1. Introduction
Endometrial carcinoma (EC) is the most prevalent gynecological malignancy worldwide, with approximately 417,000 new cases diagnosed annually [
1,
2]. Advances in surgical resection, radiotherapy, and chemotherapy have improved outcomes for early-stage EC. However, the prognosis for patients with advanced or recurrent disease remains poor due to the lack of effective targeted therapies [
3,
4,
5]. This gap highlights the critical need to identify molecular biomarkers and therapeutic targets to enhance diagnostic accuracy, prognostic assessment, and treatment outcomes.
Among the molecular pathways implicated in EC, the PI3K-AKT signaling pathway plays a central role in tumorigenesis. Aberrant activation of AKT drives cell cycle progression and proliferation by upregulating key proteins such as Cyclin D1 [
6,
7,
8]. Despite its therapeutic potential, clinical application of AKT inhibitors has been constrained by significant adverse effects, emphasizing the need to identify upstream regulators of AKT activation as alternative targets [
9].
Nucleoside Diphosphate-Linked Moiety X Motif 5 (NUDT5) is a hydrolase involved in maintaining nucleotide homeostasis and regulating cellular processes such as transcription elongation and DNA repair. Structurally, NUDT5 contains a conserved Nudix hydrolase domain critical for its enzymatic function [
10]. Previous research demonstrated that NUDT5 plays a crucial role in preventing oxidative DNA damage in TNBC cells [
11]. Recent research has also suggested a potential link between NUDT5 and hormone-driven cancers, such as breast cancer [
12]. Furthermore, a study utilizing whole-exome sequencing identified NUDT5 mutations in EC patients, suggesting that NUDT5 may be associated with the development and progression of EC [
13]. However, the precise molecular mechanisms by which NUDT5 interacts with signaling pathways in EC have yet to be fully elucidated. Investigating NUDT5’s function in EC could provide new insights into the molecular mechanisms driving tumor progression and identify therapeutic opportunities.
In this study, we aimed to investigate the expression patterns, clinical relevance, and functional role of NUDT5 in EC. By integrating analyses of public datasets with tissue microarray validation, we assessed the association between NUDT5 expression and clinical outcomes. Additionally, we explored the molecular mechanisms underlying NUDT5-mediated tumorigenesis, focusing on its interaction with the PI3K-AKT signaling pathway. Our findings establish NUDT5 as a key oncogene and therapeutic target in EC, paving the way for novel diagnostic and treatment strategies.
2. Materials and Methods
2.1. Public Datasets and Bioinformatics Analysis
Gene expression data for endometrial cancer (EC) were retrieved from The Cancer Genome Atlas (TCGA) (
https://portal.gdc.cancer.gov) and analyzed using R (version 4.3.1). Differential gene expression analysis was performed using the ‘DESeq2’ package (version 1.40.2). The cutoff thresholds for significance were set as |Fold Change| > 1 and
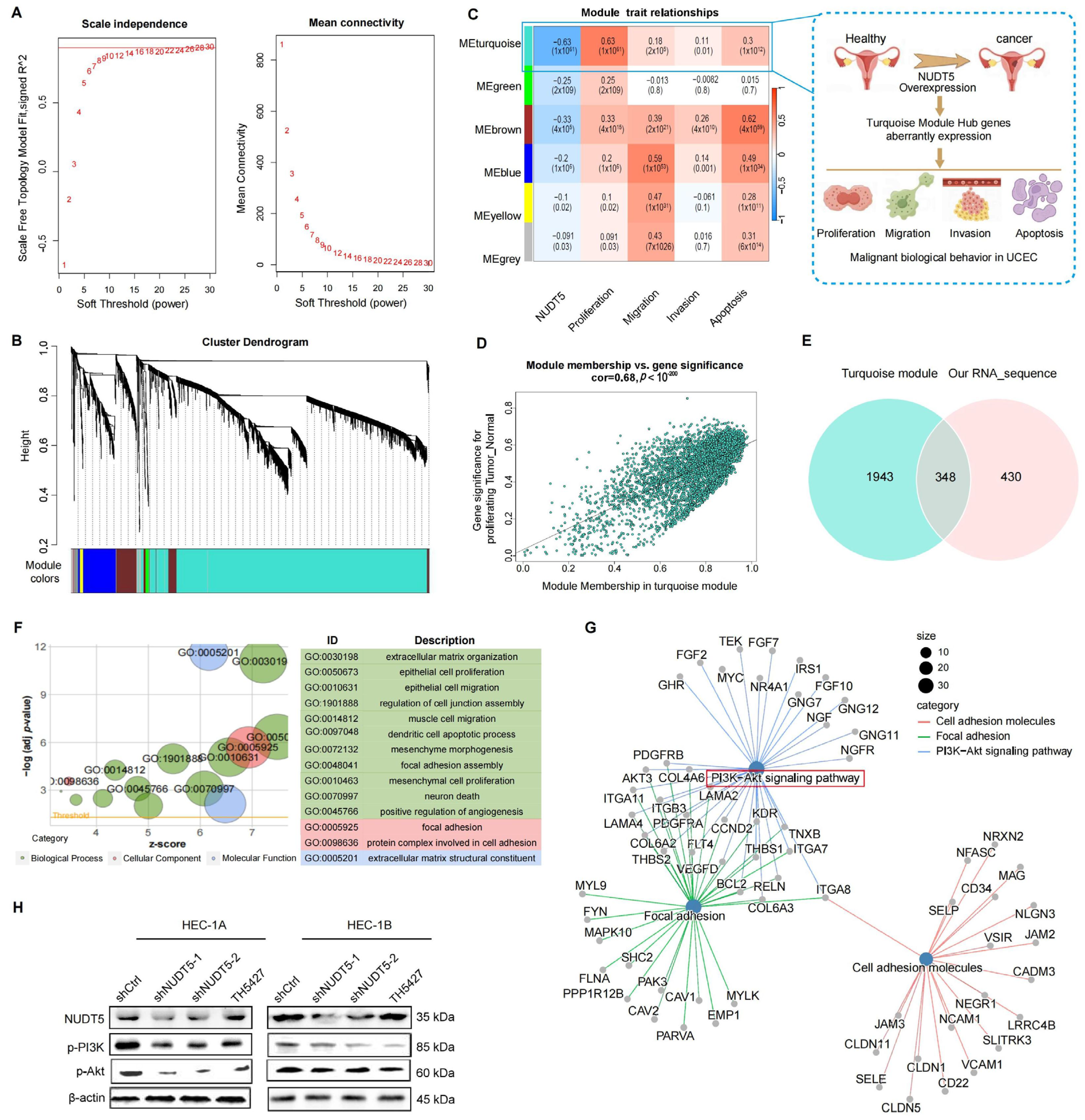
