Malignancy in Systemic Sclerosis: A Multicenter Retrospective Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Laboratory Evaluation
2.3. Collected Data
- Demographic: date of birth, age, sex, presence and numbers of pregnancy.
- Clinical: year of SSc diagnosis, SSc subtype (limited or diffuse), year of death, cause of death, year of malignancy diagnosis, malignancy type and outcome of malignancy.
- Serological: immunoserological positivity with special emphasis on ANA, anti-RNAPIII, ACA, ATA, anti-PM/Scl-75 and 100, anti-Ku, anti-fibrillarin, anti-Th/To, anti-NOR90 and anti-PDGF.
- Treatment-related: immunosuppressive treatments, therapeutic indications and duration of treatments.
- Risk and environmental factors: smoking; alcohol consumption with the subcategories of ‘no’, ‘sometimes’, ‘occasionally’ and ‘regularly’; organic solvents; silica; vinyl chloride exposures and immunosuppressive treatments.
2.4. Statistical Analysis
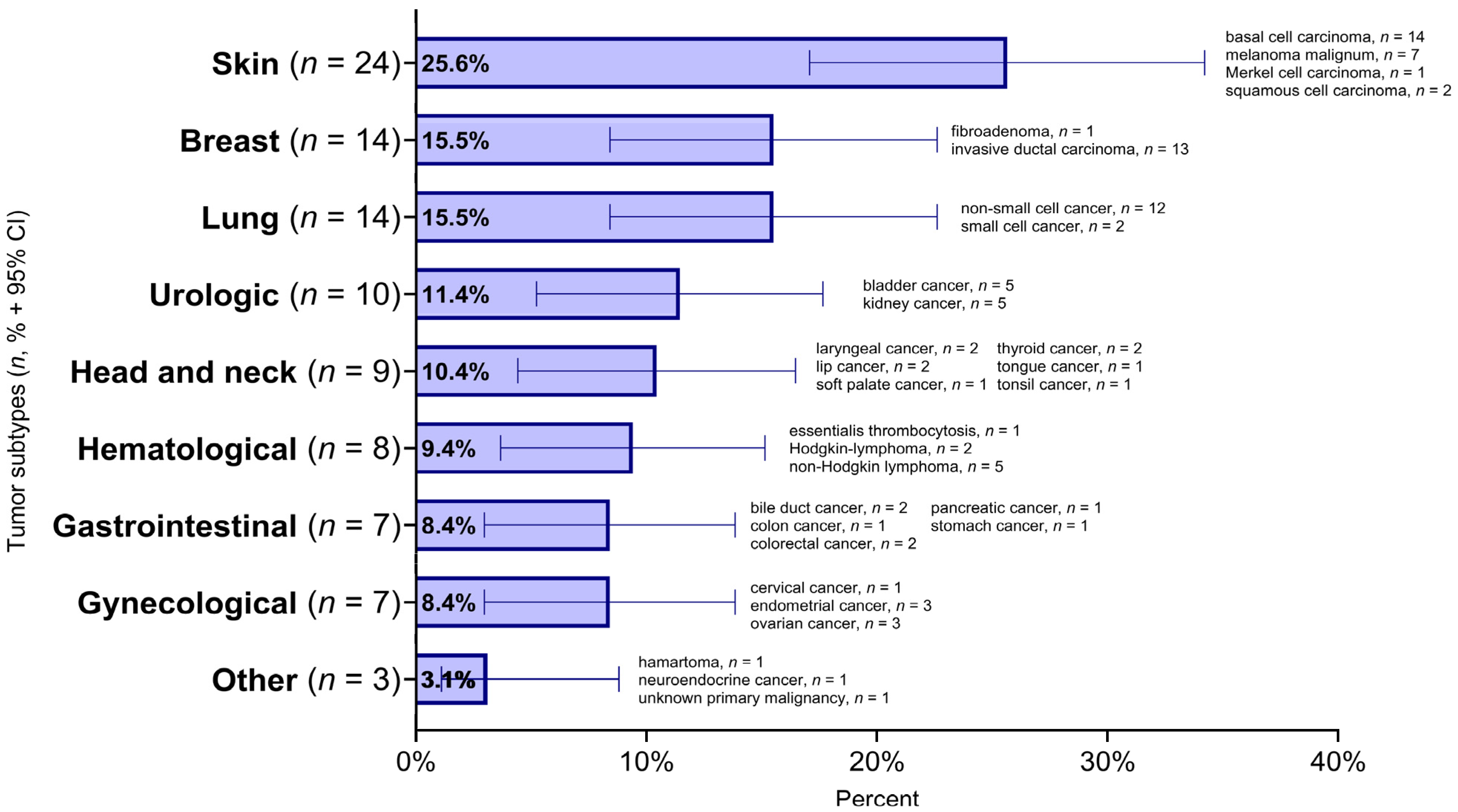
3. Results
3.1. Demographic Characteristics
3.2. Temporal Relationship of SSc and Malignancy
3.3. Autoantibodies and Malignancy
3.4. Treatment Modalities and Malignancy
3.5. Environmental Factors and Malignancy
3.6. Risk Factors for Malignancy
3.7. Survival of Patients with SSc and Malignancy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Denton, C.P.; Khanna, D. Systemic sclerosis. Lancet 2017, 390, 1685–1699. [Google Scholar] [CrossRef] [PubMed]
- Lóránd, V.; Czirják, L.; Minier, T. Musculoskeletal involvement in systemic sclerosis. Presse Méd. 2014, 43, e315–e328. [Google Scholar] [CrossRef]
- Volkmann, E.R.; Andréasson, K.; Smith, V. Systemic sclerosis. Lancet 2023, 401, 304–318. [Google Scholar] [CrossRef]
- Hughes, M.; Herrick, A.L. Systemic sclerosis. Br. J. Hosp. Med. 2019, 80, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Czirják, L.; Nagy, Z.; Szegedi, G. Systemic sclerosis in the elderly. Clin. Rheumatol. 1992, 11, 483–485. [Google Scholar] [CrossRef]
- Murdaca, G.; Contatore, M.; Gulli, R.; Mandich, P.; Puppo, F. Genetic factors and systemic sclerosis. Autoimmun. Rev. 2016, 15, 427–432. [Google Scholar] [CrossRef]
- Rubio-Rivas, M.; Moreno, R.; Corbella, X. Occupational and environmental scleroderma. Systematic review and meta-analysis. Clin. Rheumatol. 2017, 36, 569–582. [Google Scholar] [CrossRef] [PubMed]
- Steen, V.D. Occupational scleroderma. Curr. Opin. Rheumatol. 1999, 11, 490–494. [Google Scholar] [CrossRef]
