Last Resort? Rationale for Comprehensive Molecular Analysis in Treatment-Refractory R/M HNSCC: A Case Report of Remarkable Response to Sacituzumab Govitecan Following Molecular and Functional Characterization
Abstract
1. Introduction
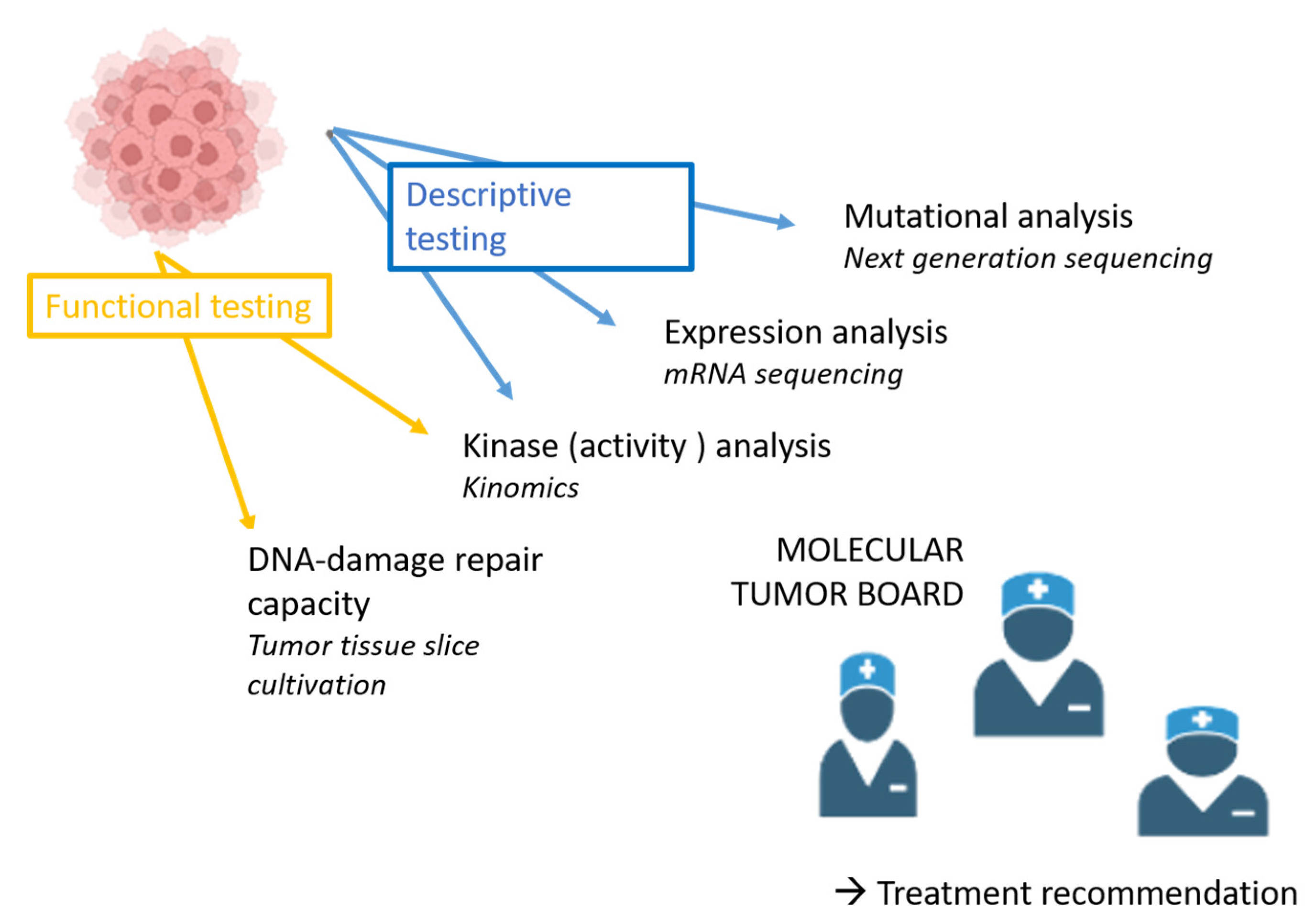
2. Materials and Methods
2.1. Genetic Analysis
2.2. mRNA Sequencing
