Bioinformatics-Based Analysis of Ferroptosis-Related Biomarkers and the Prediction of Drugs Affecting the Adipogenic Differentiation of MSCs
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Download
2.2. Identification of DEGs
2.3. GO, KEGG, and GSEA Enrichment Analysis
2.4. PPI Network Construction and Hub Gene Identification
2.5. Correlation Analysis Among Hub Ferroptosis Genes
2.6. Diagnostic Value Analysis of Hub Ferroptosis Genes
2.7. OVX Model Construction
2.8. Micro-CT Analysis
2.9. MSC Isolation and Culture
2.10. Total RNA Extraction and qRT-PCR Analysis
2.11. Alkaline Phosphatase (ALP) Assays
2.12. Oil Red O (ORO) Assays
2.13. Drug Prediction and Molecular Docking
2.14. Cell Counting Kit-8(CCK-8) Assay
2.15. Protein Extraction and Western Blotting
2.16. Statistical Analysis
3. Results
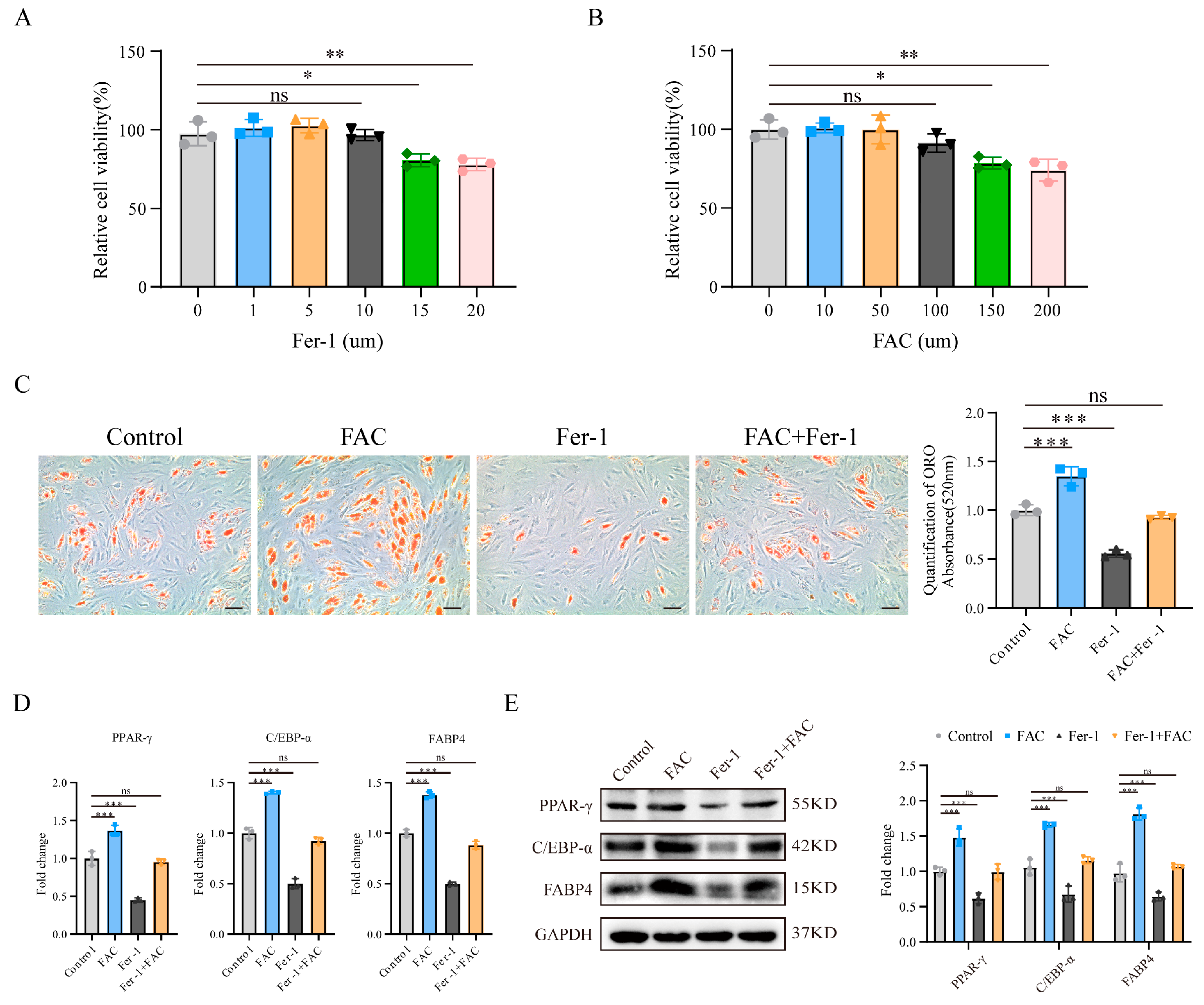
3.1. Inhibition of Ferroptosis Reduced MSC Adipogenic Differentiation Induced by Iron Overload
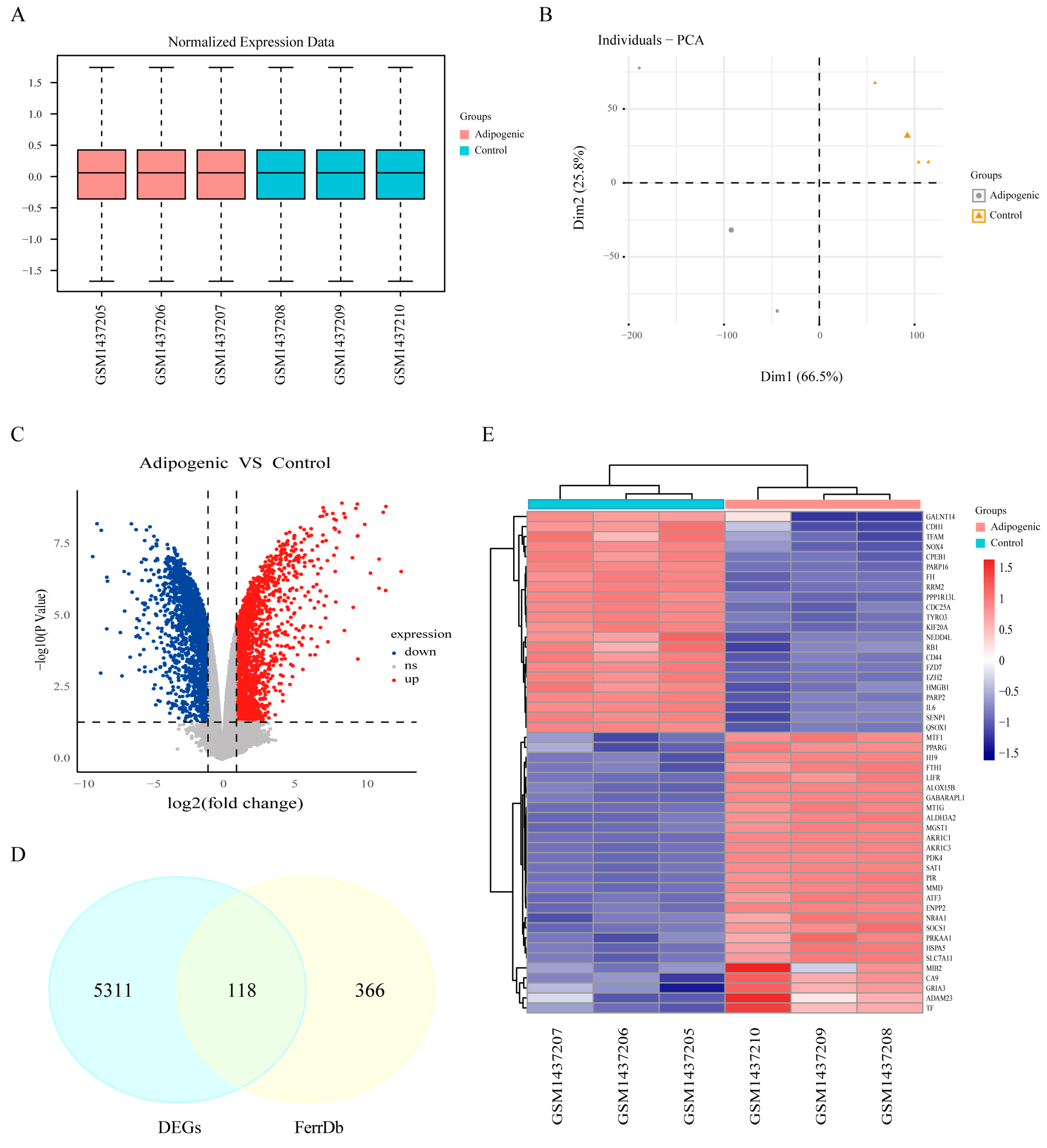
3.2. Identification of Ferroptosis-Related Differentially Expressed Genes
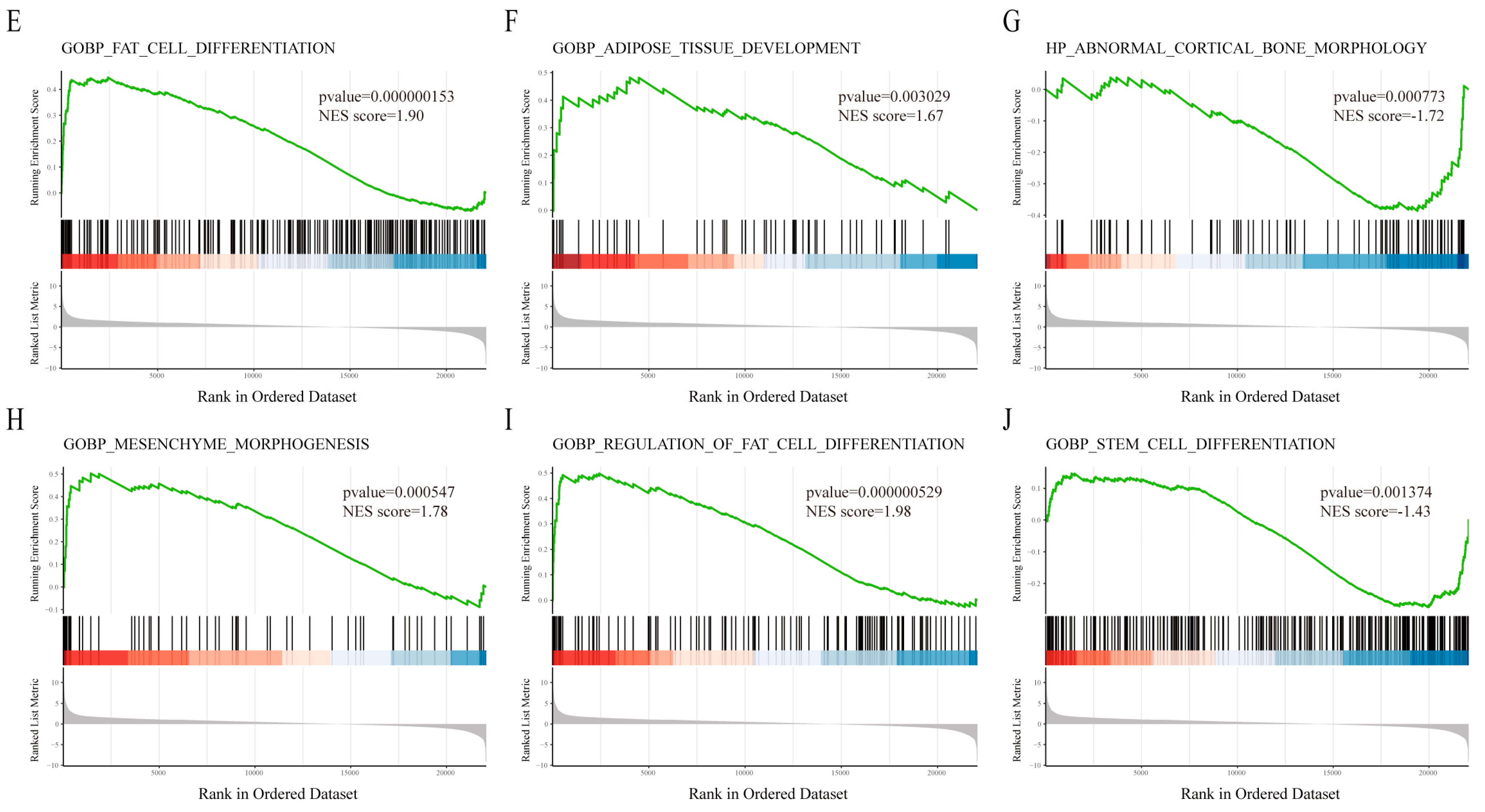
3.3. Functional Enrichment Analysis
3.4. PPI Network Construction and Identification of Hub Genes
3.5. Validation of the Diagnostic Value of Ferroptosis-Related Hub Genes
3.6. Validation of the Expression of Ferroptosis-Related Hub Genes
3.7. Prediction of Drugs Affecting the Expression of Ferroptosis-Related Hub Genes
3.8. Molecular Docking Analysis and Functional Validation of the Candidate Drugs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Han, L.; Wang, B.; Wang, R.; Gong, S.; Chen, G.; Xu, W. The shift in the balance between osteoblastogenesis and adipogenesis of mesenchymal stem cells mediated by glucocorticoid receptor. Stem Cell Res. Ther. 2019, 10, 377. [Google Scholar] [CrossRef] [PubMed]
- Infante, A.; Rodríguez, C.I. Osteogenesis and aging: Lessons from mesenchymal stem cells. Stem Cell Res. Ther. 2018, 9, 244. [Google Scholar] [CrossRef] [PubMed]