- Czirják, L.; Csiki, Z.; Nagy, Z.; Tóth, E. Exposure to chemicals and systemic sclerosis. Ann. Rheum. Dis. 1995, 54, 529. [Google Scholar] [CrossRef]
- Czirják, L.; Kumánovics, G. Exposure to Solvents in Female Patients with Scleroderma. Clin. Rheumatol. 2002, 21, 114–118. [Google Scholar] [CrossRef]
- Czirják, L.; Bokk, A.; Csontos, G.; Lörincz, G.; Szegedi, G. Clinical findings in 61 patients with progressive systemic sclerosis. Acta Derm. Venereol. 1989, 69, 533–536. [Google Scholar] [PubMed]
- Nagy, Z.; Czirják, L. Predictors of survival in 171 patients with systemic sclerosis (scleroderma). Clin. Rheumatol. 1997, 16, 454–460. [Google Scholar] [CrossRef]
- Fragoulis, G.E.; Daoussis, D.; Pagkopoulou, E.; Garyfallos, A.; Kitas, G.D.; Dimitroulas, T. Cancer risk in systemic sclerosis: Identifying risk and managing high-risk patients. Expert Rev. Clin. Immunol. 2020, 16, 1105–1113. [Google Scholar] [CrossRef] [PubMed]
- Bonifazi, M.; Tramacere, I.; Pomponio, G.; Gabrielli, B.; Avvedimento, E.V.; La Vecchia, C.; Negri, E.; Gabrielli, A. Systemic sclerosis (scleroderma) and cancer risk: Systematic review and meta-analysis of observational studies. Rheumatology 2013, 52, 143–154. [Google Scholar] [CrossRef] [PubMed]
- Romero Noboa, M.E.; Litvin, R.; Sami, F.; Tanveer, S. Nationwide analysis of adult hospitalizations with hematologic malignancies and systemic sclerosis. ARP Rheumatol. 2023, 2, 291–298. [Google Scholar] [CrossRef]
- Partouche, L.; Goulabchand, R.; Maria, A.T.J.; Rivière, S.; Jorgensen, C.; Rigau, V.; Bourgier, C.; Bessis, D.; Le Quellec, A.; Quere, I.; et al. Biphasic Temporal Relationship between Cancers and Systemic Sclerosis: A Clinical Series from Montpellier University Hospital and Review of the Literature. JCM 2020, 9, 853. [Google Scholar] [CrossRef]
- Szekanecz, É.; Szamosi, S.; Horváth, Á.; Németh, Á.; Juhász, B.; Szántó, J.; Szücs, G.; Szekanecz, Z. Malignancies associated with systemic sclerosis. Autoimmun. Rev. 2012, 11, 852–855. [Google Scholar] [CrossRef]
- Czirják, L.; Kumánovics, G.; Varjú, C.; Nagy, Z.; Pákozdi, A.; Szekanecz, Z.; Szűcs, G. Survival and causes of death in 366 Hungarian patients with systemic sclerosis. Ann. Rheum. Dis. 2008, 67, 59–63. [Google Scholar] [CrossRef]
- Di Battista, M.; Lepri, G.; Codullo, V.; Da Rio, M.; Fiorentini, E.; Della Rossa, A.; Guiducci, S. Systemic sclerosis: One year in review 2023. Clin. Exp. Rheumatol. 2023, 41, 1567–1574. [Google Scholar] [CrossRef]