2.3. Functional Kinome Profiling
2.4. Ex Vivo Tumor Tissue Slice Cultivation and Radiosensitivity Assay
2.5. Ethics
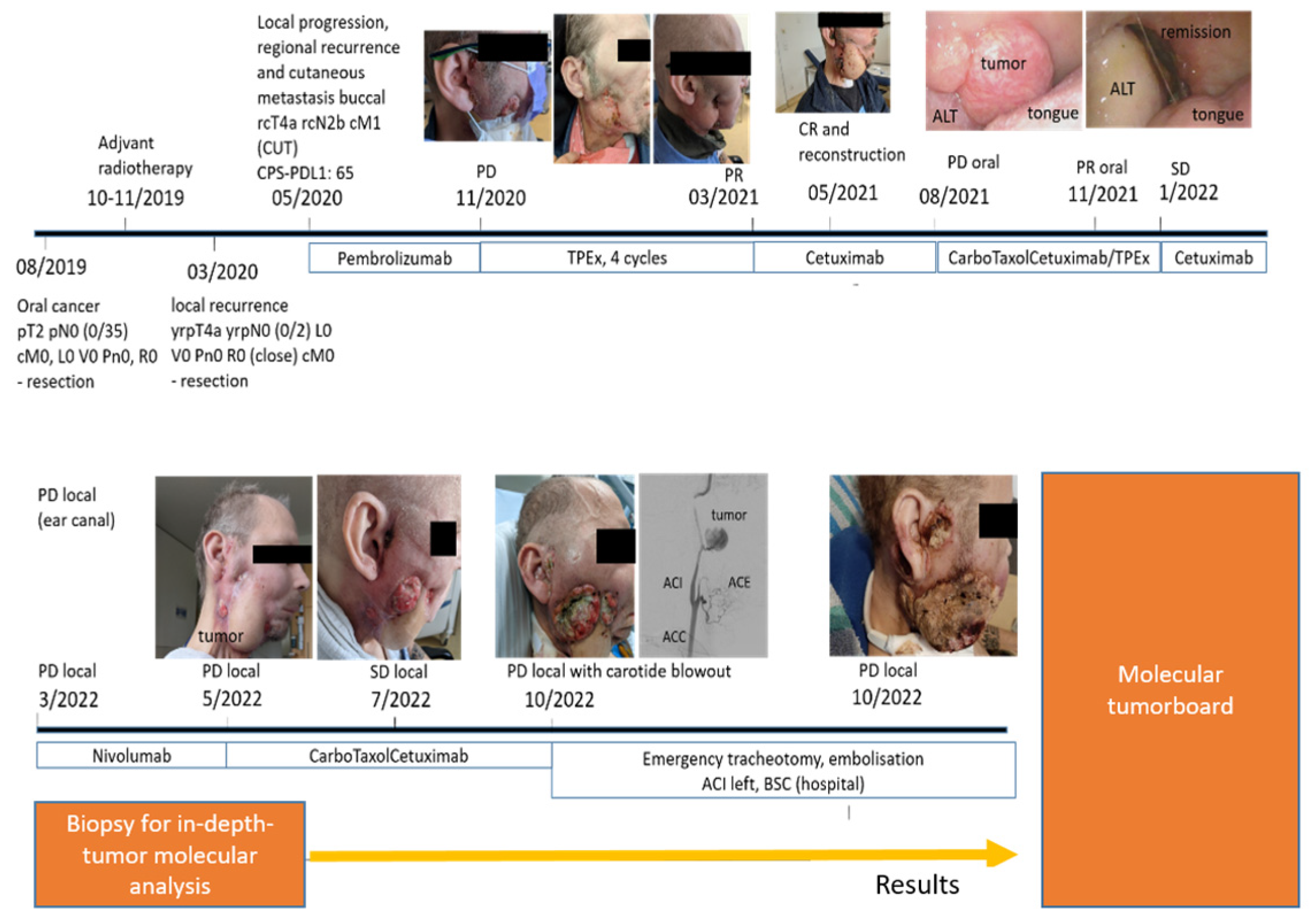
3. Results
3.1. Results of Descriptive and Functional Testing
- ○
- Genetic analysisNo targetable genetic mutations were identified while the tumor mutational burden was moderate (5.2 mutations/MB).
