Vitamin D Imbalance and Hydro-Electrolyte Disturbances in Hospitalized Children: A Comparation Between Post-COVID-19 Status and SARS-CoV-2/EBV Coinfection
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Variables
2.2. Inclusion Criteria
2.3. Exclusion Criteria
3. Results
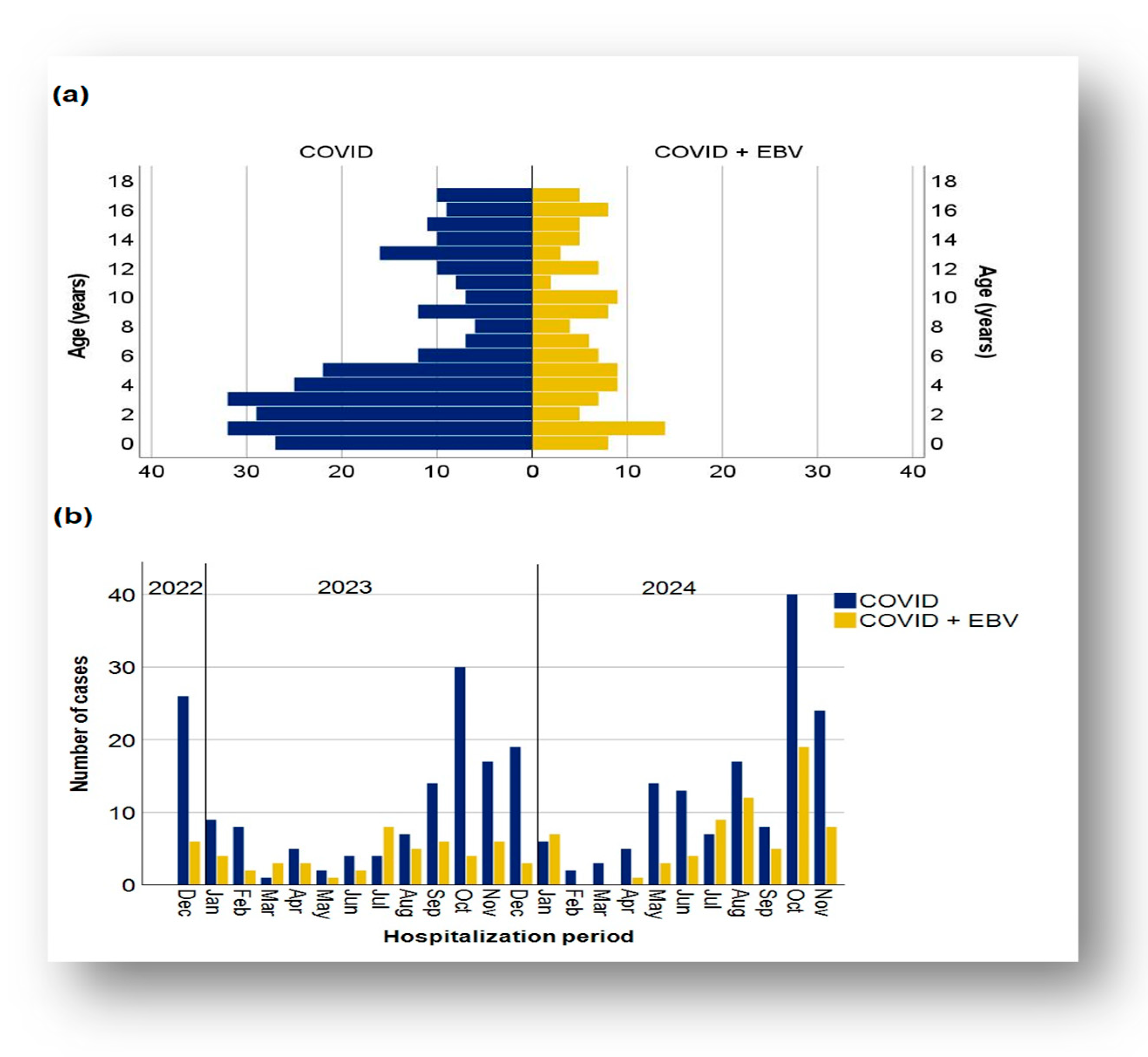
3.1. Demographic Data Distribution of Cases in the Two Subgroups
3.2. Characteristics of Clinical Evolution: Severity and Duration of Hospitalization
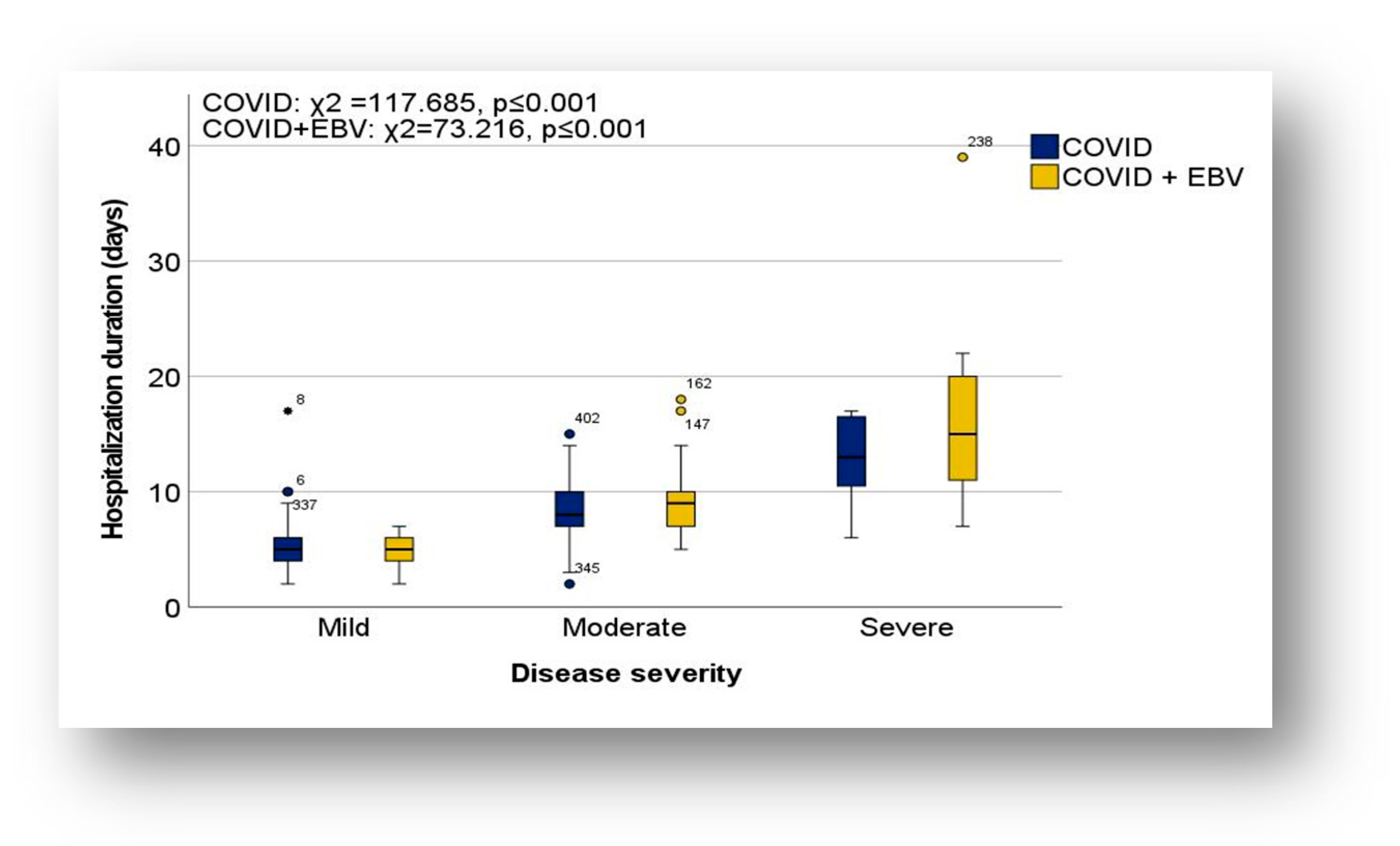
3.2.1. Severity of the Disease
Inflammatory Markers and Severity of Disease
3.2.2. Length of Hospitalization in the Analyzed Subgroups
Length of Hospitalization in the Context of Severity of Illness
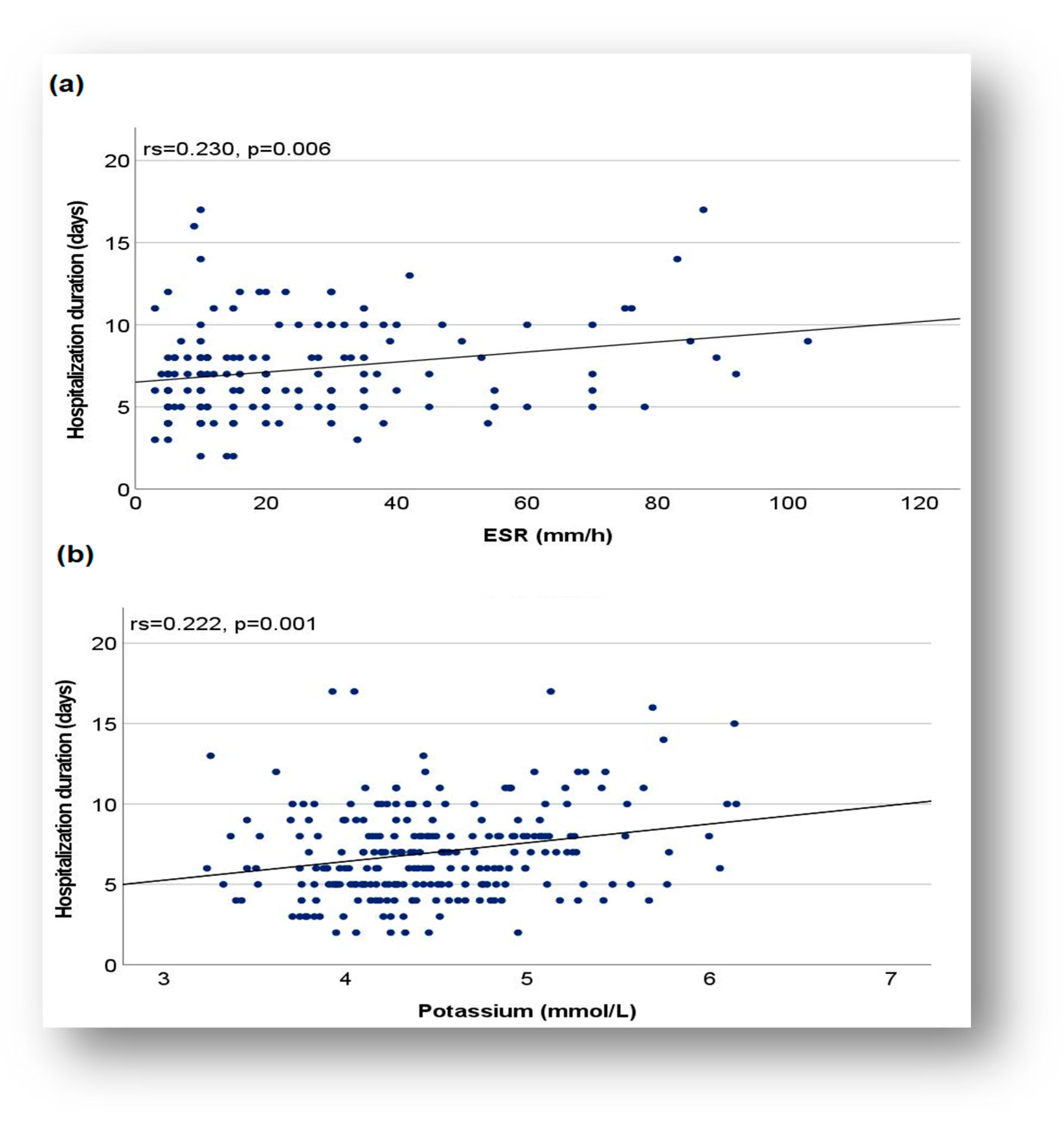
Duration of Hospitalization and Relationship with Biomarkers
3.3. Variations in Metabolic and Biochemical Status in the Clinical Context
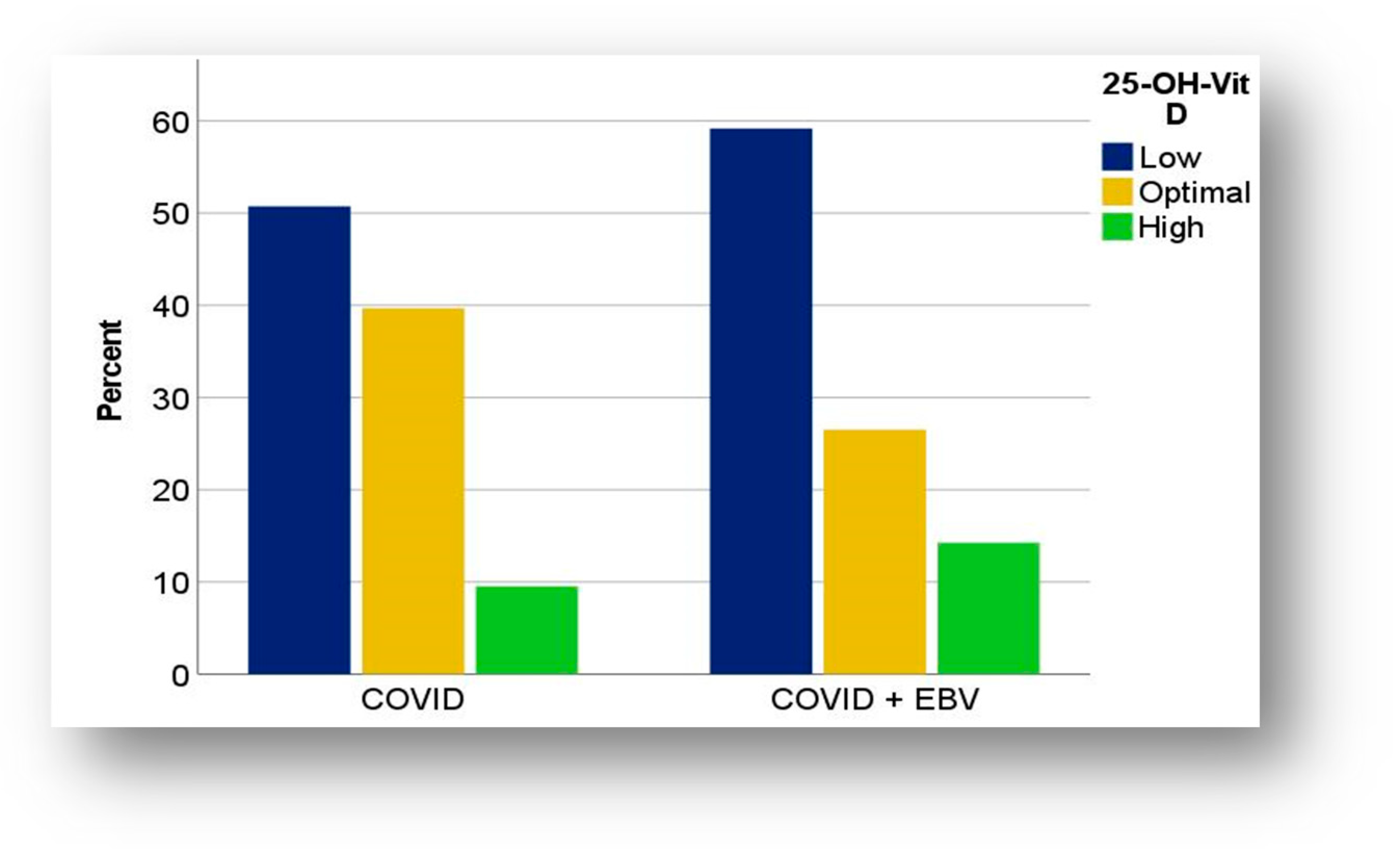
3.3.1. Distribution of Vitamin D in COVID-19 and COVID-19 + EBV Subgroups
Vitamin D and Biological Parameters in COVID-19 and COVID-19 + EBV Subgroups
Vitamin D in the Context of the Severity of the Illness
3.3.2. Serum Electrolyte Variations in COVID-19 and COVID-19 + EBV Subgroups
Hydro-Electrolyte Disorders and Variations in Coagulation Markers
4. Discussion
4.1. Vitamin D Levels and Distribution in COVID-19 and COVID-19 + EBV Subgroups
4.1.1. Hypovitaminosis D
4.1.2. Hypervitaminosis D
4.1.3. The Role of Vitamin D in the Context of Disease Severity
4.1.4. Vitamin D Levels and Biomarkers Variations
4.2. Serum Electrolyte Variations in the Analyzed Subgroups
4.3. Inflammatory Markers in the Context of Immunologic Status
4.4. Length of Hospitalization and Relation to Biomarkers
4.5. Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ADH | Antidiuretic hormone |
| COVID-19 | Coronavirus Disease 2019 |
| CRP | C-reactive protein |
| EBV | Epstein–Barr virus |
| ESR | Erythrocyte sedimentation rate |
| EBNA | Epstein–Barr nuclear Antigen |
| IgM antibodies | Immunoglobulin M antibodies |
| IgG antibodies | Immunoglobulin G antibodies |
| IL-6 | Interleukin-6 |
| 25-OH vitamin D | 25-Hydroxyvitamin D |
| SARS-CoV-2 | Severe Acute Respiratory Syndrome-Related Coronavirus 2 |
| VCA | Viral Capsid Antigen |
