Retinal Thickness in Patients with Parkinson’s Disease and Dopa Responsive Dystonia—Is There Any Difference?
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Clinical Assessment
2.3. Criteria for Exclusion
2.4. Optical Coherence Tomography Assessment
2.5. Statistical Evaluation
3. Results
3.1. Patients with PD and Healthy Control Subjects
3.2. Patients with DRD and Healthy Control Subjects
3.3. Patients with PD and DRD
4. Discussion
4.1. Thickness of GCIPL, pRNFL and Macular Segments in PD and DRD Patients
4.2. Correlation Between Retinal Thickness Measurements with Different Clinical and Demographic Parameters
4.2.1. Current Age, Sex and Disease Severity
4.2.2. Cognitive Functions
4.2.3. Affected Side
4.2.4. Levodopa Daily Dose
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CNS | Central nervous system |
| PD | Parkinson’s disease |
| OCT | Optical coherence tomography |
| RNFL | Retinal nerve fiber layer |
| DRD | Dopa responsive dystonia |
| GCIPL | Ganglion cell/inner plexiform layer |
| CMT | Central macula thickness |
| UCCS | University Clinical Center of Serbia |
| HC | Healthy controls |
| MDS | Movement Disorder Society |
| MDS-UPDRS | MDS-Unified Parkinson’s disease rating scale |
| MMSE | Mini Mental State Examination |
| ETDRS | Early Treatment of Diabetic Retinopathy Study |
| IRL | Inner retinal layer |
| SPSS | Statistical Package for Social Sciences |
| HY | Hoehn and Yahr |
| M1 | Central macula |
| M2 | Inner superior segment |
| M3 | Inner nasal segment |
| M4 | Inner inferior segment |
| M5 | Inner temporal segment |
| M6 | Outer superior segment |
| M7 | Outer nasal segment |
| M8 | Outer inferior segment |
| M9 | Outer temporal segment |
| PET | Positron Emission Tomography |
References
- Kashani, A.H.; Asanad, S.; Chan, J.W.; Singer, M.B.; Zhang, J.; Sharifi, M.; Khansari, M.M.; Abdolahi, F.; Shi, Y.; Biffi, A.; et al. Past, present and future role of retinal imaging in neurodegenerative disease. Prog. Retin. Eye Res. 2021, 83, 100938. [Google Scholar] [CrossRef] [PubMed]
- Wagner, S.K.; Romero-Bascones, D.; Cortina-Borja, M.; Williamson, D.J.; Struyven, R.R.; Zhou, Y.; Patel, S.; Weil, R.S.; Antoniades, C.A.; Topol, E.J.; et al. Retinal Optical Coherence Tomography Features Associated with Incident and Prevalent Parkinson Disease. Neurology 2023, 101, e1581–e1593. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhang, D.; Ji, J.; Wang, Y.; Zhang, R. Central retina changes in Parkinson’s disease: A systematic review and meta-analysis. J. Neurol. 2021, 268, 4646–4654. [Google Scholar] [CrossRef]
