Impact of Subthreshold Micropulse Laser on the Vascular Network in Diabetic Macular Edema: An Optical Coherence Tomography Angiography Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Exclusion Criteria
2.3. Study Procedure
2.4. Ophthalmic Assessments
2.4.1. BCVA Evaluation
2.4.2. Imaging Techniques
2.4.3. OCT Evaluation
2.4.4. OCTA Evaluation
2.5. Treatment Protocol
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics
3.2. OCTA Parameters
3.3. OCTAVA Parameters
3.4. OCT Parameters
3.5. BCVA
3.6. MA
3.7. Supplementary Analysis
3.8. Safety
4. Discussion
4.1. OCTAVA
4.2. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DME | Diabetic macular edema |
| DM | Diabetes mellitus |
| SMPL | Subthreshold micropulse laser |
| RPE | Retinal pigment epithelium |
| DC | Duty cycle |
| VEGF | Vascular endothelial growth factor |
| BCVA | Best-corrected visual acuity |
| ETDRS | Early Treatment Diabetic Retinopathy Study |
| UWF | Ultra-widefield |
| FAF | Fundus autofluorescence |
| OCT | Optical coherence tomography |
| FFA | Fundus fluorescein angiography |
| OCTA | Optical coherence tomography angiography |
| FAZ | Foveal avascular zone |
| MA | Microaneurysms |
| SCP | Superficial capillary plexus |
| DCP | Deep capillary plexus |
| DR | Diabetic retinopathy |
| RVO | Retinal vein occlusion |
| AMD | Age-related macular degeneration |
| ERM | Epiretinal membrane |
| HbA1c | Glycated hemoglobin |
| NVD | Neovascularization at the optic disk |
| NVE | Neovascularization elsewhere in the retina |
| H | Hemorrhages |
| HE | Hard exudates |
| SE | Soft exudates |
| CRT | Central retinal thickness |
| MT | Macular thickness |
| MV | Macular volume |
| ILM | Internal limiting membrane |
| IPL | Inner plexiform layer |
| INL | Inner nuclear layer |
| VD | Vessel area density |
| SAD | Skeleton area density |
| VAD | Vessel area density |
| VLD | Vessel length density |
| TVL | Total vessel length |
| MD | Mean vessel diameter |
| MEDD | Median vessel diameter |
| BD | Branchpoint density |
| MTO | Mean tortuosity |
| IQR | Interquartile range |
| SD | Standard deviation |
| BMI | Body mass index |
| EZ | Ellipsoid zone |
References
- Sakini, A.S.A.; Hamid, A.K.; Alkhuzaie, Z.A.; Al-Aish, S.T.; Al-Zubaidi, S.; Tayem, A.A.; Alobi, M.A.; Sakini, A.S.A.; Al-Aish, R.T.; Al-Shami, K.; et al. Diabetic macular edema (DME): Dissecting pathogenesis, prognostication, diagnostic modalities along with current and futuristic therapeutic insights. Int. J. Retin. Vitr. 2024, 10, 83. [Google Scholar] [CrossRef]
- Romero-Aroca, P.; Navarro-Gil, R.; Valls-Mateu, A.; Sagarra-Alamo, R.; Moreno-Ribas, A.; Soler, N. Differences in incidence of diabetic retinopathy between type 1 and 2 diabetes mellitus: A nine-year follow-up study. Br. J. Ophthalmol. 2017, 101, 1346–1351. [Google Scholar] [CrossRef]