| WHO | World Health Organization |
References
- Mahase, E. COVID-19: WHO declares pandemic because of “alarming levels” of spread, severity, and inaction. BMJ 2020, 368, m1036. [Google Scholar] [CrossRef] [PubMed]
- Skenderi, E.; Sulovari, A.; Kuli-Lito, G.; Shahini, N.; Toci, G.; Pema, A. COVID-19 and EBV Co-Infection in a Child. J. Biosci. Med. 2021, 9, 20–27. [Google Scholar] [CrossRef]
- Dong, Y.; Mo, X.; Hu, Y.; Qi, X.; Jiang, F.; Jiang, Z.; Tong, S. Epidemiology of COVID-19 Among Children in China. Pediatrics 2020, 145, e20200702. [Google Scholar] [CrossRef]
- Parri, N.; Lenge, M.; Buonsenso, D. Children with COVID-19 in Pediatric Emergency Departments in Italy. N. Engl. J. Med. 2020, 383, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Bialek, S.; Gierke, R.; Hughes, M.; McNamara, L.A.; Pilishvili, T.; Skoff, T. Coronavirus Disease 2019 in Children—United States, 12 February–2 April 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 422–426. [Google Scholar] [CrossRef]
- Rowley, A.H. Understanding SARS-CoV-2-related multisystem inflammatory syndrome in children. Nat. Rev. Immunol. 2020, 20, 453–454. [Google Scholar] [CrossRef]
- Bannister, B.A. Post-infectious disease syndrome. Postgrad. Med. J. 1988, 64, 559–567. [Google Scholar] [CrossRef]
- Moss-Morris, R.; Deary, V.; Castell, B. Chronic fatigue syndrome. Handb. Clin. Neurol. 2013, 110, 303–314. [Google Scholar] [CrossRef]
- Komaroff, A.L.; Lipkin, W.I. Insights from myalgic encephalomyelitis/chronic fatigue syndrome may help unravel the pathogenesis of postacute COVID-19 syndrome. Trends Mol. Med. 2021, 27, 895–906. [Google Scholar] [CrossRef]
- Rasizadeh, R.; Ebrahimi, F.; Kermanshahi, A.Z.; Sorkhabi, A.D.; Sarkesh, A.; Nahand, J.S.; Baghi, H.B. Viruses and thrombocytopenia. Heliyon 2024, 10, e27844. [Google Scholar] [CrossRef]
- Gold, J.E.; Okyay, R.A.; Licht, W.E.; Hurley, D.J. Investigation of Long COVID Prevalence and Its Relationship to Epstein-Barr Virus Reactivation. Pathogens 2021, 10, 763. [Google Scholar] [CrossRef] [PubMed]
- Shafiee, A.; Shamsi, S.; Gargari, O.K.; Beiky, M.; Allahkarami, M.M.; Miyanaji, A.B.; Aghajanian, S.; Mozhgani, S. EBV associated T- and NK-cell lymphoproliferative diseases: A comprehensive overview of clinical manifestations and novel therapeutic insights. Rev. Med. Virol. 2022, 32, e2328. [Google Scholar] [CrossRef] [PubMed]
