Influence of Constipation in the Behavior of Circulating Alpha- and Beta-CGRP Levels in Chronic/High-Frequency Migraine Patients After CGRP Monoclonal Antibodies
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Considerations
2.2. Study Design, Population, and Clinical Assessments
2.3. Blood Extraction and Laboratory Determinations
2.4. Statistical Analysis
3. Results
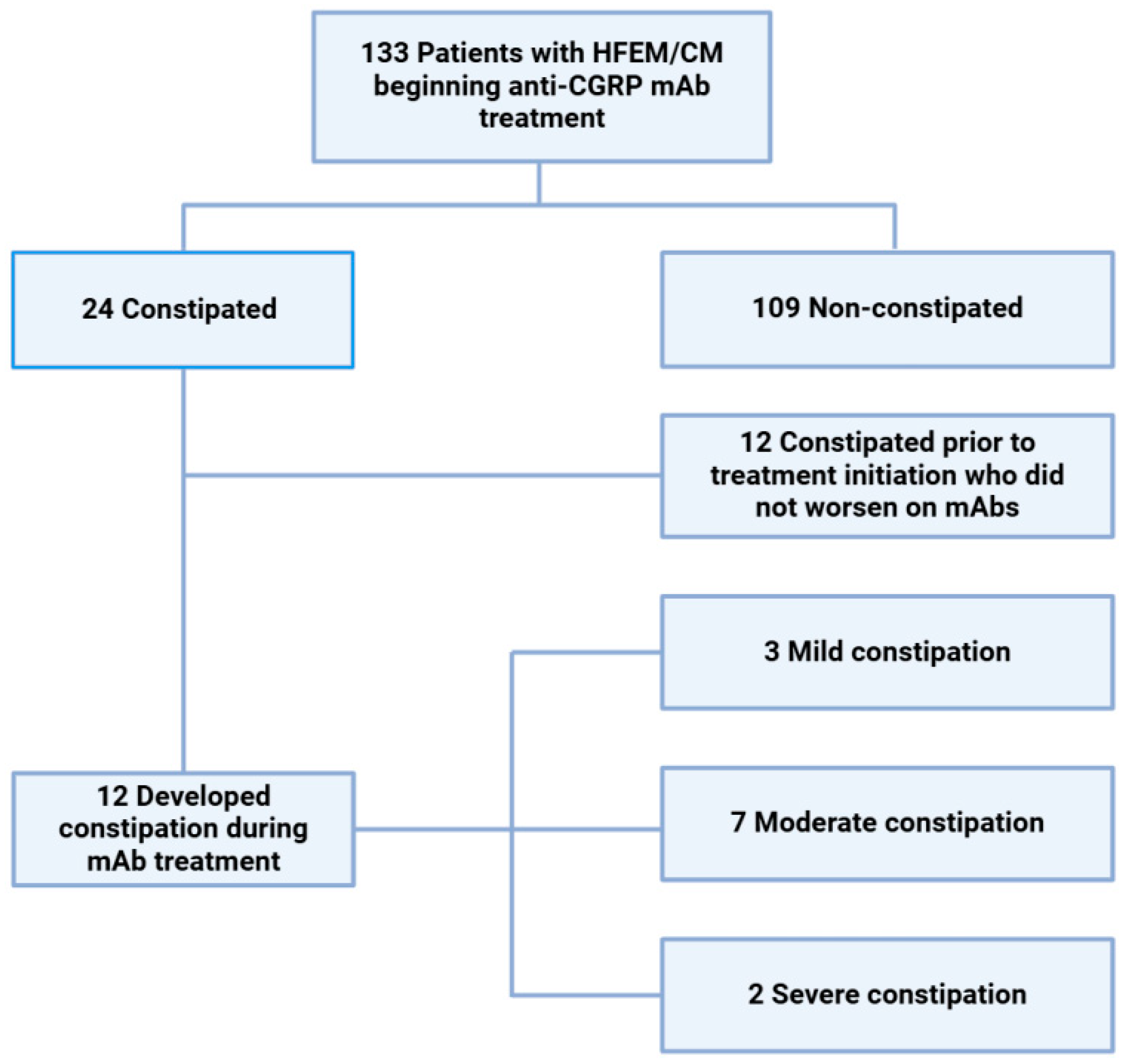
3.1. Patient Population, Response, and Constipation with mAbs
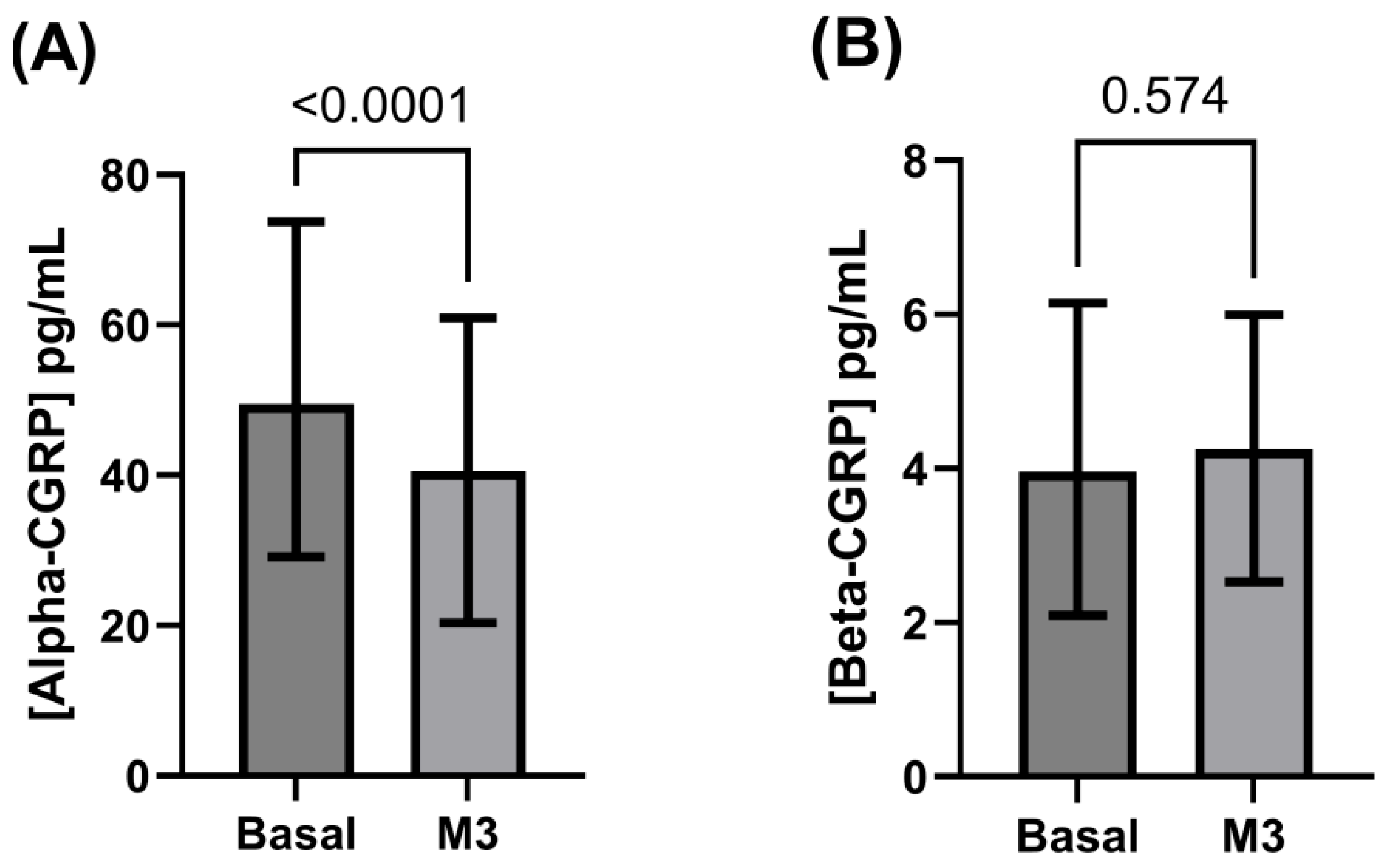
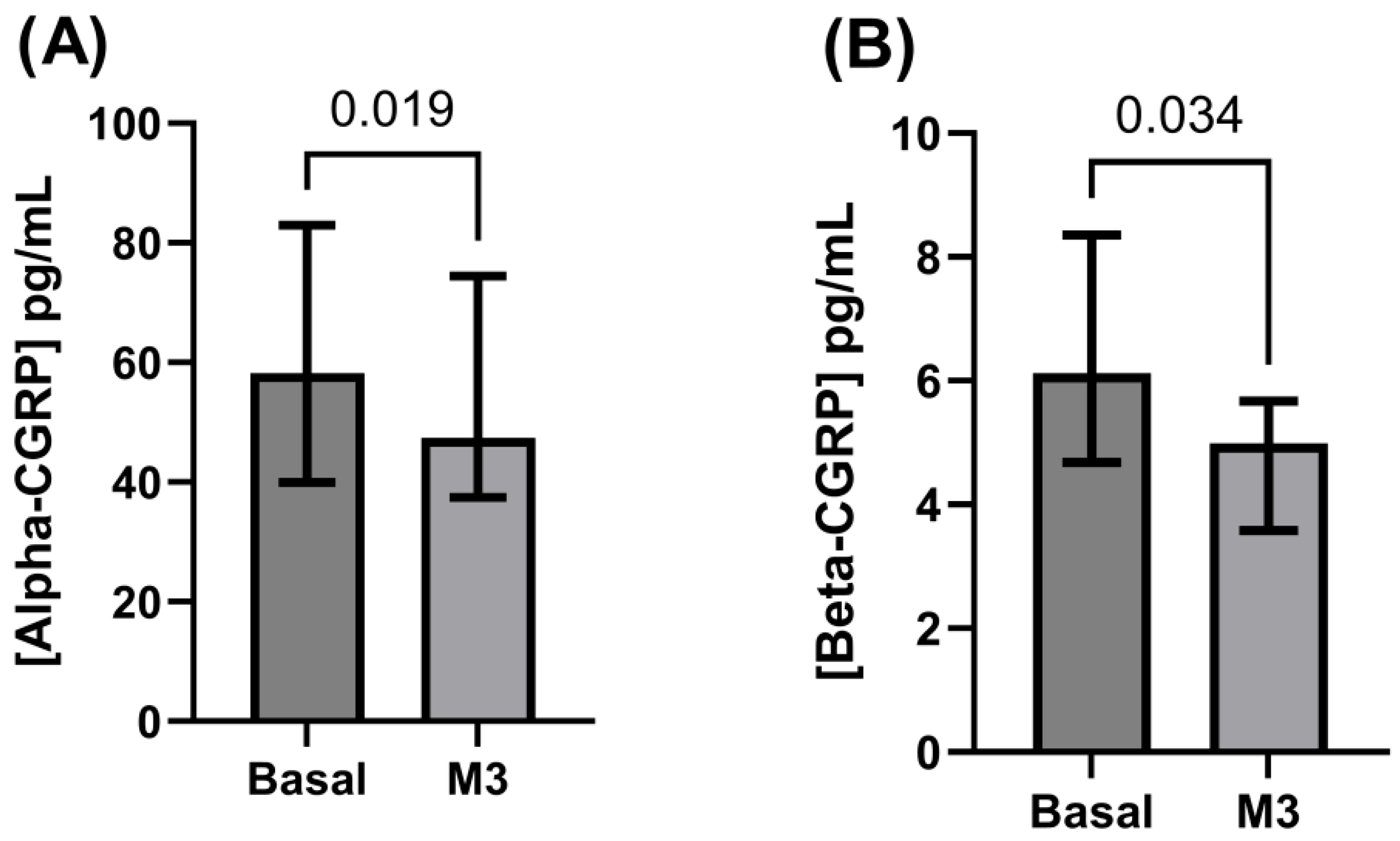
3.2. Alpha- and Beta-CGRP Levels
4. Discussion
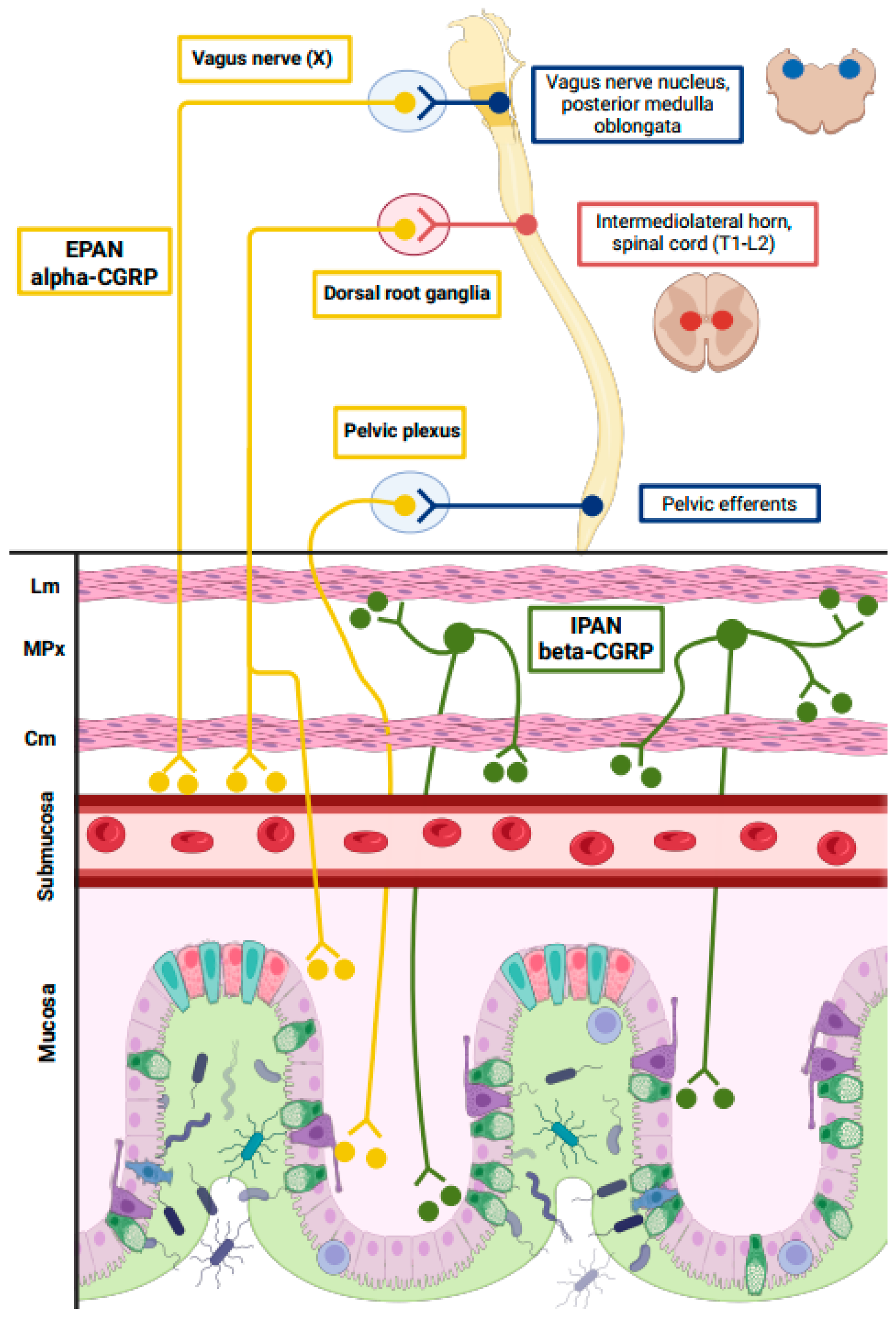
4.1. Pathophysiological and Clinical Implications
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ELISA | Enzyme-linked immunosorbent assay |
| CGRP | Calcitonin gene-related peptide |
| CM | Chronic migraine |
| HFEM | High-frequency episodic migraine |
| IQR | Interquartile range |
| mAb | CGRP monoclonal antibodies |
| PGIC | Patient Global Impression of Change |
| SD | Standard deviation |
References
- Arzani, M.; Jahromi, S.R.; Ghorbani, Z.; Vahabizad, F.; Martelleti, P.; Ghaemi, A.; Sacco, S.; Togha, M. School of Advances Studies of the European Headache Federation (EHF-SAS). Gut-Brain axis and migraine headache: A comprehensive review. J. Headache Pain 2020, 21, 15. [Google Scholar] [CrossRef] [PubMed]
- Ailani, J.; Kaiser, E.A.; Mathew, P.G.; McAllister, P.; Russo, A.F.; Vélez, C.; Pozo-Ramajo, A.; Abdrabboh, A.; Xu, C.; Rasmussen, S.; et al. Role of calcitonin gene-related peptide on the gastrointestinal symptoms of migraine-clinical considerations. A narrative review. Neurology 2022, 99, 841–853. [Google Scholar] [CrossRef] [PubMed]
