Ivosidenib Confers BRCAness Phenotype and Synthetic Lethality to Poly (ADP-Ribose) Polymerase Inhibition in BRCA1/2-Proficient Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Transfection
2.2. Colony Formation Assay
2.3. Cell Viability Assay
2.4. Immunoflurosence Assay
2.5. Immunohistochemical (IHC) Staining Assay
2.6. Western Blot Assay
2.7. Comet Assay
2.8. Xenograft Tumor Study
2.9. Homologous Recombination (HR) Repair Assay
2.10. Drug Affinity Responsive Target Stability (DARTS)
2.11. Statistics
3. Results
3.1. High-Throughput Screening Identifies Ivosidenib as a PARPi Sensitizer in BRCA1/2 Wild-Type Ovarian Cancer Cells
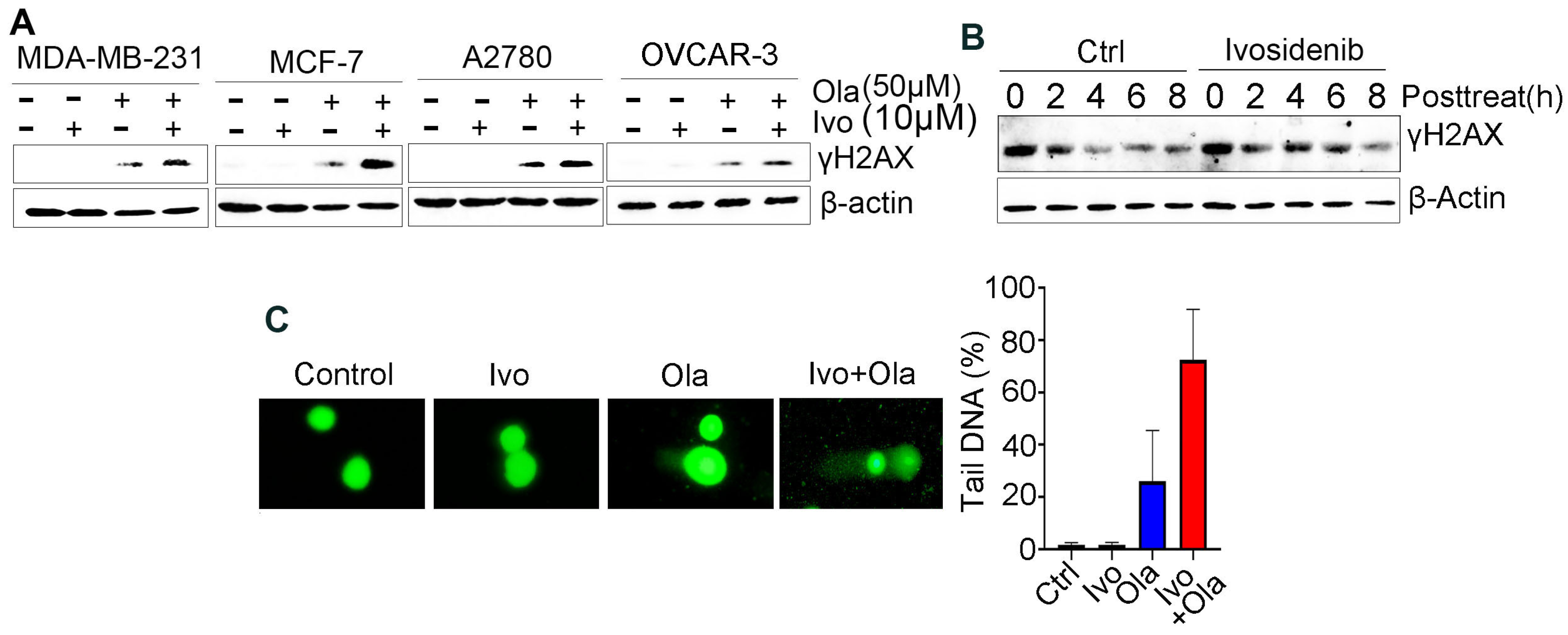
3.2. Ivosidenib Enhances Olaparib-Induced DNA Double-Strand Breaks (DSBs) in Cancer Cells
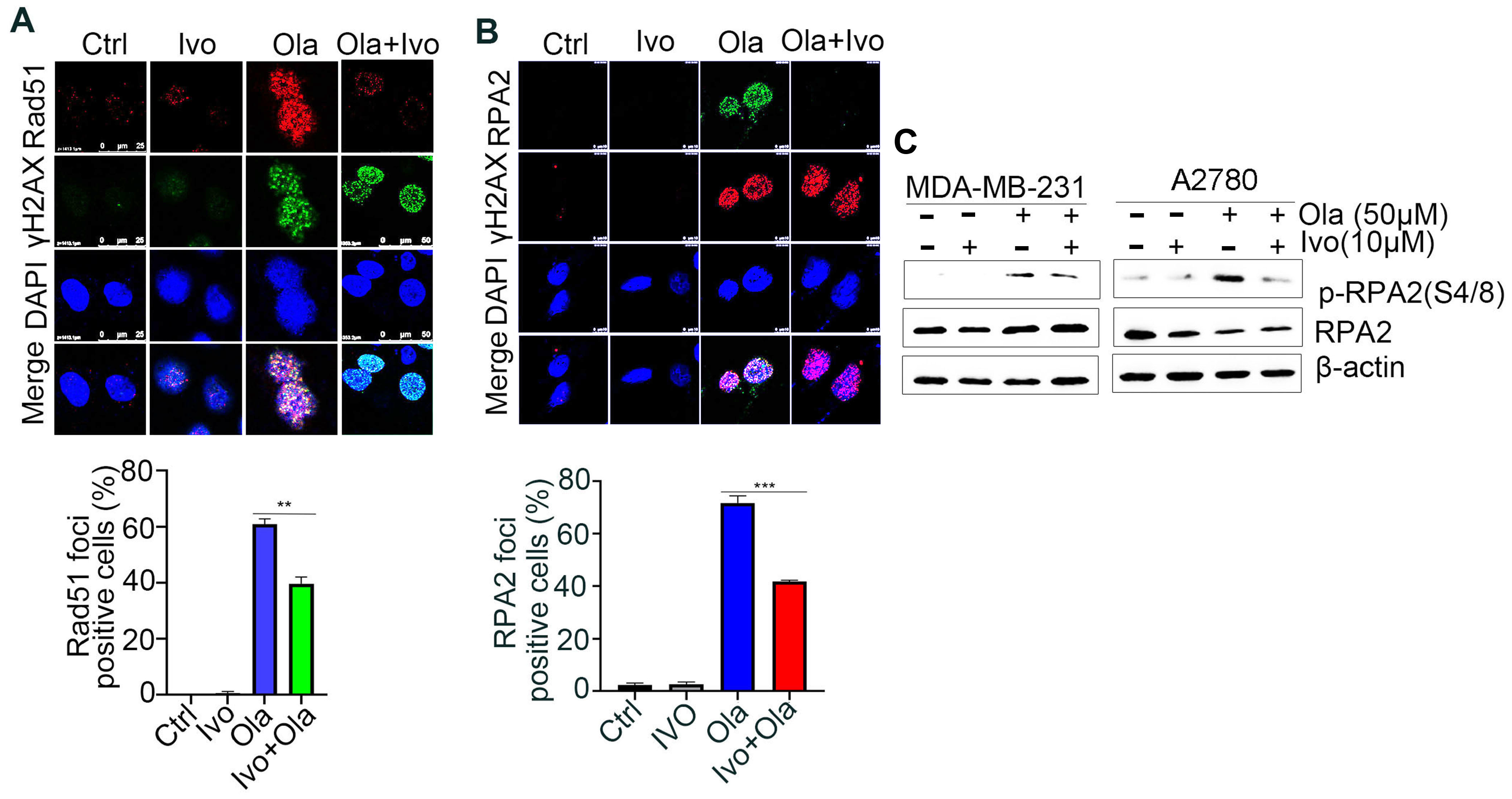
3.3. Ivosidenib Inhibits Homologous-Recombination (HR) Repair
3.4. Ivosidenib Targets YTHDC2
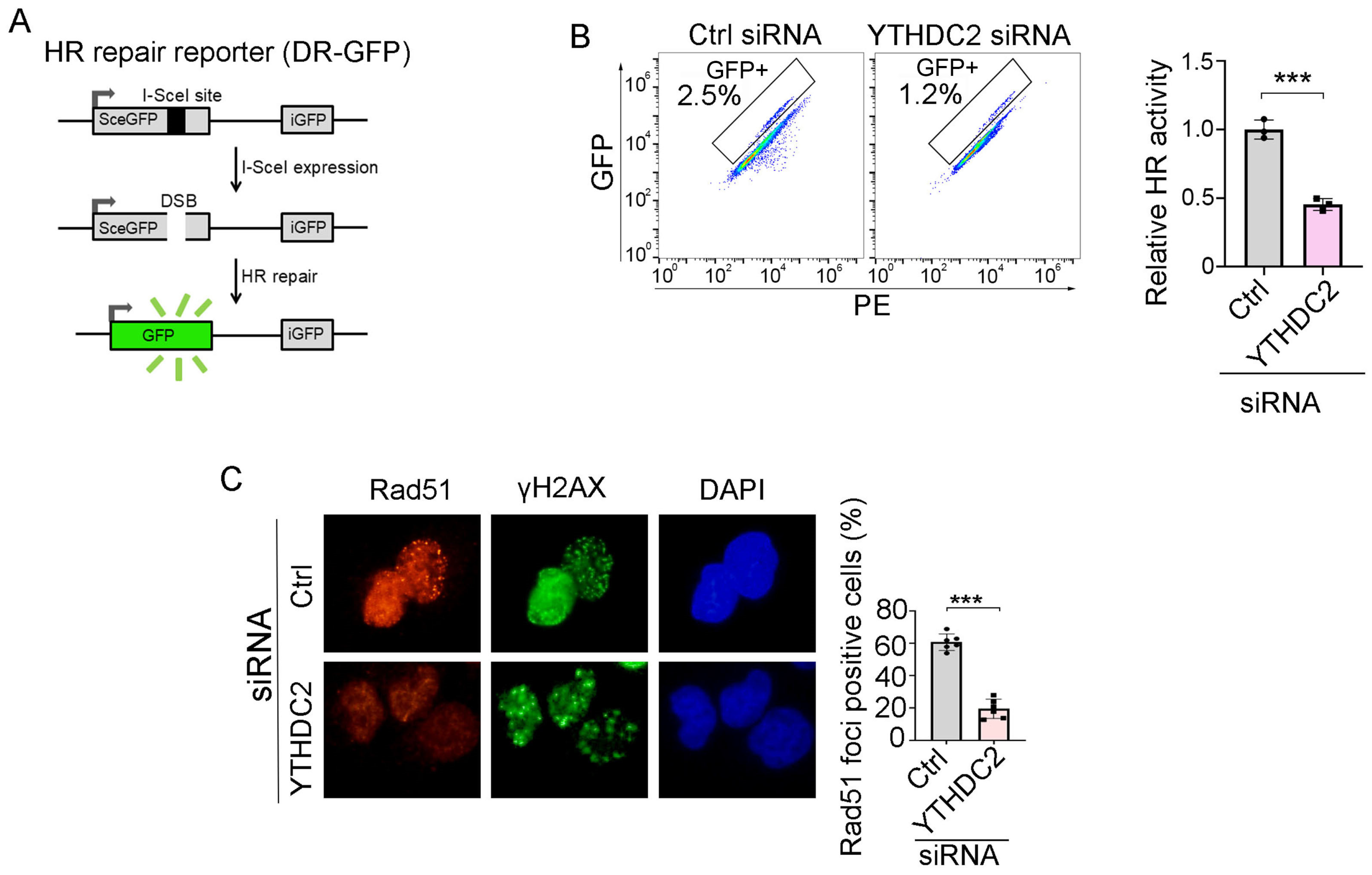
3.5. YTHDC2 Is Required for Proficient HR Repair
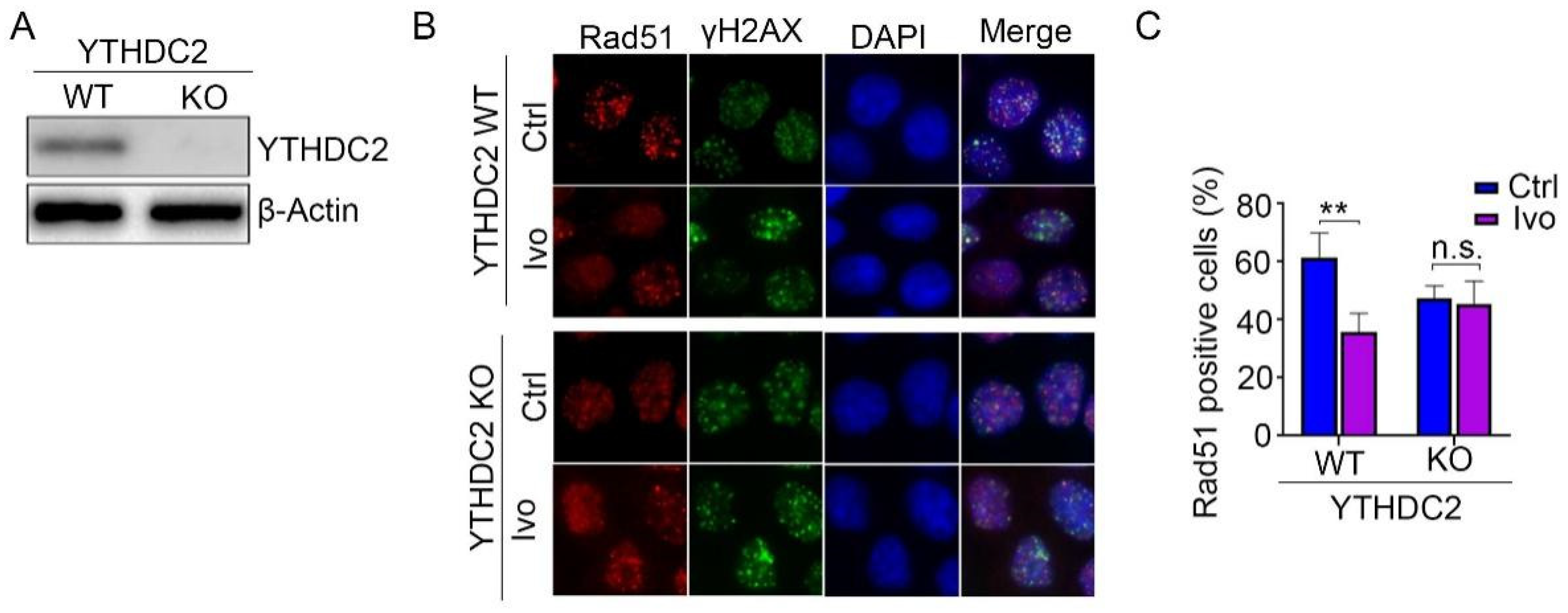
3.6. Ivosidenib Inhibits HR Repair by Targeting YTHDC2
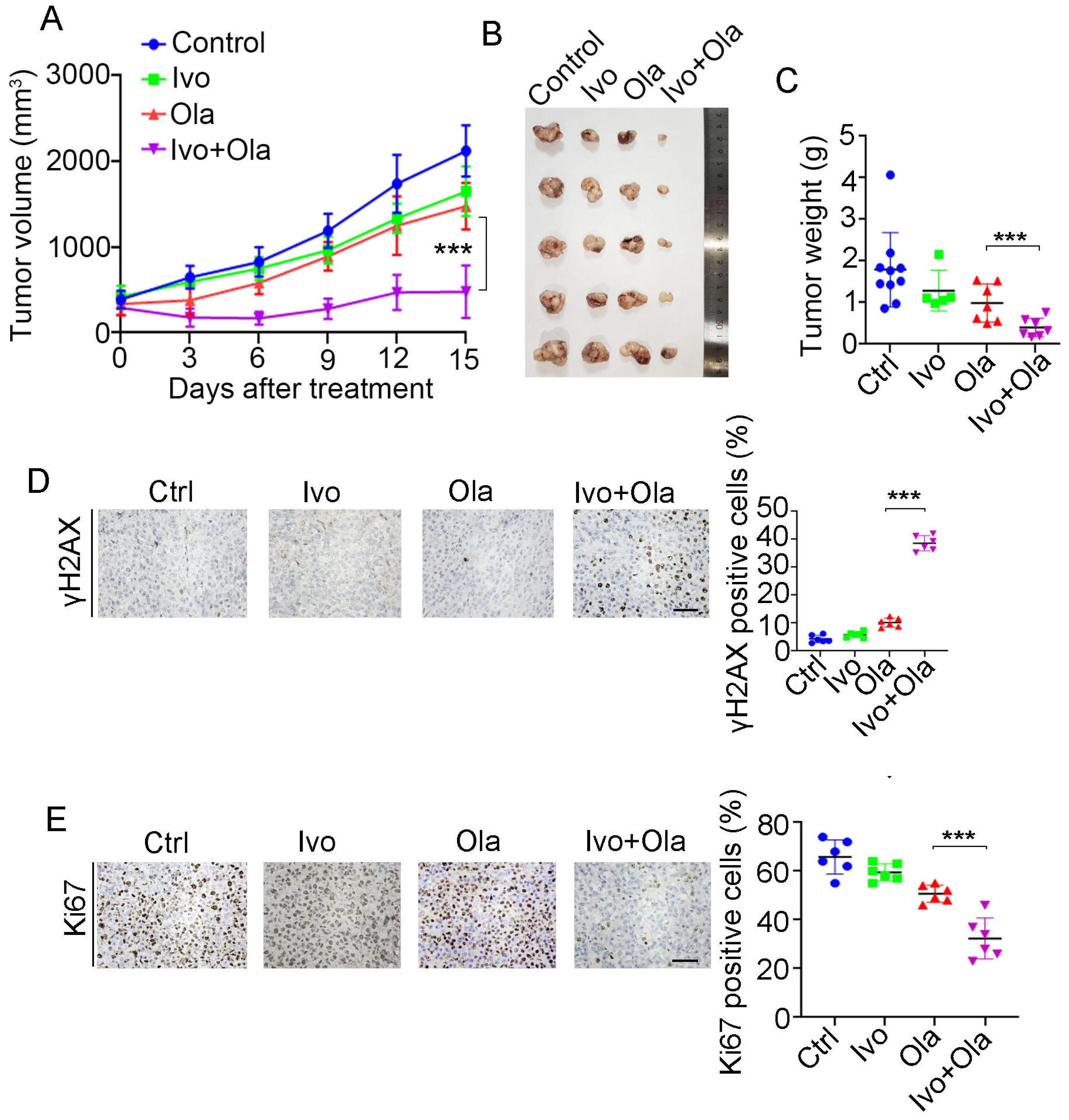
3.7. Ivosidenib Sensitizes BRCA1/2-Proficient Cancer Cells to PARPi In Vivo