- Luttrull, J.K.; Sinclair, S.H. Safety of Transfoveal Subthreshold Diode Micropulse Laser for Fovea-Involving Diabetic Macular Edema in Eyes with Good Visual Acuity. Retina 2014, 34, 2010–2020. [Google Scholar] [CrossRef]
- Sabal, B.; Teper, S.; Wylęgała, E. Subthreshold Micropulse Laser for Diabetic Macular Edema: A Review. J. Clin. Med. 2023, 12, 274. [Google Scholar] [CrossRef] [PubMed]
- Jorge, E.C.; Jorge, E.N.; Botelho, M.; Farat, J.G.; Virgili, G.; El Dib, R. Monotherapy laser photocoagulation for diabetic macular oedema. Cochrane Database Syst. Rev. 2018, 10, CD010859. [Google Scholar] [CrossRef] [PubMed]
- Schmidt-Erfurth, U.; Garcia-Arumi, J.; Bandello, F.; Berg, K.; Chakravarthy, U.; Gerendas, B.S.; Jonas, J.; Larsen, M.; Tadayoni, R.; Loewenstein, A. Guidelines for the Management of Diabetic Macular Edema by the European Society of Retina Specialists (EURETINA). Ophthalmologica 2017, 237, 185–222. [Google Scholar] [CrossRef] [PubMed]
- Inagaki, K.; Hamada, M.; Ohkoshi, K. Minimally invasive laser treatment combined with intravitreal injection of anti-vascular endothelial growth factor for diabetic macular oedema. Sci. Rep. 2019, 9, 7585. [Google Scholar] [CrossRef]
- Akhlaghi, M.; Dehghani, A.; Pourmohammadi, R.; Asadpour, L.; Pourazizi, M. Effects of subthreshold diode micropulse laser photocoagulation on treating patients with refractory diabetic macular edema. J. Curr. Ophthalmol. 2019, 31, 157–160. [Google Scholar] [CrossRef]
- Altınel, G.M.; Acikalin, B.; Guler Alis, M.; Demir, G.; Mert Mutibayraktaroglu, K.; Melike Gedar Totuk, O.; Ardagil, A. Comparison of the efficacy and safety of anti-VEGF monotherapy versus anti-VEGF therapy combined with subthreshold micropulse laser therapy for diabetic macular edema. Lasers Med. Sci. 2021, 36, 1545–1553. [Google Scholar] [CrossRef]
- El Matri, L.; Chebil, A.; El Matri, K.; Falfoul, Y.; Chebbi, Z. Subthreshold micropulse laser adjuvant to bevacizumab versus bevacizumab monotherapy in treating diabetic macular edema: One-year-follow-up. Ther. Adv. Ophthalmol. 2021, 13, 25158414211040887. [Google Scholar] [CrossRef]
- Bıçak, F.; Kayıkçıoğlu, Ö.R.; Altınışık, M.; Doğruya, S.; Kurt, E. Efficacy of subthreshold micropulse laser combined with ranibizumab in the treatment of diabetic macular edema. Int. Ophthalmol. 2022, 42, 3829–3836. [Google Scholar] [CrossRef] [PubMed]
- Khattab, A.M.; Hagras, S.M.; AbdElhamid, A.H.; Torky, M.A.; Awad, E.A.; Abdelhameed, A.G. Aflibercept with adjuvant micropulsed yellow laser versus aflibercept monotherapy in diabetic macular edema. Graefes Arch. Clin. Exp. Ophthalmol. 2019, 257, 1373–1380. [Google Scholar] [CrossRef] [PubMed]