p-value < 0.05. To account for multiple testing, False Discovery Rate was controlled using the Benjamini–Hochberg method, with an FDR threshold of 0.05. Gene Ontology (GO) analysis was conducted using the ‘clusterProfiler’ package, and survival analysis was performed using the ‘survminer’ package. Forest plots were generated with the ‘forestplot’ package. Gene set scoring was performed using the GSWA package, which informed subsequent weighted gene co-expression network analysis (WGCNA). Hub genes from the turquoise module were further analyzed for KEGG (Kyoto Encyclopedia of Genes and Genomes) and GO enrichment.
Protein expression data were sourced from the UALCAN database (
http://ualcan.path.uab.edu/) using CPTAC data to compare NUDT5 expression between tumor and corresponding normal tissues. Z-values were used for statistical evaluation. Gene Set Enrichment Analysis (GSEA) was performed using the MSigDB dataset (
https://www.gsea-msigdb.org/gsea/msigdb), with the ‘c2.cp.all.v2022.1.Hs.symbols.gmt’ dataset used to analyze the enrichment of differentially expressed genes (DEGs) between high and low NUDT5 expression groups. Enrichment analysis for KEGG and GO was conducted using the ‘clusterProfiler’ package, with |log2Fold Change| > 1 and
p < 0.05 as thresholds.
The Human Protein Atlas (
https://www.proteinatlas.org/) provides protein expression data and immunohistochemical staining images of normal and tumor tissues. NUDT5 protein expression was examined using the immunohistochemical data from the Human Protein Atlas.
2.2. Tissue Microarray
NUDT5 expression in EC tissues was evaluated using tissue microarrays, which included 276 EC tissue samples and 240 control samples from non-cancerous uterine tissues. Data were obtained from the Department of Obstetrics and Gynecology at Tianjin Medical University General Hospital, including patients who underwent surgery between January 2003 and December 2012. Inclusion criteria required pathological confirmation by at least two independent pathologists, and patients had no prior history of radiotherapy, chemotherapy, or other related adjuvant therapies before surgery. Exclusion criteria included concurrent malignant tumors in other parts of the body and incomplete medical records. All tissue samples used for the microarray were fresh-frozen to preserve the integrity of RNA, DNA, and proteins. The tissue microarray was constructed using standard frozen tissue core technology, where multiple tissue cores were included in each core block for high-throughput analysis. All experiments involving human subjects were conducted with the written consent of each participant. The study adhered to the Declaration of Helsinki and was approved by the Ethics Committee of Tianjin Medical University General Hospital (Ethics code: IRB-2019-KY-130).
2.3. Immunohistochemistry
Immunohistochemical staining was performed using the Leica BOND RX system (Leica Biosystems, Wetzlar, Germany). Tissue sections were dewaxed with Bond Dewax Solution and rehydrated. Antigen retrieval was performed with Leica Bond Epitope Retrieval Solution 2 for 20 min. Immunostaining was conducted using Bond Polymer Refine Detection, followed by incubation with primary antibodies for 15 min and secondary antibodies for 8 min. The primary antibody used was rabbit monoclonal anti-NUDT5 antibody(Abcam, ab129172, (Cambridge, UK)). The secondary antibody was HRP-conjugated goat anti-rabbit IgG (Abcam, ab6721). After washing, DAB staining was performed for 10 min, followed by nuclear staining and mounting.