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chappidi, N.; Quail, T.; Doll, S.; Vogel, L.T.; Aleksandrov, R.; Felekyan, S.; Kuhnemuth, R.; Stoynov, S.; Seidel, C.A.M.; Brugues, J.; et al. PARP1-DNA co-condensation drives DNA repair site assembly to prevent disjunction of broken DNA ends. Cell 2024, 187, 945–961.e918. [Google Scholar] [CrossRef] [PubMed]
- Jackson, S.P.; Bartek, J. The DNA-damage response in human biology and disease. Nature 2009, 461, 1071–1078. [Google Scholar] [CrossRef]
- Chatterjee, N.; Walker, G.C. Mechanisms of DNA damage, repair, and mutagenesis. Environ. Mol. Mutagen. 2017, 58, 235–263. [Google Scholar] [CrossRef]
- Alemasova, E.E.; Lavrik, O.I. Poly(ADP-ribosyl)ation by PARP1: Reaction mechanism and regulatory proteins. Nucleic Acids Res. 2019, 47, 3811–3827. [Google Scholar] [CrossRef]
- Herrmann, G.K.; Yin, Y.W. The Role of Poly(ADP-ribose) Polymerase 1 in Nuclear and Mitochondrial Base Excision Repair. Biomolecules 2023, 13, 1195. [Google Scholar] [CrossRef] [PubMed]
- Gottipati, P.; Vischioni, B.; Schultz, N.; Solomons, J.; Bryant, H.E.; Djureinovic, T.; Issaeva, N.; Sleeth, K.; Sharma, R.A.; Helleday, T. Poly(ADP-ribose) polymerase is hyperactivated in homologous recombination-defective cells. Cancer Res. 2010, 70, 5389–5398. [Google Scholar] [CrossRef]