- Boscia, F.; Veritti, D.; Iaculli, C.; Lattanzio, R.; Freda, S.; Piergentili, B.; Varano, M. Management of treatment-naïve diabetic macular edema patients: Review of real-world clinical data. Eur. J. Ophthalmol. 2024, 34, 1675–1694. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.K.; Jampol, L.M. The Diabetic Retinopathy Clinical Research Network (DRCR.Net) and Its Contributions to the Treatment of Diabetic Retinopathy. Ophthalmic Res. 2019, 62, 225–230. [Google Scholar] [CrossRef]
- Hudson, C.; Flanagan, J.G.; Turner, G.S.; Chen, H.C.; Young, L.B.; McLeod, D. Influence of laser photocoagulation for clinically significant diabetic macular oedema (DMO) on short-wavelength and conventional automated perimetry. Diabetologia 1998, 41, 1283–1292. [Google Scholar] [CrossRef]
- Vujosevic, S.; Bottega, E.; Casciano, M.; Pilotto, E.; Convento, E.; Midena, E. Microperimetry and Fundus Autofluorescence in Diabetic Macular Edema. Subthreshold Micropulse Diode Laser Versus Modified Early Treatment Diabetic Retinopathy Study Laser Photocoagulation. Retina 2010, 30, 908–916. [Google Scholar] [CrossRef]
- Fazel, F.; Bagheri, M.; Golabchi, K.; Jahanbani Ardakani, H. Comparison of subthreshold diode laser micropulse therapy versus conventional photocoagulation laser therapy as primary treatment of diabetic macular edema. J. Curr. Ophthalmol. 2016, 28, 206–211. [Google Scholar] [CrossRef]
- Bougatsou, P.; Panagiotopoulou, E.K.; Gkika, M.; Dardabounis, D.; Konstantinidis, A.; Sideroudi, H.; Perente, I.; Labiris, G. Comparison of Subthreshold 532 nm Diode Micropulse Laser with Conventional Laser Photocoagulation in the Treatment of Non-Centre Involved Clinically Significant Diabetic Macular Edema. Acta Medica 2020, 63, 25–30. [Google Scholar] [CrossRef]
- Lavinsky, D.; Cardillo, J.A.; Melo, L.A.S.; Dare, A.; Farah, M.E.; Belfort, R. Randomized clinical trial evaluating mETDRS versus normal or high-density micropulse photocoagulation for diabetic macular edema. Investig. Ophthalmol. Vis. Sci. 2011, 52, 4314–4323. [Google Scholar] [CrossRef]
- Venkatesh, P.; Ramanjulu, R.; Azad, R.; Vohra, R.; Garg, S. Subthreshold micropulse diode laser and double frequency neodymium: YAG laser in treatment of diabetic macular edema: A prospective, randomized study using multifocal electroretinography. Photomed. Laser Surg. 2011, 29, 727–733. [Google Scholar] [CrossRef]
- Chhablani, J.; Alshareef, R.; Kim, D.T.; Narayanan, R.; Goud, A.; Mathai, A. Comparison of different settings for yellow subthreshold laser treatment in diabetic macular edema. BMC Ophthalmol. 2018, 18, 168. [Google Scholar] [CrossRef] [PubMed]
