CD8+ T Cell Subsets as Biomarkers for Predicting Checkpoint Therapy Outcomes in Cancer Immunotherapy
Abstract
1. Immune Checkpoint Blockade—Still an Open Road in Cancer Therapy
2. Molecular and Cellular Drivers of Cancer Progression as Modulators of the Immune Response
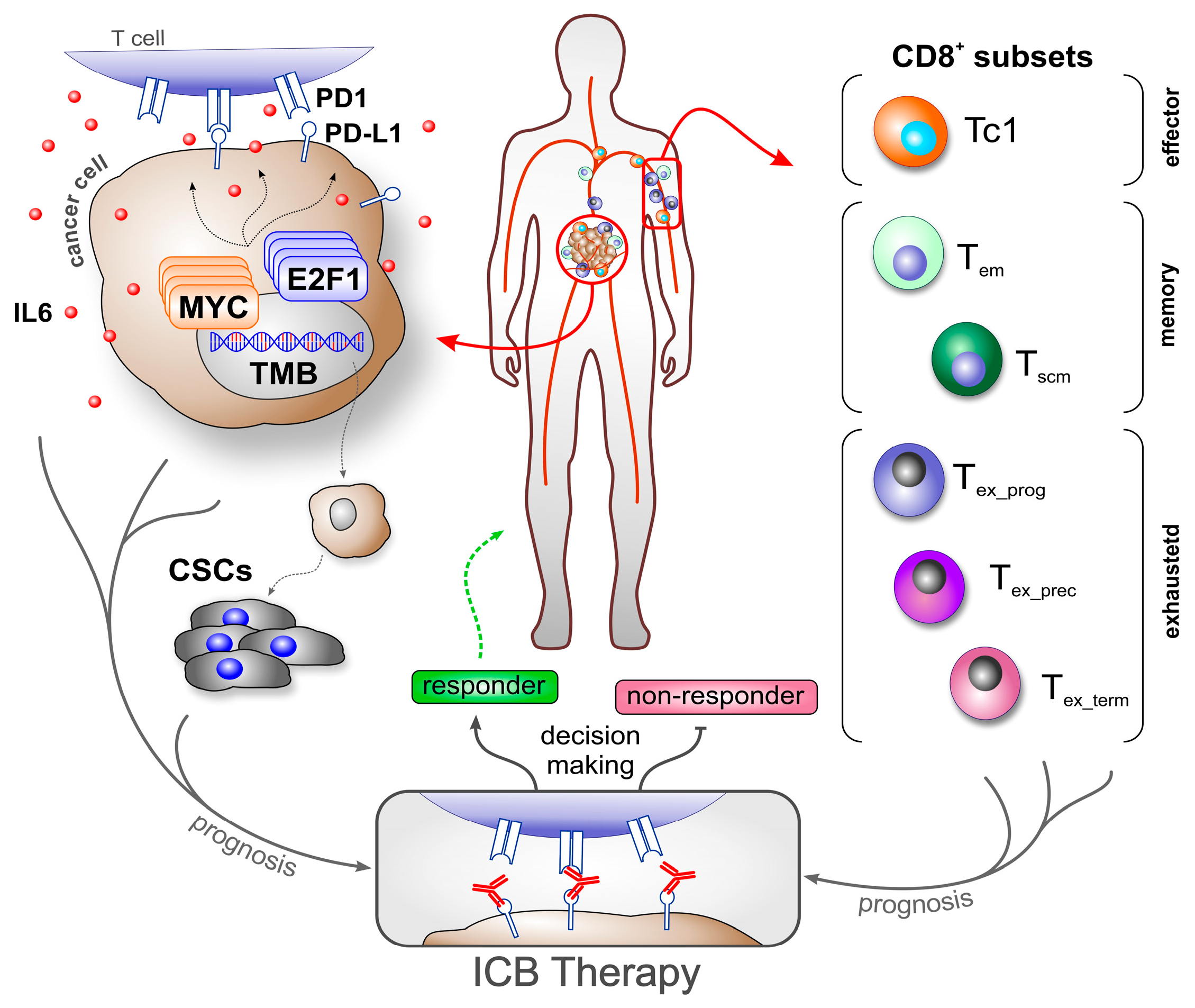
3. CD8+ T Cell Heterogeneity in Cancer—Using Population Subsets as Biomarkers
3.1. Pan CD8+ T Cells
3.2. Memory CD8+ T Cells
3.3. Exhausted CD8+ T Cells
3.4. T Cell Distribution in the TME
3.5. Bottlenecks in Biomarker Application—Conflicting Roles of CD8+ T Cells
| Entity | Therapy | Subset | Biomarker | Origin | Technique | Conclusion | Ref. |
|---|---|---|---|---|---|---|---|
| NSCLC | Anti PD1 and ChTx | Precursor exhausted | CXCL13+ TIM3- CD8+ TILs | T | scTCR-seq | Increased precursor exhausted CD8+ T cells in responsive tumors after treatment. | [53] |
| adv. SKCM | Anti PD1 and CTLA-4 | Progenitor exhausted | PD1+ TCF1+ CD8+ TILs | T | Quantitative multiplex immunofluorescence in pre- and post-treatment biopsies | No significant difference in PD1+ TCF1+ CD8+ T cell frequencies were seen in pre-treatment biopsies of responsive tumors vs. non-responsive. However, increased frequency of the studied subset was significantly associated with clinical outcome. | [9] |
| met. SKCM | ICB agents | TCF1+ CD8+ TILs | scRNA-seq and IHC of tumor samples | TCF1+ CD8+ TILs predict response to therapy and were correlated to a positive outcome in patients. TCF7+ CD8+ T cells were enriched in tumor biopsies obtained from metastatic SKCM patients responding to ICB treatment. | [78] | ||
| NSCLC | Anti PD1/PD-L1 | Terminally exhausted | HPK1+ PD1+ TIM3+ CD8+ TILs | T | FFPE-stained tissue by multiplex immunofluorescence | High infiltration of HPK1+ PD1+ TIM3+ CD8+ TILs correlated to poor prognosis in patients receiving ICB. | [84] |
| adv. NSCLC | Anti PD1 | Eomes+ PD1+ CD8+ T cells | B | FC of PB at baseline and during treatment | Low percentage of circulating Eomes+ PD1+ CD8+ associated with an improved outcome. Higher levels of CD8+ T cells correlated with longer OS and PFS. No correlation was found in patient survival and CD8+ ratio relative to a specific CD4+ Treg subset. | [97] | |
| NSCLC | Anti PD1/PD-L1 | Exhausted | CD39+ CD8+ TILs | T | Multiplex IHC or immunofluorescence | Higher proportion of CD39+ CD8+ TILs found in responders to therapy. | [89] |
| adv. NSCLC | PD1+ CD8+ T cells | B | FC of PB at baseline | Low frequencies of baseline PD-1+ CD8+ and NK cells combined with high plasma sPD-L1 was negatively associated with therapy response. | [88] | ||
| adv. NSCLC | Anti PD1 | CD39+ CD8+ T cells | FC of PB at baseline and follow-ups | Lower frequencies of CD39+ CD8+ T cells associated with better OS. Lower frequencies of both circulating CD39+ CD8+ T cells and monocytic MDSCs showed a stronger correlation with OS. | [92] | ||