- Shafiee, A.; Aghajanian, S.; Athar, M.M.T.; Gargari, O.K. Epstein–Barr virus and COVID-19. J. Med. Virol. 2022, 94, 4040. [Google Scholar] [CrossRef]
- Chen, T.; Song, J.; Liu, H.; Zheng, H.; Chen, C. Positive Epstein-Barr virus detection in coronavirus disease 2019 (COVID-19) patients. Sci. Rep. 2021, 11, 10902. [Google Scholar] [CrossRef]
- Sotodeh-Asl, N.; Tamadon, M.; Malek, F.; Zahmatkesh, M. Vitamin D deficiency and psychological disorders. J. Parathyr. Dis. 2014, 2, 21–25. [Google Scholar]
- Judd, S.E.; Tangpricha, V. Vitamin D deficiency and risk for cardiovascular disease. Am. J. Med. Sci. 2009, 338, 40–44. [Google Scholar] [CrossRef]
- Marik, P.E.; Kory, P.; Varon, J. Does vitamin D status impact mortality from SARS-CoV-2 infection? Med. Drug Discov. 2020, 6, 100041. [Google Scholar] [CrossRef]
- Xu, J.; Yang, J.; Chen, J.; Luo, Q.; Zhang, Q.; Zhang, H. Vitamin D alleviates lipopolysaccharide-induced acute lung injury via regulation of the renin-angiotensin system. Mol. Med. Rep. 2017, 16, 7432–7438. [Google Scholar] [CrossRef]
- Yılmaz, K.; Şen, V. Is vitamin D deficiency a risk factor for COVID-19 in children? Pediatr. Pulmonol. 2020, 55, 3595–3601. [Google Scholar] [CrossRef]
- Siuka, D.; Pfeifer, M.; Pinter, B. Vitamin D Supplementation During the COVID-19 Pandemic. Mayo Clin. Proc. 2020, 95, 1804–1805. [Google Scholar] [CrossRef]
- Grant, W.B.; Lahore, H.; McDonnell, S.L.; Baggerly, C.A.; French, C.B.; Aliano, J.L.; Bhattoa, H.P. Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths. Nutrients 2020, 12, 988. [Google Scholar] [CrossRef] [PubMed]
- Fu, H.; Li, Y.; Huang, H.; Wang, D. Serum Vitamin D Level and Efficacy of Vitamin D Supplementation in Children with Atopic Dermatitis: A Systematic Review and Meta-analysis. Comput. Math. Methods Med. 2022, 2022, 9407888. [Google Scholar] [CrossRef] [PubMed]
- Tomaszewska, A.; Rustecka, A.; Lipińska-Opałka, A.; Piprek, R.P.; Kloc, M.; Kalicki, B.; Kubiak, J.Z. The Role of Vitamin D in COVID-19 and the Impact of Pandemic Restrictions on Vitamin D Blood Content. Front. Pharmacol. 2022, 13, 836738. [Google Scholar] [CrossRef]