- Kawai, M.; Devlin, M.J.; Rosen, C.J. Fat targets for skeletal health. Nat. Rev. Rheumatol. 2009, 5, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Muruganandan, S.; Roman, A.A.; Sinal, C.J. Adipocyte differentiation of bone marrow-derived mesenchymal stem cells: Cross talk with the osteoblastogenic program. Cell. Mol. Life Sci. 2009, 66, 236–253. [Google Scholar] [CrossRef]
- Kwiatkowski, S.; Seliga, A.K.; Vertommen, D.; Terreri, M.; Ishikawa, T.; Grabowska, I.; Tiebe, M.; Teleman, A.A.; Jagielski, A.K.; Veiga-Da-Cunha, M.; et al. SETD3 protein is the actin-specific histidine N-methyltransferase. eLife 2018, 7, e37921. [Google Scholar] [CrossRef]
- Davydova, E.; Shimazu, T.; Schuhmacher, M.K.; Jakobsson, M.E.; Willemen, H.L.D.M.; Liu, T.; Moen, A.; Ho, A.Y.Y.; Małecki, J.; Schroer, L.; et al. The methyltransferase METTL9 mediates pervasive 1-methylhistidine modification in mammalian proteomes. Nat. Commun. 2021, 12, 891. [Google Scholar] [CrossRef]
- Lv, M.; Cao, D.; Zhang, L.; Hu, C.; Li, S.; Zhang, P.; Zhu, L.; Yi, X.; Li, C.; Yang, A.; et al. METTL9 mediated N1-histidine methylation of zinc transporters is required for tumor growth. Protein Cell 2021, 12, 965–970. [Google Scholar] [CrossRef]
- Paccou, J.; Penel, G.; Chauveau, C.; Cortet, B.; Hardouin, P. Marrow adiposity and bone: Review of clinical implications. Bone 2019, 118, 8–15. [Google Scholar] [CrossRef]
- Di Iorgi, N.; Mo, A.O.; Grimm, K.; Wren, T.A.; Dorey, F.; Gilsanz, V. Bone acquisition in healthy young females is reciprocally related to marrow adiposity. J. Clin. Endocrinol. Metab. 2010, 95, 2977–2982. [Google Scholar] [CrossRef]
- Yeung, D.K.; Griffith, J.F.; Antonio, G.E.; Lee, F.K.; Woo, J.; Leung, P.C. Osteoporosis is associated with increased marrow fat content and decreased marrow fat unsaturation: A proton MR spectroscopy study. J. Magn. Reson. Imaging 2005, 22, 279–285. [Google Scholar] [CrossRef]
- Limonard, E.J.; Veldhuis-Vlug, A.G.; van Dussen, L.; Runge, J.H.; Tanck, M.W.; Endert, E.; Heijboer, A.C.; Fliers, E.; Hollak, C.E.; Akkerman, E.M.; et al. Short-Term Effect of Estrogen on Human Bone Marrow Fat. J. Bone Miner. Res. 2015, 30, 2058–2066. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Chen, X.; Kang, R.; Kroemer, G. Ferroptosis: Molecular mechanisms and health implications. Cell Res. 2021, 31, 107–125. [Google Scholar] [CrossRef]