- ○
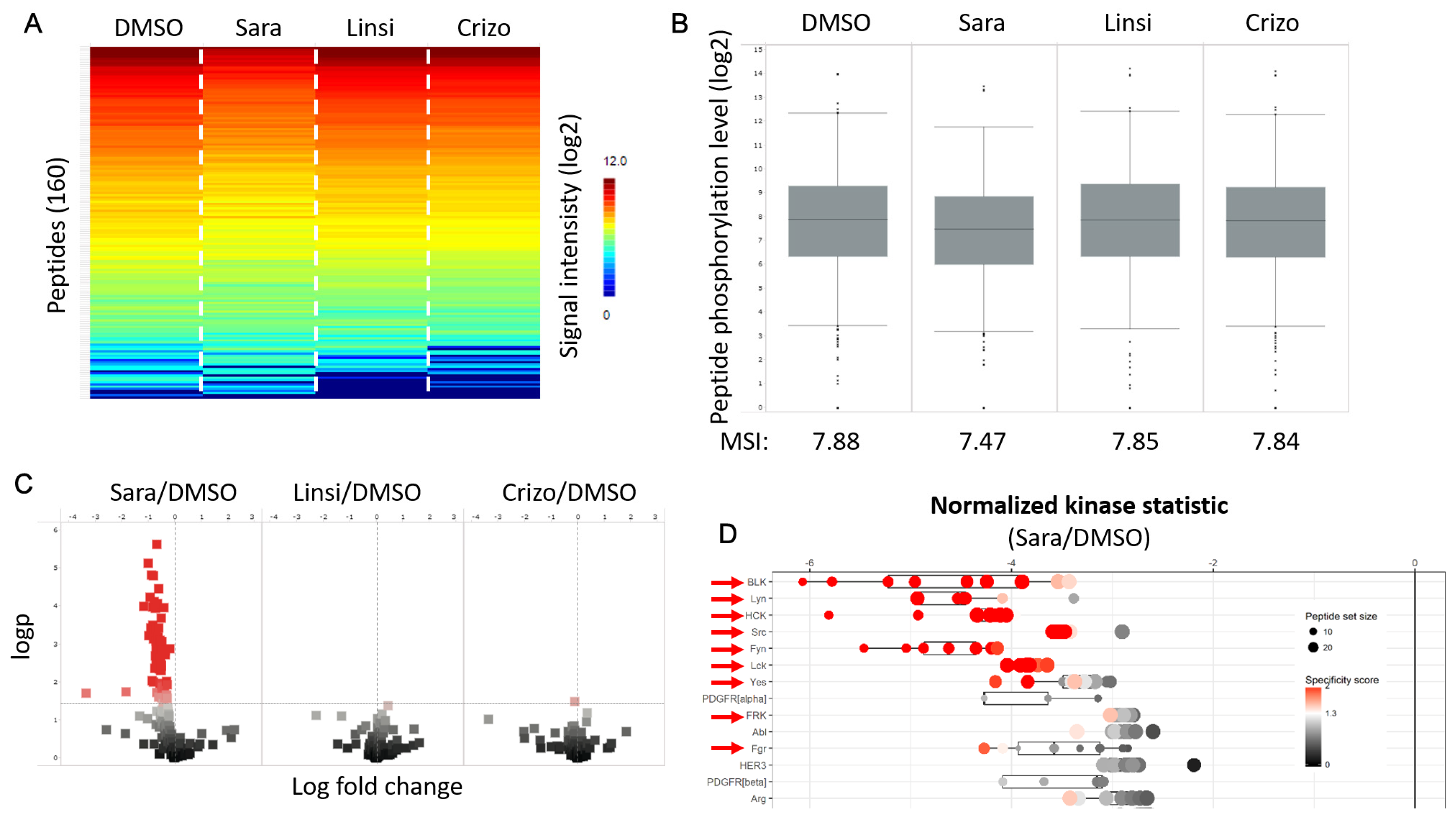
- Functional kinome profilingWe identified several kinases of the Src-family (SFK) to be more active in the tumor compared to the normal tissue, representing potential targets (Appendix A, Figure A2). A subsequent in vitro inhibition of SFK using saracatinib revealed a clear inhibition in overall as well as SFK-specific kinase activity in the tumor sample (Appendix A, Figure A2 and Figure A3). The use of alternative TKIs as negative control showed no noteworthy effect.
