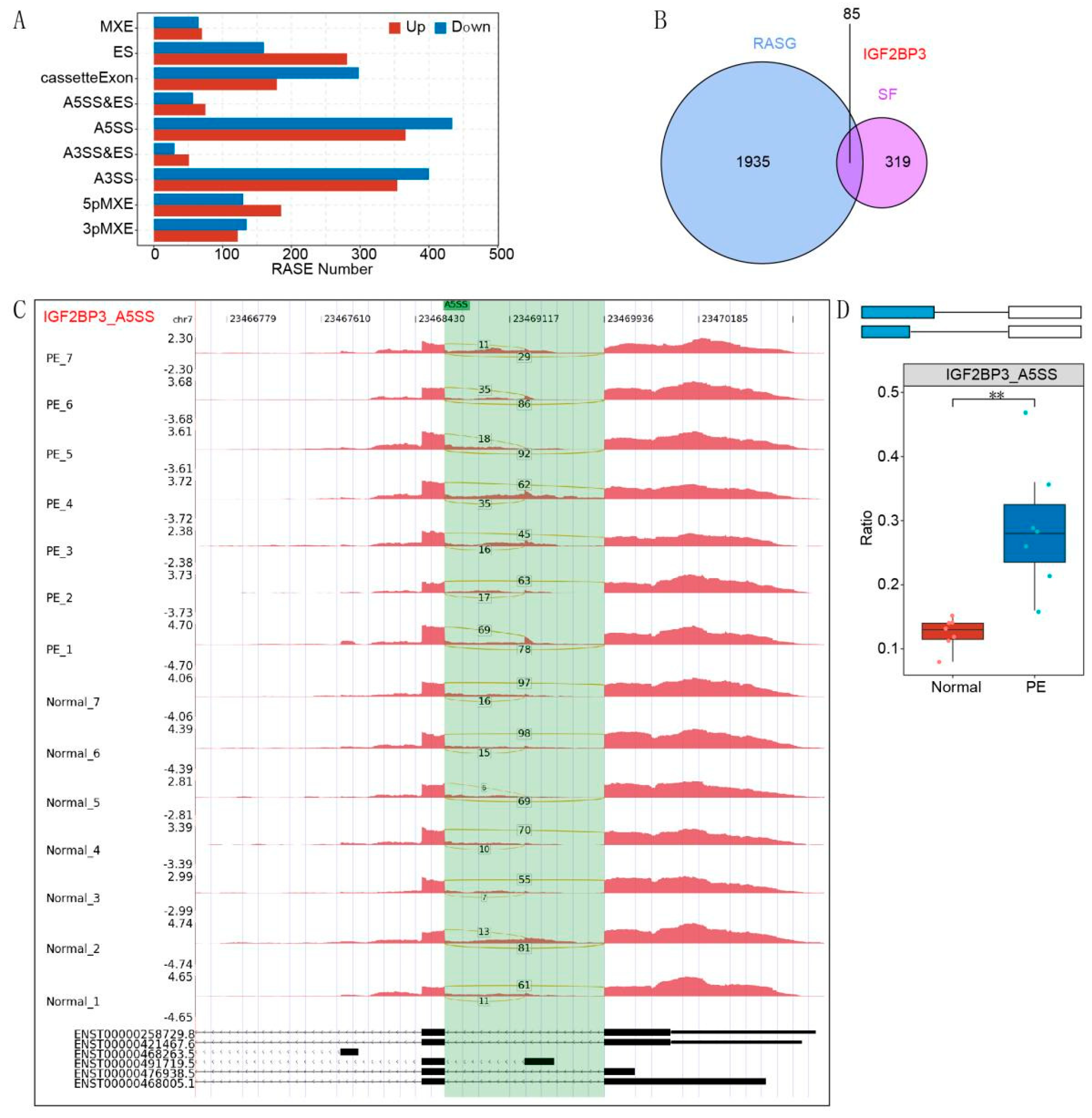
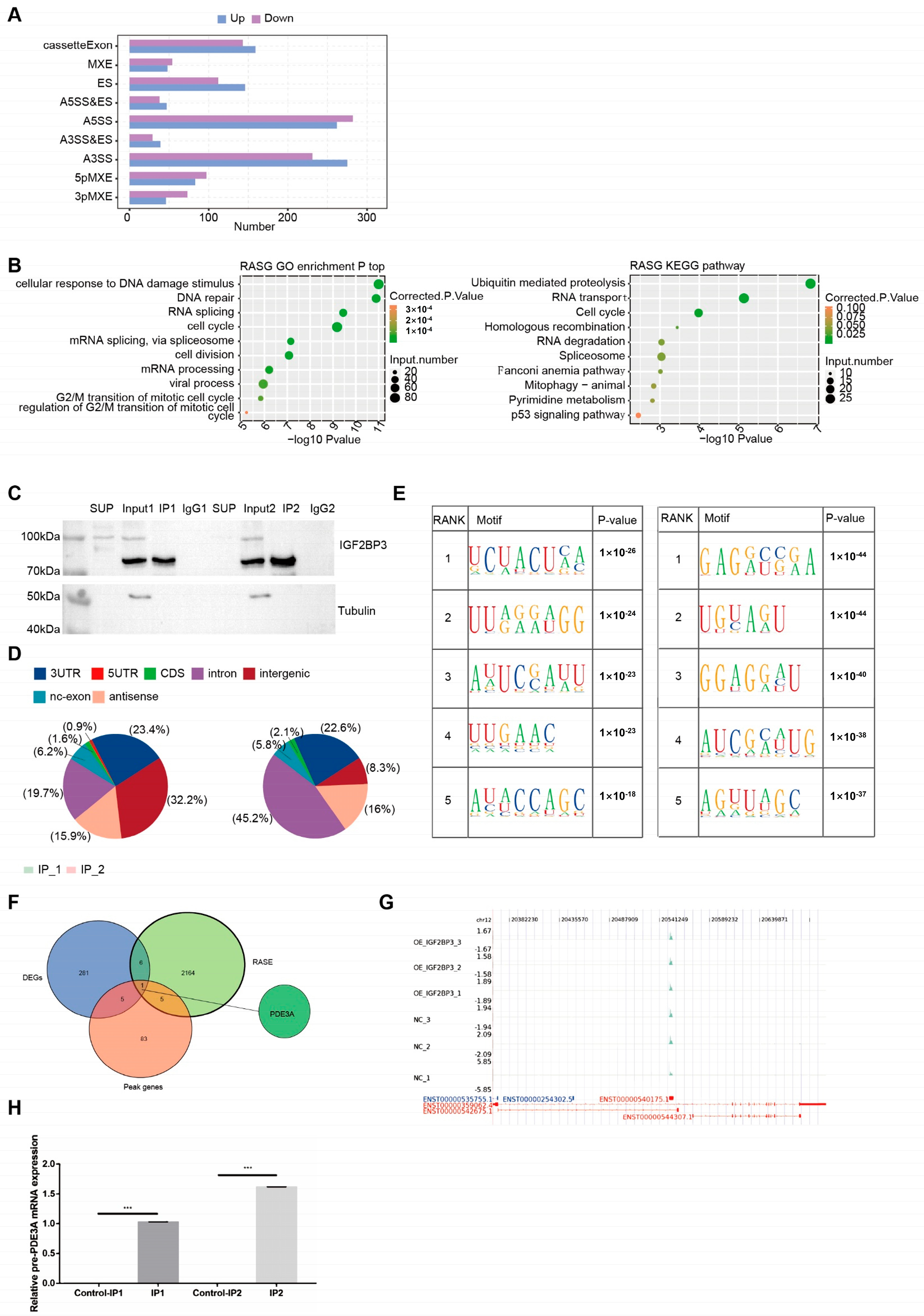
The alternative splicing events (ASEs) and regulated alternative splicing events (RASEs) between the samples were defined and quantified via the ABL as pipeline. In brief, ABL detection of ten types of ASEs was based on splice junction reads, including exon skipping (ES), alternative 5′ splice site (A5SS), alternative 3’s splice site (A3SS), mutually exclusive exons (MXE), mutually exclusive 5′UTRs (5pMXE), mutually exclusive 3′UTRs (3pMXE), cassette exons, A3SS&ES and A5SS&ES. To assess RBP -regulated ASE, Student’s t test was performed to evaluate the significance of the ratio alteration of AS events. Those events that were significant at the p value cutoff corresponding to a false discovery rate cutoff of 5% were considered RBP-regulated ASEs.
Functional enrichment analysis of DEGs was performed via the KOBAS 2.0 server [
18]. Gene Ontology (GO) terms and KEGG pathway enrichment were determined via a hypergeometric test with Benjamini-Hochberg false discovery rate (FDR) correction. Data visualization and statistical analyses were carried out via tools as below. Differential alternative splicing events were analyzed using rMATS (v4.1.0), while differential gene expression analysis was performed using DESeq2 (v1.36.0). GO and KEGG enrichment analyses were conducted with clusterProfiler (v4.2.2). Sashimi plots were visualized using IGV (v2.12.3). PCA and volcano plots were generated in R using ggplot2 and EnhancedVolcano packages, respectively. Motif analysis was carried out with the HOMER tool (v4.11). Heatmaps were plotted using ComplexHeatmap (v2.12.0), and Venn diagrams were created with the VennDiagram R package.
2.3. Coimmunoprecipitation and Library Preparation
HTR-8/SVneo cells were exposed once to ultraviolet C (UVC) radiation at 254 nm with a total energy dose of 400 mJ/cm2. After irradiation, cells were immediately lysed on ice using a chilled buffer composed of 1 × PBS, 0.1% SDS, 0.5% NP-40, and 0.5% sodium deoxycholate, supplemented with RNase inhibitor (200 U/mL, Takara) and a protease inhibitor cocktail (Roche, Basel, Switzerland). The lysates were kept on ice for 30 min. Cellular debris was cleared by centrifugation at 10,000 rpm for 10 min at 4 °C.
To degrade contaminating DNA, RQ I DNase (Promega, Madison, WI, USA, 1 U/μL) was added to the supernatant to reach a final concentration of 0.05 U/μL, followed by incubation in a 37 °C water bath for 30 min. The enzymatic reaction was then halted by the addition of a stop solution. Samples were vortexed thoroughly and centrifuged at 13,000× g for 20 min at 4 °C to remove any residual debris.