The IRS scoring system was used to evaluate immunostaining intensity and cell positivity rate. The final NUDT5 score was calculated by multiplying the staining intensity score by the cell positivity rate score. The staining intensity was scored as follows: 0, no staining; 1, pale yellow; 2, brown-yellow; 3, brownish brown. The percentage of positively stained cells was scored as follows: 0–5% (0), 6–25% (1), 26–50% (2), 51–75% (3), 76–100% (4).
2.4. Cell Culture and Transfection
EC cell lines were purchased from ATCC (Manassas, VA, USA): HEC-1-A (RRID: CVCL_0293), HEC-1-B (RRID: CVCL_0294), ECC-1 (RRID: CVCL_7260), and Ishikawa (RRID: CVCL_2529). HEC-1-A cells were cultured in McCoy’s 5A medium, while HEC-1-B, ECC-1, and Ishikawa cells were cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS). All cell lines were authenticated within the past three years using short tandem repeat profiling every six months. Mycoplasma testing was regularly performed. The primers for NUDT5 overexpression were as follows: forward, AGGTCGACTCTAGAGGATCCCGCCACCATGGAGAGCCAAGAACCAACGG; reverse, TCCTTGTAGTCCATACCAAATTTCAAG. Transfection efficiency was determined by western blot (WB) and quantitative real-time PCR. In addition, TH5427, a validated NUDT5-targeting inhibitor, was used to treat shCtrl cells to assess the effects of NUDT5 inhibition. TH5427 (15 μM) and the AKT inhibitor Perifosine (5 μM) were applied to EC cells following the manufacturer’s instructions. The experimental groups in this study were as follows: shCtrl (empty plasmid control), SH1 (NUDT5 knockdown group 1), SH2 (NUDT5 knockdown group 2), TH5427 (TH5427-treated shCtrl cells), Vector (empty plasmid overexpression control), OE (NUDT5 overexpression group), and OE treated with an AKT inhibitor.
2.5. Western Blot
Cells were lysed in RIPA buffer supplemented with 1% phosphatase inhibitors and phenylmethylsulfonyl fluoride. Total protein was extracted and denatured in loading buffer for 15 min. A sample of 40 μg was loaded onto a 10% SDS-PAGE gel, separated, and transferred to a PVDF membrane (Leica Biosystems, Wetzlar, Germany). The membrane was blocked with 5% milk and incubated overnight with primary antibodies from Abcam (Cambridge, UK) and Cell Signaling Technology (Danvers, MA, USA). The following primary antibodies were used: NUDT5 (Abcam, ab129172), PI3 Kinase (Cell Signaling Technology, 4249), AKT (Cell Signaling Technology, 9272S), phospho-AKT (Cell Signaling Technology, 4060S), E-Cadherin (Cell Signaling Technology, 14472), N-Cadherin (Cell Signaling Technology, 14215S), Vimentin (Cell Signaling Technology, 5741), Caspase-3 (Cell Signaling Technology, 9662), Cyclin D1 (Cell Signaling Technology, 2922S), and β-actin (Abcam, ab8226) as the loading control. After washing with TBST, the membranes were incubated with secondary antibodies for two hours and visualized using ECL detection reagents. Quantification of WB bands was performed using ImageJ (version 1.53t, National Institutes of Health, Bethesda, MD, USA) densitometry analysis. The intensity of the target protein bands was normalized to the β-actin loading control to ensure accurate and consistent results. To ensure the reliability and consistency of the results, all experiments were conducted in three independent replicates.
2.6. Cell Proliferation Assays
EC cells were seeded in 96-well plates at a density of 500 cells per well. A total of 110 μL of CCK-8 working solution was added to each well according to the manufacturer’s instructions, and the cells were incubated at 37 °C for 2 h. Absorbance at 450 nm was measured using a microplate reader. For the colony formation assay, 500 cells were seeded in six-well plates and cultured for 14 days. Cells were fixed with 4% paraformaldehyde, stained with crystal violet, and observed under a microscope. Colonies containing more than 10 cells were counted and analyzed using ImageJ software (version 1.53t, National Institutes of Health, Bethesda, MD, USA). All experiments were performed in triplicate to ensure the reliability and consistency of the results.
2.7. Transwell Experiment
EC cells (300,000 cells) were seeded into Transwell chambers with or without Matrigel. Culture medium containing 10% FBS was added to the lower chambers. After 24 h of incubation, cells were fixed with 4% paraformaldehyde, and non-invading cells were removed. Invading cells were observed and counted under an inverted optical microscope and analyzed using ImageJ. All experiments were performed in triplicate to ensure result consistency.
2.8. Flow Cytometric Analysis
EC cells were seeded in six-well plates and incubated for 24 h. Cells were collected and processed according to the manufacturer’s instructions for apoptosis and cell cycle assays. Flow cytometric analysis was performed using Modfit 5.0 software. Each experiment was repeated three times to confirm the reliability of the results.
2.9. mRNA Sequencing
To investigate transcriptional changes following NUDT5 knockdown in HEC-1B cells, total mRNA was extracted and sent to Genewiz (Suzhou, China) for sequencing and preliminary analysis. R (version 4.3.1) was used for further data analysis and visualization, following methods described in the previous section.