- ○
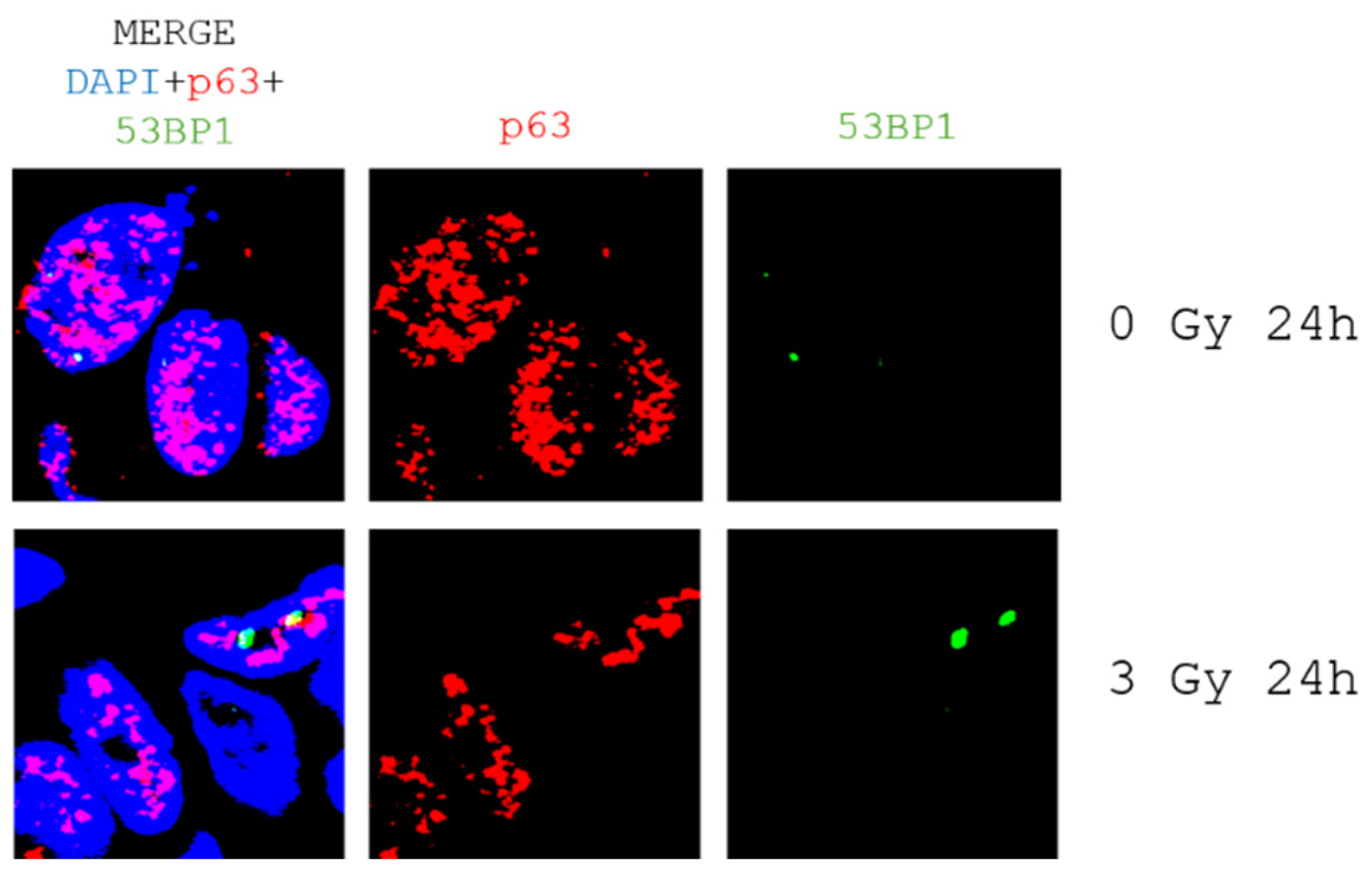
- Ex vivo tumor tissue slice cultivation and radiosensitivity assayQuantitative analysis of DNA double-strand marker 53BP1 in patient-derived tumor tissue slices revealed a low level of DNA damage 24h after radiation (Appendix A, Figure A4).
- ○
- mRNA sequencingMessenger RNA (mRNA) is transcribed from the DNA sequence of genes and then translated into proteins. Therefore, sequencing mRNA is considered as a more direct measure of the effect of DNA changes on protein products [2]. RNA analysis yielded two relevant results: 117-fold overexpression of Trop-2 (also called TACSTD-2) and 8-fold overexpression of PVRL4 (also called Nectin).
