2bRAD-M Reveals the Characteristics of Urinary Microbiota in Overweight Patients with Urinary Tract Stones
Abstract
1. Introduction
2. Methods
2.1. Patient Recruitment
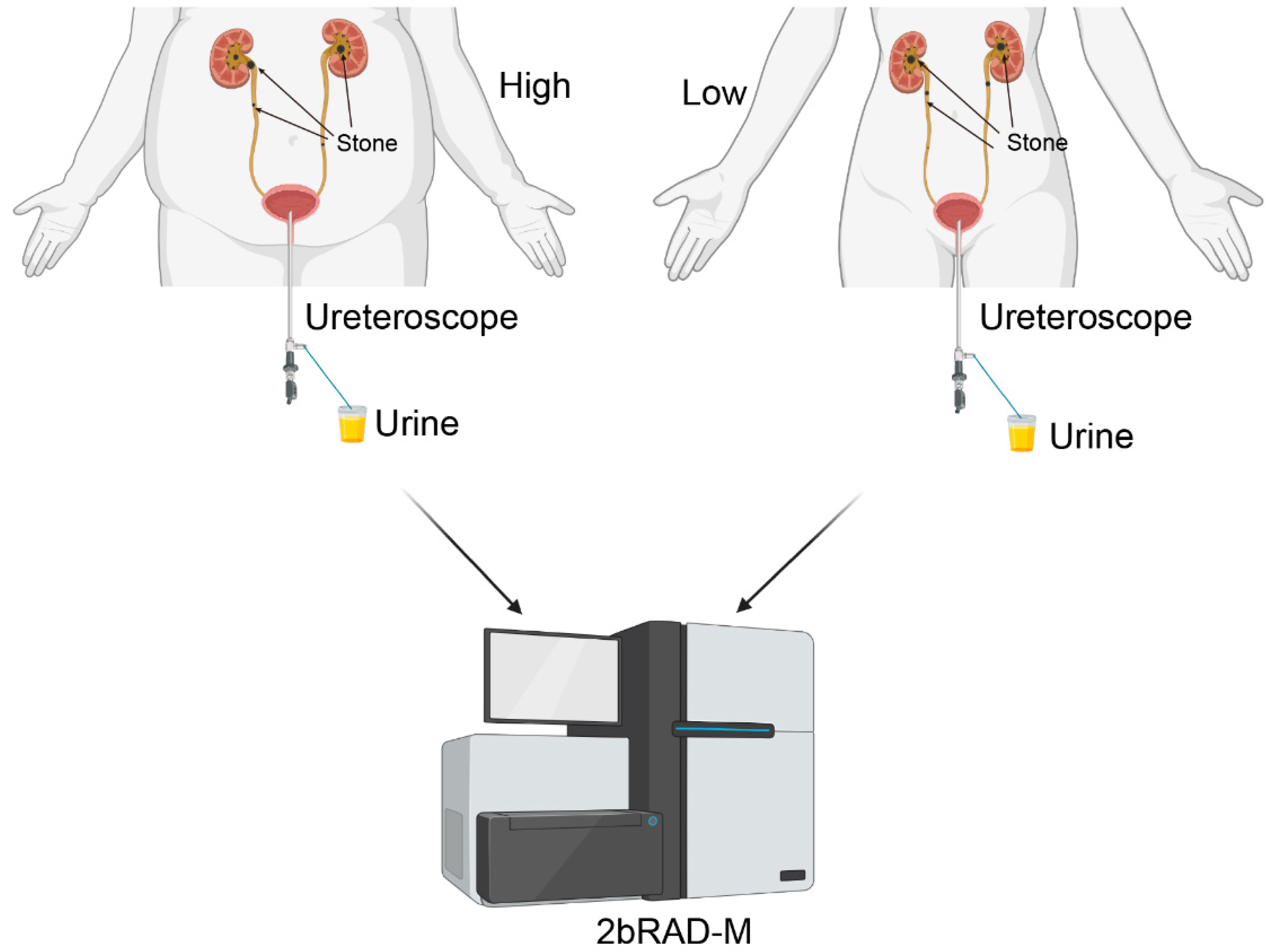
2.2. Collection and Processing of Samples
2.3. Dietary Data Collection and Analysis
2.4. DNA Extraction, Library Preparation, and Sequencing
2.5. Sequencing Processing and Quantitative Analysis
2.6. Analysis of Microbial Diversity and Identification of Differential Taxa
2.7. Statistical Analysis
3. Results
3.1. Clinical Characteristics of Selected Patients
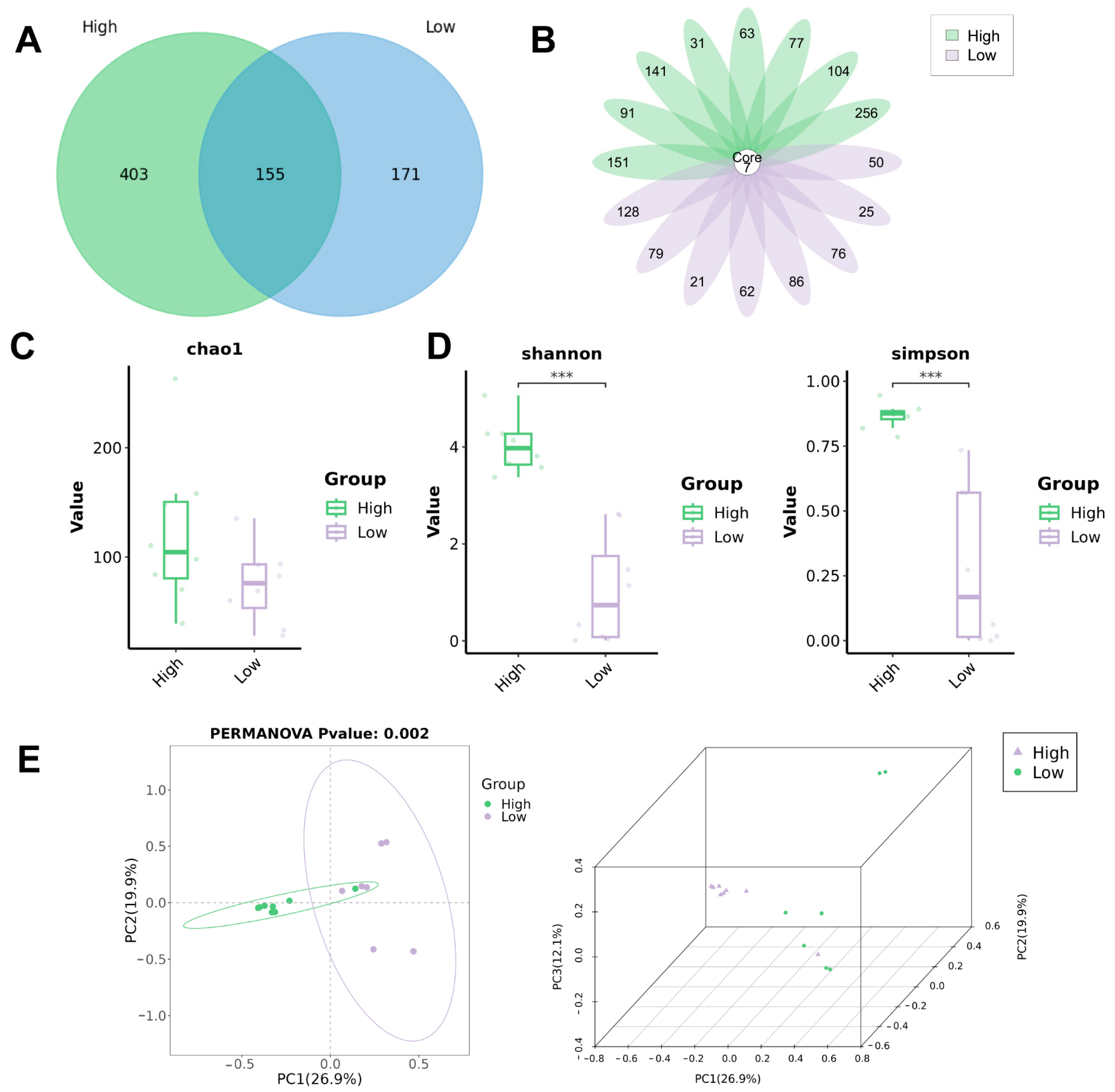
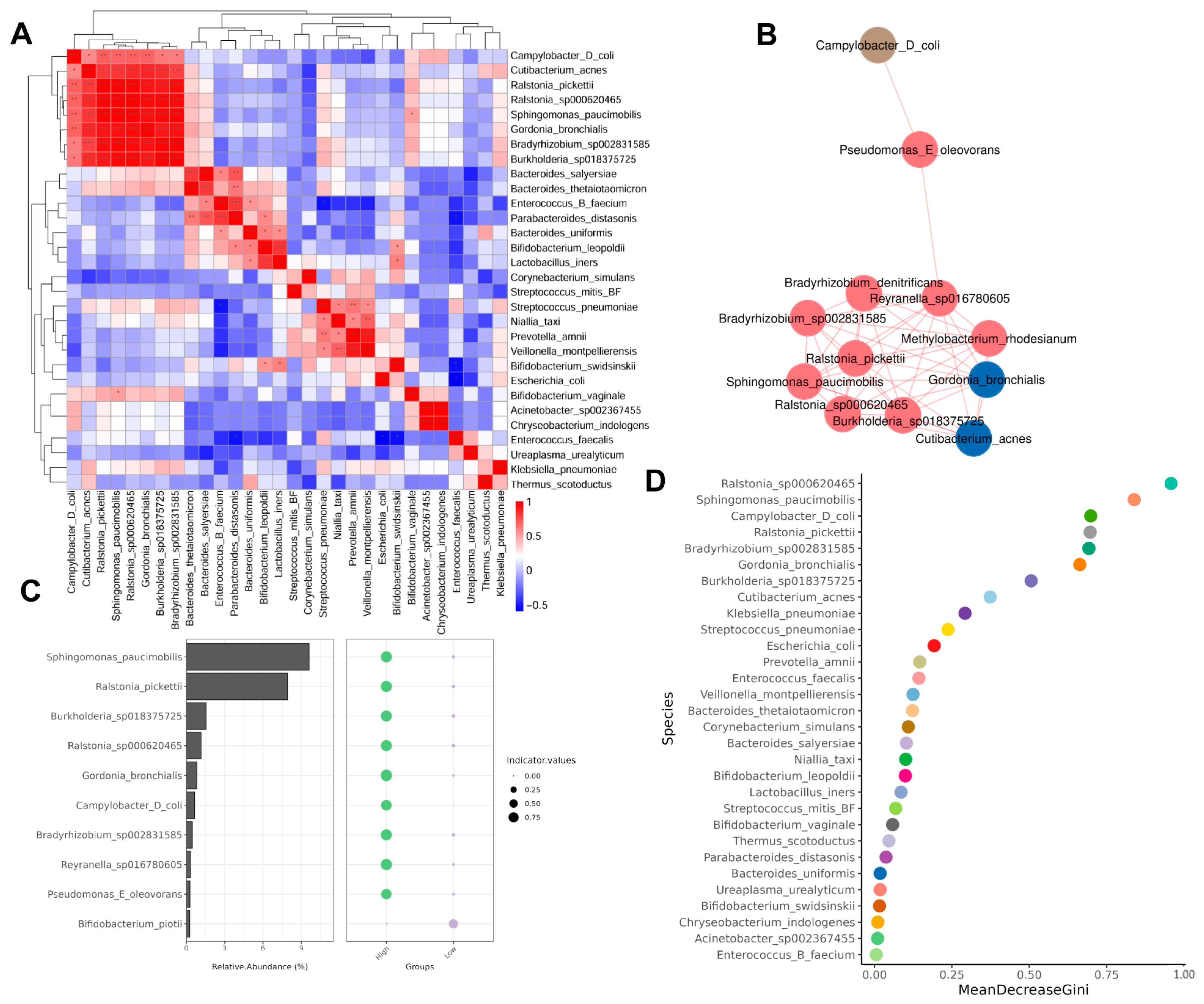
3.2. Biodiversity Analysis of Bacterial Communities in Urine
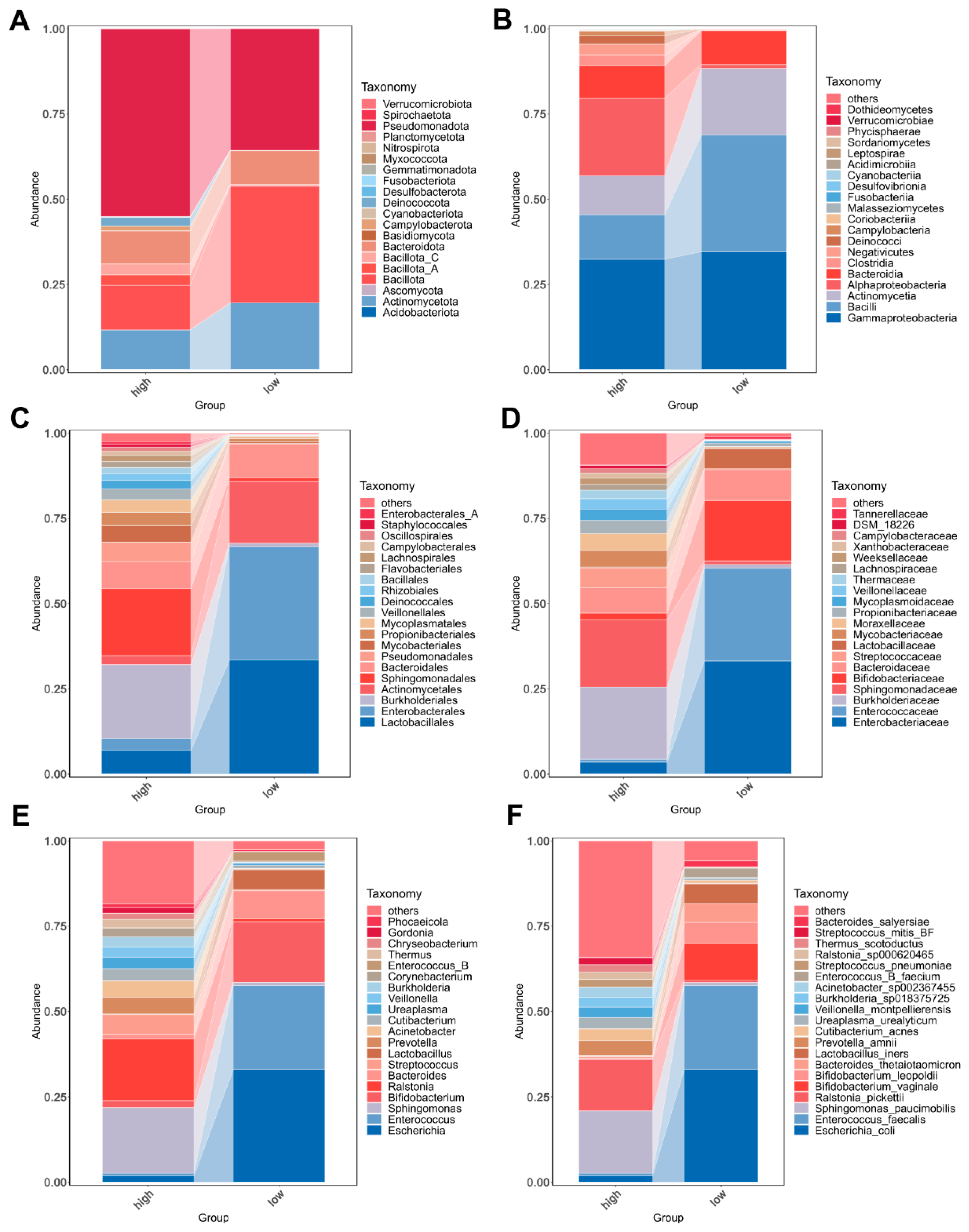
3.3. Bacterial Community Composition in Urine of Overweight and Healthy-Weight UTS Patients
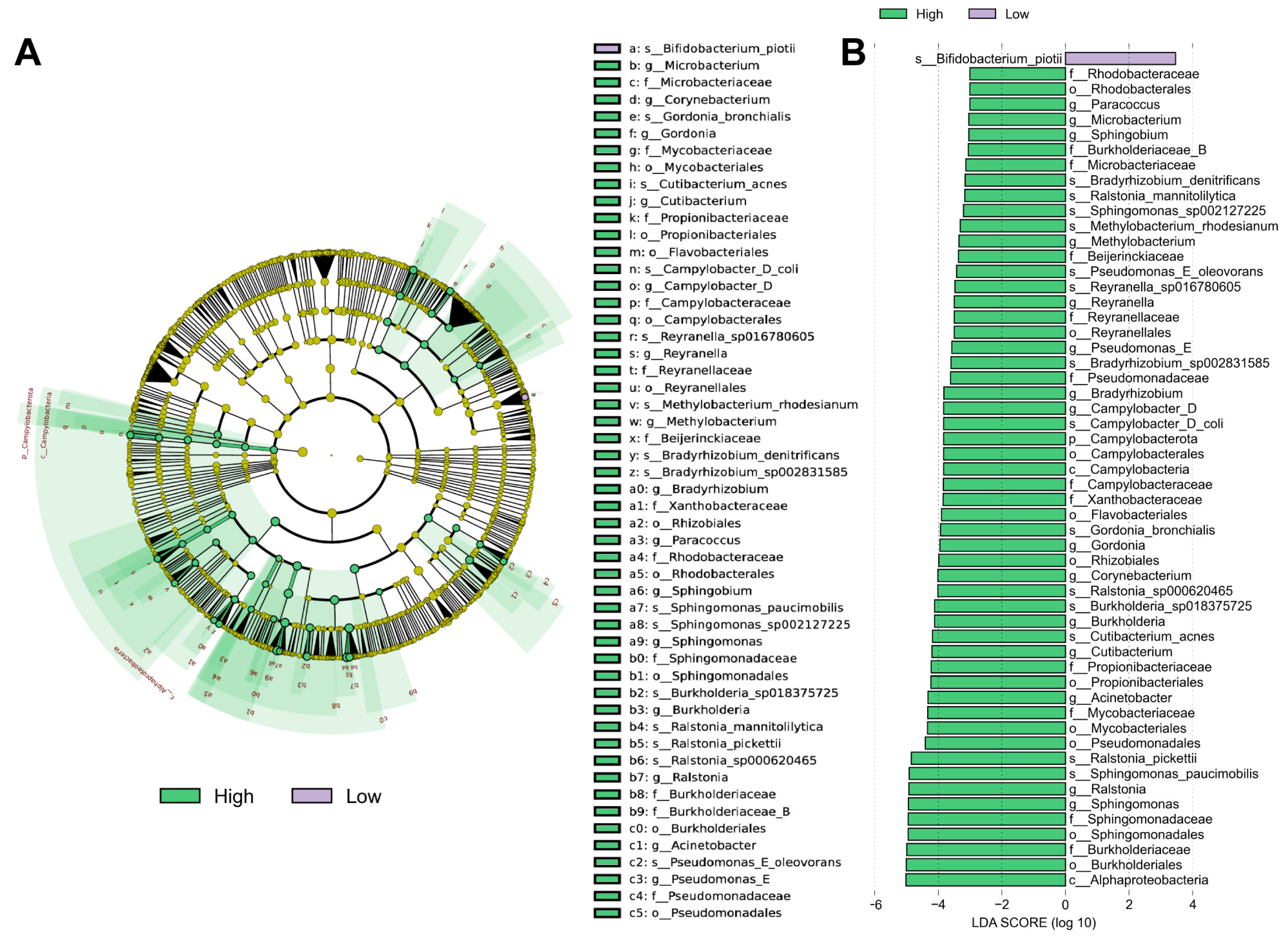
3.4. Different Abundance of Urinary Microbial Taxa in Grouped Patients
3.5. Correlation Between Microbiota
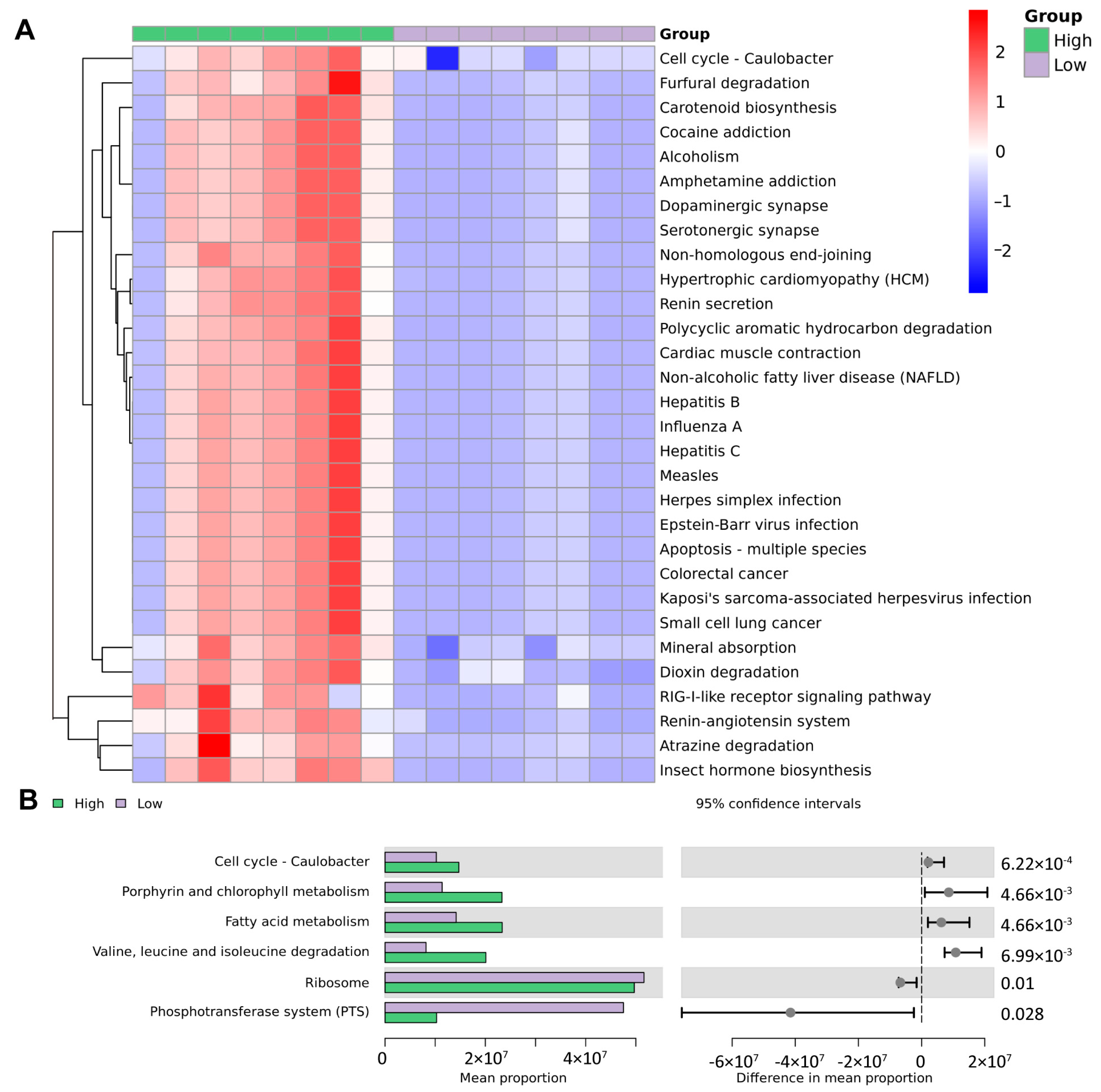
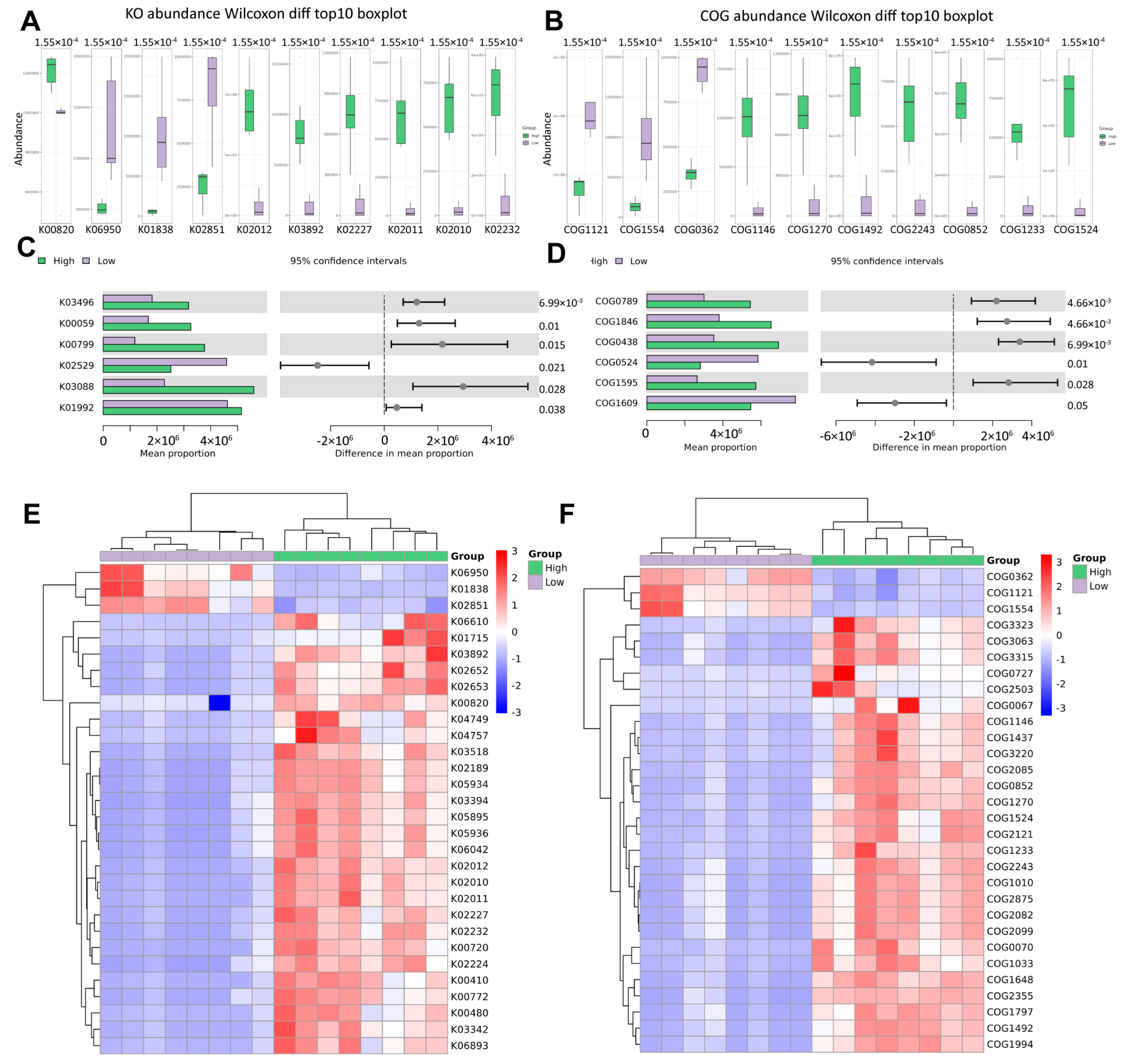
3.6. Functional Prediction Based on Microbiological Analysis Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tamborino, F.; Cicchetti, R.; Mascitti, M.; Litterio, G.; Orsini, A.; Ferretti, S.; Basconi, M.; De Palma, A.; Ferro, M.; Marchioni, M.; et al. Pathophysiology and Main Molecular Mechanisms of Urinary Stone Formation and Recurrence. Int. J. Mol. Sci. 2024, 25, 3075. [Google Scholar] [CrossRef]
- D’Ambrosio, V.; Ferraro, P.M.; Lombardi, G.; Friso, S.; Gambaro, G. Unravelling the Complex Relationship between Diet and Nephrolithiasis: The Role of Nutrigenomics and Nutrigenetics. Nutrients 2022, 14, 4961. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Zhang, J.; Deng, Q.; Liang, H. Recent advances on the mechanisms of kidney stone formation (Review). Int. J. Mol. Med. 2021, 48, 149. [Google Scholar] [CrossRef]
- Gellin, C.E. Urinary Tract Stones. Pediatr. Rev. 2019, 40, 154–156. [Google Scholar] [CrossRef]