- Wang, C.; Tian, L.; He, Q.; Lin, S.; Wu, Y.; Qiao, Y.; Zhu, B.; Li, D.; Chen, G. Targeting CK2-mediated phosphorylation of p53R2 sensitizes BRCA-proficient cancer cells to PARP inhibitors. Oncogene 2023, 42, 2971–2984. [Google Scholar] [CrossRef]
- Slade, D. PARP and PARG inhibitors in cancer treatment. Genes Dev. 2020, 34, 360–394. [Google Scholar] [CrossRef] [PubMed]
- Cortesi, L.; Rugo, H.S.; Jackisch, C. An Overview of PARP Inhibitors for the Treatment of Breast Cancer. Target. Oncol. 2021, 16, 255–282. [Google Scholar] [CrossRef]
- Mekonnen, N.; Yang, H.; Shin, Y.K. Homologous Recombination Deficiency in Ovarian, Breast, Colorectal, Pancreatic, Non-Small Cell Lung and Prostate Cancers, and the Mechanisms of Resistance to PARP Inhibitors. Front. Oncol. 2022, 12, 880643. [Google Scholar] [CrossRef]
- Nguyen, L.; WM Martens, J.; Van Hoeck, A.; Cuppen, E. Pan-cancer landscape of homologous recombination deficiency. Nat. Commun. 2020, 11, 5584. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Chen, C.; Wang, C.; Guo, Y.; Sun, B.; Tian, J.; Yan, J.; Li, D.; Chen, G. Targeting GPX4-mediated ferroptosis protection sensitizes BRCA1-deficient cancer cells to PARP inhibitors. Redox Biol. 2024, 76, 103350. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, Z.Y.; Wu, N.; Chen, Y.C.; Cheng, Q.; Wang, J. PARP inhibitor resistance: The underlying mechanisms and clinical implications. Mol. Cancer 2020, 19, 107. [Google Scholar] [CrossRef] [PubMed]