- Ucak, T.; Alagoz, A.; Cakir, B.; Celik, E.; Bozkurt, E.; Alagoz, G. Analysis of the retinal nerve fiber and ganglion cell—Inner plexiform layer by optical coherence tomography in Parkinson’s patients. Park. Relat. Disord. 2016, 31, 59–64. [Google Scholar] [CrossRef]
- Tugcu, B.; Melikov, A.; Yildiz, G.B.; Gökcal, E.; Ercan, R.; Uysal, O.; Ozdemir, H. Evaluation of retinal alterations in Parkinson disease and tremor diseases. Acta Neurol. Belg. 2020, 120, 107–113. [Google Scholar] [CrossRef]
- Sung, M.S.; Choi, S.M.; Kim, J.; Ha, J.Y.; Kim, B.C.; Heo, H.; Park, S.W. Inner retinal thinning as a biomarker for cognitive impairment in de novo Parkinson’s disease. Sci. Rep. 2019, 9, 11832. [Google Scholar] [CrossRef]
- Gulmez Sevim, D.; Unlu, M.; Gultekin, M.; Karaca, C.; Mirza, M.; Mirza, G.E. Evaluation of Retinal Changes in Progressive Supranuclear Palsy and Parkinson Disease. J. Neuroophthalmol. 2018, 38, 151–155. [Google Scholar] [CrossRef]
- Chaudhuri, K.R.; Sauerbier, A. Parkinson disease. Unravelling the nonmotor mysteries of Parkinson disease. Nat. Rev. Neurol. 2016, 12, 10–11. [Google Scholar] [CrossRef]
- Nygaard, T.G. Dopa-responsive dystonia. Curr. Opin. Neurol. 1995, 8, 310–313. [Google Scholar] [CrossRef]
- Postuma, R.; Berg, D.; Stern, M.; Poewe, W.; Olanow, C.W.; Oertel, W.; Obeso, J.; Marek, K.; Litvan, I.; Lang, A.E.; et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 2015, 30, 1591–1601. [Google Scholar] [CrossRef]
- Hoehn, M.; Yahr, M. Parkinsonism: Onset, progression and mortality. Neurology 1967, 17, 427–442. [Google Scholar] [CrossRef] [PubMed]
- Goetz, C.G.; Tilley, B.C.; Shaftman, S.R.; Stebbins, G.T.; Fahn, S.; Martinez-Martin, P.; Poewe, W.; Sampaio, C.; Stern, M.B.; Dodel, R.; et al. Movement disorder society-sponsored revision of the Unified Parkinson’s Disease Rating Scale [MDS-UPDRS): Scale presentation and clinimetric testing results. Mov. Disord. 2008, 23, 2129–2170. [Google Scholar] [CrossRef]
- Folstein, M.; Folstein, S.; McHugh, P. ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Tomlinson, C.L.; Stowe, R.; Patel, S.; Rick, C.; Gray, R.; Clarke, C.E. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov. Disord. 2010, 25, 2649–2653. [Google Scholar] [CrossRef]
- Murueta-Goyena, A.; Del Pino, R.; Reyero, P.; Galdós, M.; Arana, B.; Lucas-Jiménez, O.; Acera, M.; Tijero, B.; Ibarretxe-Bilbao, N.; Ojeda, N.; et al. Parafoveal thinning of inner retina is associated with visual dysfunction in Lewy body diseases. Mov. Disord. 2019, 34, 1315–1324. [Google Scholar] [CrossRef]
- Albrecht, P.; Müller, A.K.; Südmeyer, M.; Ferrea, S.; Ringelstein, M.; Cohn, E.; Aktas, O.; Dietlein, T.; Lappas, A.; Foerster, A.; et al. Optical coherence tomography in parkinsonian syndromes. PLoS ONE 2012, 7, e34891. [Google Scholar] [CrossRef]