- Shahin, W.; Rabie, W.; Alyossof, O.; Alasiri, M.; Alfaki, M.; Mahmoud, E.; Hijazi, M.; El Faraidi, H.; Alahmari, H. COVID-19 in children ranging from asymptomatic to a multi-system inflammatory disease A single-center study. Saudi Med. J. 2021, 42, 299–305. [Google Scholar] [CrossRef]
- Khojah, H.M.J.; Ahmed, S.A.; Al-Thagfan, S.S.; Alahmadi, Y.M.; Abdou, Y.A. The Impact of Serum Levels of Vitamin D3 and Its Metabolites on the Prognosis and Disease Severity of COVID-19. Nutrients 2022, 14, 5329. [Google Scholar] [CrossRef]
- Alpcan, A.; Tursun, S.; Kandur, Y. Vitamin D levels in children with COVID-19: A report from Turkey. Epidemiol. Infect. 2021, 149, e180. [Google Scholar] [CrossRef]
- Renieris, G.; Foutadakis, S.; Andriopoulou, T.; Spanou, V.-M.; Droggiti, D.-E.; Kafousopoulos, D.; Gkavogianni, T.; Damoraki, G.; Vatsellas, G.; Giamarellos-Bourboulis, E.J. Association of Vitamin D with Severity and Outcome of COVID-19: Clinical and Experimental Evidence. J. Innate Immun. 2023, 16, 1–11. [Google Scholar] [CrossRef]
- Tzoulis, P.; A Waung, J.; Bagkeris, E.; Hussein, Z.; Biddanda, A.; Cousins, J.; Dewsnip, A.; Falayi, K.; McCaughran, W.; Mullins, C.; et al. Dysnatremia is a Predictor for Morbidity and Mortality in Hospitalized Patients with COVID-19. J. Clin. Endocrinol. Metab. 2021, 106, 1637–1648. [Google Scholar] [CrossRef]
- Patel, J.M. Multisystem Inflammatory Syndrome in Children (MIS-C). Curr. Allergy Asthma Rep. 2022, 22, 53–60. [Google Scholar] [CrossRef]
- Post, A.; Dullaart, R.P.F.; Bakker, S.J.L. Is low sodium intake a risk factor for severe and fatal COVID-19 infection? Eur. J. Intern. Med. 2020, 75, 109. [Google Scholar] [CrossRef]
- Lab Diagnostics & Drug Development, Global Life Sciences Leader | Labcorp. Available online: https://www.labcorp.com/ (accessed on 3 May 2025).
- McPherson, R.A.; Pincus, M.R. Henry’s Clinical Diagnosis Management by Laboratory Methods; Elsevier: Amsterdam, The Netherlands, 2021; Available online: https://books.google.ro/books/about/Henry_s_Clinical_Diagnosis_and_Managemen.html?id=RW4yEAAAQBAJ&redir_esc=y (accessed on 3 May 2025).
- Coronavirus Disease 2019 (COVID-19) | COVID-19 | C.D.C. Available online: https://www.cdc.gov/covid/index.html (accessed on 8 January 2025).