- Lois, N.; Campbell, C.; Waugh, N.; Azuara-Blanco, A.; Maredza, M.; Mistry, H.; Mcauley, D.; Acharya, N.; Aslam, T.M.; Bailey, C.; et al. Diabetic Macular Edema and Diode Subthreshold Micropulse Laser A Randomized Double-Masked Noninferiority Clinical Trial. Ophthalmology 2023, 130, 14–27. [Google Scholar] [CrossRef]
- Waheed, N.K.; Rosen, R.B.; Jia, Y.; Munk, M.R.; Huang, D.; Fawzi, A.; Chong, V.; Nguyen, Q.D.; Sepah, Y.; Pearce, E. Optical coherence tomography angiography in diabetic retinopathy. Prog. Retin. Eye Res. 2023, 97, 101206. [Google Scholar] [CrossRef]
- Shiihara, H.; Terasaki, H.; Sonoda, S.; Kakiuchi, N.; Shinohara, Y.; Tomita, M.; Sakamoto, T. Objective evaluation of size and shape of superficial foveal avascular zone in normal subjects by optical coherence tomography angiography. Sci. Rep. 2018, 8, 10143. [Google Scholar] [CrossRef] [PubMed]
- Parravano, M.; Cennamo, G.; Di Antonio, L.; Grassi, M.O.; Lupidi, M.; Rispoli, M.; Savastano, M.C.; Veritti, D.; Vujosevic, S. Multimodal imaging in diabetic retinopathy and macular edema: An update about biomarkers. Surv. Ophthalmol. 2024, 69, 893–904. [Google Scholar] [CrossRef]
- Sim, D.A.; Keane, P.A.; Fung, S.; Karampelas, M.; Sadda, S.R.; Fruttiger, M.; Patel, P.J.; Tufail, A.; Egan, C.A. Quantitative analysis of diabetic macular ischemia using optical coherence tomography. Investig. Ophthalmol. Vis. Sci. 2014, 55, 417–423. [Google Scholar] [CrossRef] [PubMed]
- Takamura, Y.; Yamada, Y.; Inatani, M. Role of Microaneurysms in the Pathogenesis and Therapy of Diabetic Macular Edema: A Descriptive Review. Medicina 2023, 59, 435. [Google Scholar] [CrossRef] [PubMed]
- Ishibazawa, A.; Nagaoka, T.; Takahashi, A.; Omae, T.; Tani, T.; Sogawa, K.; Yokota, H.; Yoshida, A. Optical Coherence Tomography Angiography in Diabetic Retinopathy: A Prospective Pilot Study. Am. J. Ophthalmol. 2015, 160, 35–44.e1. [Google Scholar] [CrossRef]
- Parravano, M.; De Geronimo, D.; Scarinci, F.; Querques, L.; Virgili, G.; Simonett, J.M.; Varano, M.; Bandello, F.; Querques, G. Diabetic Microaneurysms Internal Reflectivity on Spectral-Domain Optical Coherence Tomography and Optical Coherence Tomography Angiography Detection. Am. J. Ophthalmol. 2017, 179, 90–96. [Google Scholar] [CrossRef]
- Gregori, N.Z.; Feuer, W.; Rosenfeld, P.J. Novel Method for Analyzing Snellen Visual Acuity Measurements. Retina 2010, 30, 1046–1050. [Google Scholar] [CrossRef]
- Untracht, G.R.; Matos, R.S.; Dikaios, N.; Bapir, M.; Durrani, A.K.; Butsabong, T.; Campagnolo, P.; Sampson, D.D.; Heiss, C.; Sampson, D.M. OCTAVA: An open-source toolbox for quantitative analysis of optical coherence tomography angiography images. PLoS ONE 2021, 16, e0261052. [Google Scholar] [CrossRef]