- Gárate, G.; Pascual, M.; Rivero, M.; Toriello, M.; Pérez-Pereda, S.; González-Quintanilla, V.; Madera, J.; Gutiérrez-Cuadra, M.; Fariñas, M.D.C.; Hernández, J.L.; et al. Serum calcitonin gene-related peptide alpha and beta levels are increased in COVID-19 inpatients. Arch. Med. Res. 2023, 54, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Russell, F.A.; King, R.; Smillie, S.J.; Kodji, X.; Brain, S.D. Calcitonin gene-related peptide: Physiology and pathophysiology. Physiol. Rev. 2014, 94, 1099–1142. [Google Scholar] [CrossRef]
- Russo, A.F.; Hay, D.L. CGRP physiology, pharmacology, and therapeutic targets: Migraine and beyond. Physiol. Rev. 2023, 103, 1565–1644. [Google Scholar] [CrossRef]
- Mulderry, P.K.; Ghatki, M.A.; Spokks, R.A.; Jonhs, P.M.; Pierson, A.M.; Hamid, Q.A.; Kanse, S.; Amara, S.G.; Burrik, J.M.; Legon, S.; et al. Differential expression of alpha-CGRP and beta-CGRP by primary sensory neurons and enteric autonomic neurons of the rat. Neuroscience 1988, 25, 195–205. [Google Scholar] [CrossRef]
- Falkenberg, K.; Bjerg, H.R.; Olesen, J. Two-hour CGRP infusion causes gastrointestinal hyperactivity: Possible relevance of CGRP antibody treatment. Headache 2020, 60, 929–937. [Google Scholar] [CrossRef]
- Mesina, R.; Huessler, E.M.; Puledda, F.; Haghdoost, F.; Lebedeva, E.R.; Diener, H.C. Safety and tolerability of monoclonal antibodies targeting the CGRP pathway and gepants in migraine prevention: A systematic review and network meta-analysis. Cephalalgia 2023, 43, 331024231152169. [Google Scholar] [CrossRef]