- Chen, X.; Kang, R.; Kroemer, G.; Tang, D. Broadening horizons: The role of ferroptosis in cancer. Nat. Rev. Clin. Oncol. 2021, 18, 280–296. [Google Scholar] [CrossRef]
- Chen, X.; Kang, R.; Kroemer, G.; Tang, D. Ferroptosis in infection, inflammation, and immunity. J. Exp. Med. 2021, 218, e20210518. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Chen, X.Z.; Wang, Y.H.; Cheng, X.L.; Zhao, Y.; Zhou, L.Y.; Wang, K. Emerging roles of ferroptosis in cardiovascular diseases. Cell Death Discov. 2022, 8, 394. [Google Scholar] [CrossRef]
- Yang, Y.; Lin, Y.; Wang, M.; Yuan, K.; Wang, Q.; Mu, P.; Du, J.; Yu, Z.; Yang, S.; Huang, K.; et al. Targeting ferroptosis suppresses osteocyte glucolipotoxicity and alleviates diabetic osteoporosis. Bone Res. 2022, 10, 26. [Google Scholar] [CrossRef]
- Ru, Q.; Li, Y.; Xie, W.; Ding, Y.; Chen, L.; Xu, G.; Wu, Y.; Wang, F. Fighting age-related orthopedic diseases: Focusing on ferroptosis. Bone Res. 2023, 11, 12. [Google Scholar] [CrossRef]
- Luo, C.; Xu, W.; Tang, X.; Liu, X.; Cheng, Y.; Wu, Y.; Xie, Z.; Wu, X.; He, X.; Wang, Q.; et al. Canonical Wnt signaling works downstream of iron overload to prevent ferroptosis from damaging osteoblast differentiation. Free Radic. Biol. Med. 2022, 188, 337–350. [Google Scholar] [CrossRef] [PubMed]
- Mandrekar, J.N. Receiver operating characteristic curve in diagnostic test assessment. J. Thorac. Oncol. 2010, 5, 1315–1316. [Google Scholar] [CrossRef]
- Yu, W.; Xie, Z.; Li, J.; Lin, J.; Su, Z.; Che, Y.; Ye, F.; Zhang, Z.; Xu, P.; Zeng, Y.; et al. Super enhancers targeting ZBTB16 in osteogenesis protect against osteoporosis. Bone Res. 2023, 11, 30. [Google Scholar] [CrossRef]
- Miotto, G.; Rossetto, M.; Di Paolo, M.L.; Orian, L.; Venerando, R.; Roveri, A.; Vučković, A.-M.; Travain, V.B.; Zaccarin, M.; Zennaro, L.; et al. Insight into the mechanism of ferroptosis inhibition by ferrostatin-1. Redox Biol. 2020, 28, 101328. [Google Scholar] [CrossRef] [PubMed]