- Fugger, K.; Hewitt, G.; West, S.C.; Boulton, S.J. Tackling PARP inhibitor resistance. Trends Cancer 2021, 7, 1102–1118. [Google Scholar] [CrossRef]
- Jain, A.; Barge, A.; Parris, C.N. Combination strategies with PARP inhibitors in BRCA-mutated triple-negative breast cancer: Overcoming resistance mechanisms. Oncogene 2024, 44, 193–207. [Google Scholar] [CrossRef]
- Lin, Z.; Wang, L.; Xing, Z.; Wang, F.; Cheng, X. Update on Combination Strategies of PARP Inhibitors. Cancer Control 2024, 31, 10732748241298329. [Google Scholar] [CrossRef]
- Abou-Alfa, G.K.; Macarulla, T.; Javle, M.M.; Kelley, R.K.; Lubner, S.J.; Adeva, J.; Cleary, J.M.; Catenacci, D.V.; Borad, M.J.; Bridgewater, J.; et al. Ivosidenib in IDH1-mutant, chemotherapy-refractory cholangiocarcinoma (ClarIDHy): A multicentre, randomised, double-blind, placebo-controlled, phase 3 study. Lancet Oncol. 2020, 21, 796–807. [Google Scholar] [CrossRef]
- Montesinos, P.; Recher, C.; Vives, S.; Zarzycka, E.; Wang, J.; Bertani, G.; Heuser, M.; Calado, R.T.; Schuh, A.C.; Yeh, S.P.; et al. Ivosidenib and Azacitidine in IDH1-Mutated Acute Myeloid Leukemia. N. Engl. J. Med. 2022, 386, 1519–1531. [Google Scholar] [CrossRef]