- Gárate, G.; González-Quintanilla, V.; González, A.; Pascual, M.; Pérez-Pereda, S.; Madera, J.; Pascual, J. Serum alpha and beta-CGRP levels in chromic migraine patients before and after monoclonal antibodies against CGRP or its receptor. Ann. Neurol. 2023, 94, 285–294. [Google Scholar] [CrossRef]
- Gárate, G.; Pascual, M.; Olmos, J.M.; Fariñas, J.M.; Rivero, M.; Crespo, J.; Pascual, J. Increase in serum calcitonin gene-related peptide β (CGRPβ) levels in COVID-19 patients with diarrhea: An underlying mechanism? Dig. Dis. Sci. 2022, 67, 5712–5713. [Google Scholar] [CrossRef]
- Pascual-Mato, M.; Gárate, G.; González-Quintanilla, V.; Castro, B.; García, M.J.; Crespo, J.; Pascual, J.; Rivero, M. Unravelling the role of beta-CGRP in inflammatory bowel disease and its potential role in gastrointestinal homeostasis. BMC Gastroenterol. 2024, 24, 262. [Google Scholar] [CrossRef] [PubMed]
- Headache Classification Committee, International Headache Society. Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia 2018, 38, 1–211. [Google Scholar] [CrossRef] [PubMed]
- Gárate, G.; Pascual, J.; Pascual-Mato, M.; Madera, J.; Martín, M.M.; González-Quintanilla, V. Untangling the mess of CGRP levels as a migraine biomarker: An in-depth literature review and analysis of our experimental experience. J. Headache Pain 2024, 25, 69. [Google Scholar] [CrossRef]
- Schütz, B.; Mauer, D.; Salmon, A.M.; Chnageux, J.P.; Zimmer, A. Analysis of the cellular expression pattern of beta-CGRP in alpha-CGRP-deficient mice. J. Comp. Neurol. 2004, 476, 32–43. [Google Scholar] [CrossRef] [PubMed]