- Untracht, G.R.; Durkee, M.S.; Zhao, M.; Kwok-Cheung Lam, A.; Sikorski, B.L.; Sarunic, M.V.; Andersen, P.E.; Sampson, D.D.; Chen, F.K.; Sampson, D.M. Towards standardising retinal OCT angiography image analysis with open-source toolbox OCTAVA. Sci. Rep. 2024, 14, 5979. [Google Scholar] [CrossRef] [PubMed]
- Vujosevic, S.; Gatti, V.; Muraca, A.; Brambilla, M.; Villani, E.; Nucci, P.; Rossetti, L.; De Cilla’, S. Optical Coherence Tomography Angiography Changes After Subthreshold Micropulse Yellow Laser in Diabetic Macular Edema. Retina 2020, 40, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Vujosevic, S.; Toma, C.; Villani, E.; Brambilla, M.; Torti, E.; Leporati, F.; Muraca, A.; Nucci, P.; De Cilla, S. Subthreshold Micropulse Laser in Diabetic Macular Edema: 1-Year Improvement in OCT/OCT-Angiography Biomarkers. Transl. Vis. Sci. Technol. 2020, 9, 31. [Google Scholar] [CrossRef]
- Li, G.; Ho, M.; Li, S.; Chen, L.; Iu, L.; Cheung, C.Y.; Brelen, M.; Young, A.L. Comparing Functional and Vascular Layer Outcomes of Laser Photocoagulation Versus Subthreshold Micropulse Laser for Diabetic Macular Edema: An OCT-Angiography Study. Retina 2023, 43, 823–831. [Google Scholar] [CrossRef]
- Kikushima, W.; Furuhata, Y.; Shijo, T.; Matsumoto, M.; Sakurada, Y.; Viel Tsuru, D.; Kashiwagi, K. Comparison of one-year real-world outcomes between red (670 nm) subthreshold micropulse laser treatment and intravitreal aflibercept for treatment-naïve diabetic macular edema. Photodiagn. Photodyn. Ther. 2025, 51, 104430. [Google Scholar] [CrossRef] [PubMed]
- Miwa, Y.; Murakami, T.; Suzuma, K.; Uji, A.; Yoshitake, S.; Fujimoto, M.; Yoshitake, T.; Tamura, Y.; Yoshimura, N. Relationship between Functional and Structural Changes in Diabetic Vessels in Optical Coherence Tomography Angiography. Sci. Rep. 2016, 6, 29064. [Google Scholar] [CrossRef]
- Sorour, O.A.; Sabrosa, A.S.; Yasin Alibhai, A.; Arya, M.; Ishibazawa, A.; Witkin, A.J.; Baumal, C.R.; Duker, J.S.; Waheed, N.K. Optical coherence tomography angiography analysis of macular vessel density before and after anti-VEGF therapy in eyes with diabetic retinopathy. Int. Ophthalmol. 2019, 39, 2361–2371. [Google Scholar] [CrossRef]
- Santamaría, J.; Cobos, E.; Biarnes, M.; Caminal, J.M.; Rodriguez-Leor, R.; Morwani, R.; García-Mendieta, M.; Lorenzo, D.; García-Bru, P.; Arias, L. Changes in vessel density patterns assessed with OCTA in patients with diabetic macular edema treated with anti-VEGF therapy. Acta Diabetol. 2024, 61, 1385–1392. [Google Scholar] [CrossRef]
- Korobelnik, J.F.; Gaucher, D.; Baillif, S.; Creuzot-Garcher, C.; Kodjikian, L.; Weber, M. Optical Coherence Tomography Angiography in Diabetic Macular Edema Treated with Intravitreal Aflibercept: A 48-Week Observational Study (the DOCTA Study). Ophthalmologica 2023, 246, 71–80. [Google Scholar] [CrossRef]