- Lord, C.J.; Ashworth, A. PARP inhibitors: Synthetic lethality in the clinic. Science 2017, 355, 1152–1158. [Google Scholar] [CrossRef]
- Tanaka, T.; Halicka, D.; Traganos, F.; Darzynkiewicz, Z. Cytometric analysis of DNA damage: Phosphorylation of histone H2AX as a marker of DNA double-strand breaks (DSBs). Methods Mol. Biol. 2009, 523, 161–168. [Google Scholar] [CrossRef]
- Kanev, P.B.; Atemin, A.; Stoynov, S.; Aleksandrov, R. PARP1 roles in DNA repair and DNA replication: The basi(c)s of PARP inhibitor efficacy and resistance. Semin. Oncol. 2024, 51, 2–18. [Google Scholar] [CrossRef] [PubMed]
- van Wijk, L.M.; Nilas, A.B.; Vrieling, H.; Vreeswijk, M.P.G. RAD51 as a functional biomarker for homologous recombination deficiency in cancer: A promising addition to the HRD toolbox? Expert Rev. Mol. Diagn. 2022, 22, 185–199. [Google Scholar] [CrossRef]
- Cejka, P.; Symington, L.S. DNA End Resection: Mechanism and Control. Annu. Rev. Genet. 2021, 55, 285–307. [Google Scholar] [CrossRef]
- Chen, H.; Lisby, M.; Symington, L.S. RPA coordinates DNA end resection and prevents formation of DNA hairpins. Mol. Cell 2013, 50, 589–600. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Chen, J.; Qiao, Y.; Shi, Y.; Liu, W.; Zeng, Q.; Xie, H.; Shi, X.; Sun, Y.; Liu, X.; et al. ZNF830 mediates cancer chemoresistance through promoting homologous-recombination repair. Nucleic Acids Res. 2018, 46, 1266–1279. [Google Scholar] [CrossRef] [PubMed]