Subsequently, RNA was fragmented using micrococcal nuclease (MNase, Thermo Scientific, Waltham, MA, USA). For the immunoprecipitation step, the resulting supernatant was incubated overnight at 4 °C with 10 μg of anti-Flag antibody (Proteintech, Rosemont, IL, USA, FNab03155) and a control IgG (CST, Danvers, MA, USA, 5873S). Antibody–protein complexes were then captured by incubating with protein A/G-conjugated Dynabeads (Thermo Scientific) for 2 h at 4 °C.
After bead capture, magnetic separation was performed, and the beads were washed sequentially using three buffers: the original lysis buffer, a high-salt wash solution (250 mM Tris-HCl pH 7.4, 750 mM NaCl, 10 mM EDTA, 0.1% SDS, 0.5% NP-40, 0.5% sodium deoxycholate), and PNK buffer (50 mM Tris, 20 mM EGTA, 0.5% NP-40), each applied twice.
The beads were then resuspended in elution buffer (50 mM Tris-HCl pH 8.0, 10 mM EDTA, 1% SDS) and incubated at 70 °C for 20 min to release the RNA–protein complexes. After vortexing, the magnetic beads were removed and the supernatant transferred to a clean microcentrifuge tube. Both input samples (non-immunoprecipitated) and the immunoprecipitated complexes were treated with Proteinase K (Roche) at a final concentration of 1.2 mg/mL and incubated at 55 °C for 2 h.
RNA was then extracted using Trizol reagent (Life Technologies). Subsequent library preparation was carried out using the KAPA RNA Hyper Prep Kit (KAPA, Nairobi, Kenya, KK8541) in accordance with the manufacturer’s guidelines. Finally, libraries were sequenced on the Illumina NovaSeq platform using a 150 bp paired-end read configuration
2.4. Data Analysis
After reads were aligned onto the genome with HISAT2, the unique comparison on the genome was finally obtained, and the comparison result of PCR duplicate was removed. And then two software programs, Piranha(version 1.2.1) and ABLIRC(version 0.2),were used to perform peak calling. Piranha has been described elsewhere [
19]. “ABLIRC” strategy was used to identify the binding regions of GRCh38 on genome. The peak calling procedure was carried out as follows: the entire genome was scanned using a sliding window approach, with both window size and step length set to 5 bp, starting from the beginning of each chromosome. A region was considered a candidate peak if the read depth in the initial window exceeded 2.5 times the baseline for eight consecutive windows, or if the median depth surpassed 50. The termination of a peak was defined when the depth across the eight-window segment dropped below 4% of the peak’s maximum read depth. To assess statistical significance, a randomization process was performed where reads were shuffled and reassigned to genes 500 times. The frequency distribution of peak depths from these simulations was used to calculate significance, retaining peaks with either a
p-value less than 0.05 or a maximum depth ≥10.