- van Rossum, D.; Hanisch, U.K.; Quirion, R. Neuroanatomical localization, pharmacological characterization and function of CGRP, related peptides and their receptors. Neurosci. Biobehav. Rev. 1997, 21, 649–678. [Google Scholar] [CrossRef] [PubMed]
- Sternini, C.; Anderson, K. Calcitonin gene-related peptide containing neurons supplying the rat digestive system: Differential distribution and expression pattern. Somatosens. Mot. Res. 1992, 9, 45–49. [Google Scholar] [CrossRef]
- Grider, J.R. CGRP as a transmitter in the sensory pathway mediating peristaltic reflex. Am. J. Physiol. 1994, 226, 1139–1145. [Google Scholar] [CrossRef]
- Holtzer, P.; Holzer-Petsche, U. Constipation caused by anti-calcitonin gene-related peptide migraine therapeutics explained by antagonism of calcitonin gene-related peptide’s motor-stimulating and prosecretory function in the intestine. Front. Physiol. 2022, 12, 820006. [Google Scholar] [CrossRef]
- Hanna, F.W.; Ardill, J.E.; Johnston, C.F.; Cunningham, R.T.; Curry, W.J.; Rusell, C.F.; Buchanan, K.D. Regulatory peptides and other neuroendocrine markers in medullary carcinoma of the thyroid. J. Endocrinol. 1997, 152, 275–281. [Google Scholar] [CrossRef]
- Thompson, B.J.; Washington, M.K.; Kurre, U.; Singh, M.; Rula, E.Y.; Emeson, R.B. Protective roles of alpha-calcitonin and beta-calcitonin gene-related peptide in spontaneous and experimentally induced colitis. Dig. Dis. Sci. 2008, 53, 229–241. [Google Scholar] [CrossRef]