3.2. Molecular Tumor Board
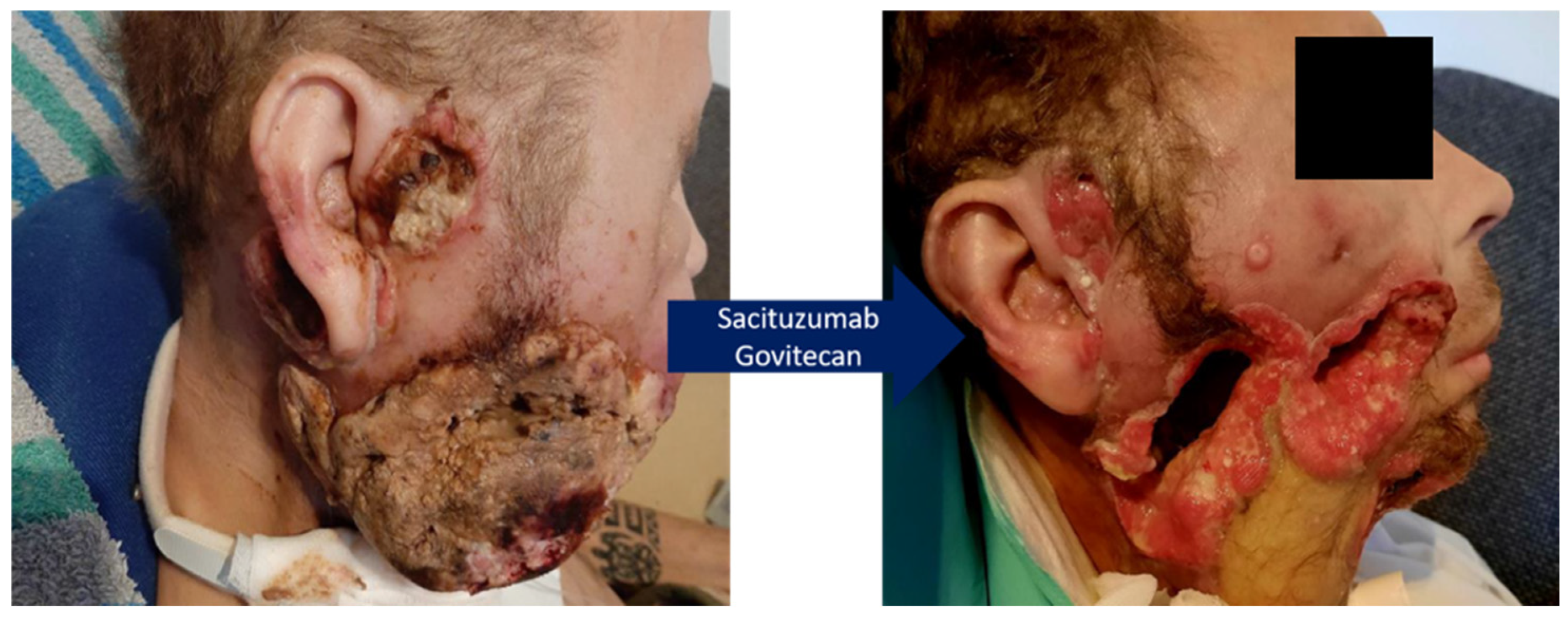
3.3. Treatment with Trop-2 Targeted Therapy
4. Discussion
4.1. Role of the MTB for Head and Neck Cancer Therapy
- -
- Patients without further guideline-based therapy options
- -
- Patients with no prospect of success through guideline-based therapies
- -
- Patients with rare tumor diseases and no available guideline-based therapy options.