- Lepri, G.; Catalano, M.; Bellando-Randone, S.; Pillozzi, S.; Giommoni, E.; Giorgione, R.; Botteri, C.; Matucci-Cerinic, M.; Antonuzzo, L.; Guiducci, S. Systemic Sclerosis Association with Malignancy. Clin. Rev. Allergy Immunol. 2022, 63, 398–416. [Google Scholar] [CrossRef]
- Krabbe, J.; Steffens, K.M.; Drießen, S.; Kraus, T. Lung cancer risk and occupational pulmonary fibrosis: Systematic review and meta-analysis. Eur. Respir. Rev. 2024, 33, 230224. [Google Scholar] [CrossRef] [PubMed]
- Bosetti, C.; La Vecchia, C.; Lipworth, L.; McLaughlin, J. Occupational exposure to vinyl chloride and cancer risk: A review of the epidemiologic literature. Eur. J. Cancer Prev. 2003, 12, 427–430. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Huang, J.; Wang, J.; Chen, Y.; Hu, N.; Cao, S. Occupational exposure to organic solvents and breast cancer risk: A systematic review and meta-analysis. Environ. Sci. Pollut. Res. 2022, 29, 1619. [Google Scholar] [CrossRef]
- Peng, H.; Wu, X.; Wen, Y.; Li, C.; Lin, J.; Li, J.; Xiong, S.; Zhong, R.; Liang, H.; Cheng, B.; et al. Association between systemic sclerosis and risk of lung cancer: Results from a pool of cohort studies and Mendelian randomization analysis. Autoimmun. Rev. 2020, 19, 102633. [Google Scholar] [CrossRef] [PubMed]
- Naccache, J.-M.; Gibiot, Q.; Monnet, I.; Antoine, M.; Wislez, M.; Chouaid, C.; Cadranel, J. Lung cancer and interstitial lung disease: A literature review. J. Thorac. Dis. 2018, 10, 3829–3844. [Google Scholar] [CrossRef]
- Migliavacca Zucchetti, B.; Peccatori, F.A.; Codacci-Pisanelli, G. Pregnancy and Lactation: Risk or Protective Factors for Breast Cancer? In Diseases of the Breast During Pregnancy and Lactation; Alipour, S., Omranipour, R., Eds.; Advances in Experimental Medicine and Biology; Springer International Publishing: Cham, Switzerland, 2020; Volume 1252, pp. 195–197. ISBN 978-3-030-41595-2. [Google Scholar]
- Kaşifoğlu, T.; Yaşar Bilge, Ş.; Yıldız, F.; Özen, G.; Pehlivan, Y.; Yılmaz, N.; Tarhan, F.; Yılmaz, S.; Küçük, A.; Emmungil, H.; et al. Risk factors for malignancy in systemic sclerosis patients. Clin. Rheumatol. 2016, 35, 1529–1533. [Google Scholar] [CrossRef]
- Költő, G.; Faludi, R.; Aradi, D.; Bartos, B.; Kumánovics, G.; Minier, T.; Czirják, L.; Komócsi, A. Impact of cardiac involvement on the risk of mortality among patients with systemic sclerosis: A 5-year follow-up of a single-center cohort. Clin. Rheumatol. 2014, 33, 197–205. [Google Scholar] [CrossRef]