- Choi, E.H.; Yoon, S.; Park, K.S.; Kim, K.P. The Homologous Recombination Machinery Orchestrates Post-replication DNA Repair During Self-renewal of Mouse Embryonic Stem Cells. Sci. Rep. 2017, 7, 11610. [Google Scholar] [CrossRef]
- Lomenick, B.; Hao, R.; Jonai, N.; Chin, R.M.; Aghajan, M.; Warburton, S.; Wang, J.; Wu, R.P.; Gomez, F.; Loo, J.A.; et al. Target identification using drug affinity responsive target stability (DARTS). Proc. Natl. Acad. Sci. USA 2009, 106, 21984–21989. [Google Scholar] [CrossRef]
- Hsu, P.J.; Zhu, Y.; Ma, H.; Guo, Y.; Shi, X.; Liu, Y.; Qi, M.; Lu, Z.; Shi, H.; Wang, J.; et al. Ythdc2 is an N6-methyladenosine binding protein that regulates mammalian spermatogenesis. Cell Res. 2017, 27, 1115–1127. [Google Scholar] [CrossRef]
- Collet, L.; Hanvic, B.; Turinetto, M.; Treilleux, I.; Chopin, N.; Le Saux, O.; Ray-Coquard, I. BRCA1/2 alterations and reversion mutations in the area of PARP inhibitors in high grade ovarian cancer: State of the art and forthcoming challenges. Front. Oncol. 2024, 14, 1354427. [Google Scholar] [CrossRef]