| NSCLC and GC | PD1+ CD8+ TILs | T | FC, CyTOF | Increased frequencies of PD1+ CD8+ T cells in the TME were associated with better outcomes. | [31] | ||
| adv. SKCM | CD73+ PD1+ CD8+; PD1+ CD8+ T | B | FC at baseline before treatment | Low frequency of circulating CD73+ PD1+ CD8+ and PD1+ CD8+ at baseline associated with clinical benefit of therapy. | [32] | ||
| met. SKCM | PD-1hi CTLA-4hi CD8+ TILs | T | FC of tumor samples pre- and post-treatment | Increased frequencies of PD1hi CTLA-4hi CD8 TILs strongly correlated with response to therapy. | [86] | ||
| adv. NSCLC | Anti PD1/PD-L1 | PD1+ CD8+ T cells | B | FC of PB at baseline | Low frequencies of baseline PD-1+ CD8+ and NK cells combined with high plasma sPD-L1 were negatively associated with therapy response. | [88] | |
| various | FC of PBMCs at baseline, and week 6 and 20 post-treatment | High frequencies of circulating PD1+ CD8+ at baseline correlated to a better outcome. | [33] | ||||
| adv. SKCM | Anti PD1 and LAG3 | CD38+ TIM3+ CD8+ T cells | B | FC at baseline and 4 weeks after treatment | Increased frequency of CD38+ TIM3+ CD8+ T cells following treatment. | [54] | |
| met. SKCM | Anti PD1 and LAG3 (+/−prior anti PD1/CTLA-4) | LAG3+ CD8+ T cells | B | scRNA, TCR-seq, FC in pre-treatment, and 4 and 12 weeks PB after therapy | Increased frequency of LAG3+ CD8+ cells in responders after treatment. | [66] | |
| NSCLC and GC | Anti PD1 | Exhausted vs. immunosuppressive | PD1 expression in CD8+ and CD4+ Tregs | T | FC, CyTOF | Higher PD1 expression in CD8+ T cells and lower expression of PD1 in CD4+ Tregs correlated to a favorable antitumor response. | [31] |
| met. SKCM | Anti CTLA-4 | Tem | CCR7- CD45RO+ CD8+ T cells | B | FC of PBMCs pre- and post-treatment | SKCM patients responding to CTLA-4 blocking therapy had a higher ratio of CCR7- CD45RO+ CD8+ memory cells compared to baseline. | [76] |
| adv. SKCM | CD27+ CD28 + CD8+ T cells | FC of PBMCs before treatment and follow-ups | High effector memory CD8+ T cell frequencies at baseline correlated with good clinical outcome. | [68] | |||
| CD45RA- CCR7- CD8+ T cells | FC of PB before, during, and at the end of treatment | Increased CD8+ Tem cell frequencies at the end of the treatment correlated with better OS and clinical response. | [77] | ||||
| various | Anti PD1/PD-L1 | FC of PBMCs at baseline, and week 6 and 20 post-treatment | Baseline CD8+ Tem cell frequencies correlate with better OS and clinical response. | [33] | |||
| adv. NSCLC | Tscm | CD45RA+ CD95+ CD62L+ CD45RO- CD8+ T cells | B | FC of PB | Therapy responders had higher counts of CD8+ Tscm prior to therapy. | [79] | |
| met. SKCM | Anti PD1 | Trm | CD103+ CD8+ TILs | T | FC and quantitative multiplex immunofluorescence on treatment-naive and undergoing tumor samples | CD103+ CD8+ T cells in the TME expanded after therapy. Patients showed improved survival. | [81] |