- Khan, S.R.; Pearle, M.S.; Robertson, W.G.; Gambaro, G.; Canales, B.K.; Doizi, S.; Traxer, O.; Tiselius, H.-G. Kidney stones. Nat. Rev. Dis. Primer 2016, 2, 16008. [Google Scholar] [CrossRef]
- Moe, O.W. Kidney stones: Pathophysiology and medical management. Lancet Lond. Engl. 2006, 367, 333–344. [Google Scholar] [CrossRef]
- Suarez Arbelaez, M.C.; Nackeeran, S.; Shah, K.; Blachman-Braun, R.; Bronson, I.; Towe, M.; Bhat, A.; Marcovich, R.; Ramasamy, R.; Shah, H.N. Association between body mass index, metabolic syndrome and common urologic conditions: A cross-sectional study using a large multi-institutional database from the United States. Ann. Med. 2023, 55, 2197293. [Google Scholar] [CrossRef]
- Powell, C.R.; Stoller, M.L.; Schwartz, B.F.; Kane, C.; Gentle, D.L.; Bruce, J.E.; Leslie, S.W. Impact of body weight on urinary electrolytes in urinary stone formers. Urology 2000, 55, 825–830. [Google Scholar] [CrossRef]
- Aune, D.; Mahamat-Saleh, Y.; Norat, T.; Riboli, E. Body fatness, diabetes, physical activity and risk of kidney stones: A systematic review and meta-analysis of cohort studies. Eur. J. Epidemiol. 2018, 33, 1033–1047. [Google Scholar] [CrossRef]
- Yuan, S.; Larsson, S.C. Assessing causal associations of obesity and diabetes with kidney stones using Mendelian randomization analysis. Mol. Genet. Metab. 2021, 134, 212–215. [Google Scholar] [CrossRef]
- Baltazar, P.; de Melo Junior, A.F.; Fonseca, N.M.; Lança, M.B.; Faria, A.; Sequeira, C.O.; Teixeira-Santos, L.; Monteiro, E.C.; Campos Pinheiro, L.; Calado, J.; et al. Oxalate (dys)Metabolism: Person-to-Person Variability, Kidney and Cardiometabolic Toxicity. Genes 2023, 14, 1719. [Google Scholar] [CrossRef]
- Akram, M.; Jahrreiss, V.; Skolarikos, A.; Geraghty, R.; Tzelves, L.; Emilliani, E.; Davis, N.F.; Somani, B.K. Urological Guidelines for Kidney Stones: Overview and Comprehensive Update. J. Clin. Med. 2024, 13, 1114. [Google Scholar] [CrossRef]
- Maalouf, N.M.; Poindexter, J.R.; Adams-Huet, B.; Moe, O.W.; Sakhaee, K. Increased production and reduced urinary buffering of acid in uric acid stone formers is ameliorated by pioglitazone. Kidney Int. 2019, 95, 1262–1268. [Google Scholar] [CrossRef]