2.10. Xenograft Nude Mouse Model
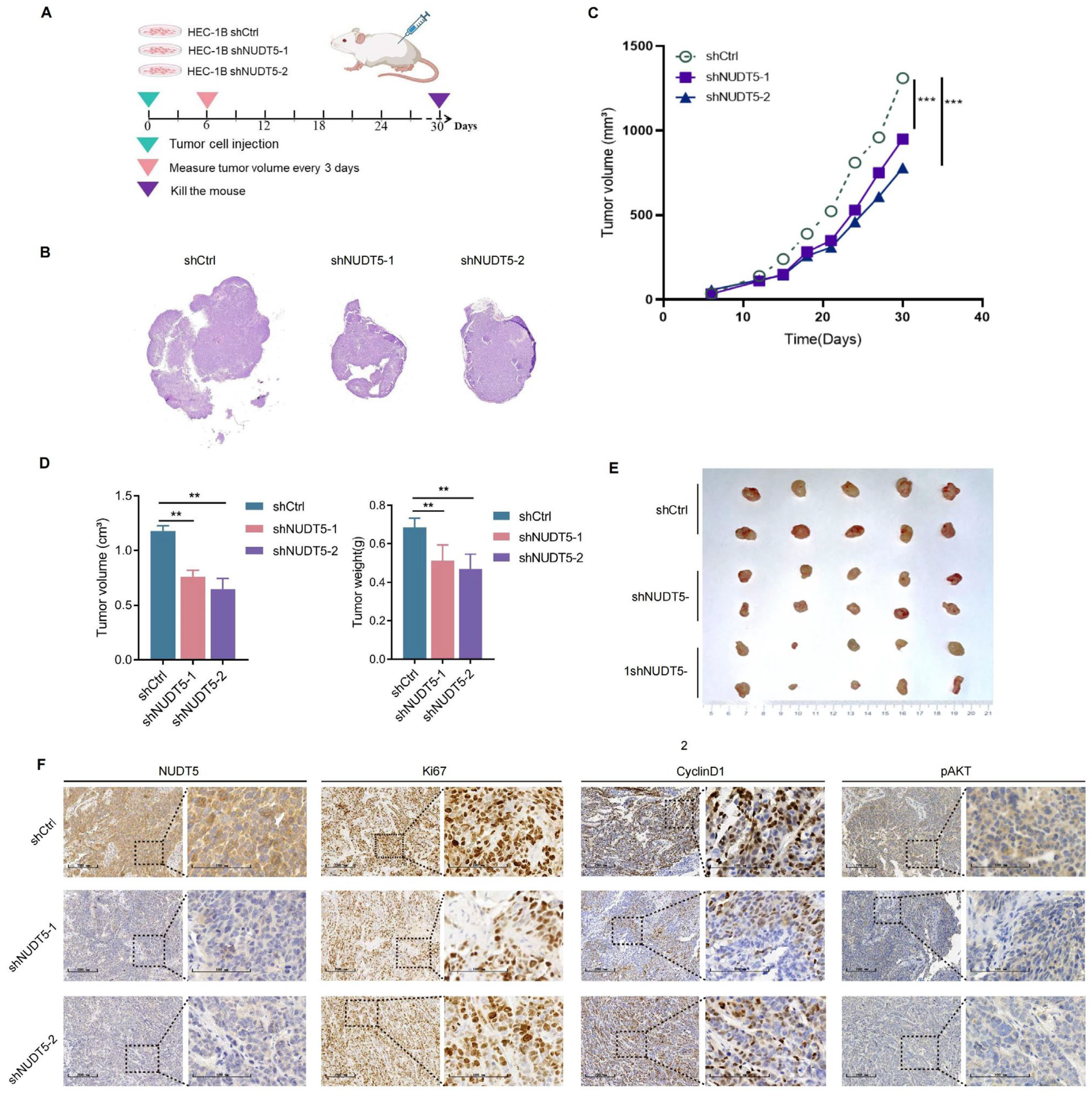
BALB/c-nu female nude mice were obtained from the Institute of Hematology, Chinese Academy of Sciences, and randomly assigned to six groups, with 10 mice per group (n = 10 per group, total n = 60): shCtrl (empty plasmid control), SH1 (NUDT5 knockdown group 1), SH2 (NUDT5 knockdown group 2), Vector (empty plasmid overexpression control), OE (NUDT5 overexpression group), and OE treated with the AKT inhibitor Perifosine. The sample size was determined based on previous literature and feasibility considerations to ensure sufficient statistical power. The animals were randomly assigned to treatment groups.
To establish xenograft models, 1 × 10⁶ HEC-1B cells were subcutaneously injected into the mice. Tumor growth and health were monitored daily. On day 7 post-injection, the AKT inhibitor group received Perifosine (30 mg/kg/day) orally, while the OE-NC and OE groups received equivalent volumes of saline. On day 30, mice were euthanized, and tumor volumes were calculated using the formula (Length × Width2)/2. Tumors were excised, photographed, and analyzed by immunohistochemistry.
All animal experiments were conducted in accordance with the guidelines set by the Animal Care and Use Committee of Tianjin Medical University (Ethics code: SYXK2016-0012).