| adv. HNSCC | Anti PD1 and ChTx | scRNA-seq, FC, multiplex immunofluorescence of FFPE tumor samples before treatment | Increased CD103+ CD8+ TIL density in patients responding to therapy. | [80] | |||
| adv. NSCLC | Anti PD1 | Migratory | CXCR4+ CD8+ T cells | B | FC of PBMCs before therapy | High frequencies of peripheral CXCR4+ CD8+ T cells in treatment-naive patients correlated to worse OS. | [98] |
| Anti PD1 and ChTx | CX3CR1+ CD8+ T cells | FC of PBMCs at baseline and follow-ups | CX3CR1+ CD8+ T cells correlate with clinical benefit. | [72] | |||
| NSCLC | Anti PD1 | At least 20% increase in circulating CX3CR1+ CD8+ T cells correlated to clinical benefit. This subset can be used as an early on-treatment biomarker. | [71] | ||||
| various | Anti PD1/PD-L1 | CD28+ CD8+ | CD28+ CD8+ T cells | B | FC of PB at baseline before Tx | Higher frequencies of circulating CD28+ CD8+ T cells were associated with responsive patients who received blocking of the PD1/PDL1 pathway. Excessive accounts of the measured subset are indicative of severe irAEs. | [69] |
| FC of PBMCs at baseline, and week 6 and 20 post-treatment | No correlation between clinical outcome and circulating CD28+ CD8 T cells. | [33] | |||||
| met. SKCM | Anti PD1 | Pan CD8 | CD8+ TILs | T | Quantitative IHC, quantitative multiplex immunofluorescence, and NGS for TCR performed pre- and during treatment tumor samples | Reduction in tumor correlates to proliferation of CD8+ TILs. Association of CD8+ TILs at the invasive margin of met. SKCM tumors and clinical benefit. Less diverse TCR repertoire associated with a better outcome. | [49] |
| NSCLC | Anti PD1 and ChTx | scRNA-seq, IHC | Higher frequencies of CD8+ T cells in responsive tumors. | [53] | |||
| adv. TNBC | Anti PD-L1 and ChTx | IHC of serial tumor biopsies | CD8+ TILs were not predictive of the therapeutic efficiency. | [100] | |||
| various | ICB agents | Meta-analysis | Higher accounts of CD8+ T cells in either intratumor and/or stroma showed a better OS and PFS in ICB-treated patients; however, stromal was a stronger biomarker. | [52] | |||
| met. SKCM | Anti CTLA-4 | CD8+ T cell ratio in TME | IHC of serial tumor biopsies | CD8+ TIL density was higher in early on-treatment tumors from responders vs. non-responders. | [51] | ||
| Anti PD1 (+/− prior CTLA-4) | Patients responsive to therapy a had higher CD8+ T cell ratio at the tumor core relative to the invasive margin in early on-treatment biopsies. | ||||||
| NSCLC | Anti PD1/PD-L1 | CD8+ T cells | B | FC and RNA-seq of PB at baseline | Fewer circulating CD8+ T cells were associated with successful therapy. | [99] | |
| adv. SKMC | Anti PD1 and LAG3 | CD8+ T cells | B/T | scRNA at baseline and 4 and 16 weeks after treatment | Combined ICB therapy improved cytotoxicity and TCR signaling despite persistence of the exhausted phenotype. | [54] | |