- Pourfridoni, M.; Abbasnia, S.M.; Shafaei, F.; Razaviyan, J.; Heidari-Soureshjani, R. Fluid and Electrolyte Disturbances in COVID-19 and Their Complications. BioMed Res. Int. 2021, 2021, 6667047. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.W.; Xu, D.; Zhang, H.; Zhou, W.; Wang, L.-H.; Cui, X.G. Identification of a potential mechanism of acute kidney injury during the COVID-19 outbreak: A study based on single-cell transcriptome analysis. Intensive Care Med. 2020, 46, 1114–1116. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, S.; Liu, H.; Li, W.; Lin, F.; Jiang, L.; Li, X.; Xu, P.; Zhang, L.; Zhao, L.; et al. SARS-CoV-2 infection of the liver directly contributes to hepatic impairment in patients with COVID-19. J. Hepatol. 2020, 73, 807–816. [Google Scholar] [CrossRef]
- Ke, C.; Xiao, J.; Wang, Z.; Yu, C.; Yang, C.; Hu, Z. Characteristics of patients with kidney injury associated with COVID-19. Int. Immunopharmacol. 2021, 96, 107794. [Google Scholar] [CrossRef]
- Lippi, G.; South, A.M.; Henry, B.M. Electrolyte imbalances in patients with severe coronavirus disease 2019 (COVID-19). Ann. Clin. Biochem. 2020, 57, 262–265. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.T.; Stenger, S.; Li, H.; Wenzel, L.; Tan, B.H.; Krutzik, S.R.; Ochoa, M.T.; Schauber, J.; Wu, K.; Meinken, C.; et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 2006, 311, 1770–1773. [Google Scholar] [CrossRef]
- Rastogi, A.; Bhansali, A.; Khare, N.; Suri, V.; Yaddanapudi, N.; Sachdeva, N.; Puri, G.D.; Malhotra, P. Short term, high-dose vitamin D supplementation for COVID-19 disease: A randomised, placebo-controlled, study (SHADE study). Postgrad. Med. J. 2022, 98, 87–90. [Google Scholar] [CrossRef]
- Su, Y.; Yuan, D.; Chen, D.G.; Ng, R.H.; Wang, K.; Choi, J.; Li, S.; Hong, S.; Zhang, R.; Xie, J.; et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell 2022, 185, 881–895.e20. [Google Scholar] [CrossRef]
- Paolucci, S.; Cassaniti, I.; Novazzi, F.; Fiorina, L.; Piralla, A.; Comolli, G.; Bruno, R.; Maserati, R.; Gulminetti, R.; Novati, S.; et al. EBV DNA increase in COVID-19 patients with impaired lymphocyte subpopulation count. Int. J. Infect. Dis. 2021, 104, 315–319. [Google Scholar] [CrossRef]
- Saade, A.; Moratelli, G.; Azoulay, E.; Darmon, M. Herpesvirus reactivation during severe COVID-19 and high rate of immune defect. Infect. Dis. Now. 2021, 51, 676–679. [Google Scholar] [CrossRef]
- Xie, Y.; Cao, S.; Dong, H.; Lv, H.; Teng, X.; Zhang, J.; Wang, T.; Zhang, X.; Qin, Y.; Chai, Y.; et al. Clinical characteristics and outcomes of critically ill patients with acute COVID-19 with Epstein-Barr virus reactivation. BMC Infect. Dis. 2021, 21, 955. [Google Scholar] [CrossRef] [PubMed]
- Bernal, K.D.E.; Whitehurst, C.B. Incidence of Epstein-Barr virus reactivation is elevated in COVID-19 patients. Virus Res. 2023, 334, 199157. [Google Scholar] [CrossRef] [PubMed]
- Martineau, A.R.; Jolliffe, D.A.; Hooper, R.L.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Raz, G.; Esposito, S.; Ganmaa, D.; Ginde, A.A.; et al. Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data. BMJ 2017, 356, i6583. [Google Scholar] [CrossRef]
- McCartney, D.M.; Byrne, D.G. Optimisation of Vitamin D Status for Enhanced Immuno-Protection Against COVID-19. Ir. Med. J. 2020, 113, 58. Available online: https://pubmed.ncbi.nlm.nih.gov/32268051/ (accessed on 26 January 2025).
- Hughes, D.A.; Norton, R. Vitamin D and respiratory health. Clin. Exp. Immunol. 2009, 158, 20–25. [Google Scholar] [CrossRef]
- Pereira, M.; Dantas Damascena, A.; Galvão Azevedo, L.M.; de Almeida Oliveira, T.; da Mota Santana, J. Vitamin D deficiency aggravates COVID-19: Systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2022, 62, 1308–1316. [Google Scholar] [CrossRef]
- Tolppanen, A.M.; Fraser, A.; Fraser, W.D.; Lawlor, D.A. Risk factors for variation in 25-hydroxyvitamin D₃ and D₂ concentrations and vitamin D deficiency in children. J. Clin. Endocrinol. Metab. 2012, 97, 1202–1210. [Google Scholar] [CrossRef]
- Choi, H.S.; Oh, H.J.; Choi, H.; Choi, W.H.; Kim, J.G.; Kim, K.M.; Kim, K.J.; Rhee, Y.; Lim, S.-K. Vitamin D insufficiency in Korea--a greater threat to younger generation: The Korea National Health and Nutrition Examination Survey (KNHANES) 2008. J. Clin. Endocrinol. Metab. 2011, 96, 643–651. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhan, J.; Shao, J.; Chen, W.; Chen, L.; Li, W.; Ji, C.; Zhao, Z. High prevalence of vitamin D deficiency among children aged 1 month to 16 years in Hangzhou, China. BMC Public Health 2012, 12, 126. [Google Scholar] [CrossRef]
- Acheampong, D.O.; Barffour, I.K.; Boye, A.; Aninagyei, E.; Ocansey, S.; Morna, M.T. Male predisposition to severe COVID-19: Review of evidence and potential therapeutic prospects. Biomed. Pharmacother. 2020, 131, 110748. [Google Scholar] [CrossRef]
- Dupuis, M.L.; Pagano, M.T.; Pierdominici, M.; Ortona, E. The role of vitamin D in autoimmune diseases: Could sex make the difference? Biol. Sex Differ. 2021, 12, 12. [Google Scholar] [CrossRef]
- Hutt-Fletcher, L.M.; Chesnokova, L.S. Integrins as triggers of Epstein-Barr virus fusion and epithelial cell infection. Virulence 2010, 1, 395. [Google Scholar] [CrossRef] [PubMed]
- Borza, C.M.; Hutt-Fletcher, L.M. Alternate replication in B cells and epithelial cells switches tropism of Epstein-Barr virus. Nat. Med. 2002, 8, 594–599. [Google Scholar] [CrossRef]