4.2. New Techniques for Precision Therapy in Recurrent and Metastatic Head and Neck Cancer (R/M HNSCC)
4.3. Trop-2: A Novel Target in R/M HNSCC?
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADC | antibody–drug conjugate |
| EMA | European Medicines Agency |
| FDA | Food and Drug Administration |
| HPV | Human papilloma virus |
| NGS | Next-generation sequencing |
| MTB | Molecular tumor board |
| R/M HNSCC | Recurrent/metastatic head and neck squamous cell carcinoma |
| SFK | Src-family kinases |
| SG | Sacituzumab govitecan |
| TEAE | Treatment-emergent adverse events |
Appendix A

References
- Horak, P.; Heining, C.; Kreutzfeldt, S.; Hutter, B.; Mock, A.; Hullein, J.; Frohlich, M.; Uhrig, S.; Jahn, A.; Rump, A.; et al. Comprehensive Genomic and Transcriptomic Analysis for Guiding Therapeutic Decisions in Patients with Rare Cancers. Cancer Discov. 2021, 11, 2780–2795. [Google Scholar] [CrossRef] [PubMed]
- Murciano-Goroff, Y.R.; Suehnholz, S.P.; Drilon, A.; Chakravarty, D. Precision Oncology: 2023 in Review. Cancer Discov. 2023, 13, 2525–2531. [Google Scholar] [CrossRef] [PubMed]
- Ortega, M.A.; Pekarek, L.; Navarro, F.; Fraile-Martinez, O.; Garcia-Montero, C.; Alvarez-Mon, M.A.; Diez-Pedrero, R.; Boyano-Adanez, M.D.C.; Guijarro, L.G.; Barrena-Blazquez, S.; et al. Updated Views in Targeted Therapy in the Patient with Non-Small Cell Lung Cancer. J. Pers. Med. 2023, 13, 167. [Google Scholar] [CrossRef]
- Guigay, J.; Auperin, A.; Fayette, J.; Saada-Bouzid, E.; Lafond, C.; Taberna, M.; Geoffrois, L.; Martin, L.; Capitain, O.; Cupissol, D.; et al. Cetuximab, docetaxel, and cisplatin versus platinum, fluorouracil, and cetuximab as first-line treatment in patients with recurrent or metastatic head and neck squamous-cell carcinoma (GORTEC 2014-01 TPExtreme): A multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 2021, 22, 463–475. [Google Scholar] [CrossRef]
- Vermorken, J.B.; Mesia, R.; Rivera, F.; Remenar, E.; Kawecki, A.; Rottey, S.; Erfan, J.; Zabolotnyy, D.; Kienzer, H.R.; Cupissol, D.; et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N. Engl. J. Med. 2008, 359, 1116–1127. [Google Scholar] [CrossRef]
- Bussmann, L.; Hoffer, K.; von Bargen, C.M.; Droste, C.; Lange, T.; Kemmling, J.; Schroder-Schwarz, J.; Vu, A.T.; Akingunsade, L.; Nollau, P.; et al. Analyzing tyrosine kinase activity in head and neck cancer by functional kinomics: Identification of hyperactivated Src family kinases as prognostic markers and potential targets. Int. J. Cancer 2021, 149, 1166–1180. [Google Scholar] [CrossRef]
- Kriegs, M.; Clauditz, T.S.; Hoffer, K.; Bartels, J.; Buhs, S.; Gerull, H.; Zech, H.B.; Bussmann, L.; Struve, N.; Rieckmann, T.; et al. Analyzing expression and phosphorylation of the EGF receptor in HNSCC. Sci. Rep. 2019, 9, 13564. [Google Scholar] [CrossRef] [PubMed]
- Rassamegevanon, T.; Lock, S.; Baumann, M.; Krause, M.; von Neubeck, C. Comparable radiation response of ex vivo and in vivo irradiated tumor samples determined by residual gammaH2AX. Radiother. Oncol. 2019, 139, 94–100. [Google Scholar] [CrossRef]
- Menegakis, A.; De Colle, C.; Yaromina, A.; Hennenlotter, J.; Stenzl, A.; Scharpf, M.; Fend, F.; Noell, S.; Tatagiba, M.; Brucker, S.; et al. Residual gammaH2AX foci after ex vivo irradiation of patient samples with known tumour-type specific differences in radio-responsiveness. Radiother. Oncol. 2015, 116, 480–485. [Google Scholar] [CrossRef]