- Sekirov, I.; Russell, S.L.; Antunes, L.C.M.; Finlay, B.B. Gut microbiota in health and disease. Physiol. Rev. 2010, 90, 859–904. [Google Scholar] [CrossRef]
- Aragón, I.M.; Herrera-Imbroda, B.; Queipo-Ortuño, M.I.; Castillo, E.; Del Moral, J.S.-G.; Gómez-Millán, J.; Yucel, G.; Lara, M.F. The Urinary Tract Microbiome in Health and Disease. Eur. Urol. Focus 2018, 4, 128–138. [Google Scholar] [CrossRef]
- Lemberger, U.; Pjevac, P.; Hausmann, B.; Berry, D.; Moser, D.; Jahrreis, V.; Özsoy, M.; Shariat, S.F.; Veser, J. The microbiome of kidney stones and urine of patients with nephrolithiasis. Urolithiasis 2023, 51, 27. [Google Scholar] [CrossRef]
- Neugent, M.L.; Hulyalkar, N.V.; Nguyen, V.H.; Zimmern, P.E.; De Nisco, N.J. Advances in Understanding the Human Urinary Microbiome and Its Potential Role in Urinary Tract Infection. mBio 2020, 11, e00218-20. [Google Scholar] [CrossRef]
- Mjaess, G.; Karam, A.; Roumeguère, T.; Diamand, R.; Aoun, F.; McVary, K.; Moul, J.W.; De Nunzio, C.; Albisinni, S. Urinary microbiota and prostatic diseases: The key for the lock? A systematic review. Prostate Cancer Prostatic Dis. 2023, 26, 451–460. [Google Scholar] [CrossRef]
- Mueller, E.R.; Wolfe, A.J.; Brubaker, L. Female urinary microbiota. Curr. Opin. Urol. 2017, 27, 282–286. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, F.; Mao, S.; Wang, R.; Chen, H.; Ran, Y.; Liu, S.; Wu, P.; Yan, Y.; Li, W.; et al. Bladder cancer-associated microbiota: Recent advances and future perspectives. Heliyon 2023, 9, e13012. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Chen, H.; Zhang, W.; Ainiwaer, A.; Mao, S.; Yao, X.; Xu, T.; Yan, Y. Urinary microbiota signatures associated with different types of urinary diversion: A comparative study. Front. Cell. Infect. Microbiol. 2024, 13, 1302870. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Huang, J.-S.; Huang, X.-J.; Peng, J.-M.; Yu, Z.; Yuan, Y.-Q.; Xiao, K.-F.; Guo, J.-N. Profiling the urinary microbiome in men with calcium-based kidney stones. BMC Microbiol. 2020, 20, 41. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhang, N.; Jiang, P.; Zhai, Q.; Li, C.; Yu, D.; Wu, Y.; Zhang, Y.; Lv, L.; Xu, X.; et al. Characteristics of the urinary microbiome in kidney stone patients with hypertension. J. Transl. Med. 2020, 18, 130. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhang, N.; Wu, Y.; Jiang, P.; Jiang, T.; Wang, Y.; Zhang, Y.; Zhai, Q.; Zou, Y.; Feng, N. The pelvis urinary microbiome in patients with kidney stones and clinical associations. BMC Microbiol. 2020, 20, 336. [Google Scholar] [CrossRef]
- Cuñé Castellana, J. Microbioma and lithiasis. Arch. Esp. Urol. 2021, 74, 157–170. [Google Scholar]
- Dornbier, R.A.; Bajic, P.; Van Kuiken, M.; Jardaneh, A.; Lin, H.; Gao, X.; Knudsen, B.; Dong, Q.; Wolfe, A.J.; Schwaderer, A.L. The microbiome of calcium-based urinary stones. Urolithiasis 2020, 48, 191–199. [Google Scholar] [CrossRef]
- Sun, Z.; Huang, S.; Zhu, P.; Tzehau, L.; Zhao, H.; Lv, J.; Zhang, R.; Zhou, L.; Niu, Q.; Wang, X.; et al. Species-resolved sequencing of low-biomass or degraded microbiomes using 2bRAD-M. Genome Biol. 2022, 23, 36. [Google Scholar] [CrossRef]
- Sun, Z.; Liu, J.; Zhang, M.; Wang, T.; Huang, S.; Weiss, S.T.; Liu, Y.-Y. Removal of false positives in metagenomics-based taxonomy profiling via targeting Type IIB restriction sites. Nat. Commun. 2023, 14, 5321. [Google Scholar] [CrossRef]
- Remer, T.; Manz, F. Potential renal acid load of foods and its influence on urine pH. J. Am. Diet. Assoc. 1995, 95, 791–797. [Google Scholar] [CrossRef]
- Alexander, R.T.; Fuster, D.G.; Dimke, H. Mechanisms Underlying Calcium Nephrolithiasis. Annu. Rev. Physiol. 2022, 84, 559–583. [Google Scholar] [CrossRef]
- Wagner, C.A. Etiopathogenic factors of urolithiasis. Arch. Esp. Urol. 2021, 74, 16–23. [Google Scholar] [PubMed]
- Schade, G.R.; Faerber, G.J. Urinary tract stones. Prim. Care 2010, 37, 565–581. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, A.J.; Toh, E.; Shibata, N.; Rong, R.; Kenton, K.; Fitzgerald, M.; Mueller, E.R.; Schreckenberger, P.; Dong, Q.; Nelson, D.E.; et al. Evidence of uncultivated bacteria in the adult female bladder. J. Clin. Microbiol. 2012, 50, 1376–1383. [Google Scholar] [CrossRef] [PubMed]