- Kaur, M.; Saxena, R.; Singh, D.; Behari, M.; Sharma, P.; Menon, V. Correlation between structural and functional retinal changes in Parkinson disease. J. Neuroophthalmol. 2015, 35, 254–258. [Google Scholar] [CrossRef]
- Zhou, W.C.; Tao, J.X.; Li, J. Optical coherence tomography measurements as potential imaging biomarkers for Parkinson’s disease: A systematic review and meta-analysis. Eur. J. Neurol. 2021, 28, 763–774. [Google Scholar] [CrossRef]
- Lee, Y.W.; Lim, M.N.; Lee, J.Y.; Yoo, Y.J. Central retina thickness measured with spectral-domain optical coherence tomography in Parkinson disease: A meta-analysis. Medicine 2023, 102, e35354. [Google Scholar] [CrossRef]
- Lee, J.Y.; Ahn, J.; Yoon, E.J.; Oh, S.; Kim, Y.K.; Jeon, B. Macular ganglion-cell-complex layer thinning and optic nerve integrity in drug-naïve Parkinson’s disease. J. Neural Transm. 2019, 126, 1695–1699. [Google Scholar] [CrossRef]
- Rascunà, C.; Russo, A.; Terravecchia, C.; Castellino, N.; Avitabile, T.; Bonfiglio, V.; Fallico, M.; Chisari, C.G.; Cicero, C.E.; Grillo, M.; et al. Retinal Thickness and Microvascular Pattern in Early Parkinson’s Disease. Front. Neurol. 2020, 11, 533375. [Google Scholar] [CrossRef] [PubMed]
- Eslami, F.; Ghiasian, M.; Mohamadrahimi, B.; Jiriaee, N.; Eslamighayour, A. Optical coherence tomography (OCT) findings in patients with Parkinson’s disease presenting to Farshchian Hospital (Sina) in 2019 compared to the normal population. J. Fr. Ophtalmol. 2024, 48, 104379. [Google Scholar] [CrossRef] [PubMed]
- Poveda, S.; Arellano, X.; Bernal-Pacheco, O.; Valencia López, A. Structural changes in the retina as a potential biomarker in Parkinson’s disease: An approach from optical coherence tomography. Front. Neuroimaging 2024, 3, 1340754. [Google Scholar] [CrossRef]
- Archibald, N.K.; Clarke, M.P.; Mosimann, U.P.; Burn, D.J. Retinal thickness in Parkinson’s disease. Park. Relat. Disord. 2011, 17, 431–436. [Google Scholar] [CrossRef]
- Mailankody, P.; Battu, R.; Khanna, A.; Lenka, A.; Yadav, R.; Pal, P.K. Optical coherence tomography as a tool to evaluate retinal changes in Parkinson’s disease. Park. Relat. Disord. 2015, 21, 1164–1169. [Google Scholar] [CrossRef]
- Bittersohl, D.; Stemplewitz, B.; Keserü, M.; Buhmann, C.; Richard, G.; Hassenstein, A. Detection of retinal changes in idiopathic Parkinson’s disease using high-resolution optical coherence tomography and heidelberg retina tomography. Acta Ophthalmol. 2015, 93, e578–e584. [Google Scholar] [CrossRef]
- Yıldız, D.; Pekel, N.B.; Yener, N.P.; Seferoğlu, M.; Günes, A.; Sığırlı, D. Assessment of Neurodegeneration by Optical Coherence Tomography and Mini-Mental Test in Parkinson’s Disease. Ann. Indian Acad. Neurol. 2019, 22, 212–216. [Google Scholar] [CrossRef]