- Meunier, P.; Aaron, J.; Edouard, C.; Vlgnon, G. Osteoporosis and the replacement of cell populations of the marrow by adipose tissue. A quantitative study of 84 iliac bone biopsies. Clin. Orthop. Relat. Res. 1971, 80, 147–154. [Google Scholar] [CrossRef]
- Moerman, E.J.; Teng, K.; Lipschitz, D.A.; Lecka-Czernik, B. Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: The role of PPAR-gamma2 transcription factor and TGF-β/BMP signaling pathways. Aging Cell 2004, 3, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, S.H.; Chen, W.; Meng, H.; Jahan, H.; Liu, Y.; Hasan, S.M. Prediction of drug-target interaction based on protein features using undersampling and feature selection techniques with boosting. Anal. Biochem. 2020, 589, 113507. [Google Scholar] [CrossRef]
- Lenneman, C.G.; Sawyer, D.B. Cardio-Oncology: An Update on Cardiotoxicity of Cancer-Related Treatment. Circ. Res. 2016, 118, 1008–1020. [Google Scholar] [CrossRef]
- Nian, Q.; Liu, R.; Zeng, J. Unraveling the pathogenesis of myelosuppression and therapeutic potential of natural products. Phytomedicine 2024, 132, 155810. [Google Scholar] [CrossRef]
- Dutta, B.J.; Rakshe, P.S.; Maurya, N.; Chib, S.; Singh, S. Unlocking the therapeutic potential of natural stilbene: Exploring pterostilbene as a powerful ally against aging and cognitive decline. Ageing Res. Rev. 2023, 92, 102125. [Google Scholar] [CrossRef] [PubMed]
- Gowri, M.S.; Reaven, G.M.; Azhar, S. Effect of masoprocol on glucose transport and lipolysis by isolated rat adipocytes. Metabolism 1999, 48, 411–414. [Google Scholar] [CrossRef]
- White, L. Osteoporosis Prevention, Screening, and Diagnosis: ACOG Recommendations. Am. Fam. Physician 2022, 106, 587–588. [Google Scholar]
- Ning, K.; Liu, S.; Yang, B.; Wang, R.; Man, G.; Wang, D.-E.; Xu, H. Update on the effects of energy metabolism in bone marrow mesenchymal stem cells differentiation. Mol. Metab. 2022, 58, 101450. [Google Scholar] [CrossRef]
- Yan, C.; Zhang, J.; An, F.; Wang, J.; Shi, Y.; Yuan, L.; Lv, D.; Zhao, Y.; Wang, Y. Research Progress of Ferroptosis Regulatory Network and Bone Remodeling in Osteoporosis. Front. Public Health 2022, 10, 910675. [Google Scholar] [CrossRef]