Next, differential enrichment analysis was conducted using input samples as controls. Peaks with immunoprecipitated (IP) read counts at least fourfold higher than those in the input (this threshold was adjustable) were defined as enriched binding sites. These final peaks were used to identify target genes of the IP protein. Furthermore, the corresponding binding motifs were identified using the HOMER (version 4.11.1) software suite. The target genes of IP were finally determined by the peaks and the binding motifs of IP protein were called by HOMER software [
20].
2.5. Immunohistochemistry
Placental samples obtained from patients with preeclampsia were promptly fixed in paraformaldehyde and embedded in paraffin. The embedded tissues were then subjected to dehydration through a graded ethanol series and subsequently cleared using xylene. After rehydration, sections were rinsed three times with Tris-buffered saline (TBS) and antigen retrieval was performed using sodium citrate buffer.
To block non-specific binding, sections were incubated with 5% bovine serum albumin (BSA) for 30 min. This was followed by overnight incubation at 4 °C with a biotin-labeled primary antibody specific to IGF2BP3. Afterward, a streptavidin-conjugated secondary antibody was applied for 30 min at ambient temperature. Immunoreactivity was visualized using 3,3′-diaminobenzidine (DAB; ZLI-9019, Beijing, China), and nuclei were counterstained with hematoxylin. Images were captured using an Olympus DP72 light microscope.
2.6. Cell Culture and Transfections
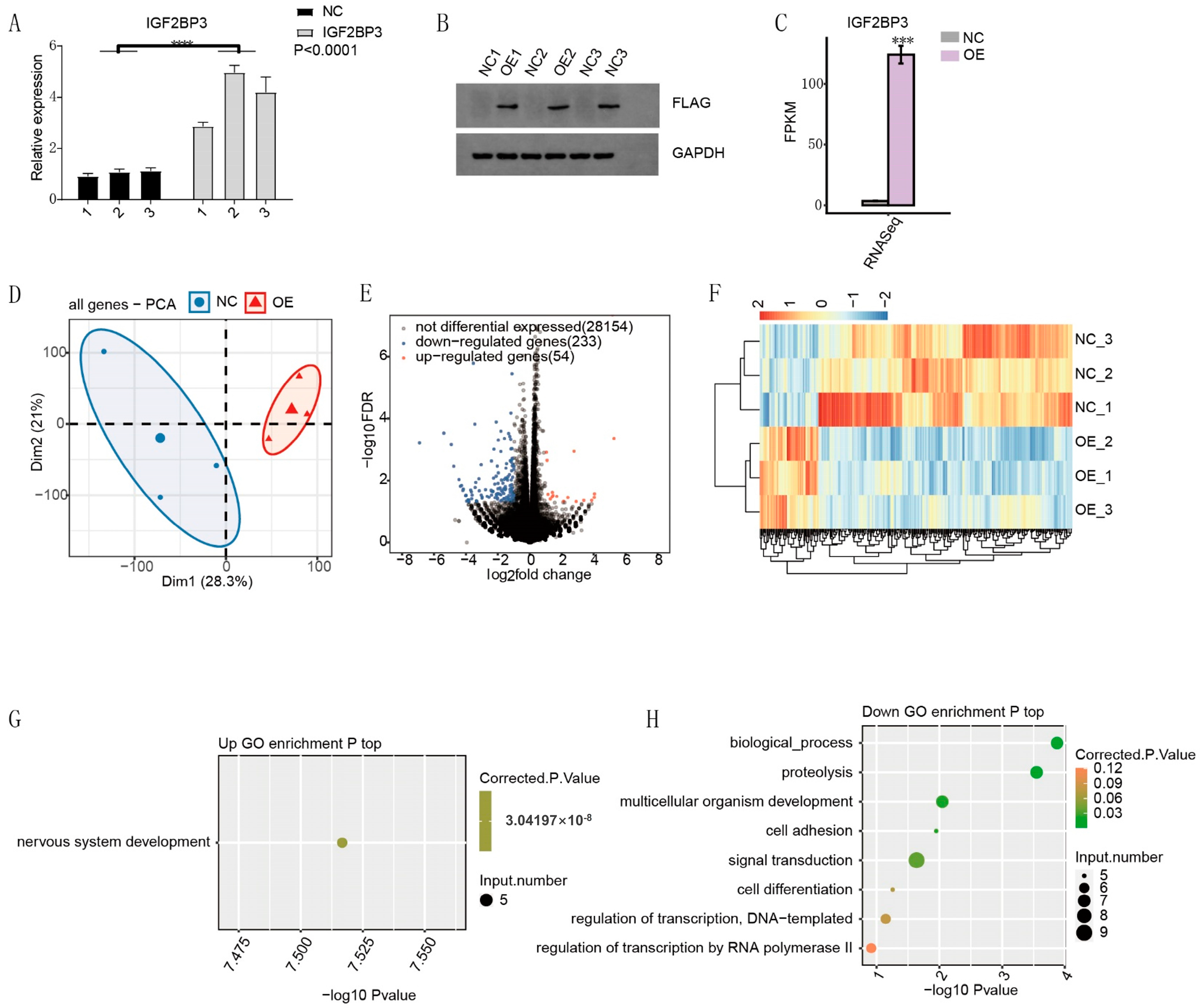
HTR-8/SVneo cells (Cell Research Science & Technology Co., Ltd., Shanghai, China) were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS), 100 U/mL penicillin, and 100 μg/mL streptomycin. Cells were incubated at 37 °C in a humidified environment with 5% CO2. For overexpression experiments, cells were transfected with the pcDNA3.1-3×Flag-IGF2BP3 plasmid (Youbio Biotech, Changsha, China) using Lipofectamine 2000 (Invitrogen) following the manufacturer’s guidelines. After 48 h of transfection, cells were collected for RT-qPCR and Western blot analyses.