- Masi, A.T.; Subcommittee for Scleroderma Criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Preliminary criteria for the classification of systemic sclerosis (scleroderma). Arthritis Rheum. 1980, 23, 581–590. [Google Scholar] [CrossRef]
- Van Den Hoogen, F.; Khanna, D.; Fransen, J.; Johnson, S.R.; Baron, M.; Tyndall, A.; Matucci-Cerinic, M.; Naden, R.P.; Medsger, T.A.; Carreira, P.E.; et al. 2013 classification criteria for systemic sclerosis: An American college of rheumatology/European league against rheumatism collaborative initiative. Ann. Rheum. Dis. 2013, 72, 1747–1755. [Google Scholar] [CrossRef]
- Sjoberg, D.D.; Whiting, K.; Curry, M.; Lavery, J.A.; Larmarange, J. Reproducible Summary Tables with the gtsummary Package. R J. 2021, 13, 570. [Google Scholar] [CrossRef]
- Onishi, A.; Sugiyama, D.; Kumagai, S.; Morinobu, A. Cancer Incidence in Systemic Sclerosis: Meta-Analysis of Population-Based Cohort Studies. Arthritis Rheum. 2013, 65, 1913–1921. [Google Scholar] [CrossRef] [PubMed]
- Morrisroe, K.; Hansen, D.; Huq, M.; Stevens, W.; Sahhar, J.; Ngian, G.; Ferdowsi, N.; Hill, C.; Roddy, J.; Walker, J.; et al. Incidence, Risk Factors, and Outcomes of Cancer in Systemic Sclerosis. Arthritis Care Res. 2020, 72, 1625–1635. [Google Scholar] [CrossRef] [PubMed]
- Carbonell, C.; Marcos, M.; Guillén-del-Castillo, A.; Rubio-Rivas, M.; Argibay, A.; Marín-Ballvé, A.; Rodríguez-Pintó, I.; Baldà-Masmiquel, M.; Callejas-Moraga, E.; Colunga, D.; et al. Standardized incidence ratios and risk factors for cancer in patients with systemic sclerosis: Data from the Spanish Scleroderma Registry (RESCLE). Autoimmun. Rev. 2022, 21, 103167. [Google Scholar] [CrossRef]
- Igusa, T.; Hummers, L.K.; Visvanathan, K.; Richardson, C.; Wigley, F.M.; Rosen, A.; Shah, A.A.; Casciola-Rosen, L. Autoantibodies and scleroderma phenotype define subgroups at high-risk and low-risk for cancer. Ann. Rheum. Dis. 2018, 77, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Tonutti, A.; Motta, F.; Isailovic, N.; Ceribelli, A.; Ragusa, R.; Nappi, E.; Bonovas, S.; Selmi, C.; De Santis, M. Autoantibodies, cutaneous subset and immunosuppressants contribute to the cancer risk in systemic sclerosis. RMD Open 2024, 10, e004492. [Google Scholar] [CrossRef]
- National Cancer Registry. Available online: https://onkol.hu/nemzeti-rakregiszter-es-biostatisztikai-kozpont/ (accessed on 2 March 2025).