- Oetting, A.; Christiansen, S.; Gatzemeier, F.; Kocher, S.; Bussmann, L.; Bottcher, A.; Stolzel, K.; Hoffmann, A.S.; Struve, N.; Kriegs, M.; et al. Impaired DNA double-strand break repair and effective radiosensitization of HPV-negative HNSCC cell lines through combined inhibition of PARP and Wee1. Clin. Transl. Radiat. Oncol. 2023, 41, 100630. [Google Scholar] [CrossRef]
- Zech, H.B.; Berger, J.; Mansour, W.Y.; Nordquist, L.; von Bargen, C.M.; Bussmann, L.; Oetting, A.; Christiansen, S.; Mockelmann, N.; Bottcher, A.; et al. Patient derived ex vivo tissue slice cultures demonstrate a profound DNA double-strand break repair defect in HPV-positive oropharyngeal head and neck cancer. Radiother. Oncol. 2022, 168, 138–146. [Google Scholar] [CrossRef] [PubMed]
- Mock, A.; Teleanu, M.V.; Kreutzfeldt, S.; Heilig, C.E.; Hullein, J.; Mohrmann, L.; Jahn, A.; Hanf, D.; Kerle, I.A.; Singh, H.M.; et al. NCT/DKFZ MASTER handbook of interpreting whole-genome, transcriptome, and methylome data for precision oncology. NPJ Precis. Oncol. 2023, 7, 109. [Google Scholar] [CrossRef] [PubMed]
- Loriot, Y.; Kalebasty, A.R.; Flechon, A.; Jain, R.K.; Gupta, S.; Bupathi, M.; Beuzeboc, P.; Palmbos, P.; Balar, A.V.; Kyriakopoulos, C.E.; et al. A plain language summary of the TROPHY-U-01 study: Sacituzumab govitecan use in people with locally advanced or metastatic urothelial cancer. Future Oncol. 2024, 20, 1621–1631. [Google Scholar] [CrossRef]
- Larroquette, M.; Lefort, F.; Domblides, C.; Heraudet, L.; Robert, G.; Ravaud, A.; Gross-Goupil, M. How Immunotherapy Has Redefined the Treatment Paradigm of Metastatic or Locally Advanced Muscle-Invasive Urothelial Bladder Carcinoma. Cancers 2024, 16, 1780. [Google Scholar] [CrossRef]
- DNPM—Informationen für Zuweisende. 2025. Available online: https://dnpm.de/de/zuweisende (accessed on 8 May 2025).
- Gladstone, B.P.; Beha, J.; Hakariya, A.; Missios, P.; Malek, N.P.; Bitzer, M. Systematic review and meta-analysis of molecular tumor board data on clinical effectiveness and evaluation gaps. NPJ Precis Oncol. 2025, 9, 96. [Google Scholar] [CrossRef]
- Marret, G.; Bièche, I.; Dupain, C.; Borcoman, E.; du Rusquec, P.; Ricci, F.; Hescot, S.; Sablin, M.P.; Tresca, P.; Bello, D.; et al. Genomic Alterations in Head and Neck Squamous Cell Carcinoma: Level of Evidence According to ESMO Scale for Clinical Actionability of Molecular Targets (ESCAT). JCO Precis. Oncol. 2021, 5, 215–226. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, P.; Filetti, M.; Falcone, R.; Altamura, V.; Paroni Sterbini, F.; Bria, E.; Fabi, A.; Giannarelli, D.; Scambia, G.; Daniele, G. Overview of Trop-2 in Cancer: From Pre-Clinical Studies to Future Directions in Clinical Settings. Cancers 2023, 15, 1744. [Google Scholar] [CrossRef]
- Shvartsur, A.; Bonavida, B. Trop2 and its overexpression in cancers: Regulation and clinical/therapeutic implications. Genes Cancer 2015, 6, 84–105. [Google Scholar] [CrossRef]
- Guerra, E.; Trerotola, M.; Alberti, S. Targeting Trop-2 as a Cancer Driver. J. Clin. Oncol. 2023, 41, 4688–4692. [Google Scholar] [CrossRef]
- Rugo, H.S.; Tolaney, S.M.; Loirat, D.; Punie, K.; Bardia, A.; Hurvitz, S.A.; O’Shaughnessy, J.; Cortes, J.; Dieras, V.; Carey, L.A.; et al. Safety analyses from the phase 3 ASCENT trial of sacituzumab govitecan in metastatic triple-negative breast cancer. NPJ Breast Cancer 2022, 8, 98. [Google Scholar] [CrossRef]
- Guerra, E.; Alberti, S. The anti-Trop-2 antibody-drug conjugate Sacituzumab Govitecan-effectiveness, pitfalls and promises. Ann. Transl. Med. 2022, 10, 501. [Google Scholar] [CrossRef] [PubMed]