2.7. Cell Proliferation and Colony Formation Assays
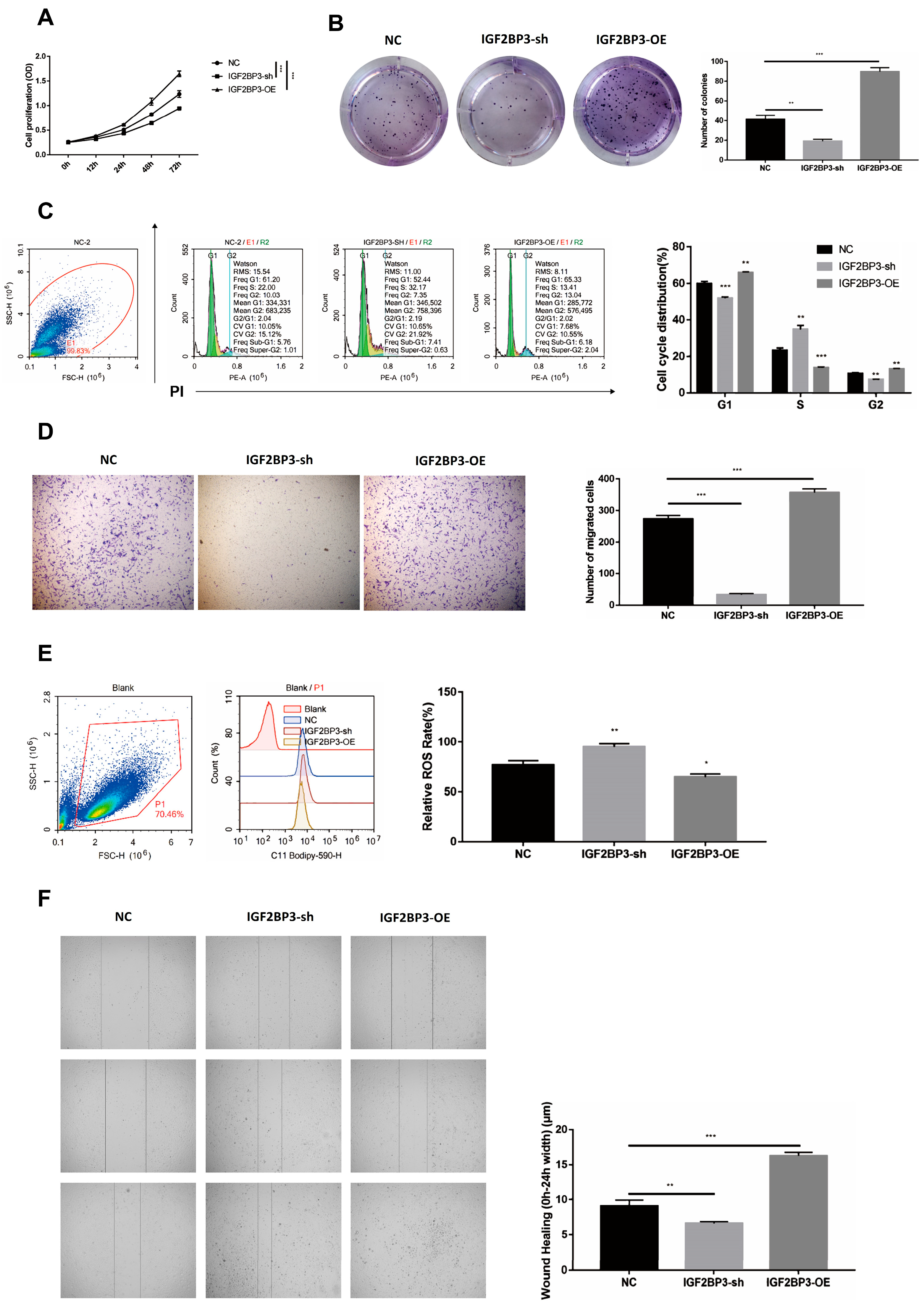
For proliferation assays, 5 × 103 HTR-8/SVneo cells were plated into 96-well plates. Cell viability was evaluated every 24 h using a CCK8 assay, with 10 μL reagent added per well and incubated at 37 °C for 1 h. Absorbance at 450 nm was measured using a microplate reader. For colony formation analysis, 1 × 103 cells were seeded in 6-well plates and cultured for 15 days. Colonies were fixed with 4% paraformaldehyde, stained with hematoxylin, and examined using a DP72 microscope (Olympus, Tokyo, Japan).
2.8. Cell Cycle Analysis
Cells were harvested, washed with PBS, and fixed in cold 70% ethanol for 30 min at 4 °C. Following fixation, cells were washed, resuspended in RNase A solution, and incubated at 37 °C for 30 min. Propidium iodide (PI) staining was performed, and cell cycle distribution was analyzed using a BD FACSCalibur Flow Cytometer (Becton Dickinson Biosciences, San Jose, CA, USA). Data were processed with ModFit LT 5.0 software.
2.9. Scratch Wound and Transwell Migration Assays
For the scratch wound assay, HTR-8/SVneo cells were plated in 6-well dishes and cultured overnight. A straight scratch was generated using a 100 μL pipette tip, and the cells were subsequently incubated for 24 h. After PBS washing, the wound area was stained with crystal violet and imaged. The extent of wound closure was analyzed using ImageJ software (version 1.53k) (NIH).
For the Transwell migration assay, cells were placed in the upper compartment of a Matrigel-coated Transwell insert filled with serum-free RPMI 1640 medium. The lower compartment contained RPMI 1640 medium supplemented with 10% FBS to act as a chemoattractant. After 24 h, non-migratory cells on the upper surface were gently removed with a cotton swab. The migrated cells on the lower surface were then fixed with 4% paraformaldehyde, stained with crystal violet, and visualized under an inverted microscope.
2.10. Reactive Oxygen Species (ROS) Activity
To assess ROS production, HTR-8/SVneo cells were incubated with 5 μM C11-BODIPY 581/591 in 5% FBS-PBS at 37 °C for 1 h. After washing with PBS, fluorescence was measured using a BD FACSCalibur Flow Cytometer (BD Biosicences, Franklin Lakes, NJ, USA) equipped with a 561 nm laser and a 581/591 nm filter.
2.11. RNA Stability Assay
HTR-8/SVneo cells were treated with 5 μg/mL actinomycin D to inhibit transcription. Total RNA was extracted at 0, 20, 40, and 60 min after the addition of actinomycin D using TRIzol reagent (Invitrogen, Carlsbad, CA, USA). The mRNA levels of IGF2BP3 were quantified by RT-qPCR, and mRNA half-lives were calculated based on the decay curves using GraphPad Prism software (v9.0).