- Bayram, D.; Yüksel, G.; Bayram, T.; Tireli, H. Optical Coherence Tomography Findings in Parkinson’s and Alzheimer’s Disease-Retinal Changes in Neurodegenerative Disease. Noro. Psikiyatr. Ars. 2019, 58, 103–107. [Google Scholar] [CrossRef]
- Batum, M.; Ak, A.K.; Arı, M.S.; Mayali, H.; Kurt, E.; Selçuki, D. Evaluation of the visual system with visual evoked potential and optical coherence tomography in patients with idiopathic Parkinson’s disease and with multiple system atrophy. Doc. Ophthalmol. 2022, 145, 99–112. [Google Scholar] [CrossRef]
- Djamgoz, M.B.; Hankins, M.W.; Hirano, J.; Archer, S.N. Neurobiology of retinal dopamine in relation to degenerative states of the tissue. Vis. Res. 1997, 37, 3509–3529. [Google Scholar] [CrossRef]
- Ahn, J.; Lee, J.Y.; Kim, T.W.; Yoon, E.J.; Oh, S.; Kim, Y.K.; Kim, J.M.; Woo, S.J.; Kim, K.W.; Jeon, B. Retinal thinning associates with nigral dopaminergic loss in de novo Parkinson disease. Neurology 2018, 91, e1003–e1012. [Google Scholar] [CrossRef] [PubMed]
- Ortuno-Lizaran, I.; Sanchez-Saez, X.; Lax, P.; Serrano, G.E.; Beach, T.G.; Adler, C.H.; Cuenca, N. Dopaminergic retinal cell loss and visual dysfunction in Parkinson disease. Ann. Neurol. 2020, 88, 893–906. [Google Scholar] [CrossRef] [PubMed]
- Bodis-Wollner, I.; Kozlowski, P.B.; Glazman, S.; Shahnaz, M. α-synuclein in the inner retina in parkinson disease. Ann. Neurol. 2014, 75, 964–966. [Google Scholar] [CrossRef]
- Marrocco, E.; Indrieri, A.; Esposito, F.; Tarallo, V.; Carboncino, A.; Alvino, F.G.; De Falco, S.; Franco, B.; De Risi, M.; De Leonibus, E. α-synuclein overexpression in the retina leads to vision impairment and degeneration of dopaminergic amacrine cells. Sci. Rep. 2020, 10, 9619. [Google Scholar] [CrossRef]
- Petzold, A.; Balcer, L.J.; Calabresi, P.A.; Costello, F.; Frohman, T.C.; Frohman, E.M.; Martinez-Lapiscina, E.H.; Green, A.J.; Kardon, R.; Outteryck, O.; et al. Retinal layer segmentation in multiple sclerosis: A systematic review and meta-analysis. Lancet Neurol. 2017, 16, 797–812. [Google Scholar] [CrossRef]
- Gulmez Sevim, D.; Unlu, M.; Sonmez, S.; Gultekin, M.; Karaca, C.; Ozturk Oner, A. Retinal vessel diameter obtained by OCT is spared in Parkinson’s disease. Int. Ophthalmol. 2019, 39, 813–819. [Google Scholar] [CrossRef]
- Garcia-Martin, E.; Pablo, L.E.; Bambo, M.P.; Alarcia, R.; Polo, V.; Larrosa, J.M.; Vilades, E.; Cameo, B.; Orduna, E.; Ramirez, T.; et al. Comparison of peripapillary choroidal thickness between healthy subjects and patients with Parkinson’s disease. PLoS ONE 2017, 12, e0177163. [Google Scholar] [CrossRef]