2.11. Statistical Analysis
Experiments were performed in triplicate, and data are presented as mean ± SD. Statistical analyses were conducted using GraphPad Prism 8 (GraphPad Software, San Diego, CA, USA). Comparisons between groups were performed using two-tailed Student’s t-test for two groups, or one-way ANOVA followed by Tukey’s post hoc test for multiple groups. p < 0.05 was considered statistically significant.
4. Discussion
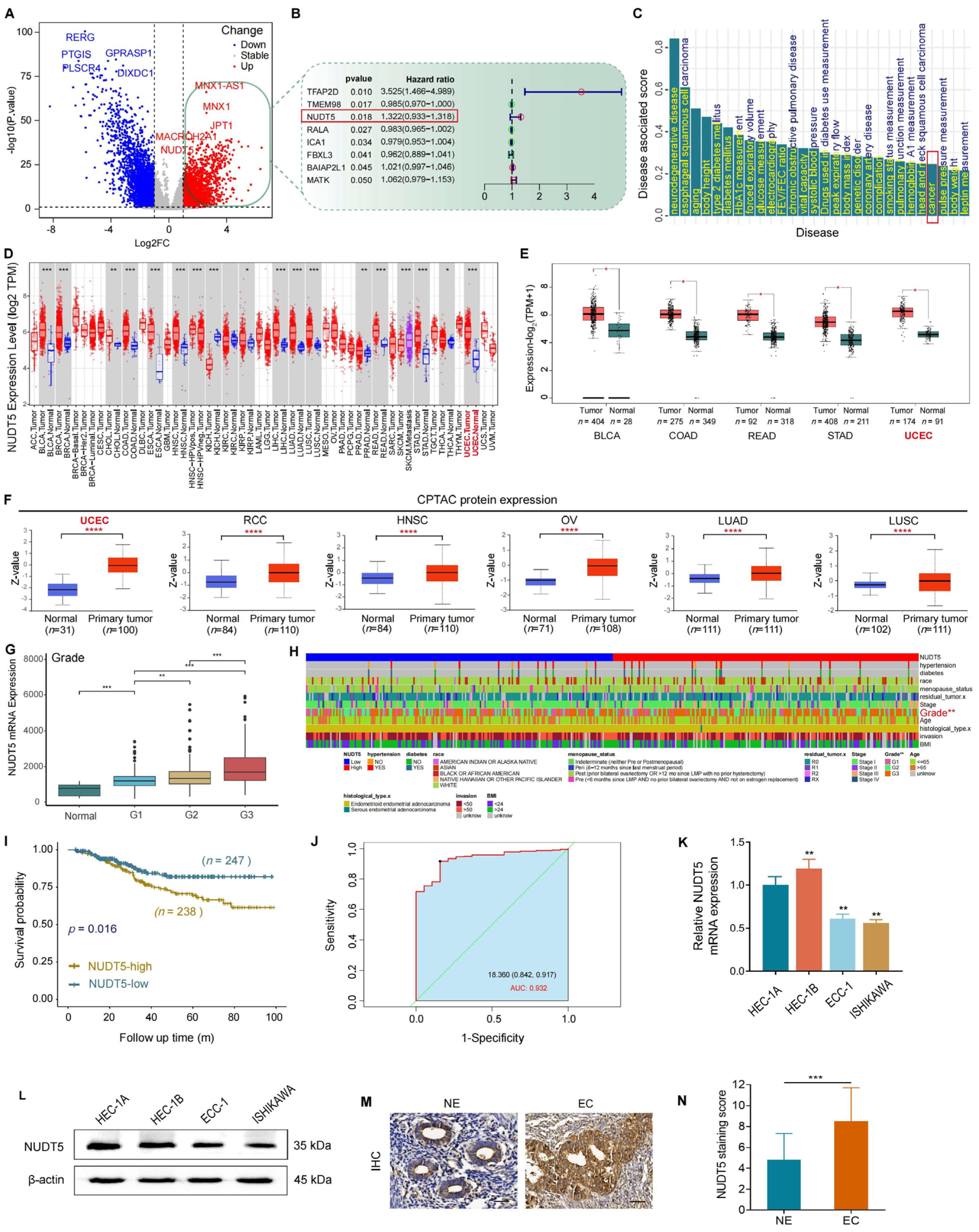
In the present study, we investigated the clinical relevance and biological function of NUDT5 in endometrial carcinoma (EC). We analyzed both publicly available datasets and our own tissue microarray data to explore the association between NUDT5 expression and clinicopathological features, as well as patient survival outcomes. We also performed functional experiments to assess the biological role of NUDT5 in EC cell lines. Our results indicate that NUDT5 is significantly upregulated in EC tissues, and its expression correlates with poorer clinical outcomes, highlighting its potential as a prognostic marker and therapeutic target in EC.