- Jaspers, J.E.; Kersbergen, A.; Boon, U.; Sol, W.; van Deemter, L.; Zander, S.A.; Drost, R.; Wientjens, E.; Ji, J.; Aly, A.; et al. Loss of 53BP1 causes PARP inhibitor resistance in Brca1-mutated mouse mammary tumors. Cancer Discov. 2013, 3, 68–81. [Google Scholar] [CrossRef]
- Zarei, M.; Hajihassani, O.; Hue, J.J.; Loftus, A.W.; Graor, H.J.; Nakazzi, F.; Naji, P.; Boutros, C.S.; Uppin, V.; Vaziri-Gohar, A.; et al. IDH1 Inhibition Potentiates Chemotherapy Efficacy in Pancreatic Cancer. Cancer Res. 2024, 84, 3072–3085. [Google Scholar] [CrossRef] [PubMed]
- Stemer, G.; Rowe, J.M.; Ofran, Y. Efficacy and Safety Profile of Ivosidenib in the Management of Patients with Acute Myeloid Leukemia (AML): An Update on the Emerging Evidence. Blood Lymphat. Cancer 2021, 11, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Lanzetti, L. Oncometabolites at the crossroads of genetic, epigenetic and ecological alterations in cancer. Cell Death Differ. 2024, 31, 1582–1594. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Huang, J.; Dong, Q.; Li, W.; Jiang, L.; Zhang, Q.; Sun, L.; Yuan, S.; He, X. Ag120-Mediated Inhibition of ASCT2-Dependent Glutamine Transport has an Anti-Tumor Effect on Colorectal Cancer Cells. Front. Pharmacol. 2022, 13, 871392. [Google Scholar] [CrossRef]