- Brîndușe, L.A.; Eclemea, I.; Neculau, A.E.; Cucu, M.A. Vitamin D Status in the Adult Population of Romania—Results of the European Health Examination Survey. Nutrients 2024, 16, 867. [Google Scholar] [CrossRef]
- Stroehlein, J.K.; Wallqvist, J.; Iannizzi, C.; Mikolajewska, A.; Metzendorf, M.-I.; Benstoem, C.; Meybohm, P.; Becker, M.; Skoetz, N.; Stegemann, M.; et al. Vitamin D supplementation for the treatment of COVID-19: A living systematic review. Cochrane Database Syst. Rev. 2021, 5, CD015043. [Google Scholar] [CrossRef]
- Brustad, N.; Yousef, S.; Stokholm, J.; Bønnelykke, K.; Bisgaard, H.; Chawes, B.L. Safety of High-Dose Vitamin D Supplementation Among Children Aged 0 to 6 Years: A Systematic Review and Meta-analysis. JAMA Netw. Open 2022, 5, E227410. [Google Scholar] [CrossRef]
- Rebelos, E.; Tentolouris, N.; Jude, E. The Role of Vitamin D in Health and Disease: A Narrative Review on the Mechanisms Linking Vitamin D with Disease and the Effects of Supplementation. Drugs 2023, 83, 665. [Google Scholar] [CrossRef] [PubMed]
- Bergman, P.; Lindh, Å.U.; Björkhem-Bergman, L.; Lindh, J.D. Vitamin D and Respiratory Tract Infections: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. PLoS ONE 2013, 8, e65835. [Google Scholar] [CrossRef]
- Xie, Z.; Chen, J.; Zheng, C.; Wu, J.; Cheng, Y.; Zhu, S.; Lin, C.; Cao, Q.; Zhu, J.; Jin, T. 1,25-dihydroxyvitamin D3 -induced dendritic cells suppress experimental autoimmune encephalomyelitis by increasing proportions of the regulatory lymphocytes and reducing T helper type 1 and type 17 cells. Immunology 2017, 152, 414–424. [Google Scholar] [CrossRef]
- García-Martínez, F.J.; Moreno-Artero, E.; Jahnke, S. SARS-CoV-2 and EBV coinfection. Med. Clin. 2020, 155, 319. [Google Scholar] [CrossRef]
- Zhang, J.-J.; Yu, H.-C.; Li, Y.; Zhang, Y.-B.; Geng, T.-T.; Lu, Q.; Liao, Y.-F.; Guo, K.-Q.; Du, L.; Ruan, H.-L.; et al. Association between serum 25-hydroxy vitamin D concentrations and mortality among individuals with metabolic dysfunction-associated fatty liver disease: A prospective cohort study. Am. J. Clin. Nutr. 2022, 116, 1409–1417. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Wang, X.; Lai, H.; Vernooij, R.W.M.; Deng, X.; Ma, N.; Li, D.; Huang, J.; Zhao, W.; Ning, J.; et al. Risk of kidney and liver diseases after COVID-19 infection: A systematic review and meta-analysis. Rev. Med. Virol. 2024, 34, e2523. [Google Scholar] [CrossRef]
- Yang, X.; Lin, B.; Shen, T. Clinical features of renal damage associated with Epstein-Barr virus infection in children. Front. Pediatr. 2023, 11, 1123941. [Google Scholar] [CrossRef]
- Dugas, B.; Delfraissy, J.F.; Calenda, A.; Peuchmaur, M.; Wallon, C.; Rannou, M.T.; Galanaud, P. Activation and Infection of B Cells by Epstein-Barr Virus. Role of Calcium Mobilization and of Protein Kinase C Translocation. J. Immunol. 1988, 141, 4344–4351. Available online: https://pubmed.ncbi.nlm.nih.gov/2848895/ (accessed on 11 February 2025). [CrossRef]
- Murdaca, G.; Tagliafico, L.; Page, E.; Paladin, F.; Gangemi, S. Gender Differences in the Interplay between Vitamin D and Microbiota in Allergic and Autoimmune Diseases. Biomedicines 2024, 12, 1023. [Google Scholar] [CrossRef]
- Zdrenghea, M.T.; Makrinioti, H.; Bagacean, C.; Bush, A.; Johnston, S.L.; Stanciu, L.A. Vitamin D modulation of innate immune responses to respiratory viral infections. Rev. Med. Virol. 2017, 27, e1909. [Google Scholar] [CrossRef]
- Bugarin, J.D.; Dosenovic, S.; Ilic, D.; Delic, N.; Saric, I.; Ugrina, I.; Stipic, S.S.; Duplancic, B.; Saric, L. Vitamin D Supplementation and Clinical Outcomes in Severe COVID-19 Patients—Randomized Controlled Trial. Nutrients 2023, 15, 1234. [Google Scholar] [CrossRef]
- Lau, F.H.; Majumder, R.; Torabi, R.; Saeg, F.; Hoffman, R.; Cirillo, J.D.; Greiffenstein, P. Vitamin D Insufficiency Is Prevalent in Severe COVID-19. medRxiv 2020. [Google Scholar] [CrossRef]
- Javed, A.; Kullo, I.J.; Balagopal, P.B.; Kumar, S. Effect of vitamin D3 treatment on endothelial function in obese adolescents. Pediatr. Obes. 2016, 11, 279–284. [Google Scholar] [CrossRef]
- Pappa, H.M.; Mitchell, P.D.; Jiang, H.; Kassiff, S.; Filip-Dhima, R.; DiFabio, D.; Quinn, N.; Lawton, R.C.; Bronzwaer, M.E.S.; Koenen, M.; et al. Maintenance of Optimal Vitamin D Status in Children and Adolescents With Inflammatory Bowel Disease: A Randomized Clinical Trial Comparing Two Regimens. J. Clin. Endocrinol. Metab. 2014, 99, 3408. [Google Scholar] [CrossRef]
- Galior, K.; Grebe, S.; Singh, R. Development of Vitamin D Toxicity from Overcorrection of Vitamin D Deficiency: A Review of Case Reports. Nutrients 2018, 10, 953. [Google Scholar] [CrossRef] [PubMed]
- Kurtoğlu, A.; Kurtoğlu, E.; Akgümüş, A.; Çar, B.; Eken, Ö.; Sârbu, I.; Ciongradi, C.I.; Alexe, D.I.; Candussi, I.L. Evaluation of electrocardiographic parameters in amputee football players. Front. Psychol. 2023, 14, 1189712. [Google Scholar] [CrossRef] [PubMed]
- LBishop, E.; Ismailova, A.; Dimeloe, S.; Hewison, M.; White, J.H. Vitamin D and Immune Regulation: Antibacterial, Antiviral, Anti-Inflammatory. JBMR Plus 2020, 5, e10405. [Google Scholar] [CrossRef]
- Stallings, V.A.; Schall, J.I.; Hediger, M.L.; Zemel, B.S.; Tuluc, F.; Dougherty, K.A.; Samuel, J.L.B.; Rutstein, R.M. High-dose vitamin D3 supplementation in children and young adults with HIV: A randomized, placebo-controlled trial. Pediatr. Infect. Dis. J. 2015, 34, e32–e40. [Google Scholar] [CrossRef]