- Hunt, M.; Wylęgała, A.; Wylęgała, E.; Teper, S. 1-Year Fixed-Regimen Bevacizumab Treatment in DME-Vascular Network Image Analysis in Optical Coherence Tomography Angiography Study. J. Clin. Med. 2022, 11, 2125. [Google Scholar] [CrossRef] [PubMed]
- Massengill, M.T.; Cubillos, S.; Sheth, N.; Sethi, A.; Lim, J.I. Response of Diabetic Macular Edema to Anti-VEGF Medications Correlates with Improvement in Macular Vessel Architecture Measured with OCT Angiography. Ophthalmol. Sci. 2024, 4, 100478. [Google Scholar] [CrossRef] [PubMed]
- Pongsachareonnont, P.; Charoenphol, P.; Hurst, C.; Somkijrungroj, T. The Effect of Anti-Vascular Endothelial Growth Factor on Retinal Microvascular Changes in Diabetic Macular Edema Using Swept-Source Optical Coherence Tomography Angiography. Clin. Ophthalmol. 2020, 14, 3871–3880. [Google Scholar] [CrossRef] [PubMed]
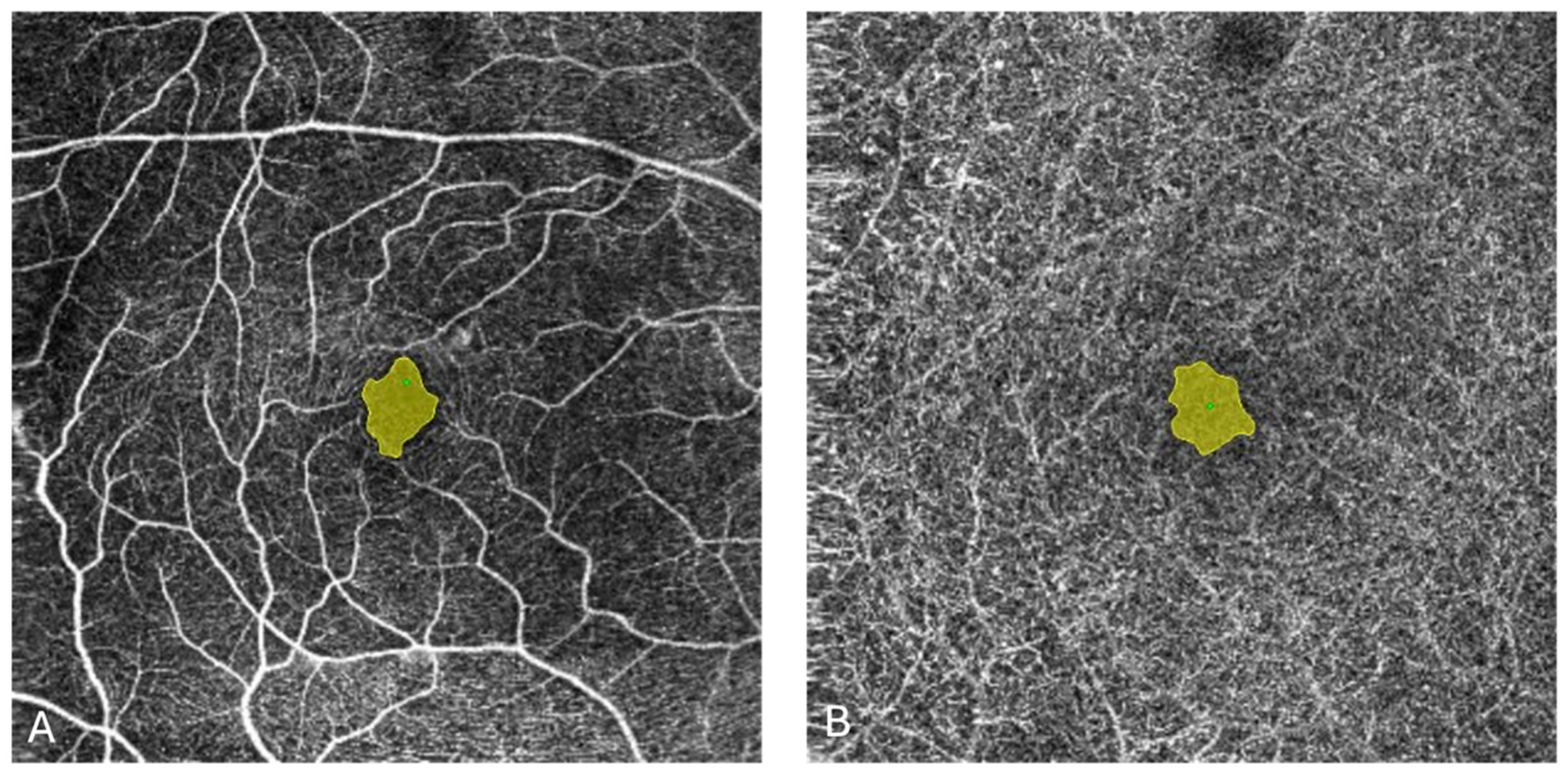
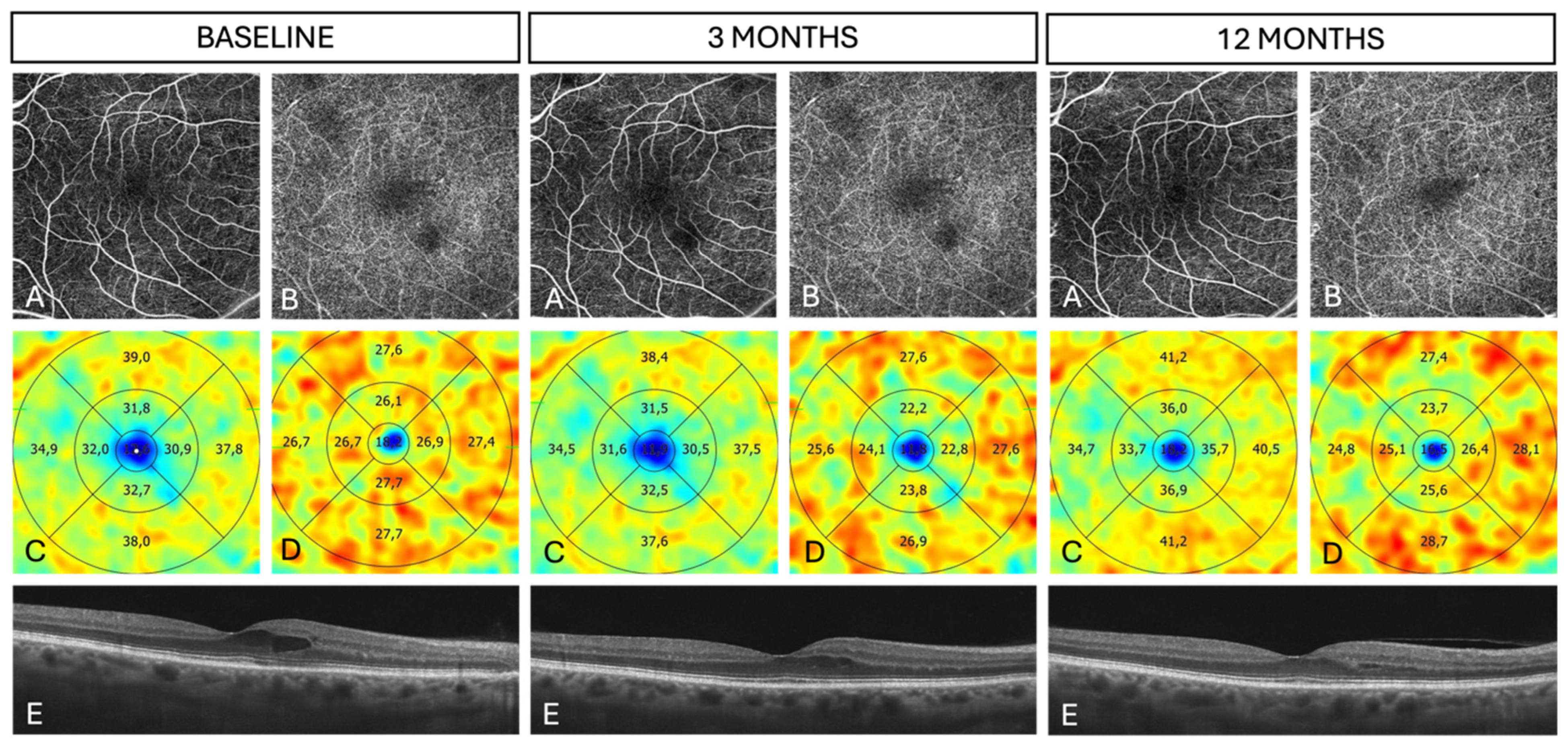
| Parameter | SMPL n = 33 | Sham n = 34 | Total n = 67 | p | |
|---|---|---|---|---|---|
| Age [years] | Mean ± SD | 63.88 ± 7.72 | 66.91 ± 8.51 | 65.42 ± 8.21 | p = 0.085 |
| Median (IQR) | 63 (59–70) | 67 (61.25–72) | 66 (59.5–71) | ||
| Sex | Female | 14 (42.42%) | 14 (41.18%) | 28 (41.79%) | p = 1 |
| Male | 19 (57.58%) | 20 (58.82%) | 39 (58.21%) | ||
| BMI | Normal weight | 4 (12.12%) | 7 (20.59%) | 11 (16.42%) | p = 0.62 |
| Overweight | 14 (42.42%) | 12 (35.29%) | 26 (38.81%) | ||
| Obesity | 15 (45.45%) | 15 (44.12%) | 30 (44.78%) | ||
| DM type | Type 1 | 2 (6.06%) | 8 (23.53%) | 10 (14.93%) | p = 0.083 |
| Type 2 | 31 (93.94%) | 26 (76.47%) | 57 (85.07%) | ||
| DM duration [years] | Mean ± SD | 17.42 ± 9.55 | 20.76 ± 10.55 | 19.12 ± 10.13 | p = 0.201 |
| Median (IQR) | 15 (10–23) | 20 (11.25–27) | 20 (10–26.5) | ||
| HbA1c [%] | Mean ± SD | 8.05 ± 1.55 | 7.8 ± 1.06 | 7.92 ± 1.32 | p = 0.754 |
| Median (IQR) | 7.9 (6.9–9) | 7.8 (7.2–8.6) | 7.8 (6.9–8.9) | ||
| Parameter | Group | n | Baseline | n | 3 Months | n | 12 Months | p |
|---|---|---|---|---|---|---|---|---|
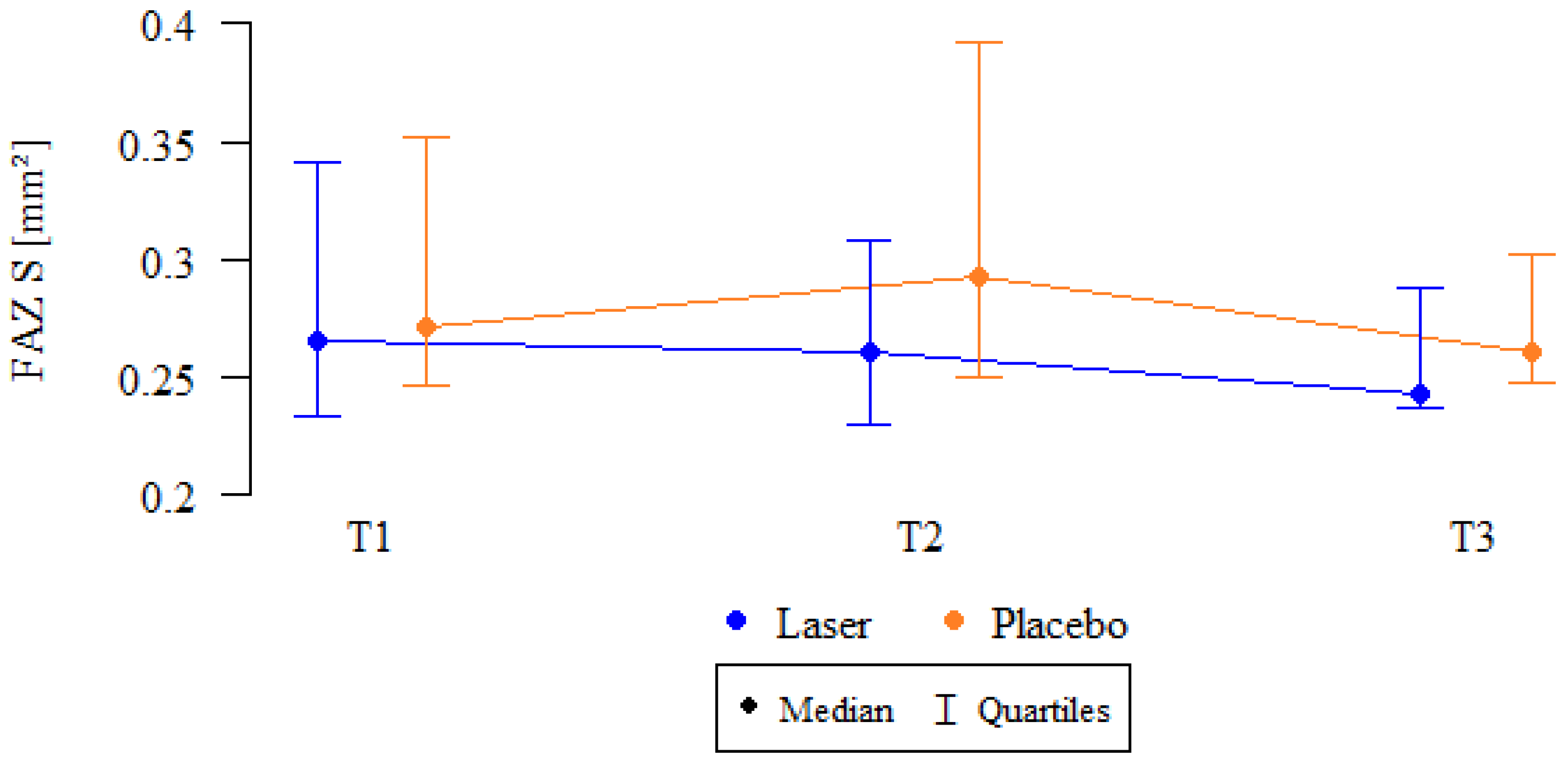
| FAZ SCP (µm2) | SMPL | 31/33 | 298 ± 85 | 30/33 | 283 ± 83 | 26/28 | 276 ± 79 | 0.435 |
| 266 (233–342) | 260 (230–309) | 243 (238–288) | ||||||
| Sham | 31/34 | 332 ± 211 | 32/34 | 351 ± 209 | 19/20 | 324 ± 239 | 0.494 | |
| 271 (246–352) | 293 (250–392) | 261 (248–302) | ||||||
| FAZ DCP (µm2) | SMPL | 24/33 | 393 ± 140 | 25/33 | 393 ± 146 | 22/28 | 366 ± 96 | 0.182 |
| 386 (293–441) | 344 (282–446) | 340 (308–398) | ||||||
| Sham | 25/34 | 434 ± 241 | 25/34 | 456 ± 258 | 12/20 | 442 ± 340 | 0.196 | |
| 384 (331–446) | 406 (342–457) | 346 (310–395) | ||||||