Our study confirmed the overexpression of NUDT5 at both the mRNA and protein levels in EC tissues compared to normal endometrial tissues. Tissue microarray analysis revealed a significant association between high NUDT5 expression and higher histological grades of EC, consistent with findings from public data analysis and earlier clinical studies in other cancers [
14,
15,
16]. Importantly, our Kaplan–Meier survival analysis demonstrated that elevated NUDT5 expression is associated with poorer overall survival, underscoring its potential as a prognostic marker in EC. These observations highlight the importance of integrating both transcriptomic and proteomic data when evaluating the clinical significance of potential biomarkers, as protein expression may provide more direct insights into tumor biology and prognosis.
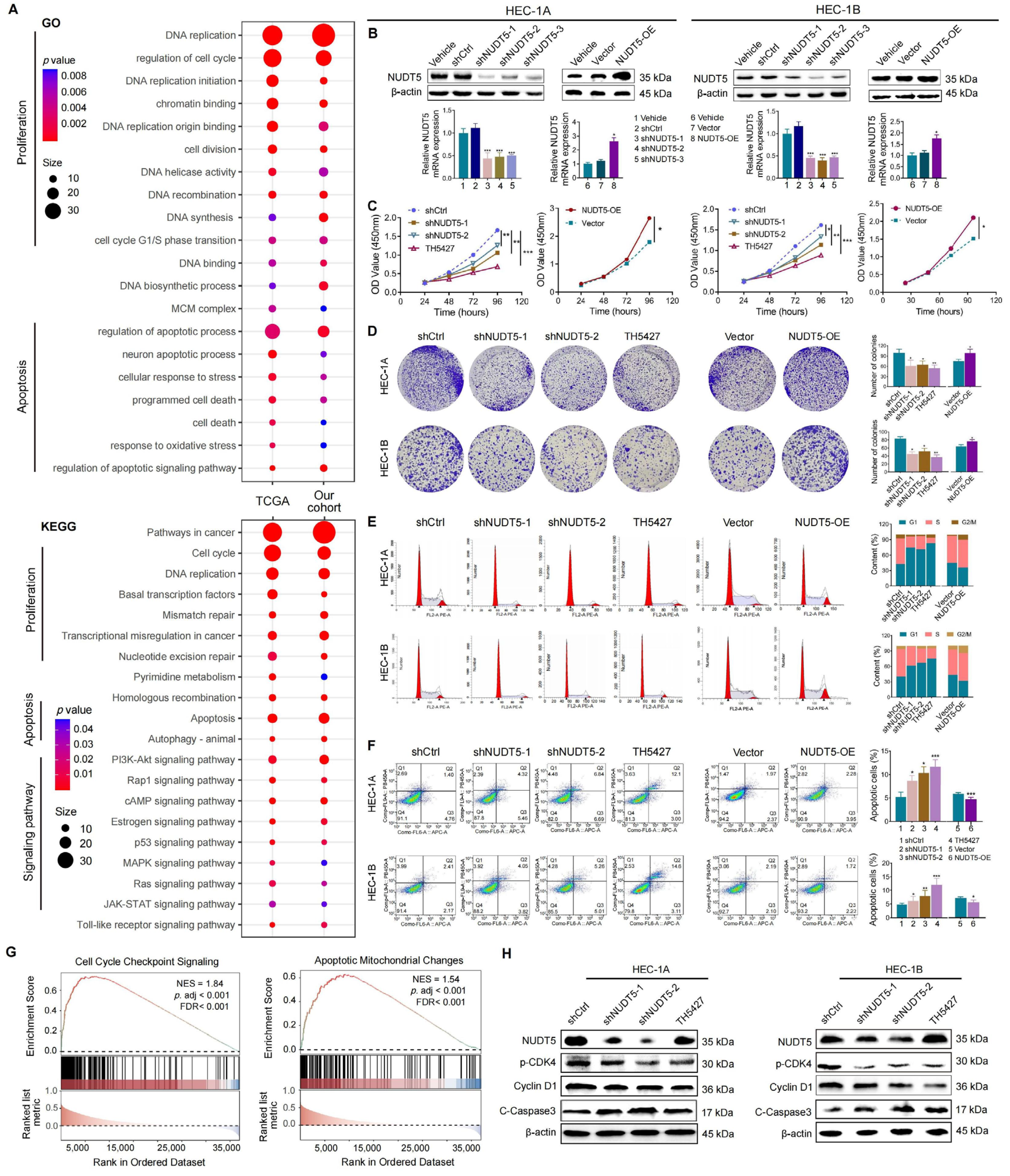
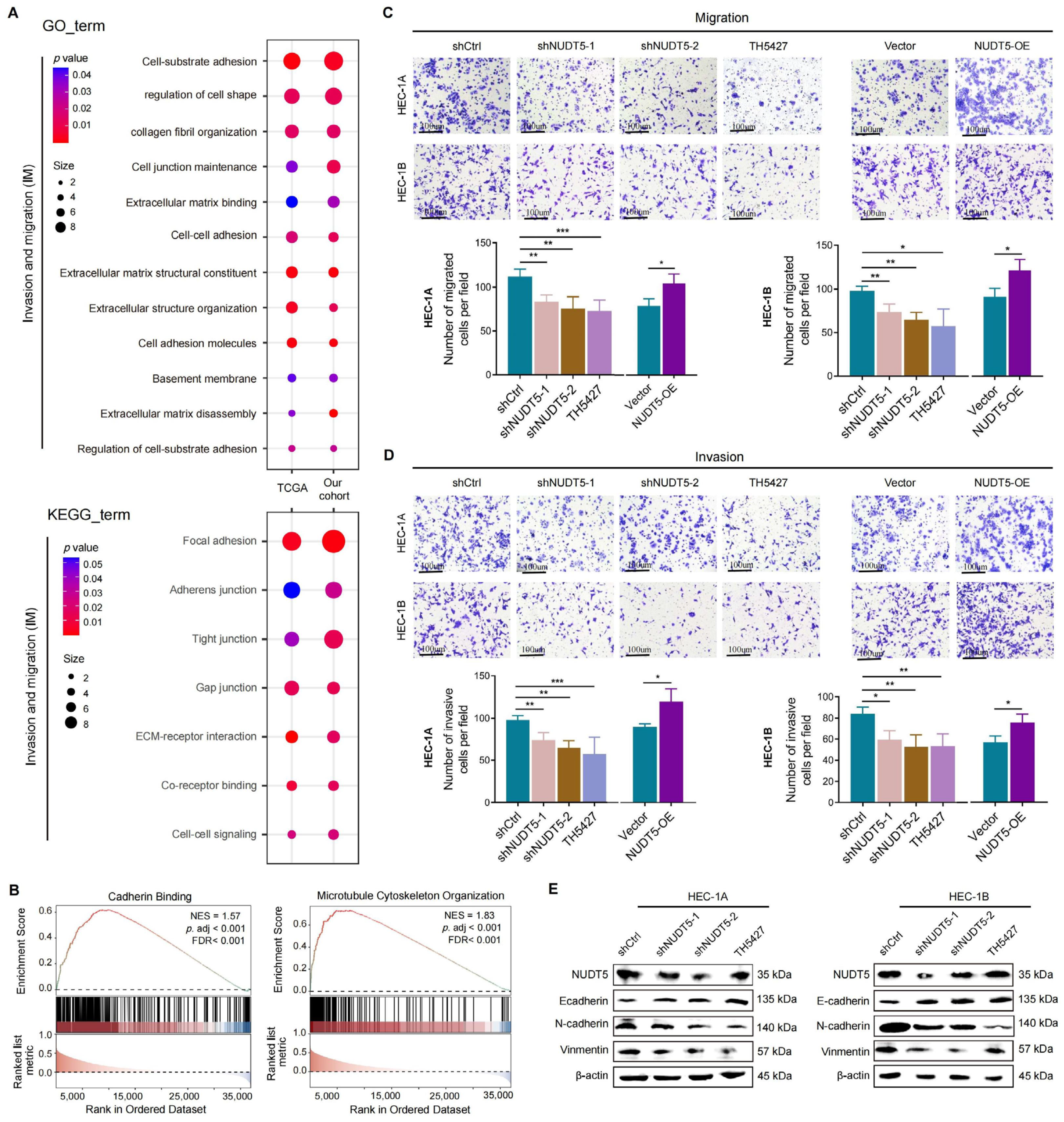
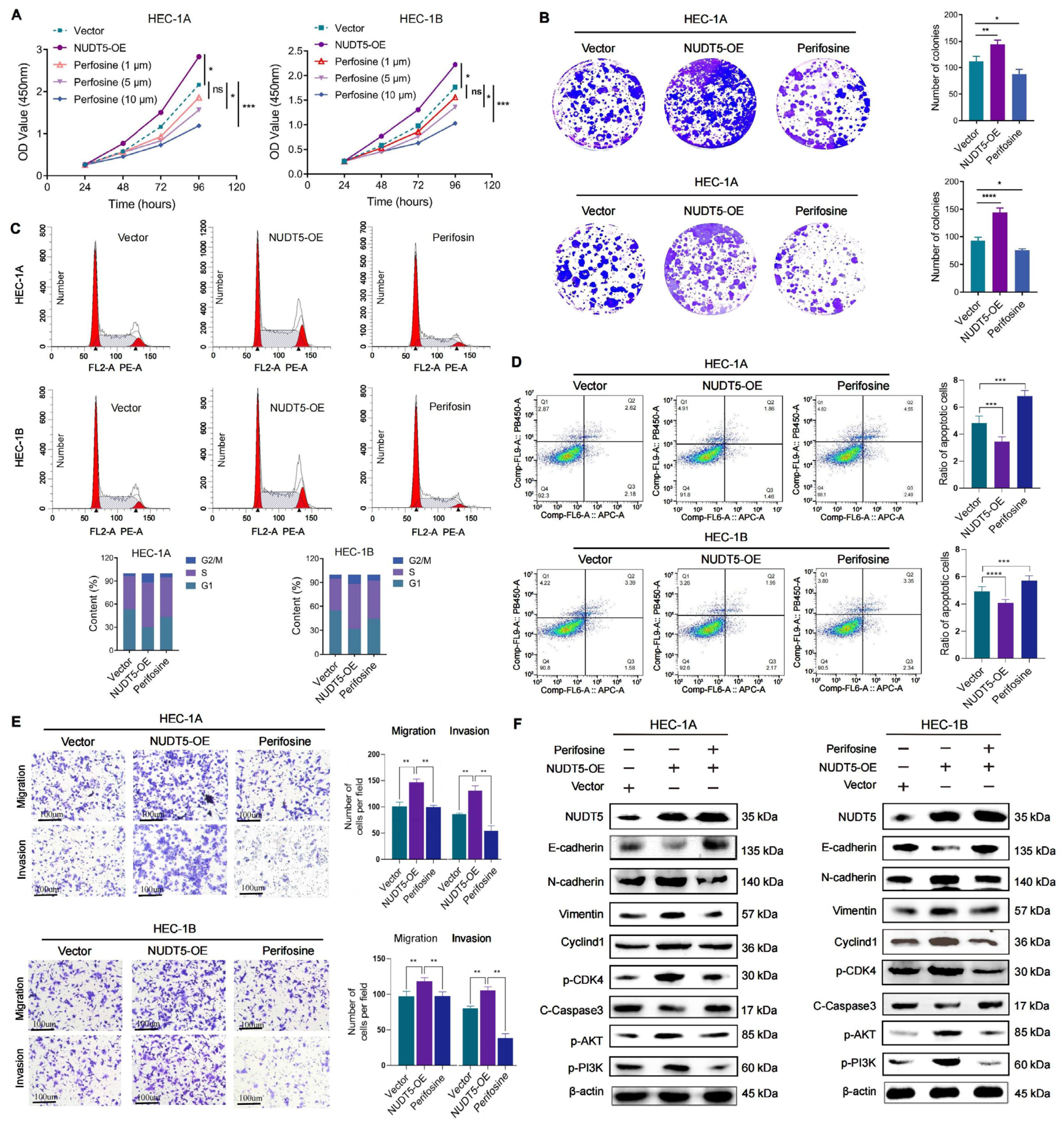
Functional analyses revealed that NUDT5 plays a critical role in promoting EC cell proliferation, survival, and migration. Knockdown of NUDT5 in EC cell lines led to a marked reduction in cell proliferation, with flow cytometry analysis showing an arrest at the G1 phase and increased apoptosis. These results suggest that NUDT5 contributes to cell cycle regulation and apoptosis evasion, which are crucial processes for tumor growth and resistance to cell death. Moreover, NUDT5 overexpression enhanced EC cell migration and invasion, indicating its role in promoting tumor metastasis. These findings are partially consistent with previous studies showing that NUDT5 functions as an oncogene in other cancers by modulating key processes involved in tumor progression [