- Graidis, S.; Papavramidis, T.S.; Papaioannou, M. Vitamin D and Acute Kidney Injury: A Two-Way Causality Relation and a Predictive, Prognostic, and Therapeutic Role of Vitamin D. Front. Nutr. 2021, 7, 630951. [Google Scholar] [CrossRef]
- Zheng, S.-Y.; Xiao, Q.-Y.; Xie, X.-H.; Deng, Y.; Ren, L.; Tian, D.-Y.; Luo, Z.-X.; Luo, J.; Fu, Z.; Huang, A.-L.; et al. Association between secondary thrombocytosis and viral respiratory tract infections in children. Sci. Rep. 2016, 6, 22964. [Google Scholar] [CrossRef]
- Atila, C.; O Sailer, C.; Bassetti, S.; Tschudin-Sutter, S.; Bingisser, R.; Siegemund, M.; Osswald, S.; Rentsch, K.; Rueegg, M.; Schaerli, S.; et al. Prevalence and outcome of dysnatremia in patients with COVID-19 compared to controls. Eur. J. Endocrinol. 2021, 184, 409. [Google Scholar] [CrossRef]
- Hu, J.; Wang, Y.; Geng, X.; Chen, R.; Zhang, P.; Lin, J.; Teng, J.; Zhang, X.; Ding, X. Dysnatremia is an Independent Indicator of Mortality in Hospitalized Patients. Med. Sci. Monit. 2017, 23, 2408–2425. [Google Scholar] [CrossRef]
- Yen, C.W.; Yu, M.C.; Lee, J. Serum electrolyte abnormalities in pediatric patients presenting to an emergency department with various diseases: Age-related differences. Pediatr. Neonatol. 2022, 63, 575–581. [Google Scholar] [CrossRef]
- Lavagno, C.; Milani, G.P.; Uestuener, P.; Simonetti, G.D.; Casaulta, C.; Bianchetti, M.G.; Fare, P.B.; Lava, S.A.G. Hyponatremia in children with acute respiratory infections: A reappraisal. Pediatr. Pulmonol. 2017, 52, 962–967. [Google Scholar] [CrossRef]
- Ciortea, D.A.; Petrea (Cliveți), C.L.; Bezman, L.B.; Vivisenco, I.C.; Berbece, S.I.; Gurău, G.; Matei, M.N.; Nechita, A. Diagnostic Utility of Copeptin in Pediatric Patients with Polyuria-Polydipsia Syndrome: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2024, 25, 10743. [Google Scholar] [CrossRef] [PubMed]
- Ciepiela, O.; Raniszewska, A.; Manda-Handzlik, A.; Kotuła, I.; Demkow, U. Pseudohyperkalemia in capillary whole-blood samples—An occasional error or a significant problem in a pediatric hospital? Clin. Chem. Lab. Med. 2017, 55, e159–e162. [Google Scholar] [CrossRef] [PubMed]
- Kovesdy, C.P.; Matsushita, K.; Sang, Y.; Brunskill, N.J.; Carrero, J.J.; Chodick, G.; Hasegawa, T.; Heerspink, H.L.; Hirayama, A.; Landman, G.W.D.; et al. Serum potassium and adverse outcomes across the range of kidney function: A CKD Prognosis Consortium meta-analysis. Eur. Heart J. 2018, 39, 1535–1542. [Google Scholar] [CrossRef]
- Choi, M.J.; Ziyadeh, F.N. The utility of the transtubular potassium gradient in the evaluation of hyperkalemia. J. Am. Soc. Nephrol. 2008, 19, 424–426. [Google Scholar] [CrossRef]
- Petrea, C.L.; Ciortea, D.A.; Candussi, I.-L.; Gurău, G.; Matei, N.M.; Bergheș, S.-E.; Chirila, S.I.; Berbece, S.I. A Study of Hydroelectrolytic and Acid–Base Disturbances in MIS-C Patients: A Perspective on Antidiuretic Hormone Secretion. Curr. Issues Mol. Biol. 2024, 46, 11438–11459. [Google Scholar] [CrossRef]
- Levi, M.; Thachil, J.; Iba, T.; Levy, J.H. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020, 7, e438–e440. [Google Scholar] [CrossRef]
- Yang, L.; Liu, S.; Liu, J.; Zhang, Z.; Wan, X.; Huang, B.; Chen, Y.; Zhang, Y. COVID-19: Immunopathogenesis and Immunotherapeutics. Signal Transduct. Target. Ther. 2020, 5, 128. [Google Scholar] [CrossRef] [PubMed]
- Füst, G. The role of the Epstein–Barr virus in the pathogenesis of some autoimmune disorders—Similarities and differences. Eur. J. Microbiol. Immunol. 2011, 1, 267. [Google Scholar] [CrossRef]
- Petrea, C.L.; Ciortea, D.A.; Miulescu, M.; Candussi, I.-L.; Chirila, S.I.; (Răuță), G.I.V.; Bergheș, S.-E.; Râșcu, M.C.; Berbece, S.I. A New Case of Paediatric Systemic Lupus Erythematosus with Onset after SARS-CoV-2 and Epstein-Barr Infection—A Case Report and Literature Review. Curr. Issues Mol. Biol. 2024, 46, 8642–8657. [Google Scholar] [CrossRef]
- Jog, N.R.; James, J.A. Epstein Barr Virus and Autoimmune Responses in Systemic Lupus Erythematosus. Front. Immunol. 2021, 11, 623944. [Google Scholar] [CrossRef]
- De Belo, I.A.C.; Gouveia, C.; Milheiro Silva, T.; Conde, M. COVID-19 infection triggered juvenile systemic lupus erythematosus-like disease. J. Paediatr. Child Health 2022, 58, 2286–2288. [Google Scholar] [CrossRef] [PubMed]
- Ghazavi, A.; Ganji, A.; Keshavarzian, N.; Rabiemajd, S.; Mosayebi, G. Cytokine profile and disease severity in patients with COVID-19. Cytokine 2020, 137, 155323. [Google Scholar] [CrossRef] [PubMed]
- Hussain, A.; Singh, L.; III, J.M.; Jo, Y.; Makaryan, T.T.; Hussain, S.; Trenschel, R.W.; Kesselman, M.M. Serum Procalcitonin as a Predictive Biomarker in COVID-19: A Retrospective Cohort Analysis. Cureus 2022, 14, e27816. [Google Scholar] [CrossRef]
- Ciortea, D.A.; Petrea, C.L.; Berbece, S.I.; Fotea, S.; Vivisenco, I.C.; Gurău, G.; Matei, M.N.; Nechita, A. Impact of Hyponatremia and ADH Secretion in MIS-C and COVID-19: An Integrative Approach of Prognostic and Diagnostic Markers. Curr. Issues Mol. Biol. 2024, 46, 11749–11771. [Google Scholar] [CrossRef]
- Gao, Y.; Li, T.; Han, M.; Li, X.; Wu, D.; Xu, Y.; Zhu, Y.; Liu, Y.; Wang, X.; Wang, L. Diagnostic utility of clinical laboratory data determinations for patients with the severe COVID-19. J. Med. Virol. 2020, 92, 791. [Google Scholar] [CrossRef]