| MA SCP | SMPL | 31/33 | 2.87 ± 2.54 | 32/33 | 3.25 ± 4.03 | 27/28 | 2.93 ± 2.32 | 0.697 |
| 2 (1–4) | 2 (1–3.25) | 2 (1–4) | ||||||
| Sham | 33/34 | 3.64 ± 3.4 | 33/34 | 3.97 ± 3.42 | 20/20 | 3 ± 2.10 | 0.157 | |
| 3 (1–4) | 3 (1–5) | 2 (1–5) | ||||||
| MA DCP | SMPL | 31/33 | 9.29 ± 9.02 | 32/33 | 8.97 ± 9.03 | 26/28 | 9.04 ± 9.37 | 0.53 |
| 7 (3–11.5) | 5.5 (3–9.25) | 4.5 (3.25–13) | ||||||
| Sham | 32/34 | 9.41 ± 9.15 | 32/34 | 10.12 ± 9.76 | 18/20 | 7.61 ± 7.06 | 0.684 | |
| 4.5 (3–14.5) | 6 (3–14.75) | 4.5 (3–9) | ||||||
| VD SCP (%) | SMPL | 27/33 | 36.68 ± 1.66 | 26/33 | 36.03 ± 1.66 | 22/28 | 35.73 ± 2.01 | 0.167 |
| 37 (34.95–37.6) | 36 (35.02–36.62) | 36.25 (34.9–37.27) | ||||||
| Sham | 24/34 | 34.94 ± 2.04 | 25/34 | 34.22 ±2.65 | 14/20 | 34.81 ± 2.54 | 0.368 | |
| 34.95 (33.3–36.7) | 34.6 (32.1–36.2) | 35 (33.3–36.45) | ||||||
| SAD SCP (%) | SMPL | 27/33 | 25.89 ± 1.21 | 26/33 | 25.74 ± 1.85 | 22/28 | 25.4 ± 1.56 | 0.842 |
| 26 (25.15–26.8) | 25.75 (24.85–26.45) | 25.8 (25.15–26.3) | ||||||
| Sham | 24/34 | 24.92 ± 2.89 | 25/34 | 24.25 ± 1.7 | 14/20 | 24.36 ± 1.76 | 0.495 | |
| 24.2 (23.58–25.88) | 24.7 (22.6–25.2) | 24.7 (22.68–25.77) |
| Parameter | Group | Baseline Values | Change in 3 Months | Change in 12 Months | |||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | p | Mean | p | Mean | p | |||
| VAD (%) | SMPL | 21.71 ± 2.26 | 21 (20–23) | 0.223 | 0.71 ± 1.9 | 0.866 | −0.56 ± 1.55 | 0.961 | |
| Sham | 20.55 ± 1.63 | 21 (19.5–21) | 0.64 ± 1.21 | −0.5 ± 1.29 | |||||
| VLD (%) | SMPL | 2.35 ± 0.25 | 2.3 (2.14–2.42) | 0.346 | 0.06 ± 0.18 | 0.925 | −0.05 ± 0.19 | 0.704 | |
| Sham | 2.27 ± 0.17 | 2.28 (2.14–2.37) | 0.06 ± 0.14 | −0.08 ± 0.14 | |||||
| TVL (mm) | SMPL | 23.49 ± 2.55 | 22.98 (21.4–24.24) | 0.329 | 0.47 ± 1.8 | 0.832 | −1.66 ± 5.6 | 0.682 | |
| Sham | 21.81 ± 3.95 | 22.8 (21.26–23.66) | 1.53 ± 4.24 | −0.8 ± 1.35 | |||||
| MD (µm) | SMPL | 5.12 ± 0.33 | 5 (5–5) | 0.67 | 0 ± 0.35 | 0.597 | 0.12 ± 0.5 | 0.635 | |
| Sham | 5.18 ± 0.4 | 5 (5–5) | 0.09 ± 0.54 | 0 ± 0 | |||||
| MEDD (µm) | SMPL | 5.29 ± 0.47 | 5 (5–6) | 0.411 | 0.12 ± 0.6 | 0.515 | 0.38 ± 1.36 | 0.747 | |
| Sham | 5.45 ± 0.52 | 5 (5–6) | 0.27 ± 0.65 | 0 ± 0.82 | |||||
| BD (nodes/mm) | SMPL | 10.79 ± 1.64 | 10.6 (10.11–11.64) | 0.384 | −0.51 ± 2.75 | 0.746 | −0.69 ± 1.98 | 1 | |
| Sham | 9.51 ± 2.85 | 10.6 (9.55–10.77) | 0.82 ± 2.8 | −0.13 ± 0.17 | |||||
| MTO | SMPL | 0.12 ± 0.01 | 0.12 (0.11–0.12) | 0.045 * | 0 ± 0.01 | 0.334 | 0 ± 0.01 | 0.578 | |
| Sham | 0.12 ± 0.01 | 0.13 (0.12–0.13) | 0 ± 0.01 | 0 ± 0 | |||||
| Parameter | Group | N | Baseline | n | 3 Months | n | 12 Months | p |
|---|---|---|---|---|---|---|---|---|
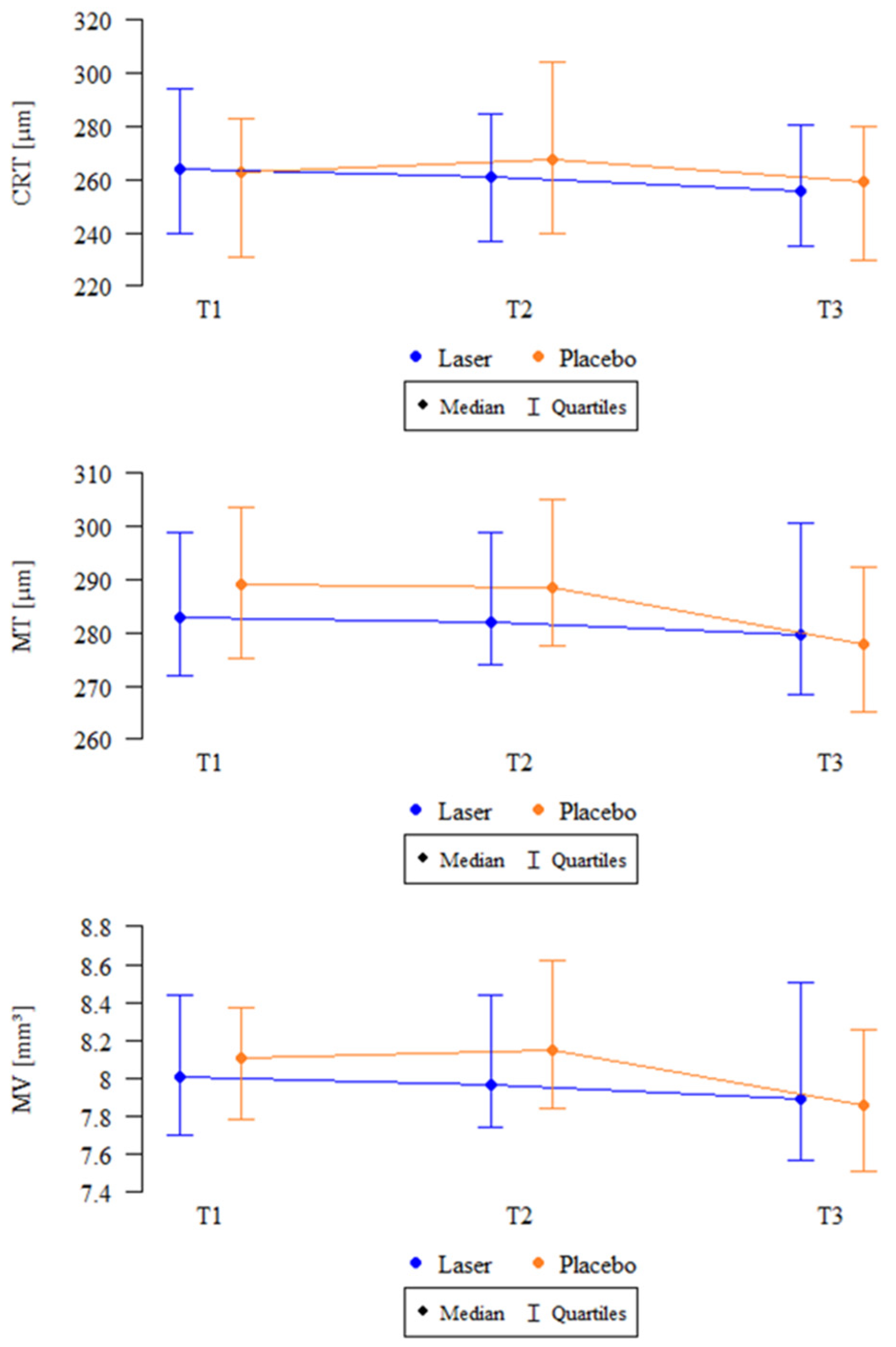