2.12. Quantitative Real-Time PCR (qRT-PCR)
Total RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s protocol, including phenol-chloroform extraction and isopropanol precipitation steps. Complementary DNA (cDNA) was synthesized from 1 μg of RNA using the PrimeScript RT reagent Kit (Takara, Shiga, Japan) in accordance with the supplier’s instructions. Quantitative PCR was conducted on a Bio-Rad S1000 thermal cycler with Bestar SYBR Green RT-PCR Master Mix (Vazyme, Nanjing, China). Primer sequences are listed in
Appendix A,
Table A1. GAPDH served as an internal reference gene, and relative expression levels were determined by the 2
−ΔΔCT method. Statistical analysis was performed using paired Student’s
t-test in GraphPad Prism software (San Diego, CA, USA).
2.13. Western Blot Analysis
Cells were lysed in ice-cold lysis buffer containing 1× PBS, 0.1% SDS, 0.5% NP-40, 0.5% sodium deoxycholate, and a protease inhibitor cocktail (Roche, Basel, Switzerland). The lysis was performed on ice for 30 min. Lysates were then mixed with SDS sample buffer and boiled at 95 °C for 10 min to denature proteins. Equal amounts of protein samples were separated by 10% SDS-PAGE and transferred onto polyvinylidene difluoride (PVDF) membranes. Membranes were blocked with 5% non-fat dry milk in TBST buffer (20 mM Tris-HCl, 150 mM NaCl, 0.1% Tween-20, pH 7.6) for 1 h at room temperature. Subsequently, membranes were incubated overnight at 4 °C with primary antibodies against FLAG (1:2000, Proteintech, 80010-1-RR) and GAPDH (1:1000, Goodhere, AB-P-R001). After washing, membranes were incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies (1:10,000, Boster, BA1054, Schwanewede, Germany) for 1 h at room temperature. Protein bands were visualized using an enhanced chemiluminescence (ECL) detection kit (Bio-Rad, Hercules, CA, USA, 170506).
2.14. Dual-Luciferase Reporter Assay
The interaction between IGF2BP3 and miR-196a-5p was evaluated using a dual-luciferase reporter assay. The pmirGLO vector (Qingke Bio, Jinghai, China) was utilized to subclone the IGF2BP3 3′-UTR containing either the wild-type (WT) or mutant (Mut) miR-196a-5p binding sequence. HTR-8/SVneo cells were transfected with these plasmids along with miR-196a-5p mimic using Lipofectamine 2000. After a 48 h incubation, luciferase activity was quantified with the Dual-Luciferase Reporter Assay System (Promega, Madison, WI, USA) following the manufacturer’s instructions. All experiments were independently repeated in triplicate.
2.15. Statistical Analysis
Experimental results are expressed as mean ± standard deviation (SD) based on a minimum of three independent replicates. Statistical analyses were conducted using GraphPad Prism version 8.0 (GraphPad Software, San Diego, CA, USA). For comparisons, unpaired Student’s t-tests and one-way analysis of variance (ANOVA, San Francisco, CA, USA) were employed, along with suitable post hoc tests when applicable. A p-value less than 0.05 was considered indicative of statistical significance. Detailed descriptions of statistical methods and corresponding tests are provided in the respective figure legends.
4. Discussion
Accumulating evidence suggests that dysregulation of mRNA metabolism and RNA editing plays a pivotal role in the pathogenesis of preeclampsia (PE) [
14]. In this study, RNA sequencing (RNA-seq) data were analyzed to investigate alternative splicing (AS) events in PE patient samples. Our findings identified IGF2BP3, a key RNA-binding protein (RBP), as a regulator of AS processes associated with PE. In vitro assays demonstrated that IGF2BP3 expression was significantly downregulated in PE placental tissues. Functional studies further revealed that IGF2BP3 overexpression promoted the proliferation and migration of HTR-8/SVneo trophoblast cells. Moreover, using iRIP-seq and Actinomycin D assays, we confirmed that IGF2BP3 directly binds to PDE3A mRNA and enhances its stability, thereby regulating alternative splicing in trophoblast cells.
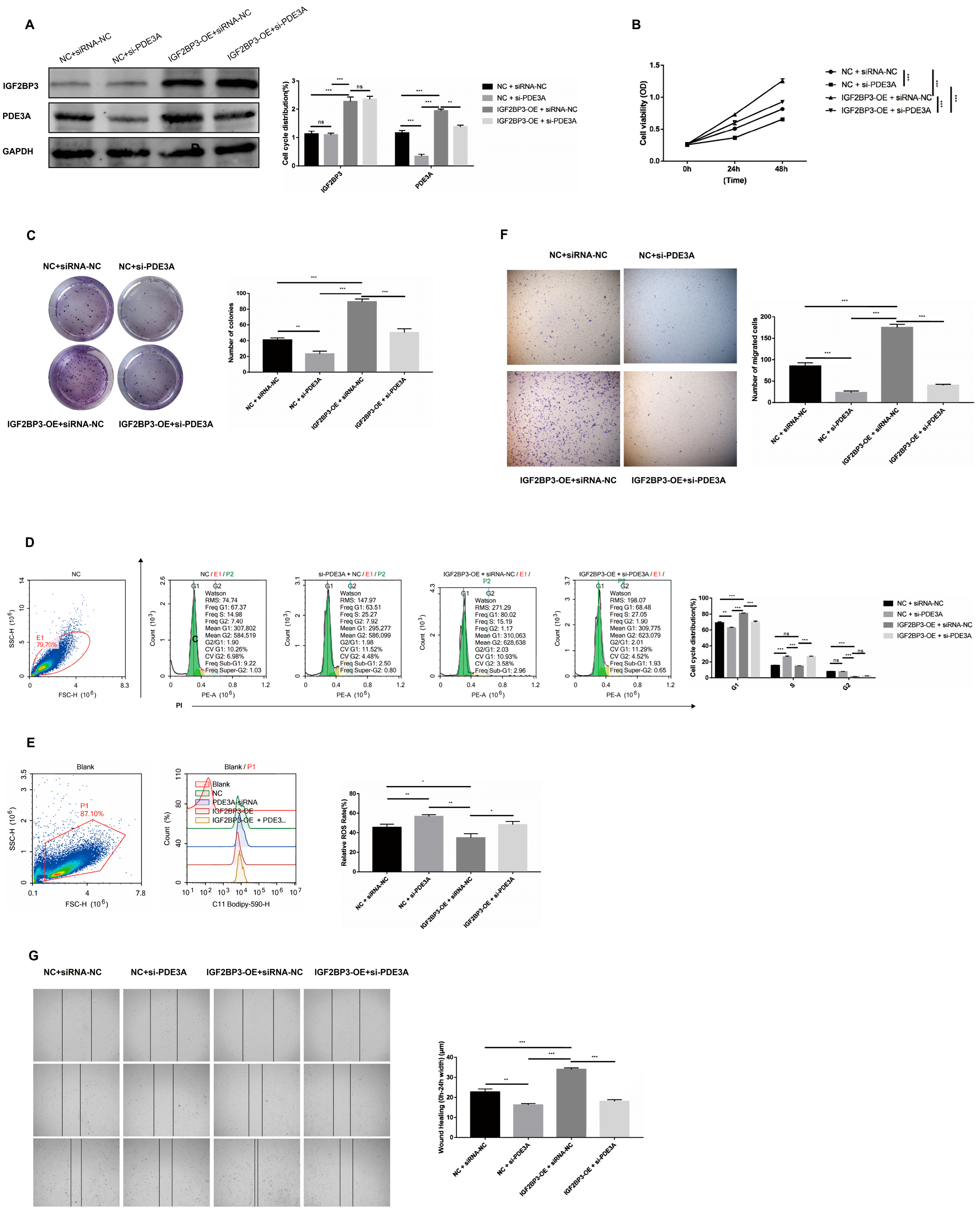
Previous studies have reported that IGF2BP3 knockdown suppresses trophoblast invasion and migration via the PI3K/AKT signaling pathway [
13]. Consistent with these findings, we observed decreased IGF2BP3 expression in PE tissues compared with normal placental tissues. Overexpression of IGF2BP3 not only enhanced proliferation, colony formation, invasion, and migration of HTR-8/SVneo trophoblast cells but also reduced reactive oxygen species (ROS) activation, further highlighting its potential as a therapeutic target for PE.