- Finkel, T. Signal transduction by reactive oxygen species. J. Cell Biol. 2011, 194, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Li, N.; Cheng, H.; Yen, M. Oxidative stress induces imbalance of adipogenic/osteoblastic lineage commitment in mesenchymal stem cells through decreasing SIRT1 functions. J. Cell. Mol. Med. 2018, 22, 786–796. [Google Scholar] [CrossRef]
- Atashi, F.; Modarressi, A.; Pepper, M.S. The role of reactive oxygen species in mesenchymal stem cell adipogenic and osteogenic differentiation: A review. Stem Cells Dev. 2015, 24, 1150–1163. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Kon, N.; Li, T.; Wang, S.-J.; Su, T.; Hibshoosh, H.; Baer, R.; Gu, W. Ferroptosis as a p53-mediated activity during tumour suppression. Nature 2015, 520, 57–62. [Google Scholar] [CrossRef]
- Boregowda, S.V.; Krishnappa, V.; Strivelli, J.; Haga, C.L.; Booker, C.N.; Phinney, D.G. Basal p53 expression is indispensable for mesenchymal stem cell integrity. Cell Death Differ. 2018, 25, 679–692. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; You, X.; Zhou, H.; Kang, A.; He, W.; Li, Z.; Li, B.; Xia, J.; Zhu, H.; Zhao, Y.; et al. p53 plays a central role in the development of osteoporosis. Aging 2020, 12, 10473–10487. [Google Scholar] [CrossRef]
- Poursaitidis, I.; Wang, X.; Crighton, T.; Labuschagne, C.; Mason, D.; Cramer, S.L.; Triplett, K.; Roy, R.; Pardo, O.E.; Seckl, M.J.; et al. Oncogene-Selective Sensitivity to Synchronous Cell Death following Modulation of the Amino Acid Nutrient Cystine. Cell Rep. 2017, 18, 2547–2556. [Google Scholar] [CrossRef]
- Chandra, A.; Lan, S.; Zhu, J.; Siclari, V.A.; Qin, L. Epidermal growth factor receptor (EGFR) signaling promotes proliferation and survival in osteoprogenitors by increasing early growth response 2 (EGR2) expression. J. Biol. Chem. 2013, 288, 20488–20498. [Google Scholar] [CrossRef]
- Liu, G.; Xie, Y.; Su, J.; Qin, H.; Wu, H.; Li, K.; Yu, B.; Zhang, X. The role of EGFR signaling in age-related osteoporosis in mouse cortical bone. FASEB J. 2019, 33, 11137–11147. [Google Scholar] [CrossRef]