- Tagawa, S.T.; Balar, A.V.; Petrylak, D.P.; Kalebasty, A.R.; Loriot, Y.; Flechon, A.; Jain, R.K.; Agarwal, N.; Bupathi, M.; Barthelemy, P.; et al. TROPHY-U-01: A Phase II Open-Label Study of Sacituzumab Govitecan in Patients With Metastatic Urothelial Carcinoma Progressing After Platinum-Based Chemotherapy and Checkpoint Inhibitors. J. Clin. Oncol. 2021, 39, 2474–2485. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Xu, H.; Zhang, S.; Wang, X.; Zhu, H.; Zhang, H.; Zhu, J.; Huang, J. Potential therapeutic target and independent prognostic marker of TROP2 in laryngeal squamous cell carcinoma. Head. Neck 2013, 35, 1373–1378. [Google Scholar] [CrossRef] [PubMed]
- Fong, D.; Spizzo, G.; Gostner, J.M.; Gastl, G.; Moser, P.; Krammel, C.; Gerhard, S.; Rasse, M.; Laimer, K. TROP2: A novel prognostic marker in squamous cell carcinoma of the oral cavity. Mod. Pathol. 2008, 21, 186–191. [Google Scholar] [CrossRef]
- Erber, R.; Spoerl, S.; Mamilos, A.; Krupar, R.; Hartmann, A.; Ruebner, M.; Taxis, J.; Wittenberg, M.; Reichert, T.E.; Spanier, G.; et al. Impact of Spatially Heterogeneous Trop-2 Expression on Prognosis in Oral Squamous Cell Carcinoma. Int. J. Mol. Sci. 2021, 23, 87. [Google Scholar] [CrossRef]
- Michel, L.; Jimeno, A.; Sukari, A.; Beck, J.T.; Chiu, J.; Ahern, E.; Hilton, J.; Even, C.; Zanetta, S.; Mekan, S.; et al. Sacituzumab Govitecan in Patients with Relapsed/Refractory Advanced Head and Neck Squamous Cell Carcinoma: Results from the Phase II TROPiCS-03 Basket Study. Clin. Cancer Res. 2025, 31, 832–838. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zech, H.B.; Schafhausen, P.; Ramke, L.; Velthaus, J.-L.; Kreutzfeldt, S.; Hübschmann, D.; Rothkamm, K.; Bokemeyer, C.; Hoffmann, A.S.; Fröhling, S.; et al. Last Resort? Rationale for Comprehensive Molecular Analysis in Treatment-Refractory R/M HNSCC: A Case Report of Remarkable Response to Sacituzumab Govitecan Following Molecular and Functional Characterization. Biomedicines 2025, 13, 1266. https://doi.org/10.3390/biomedicines13051266
Zech HB, Schafhausen P, Ramke L, Velthaus J-L, Kreutzfeldt S, Hübschmann D, Rothkamm K, Bokemeyer C, Hoffmann AS, Fröhling S, et al. Last Resort? Rationale for Comprehensive Molecular Analysis in Treatment-Refractory R/M HNSCC: A Case Report of Remarkable Response to Sacituzumab Govitecan Following Molecular and Functional Characterization. Biomedicines. 2025; 13(5):1266. https://doi.org/10.3390/biomedicines13051266
Chicago/Turabian StyleZech, Henrike Barbara, Philippe Schafhausen, Leonie Ramke, Janna-Lisa Velthaus, Simon Kreutzfeldt, Daniel Hübschmann, Kai Rothkamm, Carsten Bokemeyer, Anna Sophie Hoffmann, Stefan Fröhling, and et al. 2025. "Last Resort? Rationale for Comprehensive Molecular Analysis in Treatment-Refractory R/M HNSCC: A Case Report of Remarkable Response to Sacituzumab Govitecan Following Molecular and Functional Characterization" Biomedicines 13, no. 5: 1266. https://doi.org/10.3390/biomedicines13051266
APA StyleZech, H. B., Schafhausen, P., Ramke, L., Velthaus, J.-L., Kreutzfeldt, S., Hübschmann, D., Rothkamm, K., Bokemeyer, C., Hoffmann, A. S., Fröhling, S., Glimm, H., Betz, C. S., Kriegs, M., & Christopeit, M. (2025). Last Resort? Rationale for Comprehensive Molecular Analysis in Treatment-Refractory R/M HNSCC: A Case Report of Remarkable Response to Sacituzumab Govitecan Following Molecular and Functional Characterization. Biomedicines, 13(5), 1266. https://doi.org/10.3390/biomedicines13051266