- La Morgia, C.; Barboni, P.; Rizzo, G.; Carbonelli, M.; Savini, G.; Scaglione, C.; Capellari, S.; Bonazza, S.; Giannoccaro, M.P.; Calandra-Buonaura, G.; et al. Loss of temporal retinal nerve fibers in Parkinson disease: A mitochondrial pattern? Eur. J. Neurol. 2013, 20, 198–201. [Google Scholar] [CrossRef]
- Lee, J.-Y.; Ahn, J.; Kim, T.W.; Jeon, B.S. Optical coherence tomography in Parkinson’s disease: Is the retina a biomarker? J. Parkinson’s Dis. 2014, 4, 197–204. [Google Scholar] [CrossRef]
- Altintaş, O.; Işeri, P.; Ozkan, B.; Cağlar, Y. Correlation between retinal morphological and functional findings and clinical severity in Parkinson’s disease. Doc. Ophthalmol. 2008, 116, 137–146. [Google Scholar] [CrossRef]
- Satue, M.; Garcia-Martin, E.; Fuertes, I.; Otin, S.; Alarcia, R.; Herrero, R.; Bambo, M.P.; Pablo, L.E.; Fernandez, F.J. Use of Fourier-domain OCT to detect retinal nerve fiber layer degeneration in Parkinson’s disease patients. Eye 2013, 27, 507–514. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P.; Dutta, K.; Ghosh, S.; Mukherjee, A.; Pal, S.; Basu, D. Optical coherence tomography findings in patients of Parkinson’s disease: An Indian perspective. Ann. Indian Acad. Neurol. 2018, 21, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Satue, M.; Rodrigo, M.J.; Obis, J.; Vilades, E.; Gracia, H.; Otin, S.; Fuertes, M.I.; Alarcia, R.; Crespo, J.A.; Polo, V.; et al. Evaluation of Progressive Visual Dysfunction and Retinal Degeneration in Patients with Parkinson’s Disease. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1151–1157. [Google Scholar] [CrossRef] [PubMed]
- Subhi, Y.; Forshaw, T.; Sørensen, T.L. Macular thickness and volume in the elderly: A systematic review. Ageing Res. Rev. 2016, 29, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Quagliato, L.B.; Domingues, C.; Quagliato, E.M.A.B.; Abreu, E.B.; Kara-Junior, N. Applications of visual evoked potentials and Fourier-domain optical coherence tomography in Parkinson’s disease: A controlled study. Arq. Bras. Oftalmol. 2014, 77, 238–242. [Google Scholar] [CrossRef]
- Aydin, T.S.; Umit, D.; Nur, O.M.; Fatih, U.; Asena, K.; Nefise, O.Y.; Serpil, Y. Optical coherence tomography findings in Parkinson’s disease. Kaohsiung J. Med. Sci. 2018, 34, 166–171. [Google Scholar] [CrossRef]
- Matlach, J.; Wagner, M.; Malzahn, U.; Schmidtmann, I.; Steigerwald, F.; Musacchio, T.; Volkmann, J.; Grehn, F.; Göbel, W.; Klebe, S. Retinal changes in Parkinson’s disease and glaucoma. Park. Relat. Disord. 2018, 56, 41–46. [Google Scholar] [CrossRef]
- Bayhan, H.A.; Aslan Bayhan, S.; Tanık, N.; Gürdal, C. The association of spectral-domain optical coherence tomography determined ganglion cell complex parameters and disease severity in Parkinson’s disease. Curr. Eye Res. 2014, 39, 1117–1122. [Google Scholar] [CrossRef]