- Lin, S.; Tian, J.; He, Q.; Yang, M.; Chen, Z.; Belogurov, A.A., Jr.; Li, X.; Zhang, F.; Liu, Y.; Chen, G. SN-38 Sensitizes BRCA-Proficient Ovarian Cancers to PARP Inhibitors through Inhibiting Homologous Recombination Repair. Dis. Markers 2022, 2022, 7243146. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, D.; Liu, W.; Zhang, Y.; Li, C. Ivosidenib Confers BRCAness Phenotype and Synthetic Lethality to Poly (ADP-Ribose) Polymerase Inhibition in BRCA1/2-Proficient Cancer Cells. Biomedicines 2025, 13, 958. https://doi.org/10.3390/biomedicines13040958
Zhou D, Liu W, Zhang Y, Li C. Ivosidenib Confers BRCAness Phenotype and Synthetic Lethality to Poly (ADP-Ribose) Polymerase Inhibition in BRCA1/2-Proficient Cancer Cells. Biomedicines. 2025; 13(4):958. https://doi.org/10.3390/biomedicines13040958
Chicago/Turabian StyleZhou, Danyang, Wei Liu, Yanyan Zhang, and Chong Li. 2025. "Ivosidenib Confers BRCAness Phenotype and Synthetic Lethality to Poly (ADP-Ribose) Polymerase Inhibition in BRCA1/2-Proficient Cancer Cells" Biomedicines 13, no. 4: 958. https://doi.org/10.3390/biomedicines13040958
APA StyleZhou, D., Liu, W., Zhang, Y., & Li, C. (2025). Ivosidenib Confers BRCAness Phenotype and Synthetic Lethality to Poly (ADP-Ribose) Polymerase Inhibition in BRCA1/2-Proficient Cancer Cells. Biomedicines, 13(4), 958. https://doi.org/10.3390/biomedicines13040958