AS is a crucial post-transcriptional mechanism that generates multiple mRNA isoforms from a single gene, significantly contributing to transcriptome and proteome diversity [
13]. Extensive research has established that AS plays a critical role in tumorigenesis by modulating mRNA metabolism [
21]. However, its role in PE remains largely unexplored. One notable example is sFLT1, a soluble VEGF receptor variant, which is upregulated in PE due to an AS event and serves as a hallmark of the disease [
22]. In this study, we identified IGF2BP3 as a key regulator of AS events during PE progression and validated its functional RNA targets in vitro. IGF2BP3 has been reported to interact with m6A-modified RNAs, influencing their stability and alternative splicing [
23]. Previous studies have demonstrated that IGF2BP3 expression is reduced in PE placental tissues and that its knockdown inhibits trophoblast migration and invasion [
13]. Additionally, IGF2BP3 has been implicated in lung tumorigenesis through AS regulation of PKM [
12]. Moreover, IGF2BP3 interacts with nuclear RNA 7SK, modulating its stability and leading to aberrant splicing events, ultimately causing developmental defects in zebrafish embryos [
24]. Despite these insights, the specific RNA targets of IGF2BP3 in trophoblast cells remain largely unexplored.
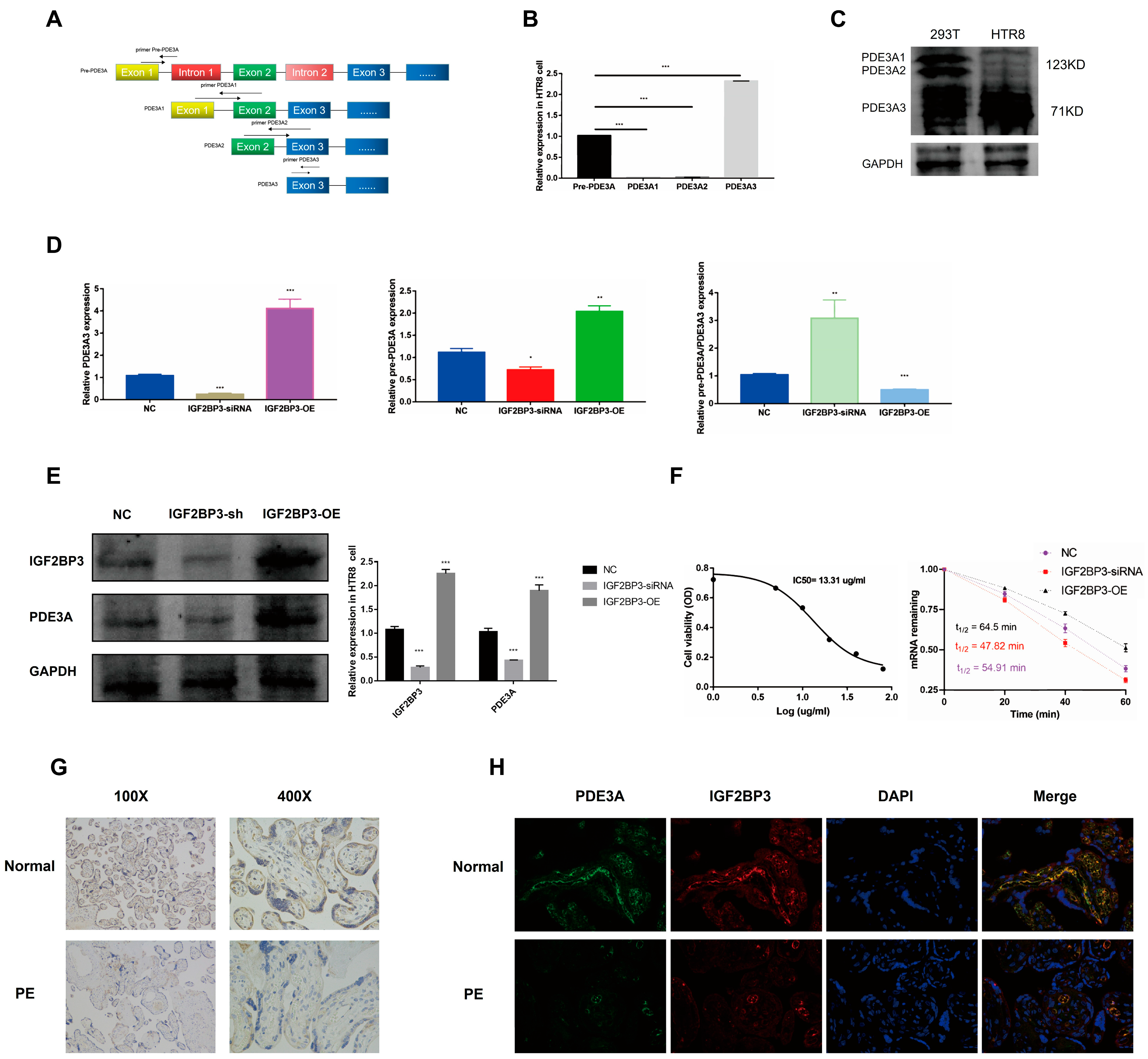
To systematically identify IGF2BP3-associated mRNAs, we performed iRIP-seq in HTR-8/SVneo trophoblast cells. Our analysis revealed that IGF2BP3 overexpression significantly altered gene expression and AS profiles. Notably, PDE3A mRNA was identified as a direct IGF2BP3 target. RNA stability assays demonstrated that IGF2BP3 overexpression prolonged the half-life of PDE3A mRNA, leading to its upregulation. Further in vitro experiments confirmed that IGF2BP3 binding enhances PDE3A mRNA stability, thereby promoting the proliferation and migration of trophoblast cells.