17,
18]. Our data also suggest that NUDT5 may influence epithelial–mesenchymal transition, a critical step in tumor metastasis. The upregulation of mesenchymal markers and the downregulation of epithelial markers in NUDT5-overexpressing EC cells further support its role in enhancing metastatic potential. These results suggest that NUDT5 could be a central player in the aggressive behavior of EC and may contribute to therapeutic resistance by promoting cellular plasticity and metastasis.
In earlier studies, NUDT5 has been implicated in ADP-ribose metabolism, which plays a key role in maintaining genomic stability and DNA damage response, processes that are crucial for tumor progression [
12]. Recent studies suggest that NUDT5 might facilitate cancer cell survival by maintaining high levels of nucleotide pools, which help cells cope with increased replication stress and DNA damage [
15]. Moreover, our pathway enrichment analysis identified NUDT5-associated genes involved in PI3K-AKT signaling, and further validation experiments demonstrated that NUDT5 knockdown led to a decrease in
p-AKT levels, suggesting a potential role in activating this pathway, which is a central regulator of cell survival, growth, and metastasis in EC and other malignancies [
19,
20]. The role of NUDT5 in modulating PI3K-AKT signaling further underscores its potential as a therapeutic target. This pathway is particularly relevant in cancer therapy, as drugs targeting this pathway, such as AKT inhibitors, have been explored [
21,
22,
23,
24,
25,
26]. However, their clinical application remains limited due to dose-dependent toxicity and off-target effects, such as hyperglycemia, immunosuppression, and gastrointestinal complications [
9,
18]. In this context, targeting NUDT5 could provide an upstream therapeutic alternative that modulates this pathway and offers a novel approach to overcome the limitations of direct AKT inhibition, highlighting the potential significance of our findings.
Our study has several limitations that must be addressed. First, while we demonstrated the biological function of NUDT5 in EC cell lines, further in vivo studies are needed to confirm its role in tumorigenesis and metastasis. Additionally, further exploration into the molecular mechanisms underlying NUDT5’s regulation of the PI3K-AKT pathway is warranted to better understand its functional relevance and therapeutic potential in EC.
In conclusion, our study demonstrated that NUDT5 is a potential oncogene and prognostic biomarker in endometrial carcinoma. High NUDT5 expression is associated with poor prognosis and aggressive biological behaviors, including increased cell proliferation, migration, and invasion. These findings suggest that NUDT5 could be a potential therapeutic target for restricting tumor progression and metastasis in EC. Further research is needed to explore the underlying molecular mechanisms and to evaluate the therapeutic efficacy of NUDT5-targeted interventions in preclinical and clinical settings.