- Jiménez, B.; Ascaso, F.J.; Cristóbal, J.A.; López del Val, J. Development of a prediction formula of Parkinson disease severity by optical coherence tomography. Mov. Disord. 2014, 29, 68–74. [Google Scholar] [CrossRef]
- Alkabie, S.; Lange, A.; Manogaran, P.; Stoessl, A.J.; Costello, F.; Barton, J.J.S. Optical coherence tomography of patients with Parkinson’s disease and progressive supranuclear palsy. Clin. Neurol. Neurosurg. 2020, 189, 105635. [Google Scholar] [CrossRef]
- Cunha, L.P.; Martins, P.N.; Martins, L.C.; Almada, F.M.D.N.; Shigaeff, N.; de Araújo, D.O.; Mello, L.G.M.; Monteiro, M.L.R.; Snyder, P.J.; Vale, T.C. Correlation between motor symptoms, cognitive function, and optical coherence tomography findings in Parkinson’s disease. Arq. Bras. Oftalmol. 2024, 88, S0004-27492025000300305. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.M.; Han, J.W.; Park, Y.J.; Bae, J.B.; Woo, S.J.; Kim, K.W. Association Between Retinal Layer Thickness and Cognitive Decline in Older Adults. JAMA Ophthalmol. 2022, 140, 683–690. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cubo, E.; Tedejo, R.P.; Rodriguez Mendez, V.; López Peña, M.J.; Trejo Gabriel, Y.; Galán, J.M. Retina thickness in Parkinson’s disease and essential tremor. Mov. Disord. 2010, 25, 2461–2462. [Google Scholar] [CrossRef]
- Pilat, A.; McLean, R.J.; Proudlock, F.A.; Maconachie, G.D.; Sheth, V.; Rajabally, Y.A.; Gottlob, I. In vivo morphology of the optic nerve and retina in patients with Parkinson’s disease. Investig. Ophthalmol. Vis. Sci. 2016, 57, 4420–4427. [Google Scholar] [CrossRef]
- Yu, J.G.; Feng, Y.F.; Xiang, Y.; Huang, J.H.; Savini, G.; Parisi, V.; Yang, W.J.; Fu, X.A. Retinal nerve fiber layer thickness changes in Parkinson disease: A meta-analysis. PLoS ONE 2014, 9, e85718. [Google Scholar] [CrossRef]
- Archibald, N.K.; Clarke, M.P.; Mosimann, U.P.; Burn, D.J. The retina in Parkinson’s disease. Brain 2009, 132 Pt 5, 1128–1145. [Google Scholar] [CrossRef]
- Harnois, C.; Di Paolo, T. Decreased dopamine in the retinas of patients with Parkinson’s disease. Investig. Ophthalmol. Vis. Sci. 1990, 31, 2473–2475. [Google Scholar]
- Brilliant, M.H.; Vaziri, K.; Connor, T.B., Jr.; Schwartz, S.G.; Carroll, J.J.; McCarty, C.A.; Schrodi, S.J.; Hebbring, S.J.; Kishor, K.S.; Flynn, H.W., Jr.; et al. Mining Retrospective Data for Virtual Prospective Drug Repurposing: L-DOPA and Age-related Macular Degeneration. Am. J. Med. 2016, 129, 292–298, PubMed: 26524704. [Google Scholar] [CrossRef]
- Figueroa, A.G.; Boyd, B.M.; Christensen, C.A.; Javid, C.G.; McKay, B.S.; Fagan, T.C.; Snyder, R.W. Levodopa Positively Affects Neovascular Age-Related Macular Degeneration. Am. J. Med. 2021, 134, 122–128. [Google Scholar] [CrossRef]