- Lai, Y.; Han, X.; Xie, B.; Xu, Y.; Yang, Z.; Wang, D.; Li, W.; Xie, Y.; Song, W.; Zhang, X.; et al. EZH2 suppresses ferroptosis in hepatocellular carcinoma and reduces sorafenib sensitivity through epigenetic regulation of TFR2. Cancer Sci. 2024, 115, 2220–2234. [Google Scholar] [CrossRef]
- Jing, H.; Liao, L.; An, Y.; Su, X.; Liu, S.; Shuai, Y.; Zhang, X.; Jin, Y. Suppression of EZH2 Prevents the Shift of Osteoporotic MSC Fate to Adipocyte and Enhances Bone Formation During Osteoporosis. Mol. Ther. 2016, 24, 217–229. [Google Scholar] [CrossRef]
- Li, H.; Li, D.; Ma, Z.; Qian, Z.; Kang, X.; Jin, X.; Li, F.; Wang, X.; Chen, Q.; Sun, H.; et al. Defective autophagy in osteoblasts induces endoplasmic reticulum stress and causes remarkable bone loss. Autophagy 2018, 14, 1726–1741. [Google Scholar] [CrossRef] [PubMed]
- He, S.; He, Y.; Deng, L.; Guo, Y.; Wang, X.; Wang, Q.; Luo, L.; Liu, Q. Identification of RRM2 as a key ferroptosis-related gene in sepsis. Inflamm. Res. 2024, 73, 459–473. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lin, J.; Guo, S.; Xue, X.; Wang, Y.; Qiu, S.; Cui, J.; Ma, L.; Zhang, X.; Wang, J. RRM2 protects against ferroptosis and is a tumor biomarker for liver cancer. Cancer Cell Int. 2020, 20, 587. [Google Scholar] [CrossRef]
- Cai, H.; Guo, H.; Deng, Y.; Jiang, J.; Liu, S.; He, W.; Jian, H. RRM2 regulates osteogenesis of mouse embryo fibroblasts via the Wnt/β-catenin signaling pathway. Exp. Ther. Med. 2022, 24, 605. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Tian, F. STAT3-mediated osteogenesis and osteoclastogenesis in osteoporosis. Cell Commun. Signal 2022, 20, 112. [Google Scholar] [CrossRef]
- Lerbs, T.; Cui, L.; Muscat, C.; Saleem, A.; van Neste, C.; Domizi, P.; Chan, C.; Wernig, G. Expansion of Bone Precursors through Jun as a Novel Treatment for Osteoporosis-Associated Fractures. Stem Cell Rep. 2020, 14, 603–613. [Google Scholar] [CrossRef]