- Li, F.J.; Zou, Y.Y.; Cui, Y.; Yin, Y.; Guo, G.; Lu, F.G. Calcitonin gene-related peptide is a promising marker in ulcerative colitis. Dig. Dis. Sci. 2013, 58, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Ofalti, H.; Mirmosayyeb, O.; Hosseinabadi, A.L.; Ghajarzadeh, M. The prevalence of migraine in inflammatory bowel disease, a systematic review and meta-analysis. Int. J. Prev. Med. 2023, 14, 66. [Google Scholar] [CrossRef]
- Johnson, K.W.; Li, X.; Huang, X.; Heinz, B.A.; Yu, J.Y.; Li, B. Characterization of transit rates in the large intestine of mice following treatment with a CGRP antibody, CGRP receptor antibody, and small molecule CGRP receptor antagonists. Headache 2022, 62, 848–857. [Google Scholar] [CrossRef] [PubMed]
- Lau, D.C.W.; Erichsen, L.; Francisco, A.M.; Satylganova, A.; le Roux, C.W.; McGowan, B.; Pedersen, S.D.; Pietilänen, K.H.; Rubino, D.; Batterhan, R.L. Once-weekly cagrilintide for weight management in people with overweight and obesity: A multicentre, randomized, double-blind, placebo-controlled and active-controlled, dose-finding phase 2 trial. Lancet 2021, 398, 2160–2172. [Google Scholar] [CrossRef]
- Whitehouse, F.; Kruger, D.F.; Fineman, M.; Shen, L.; Ruggles, J.A.; Maggs, D.G.; Weyer, C.; Kolterman, O.G. A randomized study and open-label extension evaluating the long-term efficacy of pramlintide as an adjunct to insulin therapy in type 1 diabetes. Diabetes Care 2002, 25, 724–730. [Google Scholar] [CrossRef]
| Mean age, range | 47.7 ± 10.7, 19–69 years |
| Women (%) | 128 (96.2%) |
| Diagnosis | |
| Chronic migraine | 111 (84.1%) |