| CRT (µm) | SMPL | 33 | 264.76 ± 31.11 264 (240–294) | 33 | 259.91 ± 28.33 261 (237–285) | 28 | 256.32 ± 30.90 255.5 (235–280.5) | 0.023 * T2 > T3 |
| Sham | 34 | 261.41 ± 33.9 263 (231–283) | 34 | 276.32 ± 51.17 267.5 (240–304.5) | 20 | 254.5 ± 34.16 259.5 (229.75–280) | 0.669 | |
| MT (µm) | SMPL | 33 | 287.27 ± 19.02 283 (272–299) | 33 | 287.33 ± 20.55 282 (274–299) | 28 | 285 ± 22.28 279.5 (268.25–300.75) | 0.006 * T2 > T3 |
| Sham | 34 | 287.97 ± 21.62 289 (275.25–303.5) | 34 | 292.04 ± 26.87 288.5 (277.5–305) | 20 | 282.65 ± 28.6 278 (265.25–292.5) | 0.238 | |
| MV (mm2) | SMPL | 33 | 8.12 ± 0.54 8.01 (7.7–8.44) | 33 | 8.12 ± 0.58 7.97 (7.74–8.44) | 28 | 8.06 ± 0.63 7.89 (7.57–8.51) | 0.007 * T2 > T3 |
| Sham | 34 | 8.12 ± 0.61 8.11 (7.78–8.38) | 34 | 8.26 ± 0.76 8.14 (7.84–8.62) | 20 | 7.99 ± 0.81 7.86 (7.51–8.26) | 0.311 |
| Group | Time | n | Mean ± SD | Median (IQR) | p |
|---|---|---|---|---|---|
| SMPL | Baseline | 33 | 82.97 ± 2.16 | 83 (81–85) | p = 0.003 * T2 > T1 |
| 3 months | 33 | 83.3 ± 4.74 | 85 (83–85) | ||
| 12 months | 28 | 83.5 ± 4.06 | 85 (83–85) | ||
| Sham | Baseline | 34 | 82.88 ± 2.21 | 83 (80–85) | p = 0.423 |
| 3 months | 34 | 81.82 ± 2.98 | 82.5 (80–84.75) | ||
| 12 months | 20 | 82.20 ± 3.62 | 84 (80–85) |
| Group | Time | n | Mean ± SD | Median (IQR) | p |
|---|---|---|---|---|---|
| SMPL | Baseline | 33 | 9.21 ± 11.70 | 5 (1–13) | p = 0.96 |
| 3 months | 33 | 9.36 ± 12.81 | 4 (2–12) | ||
| 12 months | 28 | 9.5 ± 11.03 | 4.5 (2–12.75) | ||
| Sham | Baseline | 33 | 10.97 ± 15.79 | 4 (2–11) | p = 0.721 |
| 3 months | 34 | 14.06 ± 18.59 | 6 (1.25–17.25) | ||
| 12 months | 20 | 8.05 ± 11.98 | 3 (2–7) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sabal, B.; Wylęgała, E.; Teper, S. Impact of Subthreshold Micropulse Laser on the Vascular Network in Diabetic Macular Edema: An Optical Coherence Tomography Angiography Study. Biomedicines 2025, 13, 1194. https://doi.org/10.3390/biomedicines13051194
Sabal B, Wylęgała E, Teper S. Impact of Subthreshold Micropulse Laser on the Vascular Network in Diabetic Macular Edema: An Optical Coherence Tomography Angiography Study. Biomedicines. 2025; 13(5):1194. https://doi.org/10.3390/biomedicines13051194
Chicago/Turabian StyleSabal, Barbara, Edward Wylęgała, and Sławomir Teper. 2025. "Impact of Subthreshold Micropulse Laser on the Vascular Network in Diabetic Macular Edema: An Optical Coherence Tomography Angiography Study" Biomedicines 13, no. 5: 1194. https://doi.org/10.3390/biomedicines13051194
APA StyleSabal, B., Wylęgała, E., & Teper, S. (2025). Impact of Subthreshold Micropulse Laser on the Vascular Network in Diabetic Macular Edema: An Optical Coherence Tomography Angiography Study. Biomedicines, 13(5), 1194. https://doi.org/10.3390/biomedicines13051194