- Chen, S.; Pan, Y.; Guo, Y.; Sun, X.; Bai, X.; Liu, M.; Wang, L.; Xiao, J.; Chen, C.; Ma, Y. Integrative bioinformatics and experimental analysis of curcumin’s role in regulating ferroptosis to combat osteoporosis. Biochem. Biophys. Res. Commun. 2024, 739, 150949. [Google Scholar] [CrossRef]
- Remsberg, C.M.; Yáñez, J.A.; Ohgami, Y.; Vega-Villa, K.R.; Rimando, A.M.; Davies, N.M. Pharmacometrics of pterostilbene: Preclinical pharmacokinetics and metabolism, anticancer, antiinflammatory, antioxidant and analgesic activity. Phytother. Res. 2008, 22, 169–179. [Google Scholar] [CrossRef]
- Yu, Z.; Wang, S.; Zhang, X.; Li, Y.; Zhao, Q.; Liu, T. Pterostilbene protects against myocardial ischemia/reperfusion injury via suppressing oxidative/nitrative stress and inflammatory response. Int. Immunopharmacol. 2017, 43, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Pari, L.; Satheesh, M.A. Effect of pterostilbene on hepatic key enzymes of glucose metabolism in streptozotocin- and nicotinamide-induced diabetic rats. Life Sci. 2006, 79, 641–645. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-C.; Chang, Y.-T.; Cheng, Y.-H.; Pranata, R.; Hsu, H.-H.; Chen, Y.-L.; Chen, R.-J. Pterostilbene Protects against Osteoarthritis through NLRP3 Inflammasome Inactivation and Improves Gut Microbiota as Evidenced by In Vivo and In Vitro Studies. J. Agric. Food Chem. 2024, 72, 9150–9163. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Chuang, T.; Cheung, J.; Quan, J.; Tsai, J.; Sullivan, C.; Hector, R.F.; Reed, M.J.; Meszaros, K.; King, S.R.; et al. Masoprocol (nordihydroguaiaretic acid): A new antihyperglycemic agent isolated from the creosote bush (Larrea tridentata). Eur. J. Pharmacol. 1998, 346, 77–79. [Google Scholar] [CrossRef]
- Gowri, M.S.; Reaven, G.M.; Azhar, S. Masoprocol lowers blood pressure in rats with fructose-induced hypertension. Am. J. Hypertens. 1999, 12, 744–746. [Google Scholar] [CrossRef]






| Drug Names | p-Value | Adjusted p-Value | Genes |
|---|---|---|---|
| Gefitinib CTD 00003879 | 1.65 × 10−8 | 1.24 × 10−5 | STAT3; PTGS2; ATG7; TP53 |
| Doxorubicin CTD 00005874 | 1.85 × 10−8 | 1.24 × 10−5 | RRM2; STAT3; PTGS2; ATG7; TP53; EZH2 |