| adv. HNSCC | Anti-PD1 +/− LAG-3/CTLA-4 | CD8+ TILs | T | scRNA-seq, scTCR-seq, CITE-seq, and mIF of PPFE at baseline and post-treatment | Anti PD1 and LAG-3 therapy reactivates exhausted CD8+ TILs and increases TCR diversity and CD8+ TILs. Anti-PD1 and CTLA-4 therapy does not change the exhausted phenotype, and rather increases Tem and Trm CD8+ TILs. | [59] | |
| NSCLC | Anti TIGIT and PD-L1 | CD8+ T cells | B/T | Bulk RNA-seq of pre-treatment tumor samples; scRNA-seq of pre-treatment PBMCs, and 2, 3, and 9 weeks after treatment | Treatment resulted in increased frequency of circulating non-naive CD8+ T cells. Improved OS and PFS after combined ICB therapy associated with CD8+ effector TILs. | [55] | |
| various | Anti PD1/PD-L1 and/or CTLA-4 | CD8+ cells (mostly T cells) | 89Zr-labeled CD8+ cells | T * | PET scans tracking 89Zr-labeled CD8+ T cells before and approx. 30 days after treatment. Corroboration by IHC staining of CD8 T cells in tumors before and during treatment | Tracking biodistribution of 89Zr-labeled CD8 T cells in cancer patients. Patients with higher tracker uptake had better OS. | [101] |
| NSCLC | Anti PD1 | PD1+ cells (mostly T cells) | 89Zr-labeled PD1+ cells | PET scans tracking 89Zr-labeled PD1-expressing cells at 2, 4 and 7 days post injection. | Uptake of 89Zr-labeled PD1 correlated with OS, PFS and response to therapy. | [102] | |
| PET scans tracking 89Zr-labeled PD1-expressing cells | 89Zr-labeled anti-PD1 uptake correlated to clinical response without statistical significance. | [103] |
4. Converging Pathways: Novel Roads and Integrative Strategies to Enhance T Cell Response Prediction in ICB Therapy
4.1. Determination of T Cell Subset Ratios in Cancer Patients
4.2. TCR Profiling
4.3. PET Imaging of CD8+ T Cells Biodistribution
4.4. Three-Dimensional Co-Culture Systems: Advancing TME Modeling and Cancer–Immune Crosstalk
5. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AdV | Adenoviral vector |
| CAF | Cancer-associated fibroblast |
| CSC | Cancer stem cell |
| CyTOF | Cytometry by time of flight |
| DC | Dendritic cell |
| FC | Flow cytometry |
| FFPE | Formalin-fixed paraffin-embedded |
| GC | Gastric cancer |
| HNSCC | Head and neck squamous cell carcinoma |
| IHC | Immunohistochemistry |
| irAE | Immune-related adverse events |
| MDSCs | Myeloid-derived suppressor cells |
| NK | Natural killer cell |
| NSCLC | Non-small cell lung cancer |
| PB | Peripheral blood |
| PBMCs | peripheral blood mononuclear cells |
| PET | Positron emission tomography |
| scRNA-seq | Single cell RNA sequencing |
| SKCM | Skin cutaneous melanoma |
| TCR | T cell receptor |
| TILs | Tumor infiltrating lymphocytes |
| TME | Tumor microenvironment |
| TNBC | Triple-negative breast cancer |
References