- Lopez, L.; Barnetche, T.; Galli, G.; Seneschal, J.; Blanchard, E.; Shipley, E.; Pellegrin, J.-L.; Lazaro, E.; Constans, J.; Duffau, P.; et al. Clinical and immunological features of patients with cancer-associated systemic sclerosis: An observational study. Jt. Bone Spine 2023, 90, 105555. [Google Scholar] [CrossRef]
- Launay, D.; Le Berre, R.; Hatron, P.-Y.; Peyrat, J.-P.; Hachulla, E.; Devulder, B.; Hebbar, M. Association between systemic sclerosis and breast cancer: Eight new cases and review of the literature. Clin. Rheumatol. 2004, 23, 516–522. [Google Scholar] [CrossRef]
- Olesen, A.B.; Svaerke, C.; Farkas, D.K.; Sørensen, H.T. Systemic sclerosis and the risk of cancer: A nationwide population-based cohort study: Systemic sclerosis and the risk of cancer. Br. J. Dermatol. 2010, 163, 800–806. [Google Scholar] [CrossRef]
- Subramani, R.; Lakshmanaswamy, R. Pregnancy and Breast Cancer. In Progress in Molecular Biology and Translational Science; Elsevier: Amsterdam, The Netherlands, 2017; Volume 151, pp. 81–111. ISBN 978-0-12-812772-8. [Google Scholar]
- Hoa, S.; Lazizi, S.; Baron, M.; Wang, M.; Fritzler, M.J.; Hudson, M.; Canadian Scleroderma Research Group. Association between autoantibodies in systemic sclerosis and cancer in a national registry. Rheumatology 2022, 61, 2905–2914. [Google Scholar] [CrossRef]
- Lazzaroni, M.-G.; Cavazzana, I.; Colombo, E.; Dobrota, R.; Hernandez, J.; Hesselstrand, R.; Varju, C.; Nagy, G.; Smith, V.; Caramaschi, P.; et al. Malignancies in Patients with Anti-RNA Polymerase III Antibodies and Systemic Sclerosis: Analysis of the EULAR Scleroderma Trials and Research Cohort and Possible Recommendations for Screening. J. Rheumatol. 2017, 44, 639–647. [Google Scholar] [CrossRef]
- Shah, A.A.; Rosen, A.; Hummers, L.; Wigley, F.; Casciola-Rosen, L. Close temporal relationship between onset of cancer and scleroderma in patients with RNA polymerase I/III antibodies. Arthritis Rheum. 2010, 62, 2787–2795. [Google Scholar] [CrossRef] [PubMed]
- Moinzadeh, P.; Fonseca, C.; Hellmich, M.; Shah, A.A.; Chighizola, C.; Denton, C.P.; Ong, V.H. Association of anti-RNA polymerase III autoantibodies and cancer in scleroderma. Arthritis Res. Ther. 2014, 16, R53. [Google Scholar] [CrossRef] [PubMed]
- Bernal-Bello, D.; De Tena, J.G.; Guillén-del Castillo, A.; Selva-O’Callaghan, A.; Callejas-Moraga, E.L.; Marín-Sánchez, A.M.; Fonollosa-Pla, V.; Simeón-Aznar, C.P. Novel risk factors related to cancer in scleroderma. Autoimmun. Rev. 2017, 16, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Elhai, M.; Meune, C.; Boubaya, M.; Avouac, J.; Hachulla, E.; Balbir-Gurman, A.; Riemekasten, G.; Airò, P.; Joven, B.; Vettori, S.; et al. Mapping and predicting mortality from systemic sclerosis. Ann. Rheum. Dis. 2017, 76, 1897–1905. [Google Scholar] [CrossRef]
- Martínez-Urbistondo, M.; González-Guzmán, A.; Fernández-Guitián, R.; Blanco-Valencia, X.P.; Esteban-Sampedro, J.; Martín-Portugués, M.; Durán-del Campo, P.; Tutor, P.; Mellor-Pita, S.; Ortega-de La Puente, A.; et al. Neoplasm related mortality risk in Systemic Sclerosis: A nationwide study. BMC Rheumatol. 2025, 9, 27. [Google Scholar] [CrossRef]