| Thalidomide CTD 00006858 | 8.15 × 10−8 | 3.65 × 10−5 | RRM2; STAT3; PTGS2; TP53 |
| Staurosporine CTD 00007273 | 1.73 × 10−7 | 5.80 × 10−5 | STAT3; PTGS2; ATG7; TP53 |
| Piroxicam CTD 00006571 | 3.07 × 10−7 | 8.25 × 10−5 | RRM2; STAT3; PTGS2; TP53; EZH2 |
| EINECS 250-892-2 CTD 00001193 | 5.13 × 10−7 | 1.15 × 10−4 | RRM2; STAT3; PTGS2; TP53 |
| Pterostilbene CTD 00003490 | 8.46 × 10−7 | 1.42 × 10−4 | STAT3; PTGS2; TP53 |
| Masoprocol CTD 00006416 | 1.19 × 10−6 | 1.78 × 10−4 | STAT3; PTGS2; TP53 |
| 3-Methyladenine BOSS | 8.46 × 10−7 | 1.42 × 10−4 | STAT3; ATG7; TP53 |
| 170449-18-0 CTD 00003361 | 1.42 × 10−6 | 1.91 × 10−4 | STAT3; PTGS2; TP53 |
| Compound | PTGS2 | TP53 | STAT3 |
|---|---|---|---|
| Masoprocol | −8.8 | −8.3 | −6.6 |
| Pterostilbene | −8.1 | −7.4 | −6.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, J.; Yuan, Z.; Wang, X.; Li, Q.; Zhang, Y.; Zhang, Y.; Ji, P.; Wu, Y.; Wang, P.; Liu, W. Bioinformatics-Based Analysis of Ferroptosis-Related Biomarkers and the Prediction of Drugs Affecting the Adipogenic Differentiation of MSCs. Biomedicines 2025, 13, 940. https://doi.org/10.3390/biomedicines13040940
Jin J, Yuan Z, Wang X, Li Q, Zhang Y, Zhang Y, Ji P, Wu Y, Wang P, Liu W. Bioinformatics-Based Analysis of Ferroptosis-Related Biomarkers and the Prediction of Drugs Affecting the Adipogenic Differentiation of MSCs. Biomedicines. 2025; 13(4):940. https://doi.org/10.3390/biomedicines13040940
Chicago/Turabian StyleJin, Jiahao, Zihao Yuan, Xinglang Wang, Quanfeng Li, Yunhui Zhang, Yibin Zhang, Pengfei Ji, Yanfeng Wu, Peng Wang, and Wenjie Liu. 2025. "Bioinformatics-Based Analysis of Ferroptosis-Related Biomarkers and the Prediction of Drugs Affecting the Adipogenic Differentiation of MSCs" Biomedicines 13, no. 4: 940. https://doi.org/10.3390/biomedicines13040940
APA StyleJin, J., Yuan, Z., Wang, X., Li, Q., Zhang, Y., Zhang, Y., Ji, P., Wu, Y., Wang, P., & Liu, W. (2025). Bioinformatics-Based Analysis of Ferroptosis-Related Biomarkers and the Prediction of Drugs Affecting the Adipogenic Differentiation of MSCs. Biomedicines, 13(4), 940. https://doi.org/10.3390/biomedicines13040940