- Hilt, E.E.; McKinley, K.; Pearce, M.M.; Rosenfeld, A.B.; Zilliox, M.J.; Mueller, E.R.; Brubaker, L.; Gai, X.; Wolfe, A.J.; Schreckenberger, P.C. Urine is not sterile: Use of enhanced urine culture techniques to detect resident bacterial flora in the adult female bladder. J. Clin. Microbiol. 2014, 52, 871–876. [Google Scholar] [CrossRef]
- Whiteside, S.A.; Razvi, H.; Dave, S.; Reid, G.; Burton, J.P. The microbiome of the urinary tract--a role beyond infection. Nat. Rev. Urol. 2015, 12, 81–90. [Google Scholar] [CrossRef]
- Karam, A.; Mjaess, G.; Albisinni, S.; El Daccache, Y.; Farah, M.; Daou, S.; Kazzi, H.; Hassoun, R.; Bou Kheir, G.; Aoun, F.; et al. Uncovering the role of urinary microbiota in urological tumors: A systematic review of literature. World J. Urol. 2022, 40, 951–964. [Google Scholar] [CrossRef]
- Sarica, K. Obesity and stones. Curr. Opin. Urol. 2019, 29, 27–32. [Google Scholar] [CrossRef]
- Wollin, D.A.; Skolarikos, A.; Preminger, G.M. Obesity and metabolic stone disease. Curr. Opin. Urol. 2017, 27, 422–427. [Google Scholar] [CrossRef]
- Johnson, J.S.; Spakowicz, D.J.; Hong, B.-Y.; Petersen, L.M.; Demkowicz, P.; Chen, L.; Leopold, S.R.; Hanson, B.M.; Agresta, H.O.; Gerstein, M.; et al. Evaluation of 16S rRNA gene sequencing for species and strain-level microbiome analysis. Nat. Commun. 2019, 10, 5029. [Google Scholar] [CrossRef]
- Hong, S.-Y.; Yang, Y.-Y.; Xu, J.-Z.; Xia, Q.-D.; Wang, S.-G.; Xun, Y. The renal pelvis urobiome in the unilateral kidney stone patients revealed by 2bRAD-M. J. Transl. Med. 2022, 20, 431. [Google Scholar] [CrossRef]
- Sun, J.-X.; Xia, Q.-D.; Zhong, X.-Y.; Liu, Z.; Wang, S.-G. The bladder microbiome of NMIBC and MIBC patients revealed by 2bRAD-M. Front. Cell Infect. Microbiol. 2023, 13, 1182322. [Google Scholar] [CrossRef] [PubMed]