- Bai, R.; Lv, Z.; Xu, D.; Cui, J. Predictive biomarkers for cancer immunotherapy with immune checkpoint inhibitors. Biomark. Res. 2020, 8, 34. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Olaoba, O.T.; Zhang, C.; Kimchi, E.T.; Staveley-O’Carroll, K.F.; Li, G. Cancer Immunotherapy and Delivery System: An Update. Pharmaceutics 2022, 14, 1630. [Google Scholar] [CrossRef]
- Spitschak, A.; Gupta, S.; Singh, K.P.; Logotheti, S.; Pützer, B.M. Drug Repurposing at the Interface of Melanoma Immunotherapy and Autoimmune Disease. Pharmaceutics 2022, 15, 83. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.-J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2019, 381, 1535–1546. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; He, Y.; He, W.; Wu, G.; Zhou, X.; Sheng, Q.; Zhong, W.; Lu, Y.; Ding, Y.; Lu, Q.; et al. Exhausted CD8+T Cells in the Tumor Immune Microenvironment: New Pathways to Therapy. Front. Immunol. 2021, 11, 622509. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, H.; Hsu, J.-M.; Sun, L.; Wang, S.-C.; Hung, M.-C. Advances and prospects of biomarkers for immune checkpoint inhibitors. Cell Rep. Med. 2024, 5, 101621. [Google Scholar] [CrossRef]
- Shiravand, Y.; Khodadadi, F.; Kashani, S.M.A.; Hosseini-Fard, S.R.; Hosseini, S.; Sadeghirad, H.; Ladwa, R.; O’Byrne, K.; Kulasinghe, A. Immune Checkpoint Inhibitors in Cancer Therapy. Curr. Oncol. 2022, 29, 3044–3060. [Google Scholar] [CrossRef]
- Shen, H.; Yang, E.S.-H.; Conry, M.; Fiveash, J.; Contreras, C.; Bonner, J.A.; Shi, L.Z. Predictive biomarkers for immune checkpoint blockade and opportunities for combination therapies. Genes Dis. 2019, 6, 232–246. [Google Scholar] [CrossRef]
- Miller, B.C.; Sen, D.R.; Al Abosy, R.; Bi, K.; Virkud, Y.V.; LaFleur, M.W.; Yates, K.B.; Lako, A.; Felt, K.; Naik, G.S.; et al. Subsets of exhausted CD8+ T cells differentially mediate tumor control and respond to checkpoint blockade. Nat. Immunol. 2019, 20, 326–336. [Google Scholar] [CrossRef]
- Wang, J.; Ma, Y.; Lin, H.; Wang, J.; Cao, B. Predictive biomarkers for immune-related adverse events in cancer patients treated with immune-checkpoint inhibitors. BMC Immunol. 2024, 25, 8. [Google Scholar] [CrossRef]
- Lamichhane, P.; Deshmukh, R.; Brown, J.A.; Jakubski, S.; Parajuli, P.; Nolan, T.; Raja, D.; Badawy, M.; Yoon, T.; Zmiyiwsky, M.; et al. Novel Delivery Systems for Checkpoint Inhibitors. Medicines 2019, 6, 74. [Google Scholar] [CrossRef] [PubMed]
- Spitschak, A.; Dhar, P.; Singh, K.P.; Casalegno Garduño, R.; Gupta, S.K.; Vera, J.; Musella, L.; Murr, N.; Stoll, A.; Pützer, B.M. E2F1-induced autocrine IL-6 inflammatory loop mediates cancer-immune crosstalk that predicts T cell phenotype switching and therapeutic responsiveness. Front. Immunol. 2024, 15, 1470368. [Google Scholar] [CrossRef] [PubMed]
- Luke, J.J.; Patel, M.R.; Blumenschein, G.R.; Hamilton, E.; Chmielowski, B.; Ulahannan, S.V.; Connolly, R.M.; Santa-Maria, C.A.; Wang, J.; Bahadur, S.W.; et al. The PD-1- and LAG-3-targeting bispecific molecule tebotelimab in solid tumors and hematologic cancers: A phase 1 trial. Nat. Med. 2023, 29, 2814–2824. [Google Scholar] [CrossRef] [PubMed]