| High-frequency episodic migraine | 22 (15.9%) |
| History of aura | 42 (31.6%) |
| Analgesic overuse | 114 (83.5%) |
| Main comorbidities | |
| Anxiety/depression | 70 (52.6%) |
| Obesity | 25 (18.9%) |
| Fibromyalgia | 23 (17.3%) |
| Arterial hypertension | 23 (17.3%) |
| Preventive treatments | |
| Oral preventatives | 101 (75.9%) |
| OnabotulinumtoxinA | 47 (35.3%) |
| Efficacy measures at month 3 | |
| Reduction in monthly headache days | −11.64 (p < 0.0001) |
| Reduction in monthly migraine days | −10.33 (p < 0.0001) |
| Monthly headache response (<50%) rate | 81 (60.9%) |
| Monthly headache response (<50%) rate | 87 (65.4%) |
| Constipation at month 3 | 12 (9.0%) |
| Variable | Constipation | No Constipation | p |
|---|---|---|---|
| Mean age ± SD | 51.25 ± 11.33 | 47.29 ± 10.56 | 0.330 |
| Women (%) | 11/12 (91.7%) | 117/121 (96.7%) | 0.382 |
| Chronic migraine | 11/12 (91.7%) | 100/121 (82.6%) | 0.422 |
| HFEM | 1/12 (8.3%) | 21/121 (17.93%) | 0.422 |
| History of aura | 3/12 (25%) | 39/121 (32.2%) | 0.607 |
| Analgesic overuse | 11/12 (91.7%) | 103/121 (85.1%) | 0.537 |
| Anxiety/depression | 10/12 (83.3%) | 60/121 (49.6%) | 0.026 |
| Obesity | 2/12 (16.7%) | 23/121 (19%) | 0.833 |
| Fibromyalgia | 2/12 (16.7%) | 21/121 (17.3%) | 0.952 |
| Arterial hypertension | 1/12 (8.3%) | 2/121 (18.2%) | 0.390 |
| Oral preventives | 10/12 (83.3%) | 91/121 (75.2%) | 0.530 |
| OnabotulinumtoxinA | 7/12 (58.3%) | 40/121 (33.1%) | 0.081 |
| Response (headache days) | 5/12 (41.7%) | 76/121 (62.8%) | 0.152 |
| Response (migraine days) | 5/12 (41.7%) | 82/121 (67.8%) | 0.070 |