- Shiue, I. Urinary polyaromatic hydrocarbons are associated with adult celiac disease and kidney stones: USA NHANES, 2011-2012. Environ. Sci. Pollut. Res. Int. 2016, 23, 3971–3977. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, A.; Tawashi, R. Growth retardation of calcium oxalate by sodium copper chlorophyllin. Eur. Urol. 1978, 4, 294–297. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Jin, K.; Qiu, S.; Yang, Q.; Wang, P.; Zhan, Y.; Huang, X.; Jiang, Z.; Hu, D.; Yang, L.; et al. Associations of exposure to polycyclic aromatic hydrocarbons and kidney stones in U.S. general population: Results from the National Health and Nutrition Examination Survey 2007-2016. World J. Urol. 2022, 40, 545–552. [Google Scholar] [CrossRef]
- Macchi, M.; Martinez, M.; Tauil, R.M.N.; Valacco, M.P.; Morelli, I.S.; Coppotelli, B.M. Insights into the genome and proteome of Sphingomonas paucimobilis strain 20006FA involved in the regulation of polycyclic aromatic hydrocarbon degradation. World J. Microbiol. Biotechnol. 2018, 34, 7. [Google Scholar] [CrossRef]
- Primiano, A.; Persichilli, S.; Ferraro, P.M.; Calvani, R.; Biancolillo, A.; Marini, F.; Picca, A.; Marzetti, E.; Urbani, A.; Gervasoni, J. A Specific Urinary Amino Acid Profile Characterizes People with Kidney Stones. Dis. Markers 2020, 2020, 8848225. [Google Scholar] [CrossRef]
- Seravalle, G.; Grassi, G. Obesity and hypertension. Pharmacol. Res. 2017, 122, 1–7. [Google Scholar] [CrossRef]
- Bayomy, O.; Zaheer, S.; Williams, J.S.; Curhan, G.; Vaidya, A. Disentangling the Relationships Between the Renin-Angiotensin-Aldosterone System, Calcium Physiology, and Risk for Kidney Stones. J. Clin. Endocrinol. Metab. 2020, 105, 1937–1946. [Google Scholar] [CrossRef]
- Bikulčienė, I.; Vasiliauskaitė, L.; Kučinskienė, Z.A.; Kaminskas, A.; Hendrixson, V. Investigation of Adipose Tissue Fatty Acid Composition in Men with Uronephrolithiasis and Metabolic Syndrome. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2018, 24, 818–826. [Google Scholar] [CrossRef]
- Siener, R.; Jansen, B.; Watzer, B.; Hesse, A. Effect of n-3 fatty acid supplementation on urinary risk factors for calcium oxalate stone formation. J. Urol. 2011, 185, 719–724. [Google Scholar] [CrossRef]