- Mocan, M.; Chiorescu, R.M.; Tirnovan, A.; Buksa, B.S.; Farcaș, A.D. Severe Thrombocytopenia as a Manifestation of COVID-19 Infection. J. Clin. Med. 2022, 11, 1088. [Google Scholar] [CrossRef]
- Iba, T.; Levy, J.H. The roles of platelets in COVID-19-associated coagulopathy and vaccine-induced immune thrombotic thrombocytopenia. Trends Cardiovasc. Med. 2021, 32, 1. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Chijioke, O.; Azzi, T.; Nadal, D.; Münz, C. Innate immune responses against Epstein Barr virus infection. J. Leukoc. Biol. 2013, 94, 1185. [Google Scholar] [CrossRef] [PubMed]
- Luțenco, V.; Țocu, G.; Guliciuc, M.; Moraru, M.; Candussi, I.L.; Dănilă, M.; Luțenco, V.; Dimofte, F.; Mihailov, O.M.; Mihailov, R. New Horizons of Artificial Intelligence in Medicine and Surgery. J. Clin. Med. 2024, 13, 2532. [Google Scholar] [CrossRef]



| Biological Parameters | Age Group | Level Range |
|---|---|---|
| Sodium (mmol/L) | <3 years 3–<6 years 6–<16 years >16 years (females) >16 years (males) | 135–142 135–142 136–143 137–142 137–143 |
| Potassium (mmol/L) | 0–<6 years (females/males) >6 years (females/males) | 3.9–4.6 3.8–4.9 |
| 25-OH vitamin D (ng/dL) | 0–18 years | <20 deficiency 20–30 insufficient 30–50-normal 50–150 increased level >150 toxic level |
| Anti-SARS-CoV-2 IgG antibodies (AU/mL) | 0–18 years | 0–10 |
| Anti-SARS-CoV-2 IgM antibodies (AU/mL) | 0–18 years | 0–10 |
| Anti-EBV(VCA) IgG antibodies (AU/mL) | 0–18 years | 0–5 |
| Anti-EBV(VCA) IgM antibodies (AU/mL) | 0–18 years | 0–10 |
| Anti-EBV(EBNA) IgG antibodies (AU/mL) | 0–18 years | 0–5 |
| Variables | COVID-19 Subgroup | COVID-19 + EBV Subgroup | p-Value |
|---|---|---|---|
| Categorical variables % (N) | |||
| Disease severity | 0.06 | ||
| Mild | 44.91 (128) | 41.32 (50) | |
| Moderate | 52.63 (150) | 51.24 (62) | |
| Severe | 2.46 (7) | 7.44 (9) | |
| Numerical variables % (N) | |||
| Hospitalization duration (days) | 0.045 | ||
| 1–7 days | 62.11 (177) | 57.85 (70) | |
| 8–14 days | 35.14 (103) | 35.54 (43) | |
| >14 days | 1.75 (5) | 6.61 (8) | |
| Biological Markers | COVID-19 Subgroup | COVID-19 + EBV Subgroup | ||
|---|---|---|---|---|
| Mean Value (SD; CI: []) | Median | Mean Value (SD; CI: []) | Median | |
| Erythrocyte sedimentation rate (ESR) [2–12 mm/h] | 25.44 (21.99; [21.85;29.02]) | 19 | 30.92 (24.76; [25.79;36.13]) | 21.00 |
| C-reactive protein [0–0.5 mg/dL] | 2.37 (4.50; [1.83;2.90]) | 0.63 | 4.13 (6.64; [2.91;5.33]) | 1.62 |
| Platelets [150–450 × 10³/µL] | 372 (147.57; [354.77;389.24)] | 344 | 378.43 (196.52; [342.75;414.10)] | 343.3 |
| Procalcitonin [0–0.5 ng/mL] | 0.95 (2.90; [0.23;1.65]) | 0.13 | 0.63 (1.57; [0.19;1.05]) | 0.16 |
| Ferritin [13–68 ng/mL] | 98.33 (147.33; [72.35;124.30]) | 53.51 | 140.61 (212.30; [90.72;190.50] | 54.80 |
| IL-6 [0–17 pg/mL] | 48.93 (9.02; [34.56;63.28]) | 47.39 | 27.91 (28.50; [0;73.26]) | 20.45 |
| D-dimers [0–0.55 ng/mL] | 1.74 (4.66; [0.54;2.9]) | 0.62 | 2.26 (3.91; [1.20;3.32]) | 0.91 |
| Anti SARS-CoV-2 IgG antibodies [0–10 AU/mL] | 70.08 (48.49; [64.42;75.72]) | 62.22 | 71.51 (56.55; [61.33;81.69]) | 64.10 |
| Anti-SARS-CoV-2 IgM antibodies [0–10 AU/mL] | 5.36 (38.72; [0.12;10.50]) | 0.61 | 3.72 (18.57; [0.02;7.43]) | 0.46 |
| Anti-EBV(VCA) IgG antibodies [0–5 AU/mL] | -- | -- | 57.24 (39.77; [49.99; 64.49]) | 46.45 |
| Anti-EBV(VCA) IgM antibodies [0–10 AU/mL] | -- | -- | 13.09 (19.65; [8.51;17.68] | 5.00 |
| Anti-EBV(EBNA)IgG antibodies [0–5 AU/mL] | -- | -- | 73.08 (48.03; [59.14; 87.03] | 99.85 |
| 25-hydroxyvitamin D [30–50 ng/dL] | 33.05 (13.03; [30.84;35.26]) | 29.80 | 32.14 (15.39; [27.72;36.56]) | 28.30 |
| Sodium [mmol/L] | 139.72 (3.38; [139.29;140.14]) | 140 | 138.65 (3.17; [138.01;139.30]) | 139 |
| Potassium [mmol/L] | 4.48 (0.59; [4.40;4.56]) | 4.39 | 4.52 (0.63; [4.39;4.65]) | 4.48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrea, C.L.; Ciortea, D.-A.; Gurău, G.; Matei, N.M.; Dinu, C.A.; Bergheș, S.-E.; Verga, G.I.; Berbece, S.I. Vitamin D Imbalance and Hydro-Electrolyte Disturbances in Hospitalized Children: A Comparation Between Post-COVID-19 Status and SARS-CoV-2/EBV Coinfection. Biomedicines 2025, 13, 1233. https://doi.org/10.3390/biomedicines13051233
Petrea CL, Ciortea D-A, Gurău G, Matei NM, Dinu CA, Bergheș S-E, Verga GI, Berbece SI. Vitamin D Imbalance and Hydro-Electrolyte Disturbances in Hospitalized Children: A Comparation Between Post-COVID-19 Status and SARS-CoV-2/EBV Coinfection. Biomedicines. 2025; 13(5):1233. https://doi.org/10.3390/biomedicines13051233
Chicago/Turabian StylePetrea (Cliveți), Carmen Loredana, Diana-Andreea Ciortea, Gabriela Gurău, Nicoleta Mădălina Matei, Ciprian Adrian Dinu, Simona-Elena Bergheș (Oprea), Gabriela Isabela Verga (Răuță), and Sorin Ion Berbece. 2025. "Vitamin D Imbalance and Hydro-Electrolyte Disturbances in Hospitalized Children: A Comparation Between Post-COVID-19 Status and SARS-CoV-2/EBV Coinfection" Biomedicines 13, no. 5: 1233. https://doi.org/10.3390/biomedicines13051233
APA StylePetrea, C. L., Ciortea, D.-A., Gurău, G., Matei, N. M., Dinu, C. A., Bergheș, S.-E., Verga, G. I., & Berbece, S. I. (2025). Vitamin D Imbalance and Hydro-Electrolyte Disturbances in Hospitalized Children: A Comparation Between Post-COVID-19 Status and SARS-CoV-2/EBV Coinfection. Biomedicines, 13(5), 1233. https://doi.org/10.3390/biomedicines13051233