| PGIC scale score | 4.25 ± 1.77 | 2.67 ± 1.54 | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gárate, G.; Polanco, M.; Madera, J.; Muñoz-San Martín, M.; Pascual-Mato, M.; González-Quintanilla, V.; Pascual, J. Influence of Constipation in the Behavior of Circulating Alpha- and Beta-CGRP Levels in Chronic/High-Frequency Migraine Patients After CGRP Monoclonal Antibodies. Biomedicines 2025, 13, 1254. https://doi.org/10.3390/biomedicines13051254
Gárate G, Polanco M, Madera J, Muñoz-San Martín M, Pascual-Mato M, González-Quintanilla V, Pascual J. Influence of Constipation in the Behavior of Circulating Alpha- and Beta-CGRP Levels in Chronic/High-Frequency Migraine Patients After CGRP Monoclonal Antibodies. Biomedicines. 2025; 13(5):1254. https://doi.org/10.3390/biomedicines13051254
Chicago/Turabian StyleGárate, Gabriel, Marcos Polanco, Jorge Madera, María Muñoz-San Martín, Marta Pascual-Mato, Vicente González-Quintanilla, and Julio Pascual. 2025. "Influence of Constipation in the Behavior of Circulating Alpha- and Beta-CGRP Levels in Chronic/High-Frequency Migraine Patients After CGRP Monoclonal Antibodies" Biomedicines 13, no. 5: 1254. https://doi.org/10.3390/biomedicines13051254
APA StyleGárate, G., Polanco, M., Madera, J., Muñoz-San Martín, M., Pascual-Mato, M., González-Quintanilla, V., & Pascual, J. (2025). Influence of Constipation in the Behavior of Circulating Alpha- and Beta-CGRP Levels in Chronic/High-Frequency Migraine Patients After CGRP Monoclonal Antibodies. Biomedicines, 13(5), 1254. https://doi.org/10.3390/biomedicines13051254