PDE3A encodes a member of the cGMP-inhibited cyclic nucleotide phosphodiesterase (cGI-PDE) family, which plays a critical role in various cellular processes by regulating cyclic nucleotide signaling [
25]. Originally cloned from human myocardial tissues, PDE3A is also expressed in vascular smooth muscle cells, platelets, oocytes, kidneys, and the cervix [
26]. Three PDE3A isoforms—PDE3A1, PDE3A2, and PDE3A3—have been identified, sharing identical sequences except for their terminal regions. Recent studies have revealed significant differences in protein interactions among these isoforms [
27]. Notably, PDE3A3 is exclusively expressed in placental tissues, whereas PDE3A1 is predominantly found in myocardial cells, a finding that aligns with our results [
28]. Our data further confirmed that PDE3A3 is highly expressed in HTR-8/SVneo trophoblast cells, whereas PDE3A1/2 is primarily expressed in HEK293T cells. Previous research has demonstrated that PDE3A inhibition modulates cell cycle progression via the cAMP/PKA/MAPK signaling pathway in vascular smooth muscle cells and oocytes [
29]. In this study, we found that PDE3A knockdown suppressed trophoblast proliferation and migration and induced G1-phase cell cycle arrest, suggesting that PDE3A plays a crucial role in cell cycle regulation.
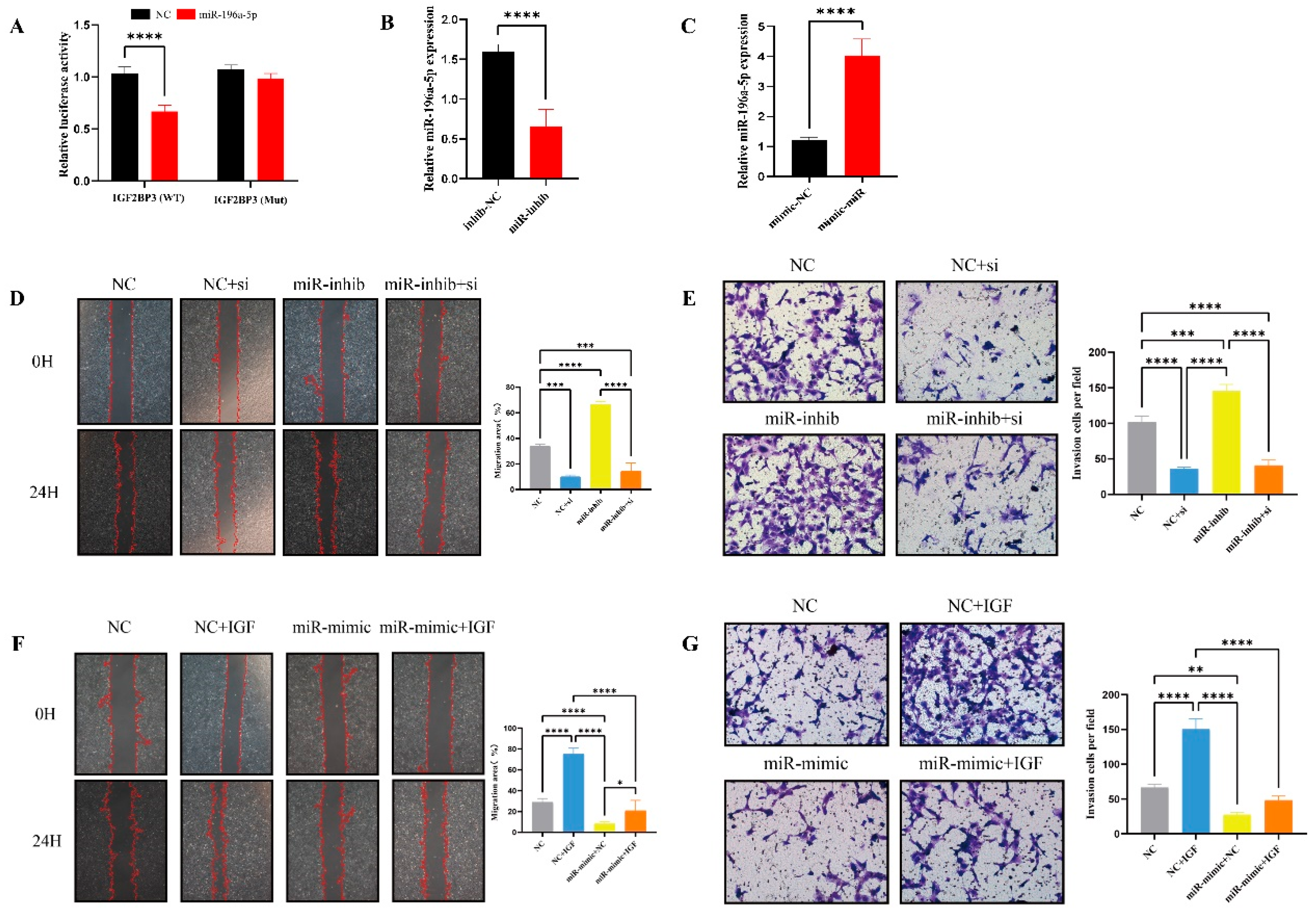
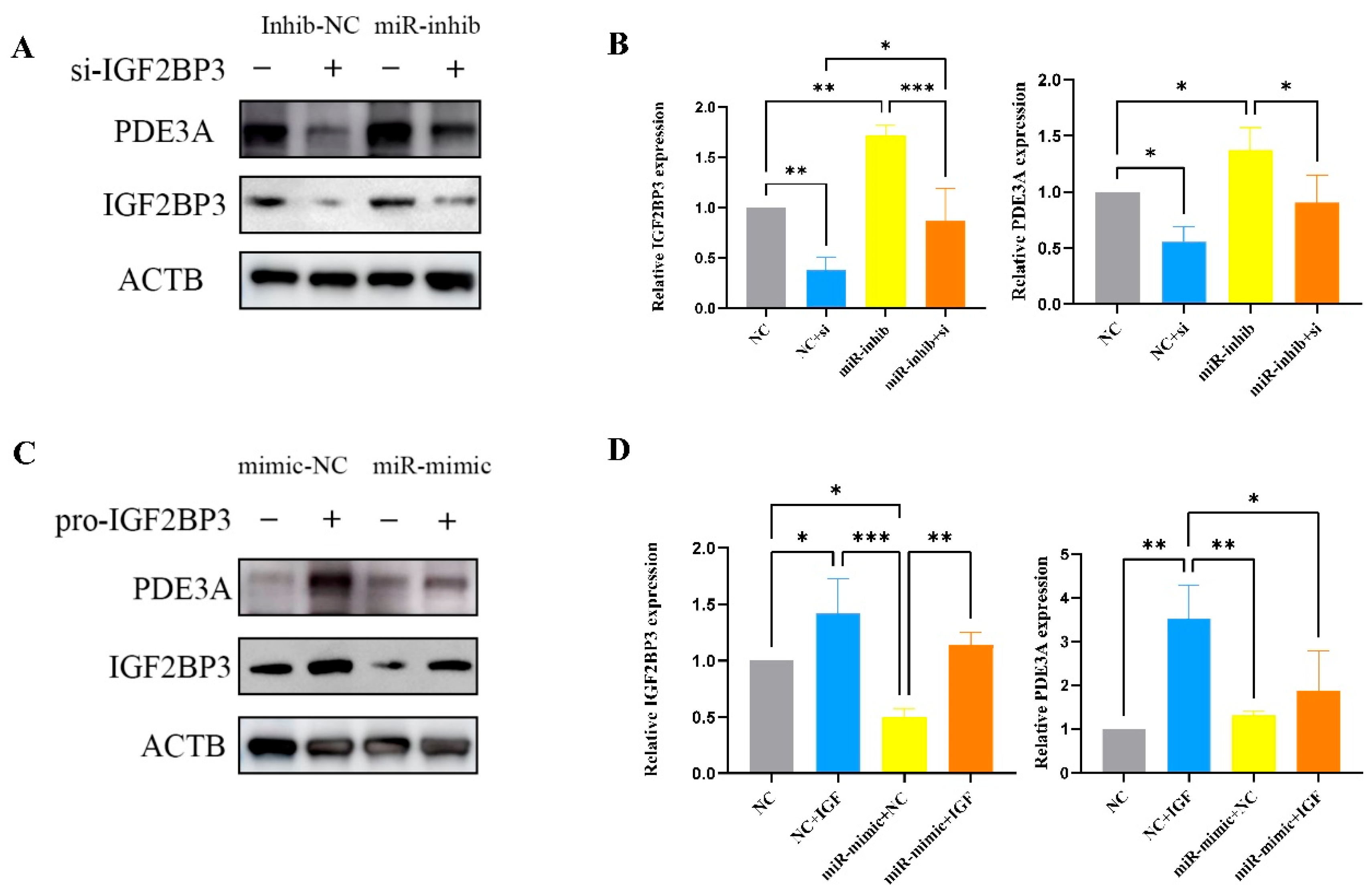
Bioinformatics analysis predicted that IGF2BP3 is directly inhibited by miR-196a-5p, a microRNA transcribed from the HOXC gene cluster, known to play critical roles in cell proliferation, differentiation, apoptosis, and migration across various tissues [
28]. Dysregulation of miR-196a-5p has been implicated in the progression of multiple cancers and pregnancy-related disorders. This prediction was experimentally validated through luciferase reporter assays, qRT-PCR, and Western blotting. Furthermore, IGF2BP3 knockdown via siRNA mimicked the effects of miR-196a-5p overexpression, significantly reducing trophoblast invasion and migration. Conversely, reintroducing IGF2BP3 expression partially rescued the inhibitory effects of miR-196a-5p on trophoblast motility. Notably, miR-196a-5p was highly expressed in PE placental tissues, further supporting its role in PE pathogenesis. These findings collectively indicate that miR-196a-5p suppresses cytotrophoblast invasion and migration by directly targeting IGF2BP3.