- Baggio, B.; Budakovic, A. Fatty acids and idiopathic calcium nephrolithiasis. Urol. Int. 2005, 75, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Alvarado, O.; Miyaoka, R.; Kriedberg, C.; Leavitt, D.A.; Moeding, A.; Stessman, M.; Monga, M. Omega-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid in the management of hypercalciuric stone formers. Urology 2012, 79, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xie, Y.; Cao, F.; Song, X. Gut microbiota-derived fatty acid and sterol metabolites: Biotransformation and immunomodulatory functions. Gut Microbes 2024, 16, 2382336. [Google Scholar] [CrossRef]
- Hall, J.A.; Brockman, J.A.; Davidson, S.J.; MacLeay, J.M.; Jewell, D.E. Increased dietary long-chain polyunsaturated fatty acids alter serum fatty acid concentrations and lower risk of urine stone formation in cats. PLoS ONE 2017, 12, e0187133. [Google Scholar] [CrossRef]
- McKenzie, C.I.; Mackay, C.R.; Macia, L. GPR43—A Prototypic Metabolite Sensor Linking Metabolic and Inflammatory Diseases. Trends Endocrinol. Metab. 2015, 26, 511–512. [Google Scholar] [CrossRef]
- Segev, Y.; Livne, A.; Mints, M.; Rosenblum, K. Concurrence of High Fat Diet and APOE Gene Induces Allele Specific Metabolic and Mental Stress Changes in a Mouse Model of Alzheimer’s Disease. Front. Behav. Neurosci. 2016, 10, 170. [Google Scholar] [CrossRef]
| Variables | Group | p Value | |
|---|---|---|---|
| Healthy-Weight (n = 8) | Overweight (n = 8) | ||
| BMI | 22.15 (21.18, 22.93) | 27.02 (26.67, 27.70) | <0.001 * |
| Age | 60 (52, 65) | 60.5 (46.75, 68.5) | 1 * |
| Gender | 0.200 † | ||
| Female | 3 (100) | 0 | |
| Male | 5 (38.46) | 8 (61.53) | |
| Hypertesion | 3 (42.85) | 4 (57.14) | 1 † |
| Diabetes | 1 (33.33) | 2 (66.67) | 1 † |
| Stone count | 1 † | ||
| Solitary | 6 (50) | 6 (50) | |
| Multiple | 2 (50) | 2 (50) | |
| Stone side | 0.688 ‡ | ||
| Left ureter | 4 (57.14) | 3 (42.86) | |
| Left ureter and kidney | 1 (50) | 1 (50) | |
| Right ureter | 1 (33.33) | 3 (66.67) | |
| Right ureter and kidney | 2 (66.67) | 1 (33.33) | |