| Patients with PD | |
|---|---|
| UPDRS I | 5.0 ± 4.9 |
| UPDRS II | 8.4 ± 6.3 |
| UPDRS III | 26.3 ± 13.6 |
| UPDRS IV | 1.0 ± 2.6 |
| Total UPDRS | 40.7 ± 22.5 |
| HY stage | I-6 |
| II-20 | |
| III-35 | |
| IV-15 | |
| V-0 | |
| MMSE | 28.6 ± 1.8 |
| Patients with PD | Healthy Control Subjects | p | |
|---|---|---|---|
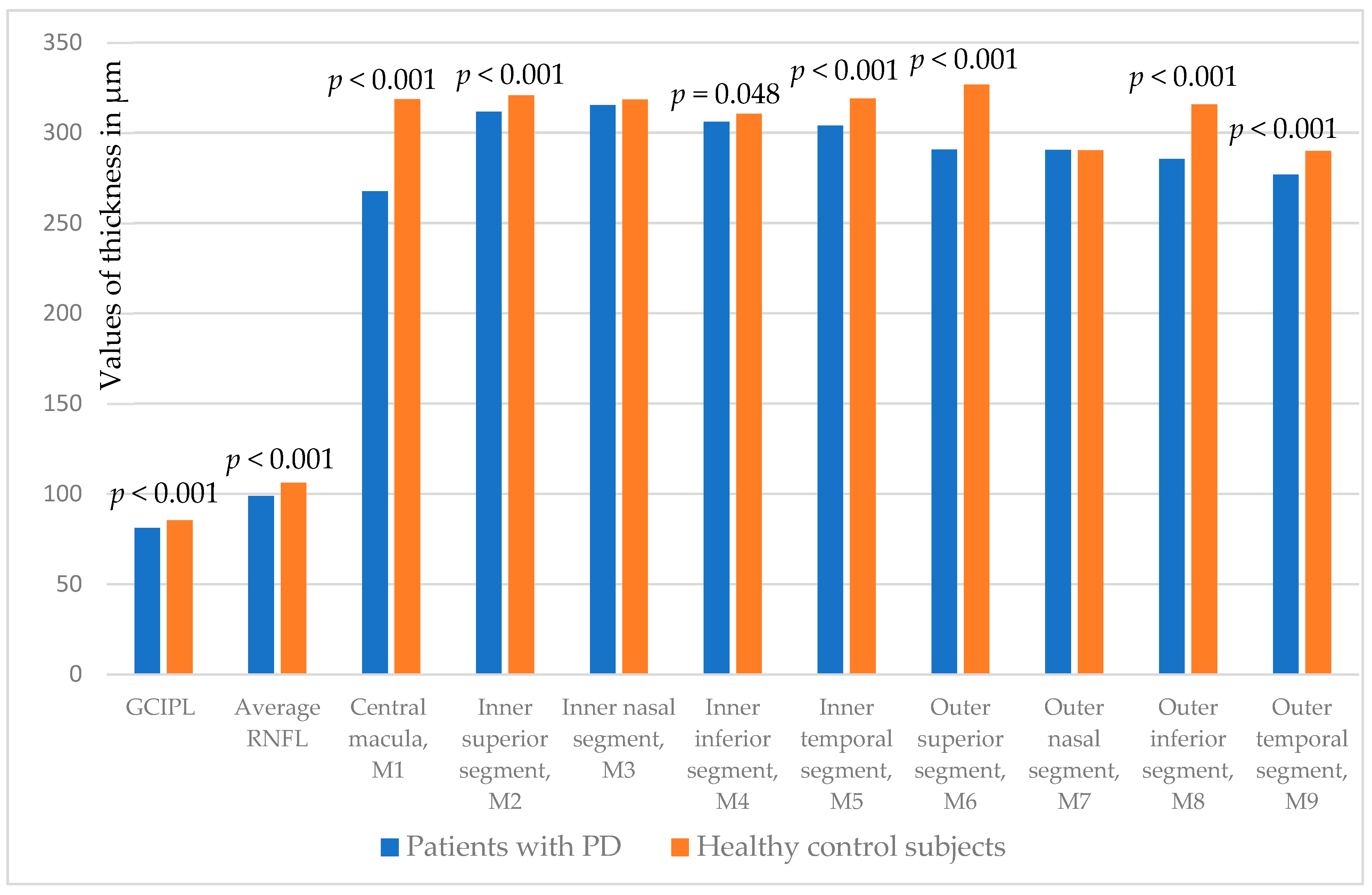
| GCIPL | 81.1 ± 7.2 | 85.4 ± 7.9 | <0.001 |
| Average RNFL | 98.9 ± 10.0 | 106.1 ± 7.8 | <0.001 |
| Central macula, M1 | 267.7 ± 32.4 | 318.6 ± 16.6 | <0.001 |
| Inner superior segment, M2 | 311.7 ± 23.5 | 320.6 ± 12.4 | <0.001 |
| Inner nasal segment, M3 | 315.2 ± 26.7 | 318.4 ± 17.8 | 0.191 |
| Inner inferior segment, M4 | 306.0 ± 22.1 | 310.5 ± 19.4 | 0.048 |
| Inner temporal segment, M5 | 303.9 ± 24.8 | 319.0 ± 17.2 | <0.001 |
| Outer superior segment, M6 | 290.6 ± 23.6 | 326.7 ± 11.7 | <0.001 |
| Outer nasal segment, M7 | 290.5 ± 25.2 | 290.3 ± 35.7 | 0.958 |
| Outer inferior segment, M8 | 285.5 ± 28.2 | 315.6 ± 15.2 | <0.001 |
| Outer temporal segment, M9 | 276.7 ± 26.9 | 289.9 ± 36.2 | <0.001 |
| GCIPL | RNFL | M1 | M2 | M3 | M4 | M5 | M6 | M7 | M8 | M9 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Current age | −0.219 * | −0.358 ** | −0.042 | −0.161 * | −0.186 * | −0.287 ** | −0.262 ** | −0.290 ** | −0.336 ** | −0.226 ** | −0.079 |
| Levodopa daily dose | 0.244 * | −0.159 * | 0.016 | −0.019 | 0.077 | 0.069 | −0.001 | 0.034 | 0.021 | 0.025 | 0.223 ** |
| LEDD | 0.175 | −0.008 | 0.147 | 0.011 | 0.102 | 0.068 | 0.115 | 0.185 * | 0.159 * | 0.171 * | 0.102 |
| UPDRS I | −0.172 | −0.001 | −0.050 | −0.153 * | −0.057 | −0.080 | 0.009 | −0.032 | −0.006 | −0.021 | −0.030 |
| UPDRS II | 0.256 * | −0.031 | 0.153 * | −0.081 | −0.033 | −0.109 | 0.014 | 0.093 | 0.039 | 0.128 | −0.078 |
| UPDRS III | 0.183 | −0.012 | 0.131 | −0.144 | 0.016 | −0.076 | 0.089 | 0.191 * | 0.104 | 0.301 ** | −0.090 |
| UPDRS IV | −0.069 | 0.064 | −0.028 | −0.006 | −0.062 | −0.077 | −0.060 | −0.066 | −0.044 | −0.101 | −0.022 |
| Total UPDRS | 0.143 | −0.007 | 0.108 | −0.144 | −0.019 | −0.103 | 0.053 | 0.124 | 0.065 | 0.199 ** | −0.085 |
| HY | 0.230 * | −0.049 | 0.098 | −0.113 | 0.048 | −0.013 | 0.024 | −0.178 * | −0.116 | −0.155 * | 0.026 |
| MMSE | 0.198 | 0.016 | 0.171 * | 0.124 | 0.075 | 0.147 | 0.081 | 0.066 | 0.025 | 0.033 | 0.043 |
| Patients with DRD | Healthy Control Subjects | p | |
|---|---|---|---|
| GCIPL | 79.4 ± 7.0 | 85.6 ± 7.9 | 0.012 |
| RNFL | 100.5 ± 10.8 | 106.1 ± 6.6 | 0.053 |
| Central macula, M1 | 293.4 ± 37.8 | 326.2 ± 12.6 | 0.001 |
| Inner superior segment, M2 | 297.1 ± 28.4 | 319.1 ± 7.1 | 0.003 |