MiRNAs play critical roles in cell proliferation, differentiation, and apoptosis, acting as either oncogenic or tumor-suppressive regulators. Extensive research has demonstrated that miRNAs regulate trophoblast proliferation, migration, and apoptosis during pregnancy [
30]. Several studies have identified miR-196a-5p as an oncogenic factor in various cancers, promoting disease progression [
31]. Interestingly, miR-196a-5p expression was significantly downregulated in placenta accreta spectrum (PAS) but upregulated in PE placenta [
32]. Consistent with these findings, our study revealed that miR-196a-5p is upregulated in PE and suppresses trophoblast proliferation and migration by downregulating IGF2BP3, thereby contributing to PE pathogenesis, along with the elucidation of the miR-196a-5p/IGF2BP3/PDE3A regulatory axis, providing novel mechanistic insights into the pathogenesis of preeclampsia (PE).
However, certain limitations should be acknowledged. Firstly, the current findings are primarily based on in vitro cellular models, lacking validation in in vivo animal studies and clinical patient cohorts, which limits the translational applicability of the results. Secondly, while focused on PDE3A as a key downstream effector of IGF2BP3, we acknowledge the emerging significance of apoptotic and autophagic regulators such as BCL-2 and Beclin-1 in preeclampsia and HELLP syndrome [
33]. Given the emerging evidence linking IGF2BP3 to cell survival and stress response pathways, future studies might explore its role in modulating apoptosis and autophagy in the context of PE. Additionally, the heterogeneity of PE subtypes and the complexity of tropho-blast-immune-endothelial interactions warrant further investigation to delineate the precise contribution of IGF2BP3 in PE pathogenesis.
In conclusion, our study identifies IGF2BP3 as a novel regulator of alternative splicing and mRNA stabilization in trophoblast cells, contributing to PE progression through its downstream target PDE3A. These findings provide a foundation for future research into the post-transcriptional regulatory networks in PE and highlight IGF2BP3 as a potential therapeutic target for the treatment of hypertensive pregnancy disorders.