| Overall | Tumor | Sex | |||
|---|---|---|---|---|---|
| Characteristic | With tumor | Without tumor | female | male | |
| n = 541 | n = 85 | n = 456 | n = 463 | n = 78 | |
| Smoking | 142/353 (40.2%) | 24/57 (42.1%) | 118/296 (39.9%) | 103/292 (35.3%) | 39/61 (63.9%) *** |
| Alcohol consumption | n= 326 | n = 54 | n = 272 | n = 270 | n = 56 |
| no | 289 (88.7%) | 46 (85.2%) | 243 (89.3%) | 243 (90.0%) | 46 (82.1%) |
| sometimes | 1 (0.3%) | 1 (1.9%) | 0 (0%) | 1 (0.4%) | 0 (0%) |
| occasionally | 4 (1.2%) | 4 (7.4%) | 0 (0%) | 4 (1.5%) | 1 (1.8%) |
| regularly | 32 (9.8%) | 3 (5.6%) | 29 (10.7%) *** | 23 (8.5%) | 9 (16.1%) |
| Organic solvents | 12/156 (7.7%) | 1/35 (2.9%) | 11/121 (9.1%) | 8/132 (6.1%) | 4/24 (16.7%) |
| Silica exp. | 0/153 (0%) | 0/35 (0%) | 0/118 (0%) | 0/129 (0%) | 0/24 (0%) |
| Vinyl-cloride exp. | 0/153 (0%) | 0/35 (0%) | 0/118 (0%) | 0/129 (0%) | 0/24 (0%) |
| Pregnancy | 282/304 (92.8%) | 45/49 (91.8%) | 237/255 (92.9%) | ||
| Variable | Model 1 OR (95% CI) | Model 2 OR (95% CI) | Model 3 OR (95% CI) | |
|---|---|---|---|---|
| Demographics and SSc characteristics | Female (reference: male) | 1.20 (0.52, 3.27) | ||
| dcSSc (reference: lcSSc) | 1.44 (0.78, 2.63) | |||
| Age at the diagnosis of SSc | 1.01 (0.99, 1.04) | |||
| Duration of SSc (outcome) | 1.00 (0.96, 1.03) | |||
| Antibody profile | ANA (+) | 2.17 (0.96, 5.84) | 1.57 (0.51, 5.93) | 1.55 (0.50, 5.88) |
| Anti-topoisomerase I (+) | 1.36 (0.69, 2.58) | 2.34 (0.94, 5.84) | 2.44 (0.92, 6.58) | |
| Anti-RNAPIII (+) | 3.18 (1.09, 8.20) | 4.33 (1.08, 15.1) | 4.72 (1.12, 17.6) | |
| Anti-centromere (+) | 0.68 (0.23, 1.66) | 1.25 (0.26, 4.40) | 1.22 (0.23, 5.02) | |
| Anti-Ro52 (+) | 0.75 (0.30, 1.65) | 1.01 (0.32, 2.76) | 1.08 (0.33, 2.99) | |
| Treatment | MTX | 0.66 (0.31, 1.29) | 0.49 (0.16, 1.25) | 0.53 (0.18, 1.39) |
| CYC | 0.92 (0.42, 1.86) | 0.97 (0.34, 2.47) | 1.09 (0.38, 2.83) | |
| Exposure | Smoking | 1.05 (0.47, 2.27) | 1.26 (0.54, 2.91) | 1.27 (0.53, 3.00) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nemes-Tömöri, D.; Jász, D.K.; Tari, D.; Bói, B.; Ágoston-Szabó, Á.; Szűcs, G.; Majai, G.E. Malignancy in Systemic Sclerosis: A Multicenter Retrospective Study. Biomedicines 2025, 13, 993. https://doi.org/10.3390/biomedicines13040993
Nemes-Tömöri D, Jász DK, Tari D, Bói B, Ágoston-Szabó Á, Szűcs G, Majai GE. Malignancy in Systemic Sclerosis: A Multicenter Retrospective Study. Biomedicines. 2025; 13(4):993. https://doi.org/10.3390/biomedicines13040993
Chicago/Turabian StyleNemes-Tömöri, Dóra, Dávid Kurszán Jász, Dóra Tari, Bernadett Bói, Ágnes Ágoston-Szabó, Gabriella Szűcs, and Gyöngyike Emese Majai. 2025. "Malignancy in Systemic Sclerosis: A Multicenter Retrospective Study" Biomedicines 13, no. 4: 993. https://doi.org/10.3390/biomedicines13040993
APA StyleNemes-Tömöri, D., Jász, D. K., Tari, D., Bói, B., Ágoston-Szabó, Á., Szűcs, G., & Majai, G. E. (2025). Malignancy in Systemic Sclerosis: A Multicenter Retrospective Study. Biomedicines, 13(4), 993. https://doi.org/10.3390/biomedicines13040993