| Inner nasal segment, M3 | 307.4 ± 21.7 | 314.5 ± 16.1 | 0.247 |
| Inner inferior segment, M4 | 314.3 ± 22.0 | 305.2 ± 26.8 | 0.247 |
| Inner temporal segment, M5 | 313.3 ± 15.9 | 315.1 ± 14.4 | 0.710 |
| Outer superior segment, M6 | 317.5 ± 26.8 | 329.0 ± 13.1 | 0.091 |
| Outer nasal segment, M7 | 323.4 ± 23.8 | 284.3 ± 33.2 | 0.003 |
| Outer inferior segment, M8 | 322.3 ± 22.2 | 284.7 ± 30.9 | 0.583 |
| Outer temporal segment, M9 | 321.6 ± 21.7 | 284.7 ± 30.9 | 0.002 |
| Patients with PD | Patients with DRD | p | |
|---|---|---|---|
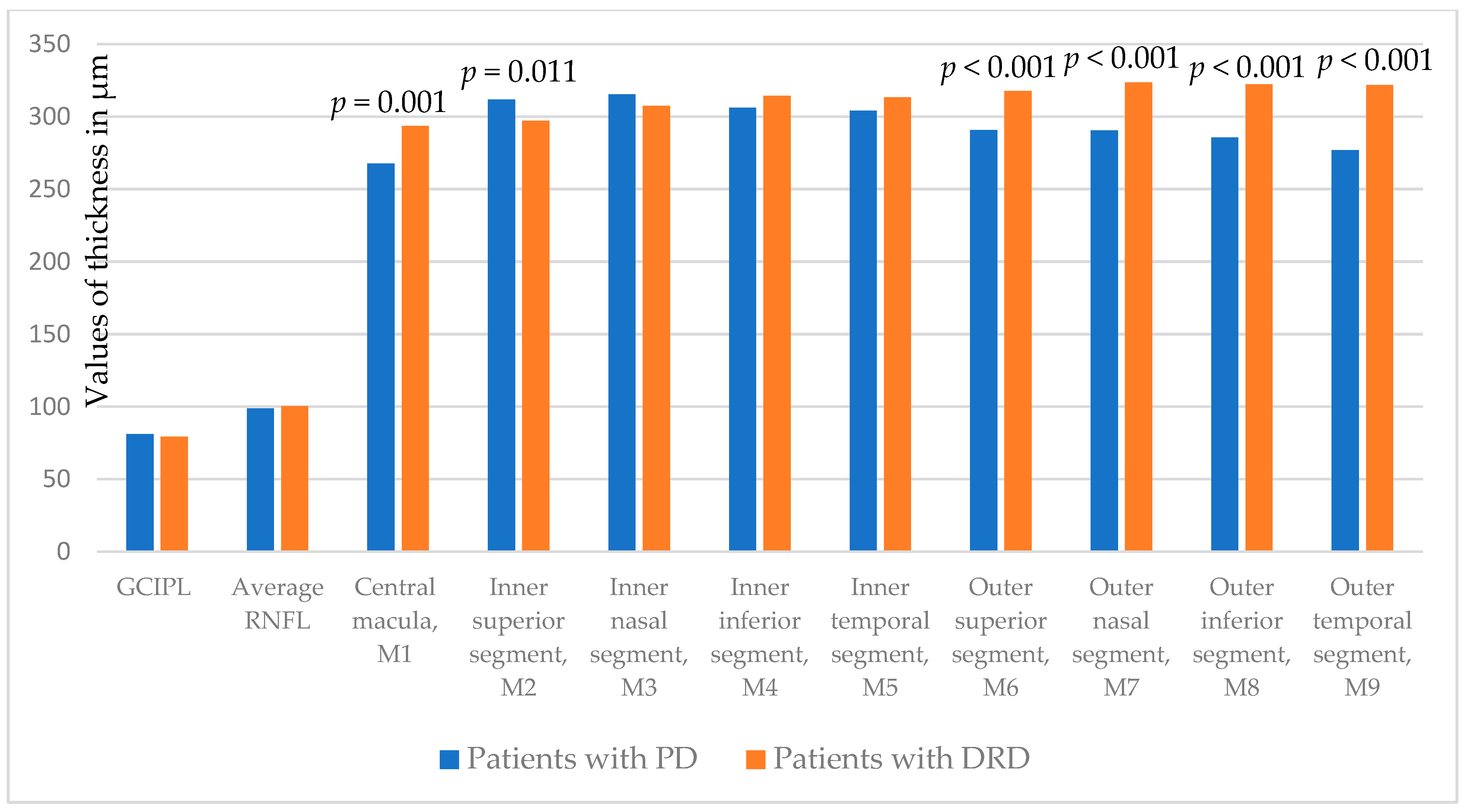
| GCIPL | 81.1 ± 7.2 | 79.4 ± 7.0 | 0.331 |
| Average RNFL | 98.9 ± 10.0 | 100.5 ± 10.8 | 0.519 |
| Central macula, M1 | 267.7 ± 32.4 | 293.4 ± 37.8 | 0.001 |
| Inner superior segment, M2 | 311.7 ± 23.5 | 297.1 ± 28.4 | 0.011 |
| Inner nasal segment, M3 | 315.2 ± 26.7 | 307.4 ± 21.7 | 0.208 |
| Inner inferior segment, M4 | 306.0 ± 22.1 | 314.3 ± 22.0 | 0.118 |
| Inner temporal segment, M5 | 303.9 ± 24.8 | 313.3 ± 15.9 | 0.102 |
| Outer superior segment, M6 | 290.6 ± 23.6 | 317.5 ± 26.8 | <0.001 |
| Outer nasal segment, M7 | 290.5 ± 25.2 | 323.4 ± 23.8 | <0.001 |
| Outer inferior segment, M8 | 285.5 ± 28.2 | 322.3 ± 22.2 | <0.001 |
| Outer temporal segment, M9 | 276.7 ± 26.9 | 321.6 ± 21.7 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Svetel, M.; Marić, G.; Božić, M.; Lazić, U.; Milovanović, A.; Jakšić, J.; Petrović, I.; Dimitrijević, A.; Knežević, M.; Pekmezović, T. Retinal Thickness in Patients with Parkinson’s Disease and Dopa Responsive Dystonia—Is There Any Difference? Biomedicines 2025, 13, 1227. https://doi.org/10.3390/biomedicines13051227
Svetel M, Marić G, Božić M, Lazić U, Milovanović A, Jakšić J, Petrović I, Dimitrijević A, Knežević M, Pekmezović T. Retinal Thickness in Patients with Parkinson’s Disease and Dopa Responsive Dystonia—Is There Any Difference? Biomedicines. 2025; 13(5):1227. https://doi.org/10.3390/biomedicines13051227
Chicago/Turabian StyleSvetel, Marko, Gorica Marić, Marija Božić, Una Lazić, Andona Milovanović, Jana Jakšić, Igor Petrović, Ana Dimitrijević, Milica Knežević, and Tatjana Pekmezović. 2025. "Retinal Thickness in Patients with Parkinson’s Disease and Dopa Responsive Dystonia—Is There Any Difference?" Biomedicines 13, no. 5: 1227. https://doi.org/10.3390/biomedicines13051227
APA StyleSvetel, M., Marić, G., Božić, M., Lazić, U., Milovanović, A., Jakšić, J., Petrović, I., Dimitrijević, A., Knežević, M., & Pekmezović, T. (2025). Retinal Thickness in Patients with Parkinson’s Disease and Dopa Responsive Dystonia—Is There Any Difference? Biomedicines, 13(5), 1227. https://doi.org/10.3390/biomedicines13051227