| Maximum diameter of stones | 10 (8, 14.75) | 10 (7.25, 22.25) | 0.958 * |
| Stone composition | 0.642 ‡ | ||
| CaOx | 3 (60) | 2 (40) | |
| CaOx + CaP | 1 (100) | 0 | |
| Urine acid | 1 (33.33) | 2 (66.67) | |
| None? | 3 (42.86) | 4 (57.14) | |
| Stone medical history | 5 (50) | 5 (50) | 1 ‡ |
| Variables | Group | p Value | |
|---|---|---|---|
| Healthy-Weight (n = 8) | Overweight (n = 8) | ||
| BMI | 22.15 (21.18, 22.93) | 27.02 (26.67, 27.70) | <0.001 |
| Calorie intake | 2850 (2350, 3230) | 1991.25 (1650, 2220) | <0.001 |
| Protein intake | 92.75 (79, 106) | 75.88 (64, 89) | 0.0019 |
| Fiber intake | 18 (14, 23) | 29.5 (25, 34) | <0.001 |
| PRAL value | 13.71 (5.2, 18.7) | −3.24 (−7.5, 3.8) | <0.001 |
| Fermented food consumption | 0.96 (0, 1.8) | 1.21 (0, 2.7) | 0.5156 |
| Hydration level | 2.04 (1.5, 3.2) | 2.62 (1.3, 3.4) | 0.0727 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, P.; Zhang, J.; Zhang, W.; Yang, F.; Yu, Y.; Zhang, Y.; Wang, G.; Zhang, H.; Xu, Y.; Yao, X. 2bRAD-M Reveals the Characteristics of Urinary Microbiota in Overweight Patients with Urinary Tract Stones. Biomedicines 2025, 13, 1197. https://doi.org/10.3390/biomedicines13051197
Wu P, Zhang J, Zhang W, Yang F, Yu Y, Zhang Y, Wang G, Zhang H, Xu Y, Yao X. 2bRAD-M Reveals the Characteristics of Urinary Microbiota in Overweight Patients with Urinary Tract Stones. Biomedicines. 2025; 13(5):1197. https://doi.org/10.3390/biomedicines13051197
Chicago/Turabian StyleWu, Pengfei, Jingcheng Zhang, Wentao Zhang, Fuhang Yang, Yang Yu, Yuke Zhang, Guangchun Wang, Haimin Zhang, Yunfei Xu, and Xudong Yao. 2025. "2bRAD-M Reveals the Characteristics of Urinary Microbiota in Overweight Patients with Urinary Tract Stones" Biomedicines 13, no. 5: 1197. https://doi.org/10.3390/biomedicines13051197
APA StyleWu, P., Zhang, J., Zhang, W., Yang, F., Yu, Y., Zhang, Y., Wang, G., Zhang, H., Xu, Y., & Yao, X. (2025). 2bRAD-M Reveals the Characteristics of Urinary Microbiota in Overweight Patients with Urinary Tract Stones. Biomedicines, 13(5), 1197. https://doi.org/10.3390/biomedicines13051197