- Berezhnoy, A.; Sumrow, B.J.; Stahl, K.; Shah, K.; Liu, D.; Li, J.; Hao, S.-S.; de Costa, A.; Kaul, S.; Bendell, J.; et al. Development and Preliminary Clinical Activity of PD-1-Guided CTLA-4 Blocking Bispecific DART Molecule. Cell Rep. Med. 2020, 1, 100163. [Google Scholar] [CrossRef]
- Dovedi, S.J.; Elder, M.J.; Yang, C.; Sitnikova, S.I.; Irving, L.; Hansen, A.; Hair, J.; Des Jones, C.; Hasani, S.; Wang, B.; et al. Design and Efficacy of a Monovalent Bispecific PD-1/CTLA4 Antibody That Enhances CTLA4 Blockade on PD-1+ Activated T Cells. Cancer Discov. 2021, 11, 1100–1117. [Google Scholar] [CrossRef]
- Fenis, A.; Demaria, O.; Gauthier, L.; Vivier, E.; Narni-Mancinelli, E. New immune cell engagers for cancer immunotherapy. Nat. Rev. Immunol. 2024, 24, 471–486. [Google Scholar] [CrossRef]
- Agudo, J.; Miao, Y. Stemness in solid malignancies: Coping with immune attack. Nat. Rev. Cancer 2025, 25, 27–40. [Google Scholar] [CrossRef]
- Bayik, D.; Lathia, J.D. Cancer stem cell-immune cell crosstalk in tumour progression. Nat. Rev. Cancer 2021, 21, 526–536. [Google Scholar] [CrossRef]
- Li, J.; Dong, T.; Wu, Z.; Zhu, D.; Gu, H. The effects of MYC on tumor immunity and immunotherapy. Cell Death Discov. 2023, 9, 103. [Google Scholar] [CrossRef]
- Yang, C.; Liu, Y.; Hu, Y.; Fang, L.; Huang, Z.; Cui, H.; Xie, J.; Hong, Y.; Chen, W.; Xiao, N.; et al. Myc inhibition tips the immune balance to promote antitumor immunity. Cell. Mol. Immunol. 2022, 19, 1030–1041. [Google Scholar] [CrossRef]
- Wang, D.; Quiros, J.; Mahuron, K.; Pai, C.-C.; Ranzani, V.; Young, A.; Silveria, S.; Harwin, T.; Abnousian, A.; Pagani, M.; et al. Targeting EZH2 Reprograms Intratumoral Regulatory T Cells to Enhance Cancer Immunity. Cell Rep. 2018, 23, 3262–3274. [Google Scholar] [CrossRef] [PubMed]
- Jayson, G.C.; Kerbel, R.; Ellis, L.M.; Harris, A.L. Antiangiogenic therapy in oncology: Current status and future directions. Lancet 2016, 388, 518–529. [Google Scholar] [CrossRef] [PubMed]
- Voron, T.; Colussi, O.; Marcheteau, E.; Pernot, S.; Nizard, M.; Pointet, A.-L.; Latreche, S.; Bergaya, S.; Benhamouda, N.; Tanchot, C.; et al. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J. Exp. Med. 2015, 212, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Voron, T.; Marcheteau, E.; Pernot, S.; Colussi, O.; Tartour, E.; Taieb, J.; Terme, M. Control of the immune response by pro-angiogenic factors. Front. Oncol. 2014, 4, 70. [Google Scholar] [CrossRef]
- Terme, M.; Pernot, S.; Marcheteau, E.; Sandoval, F.; Benhamouda, N.; Colussi, O.; Dubreuil, O.; Carpentier, A.F.; Tartour, E.; Taieb, J. VEGFA-VEGFR pathway blockade inhibits tumor-induced regulatory T-cell proliferation in colorectal cancer. Cancer Res. 2013, 73, 539–549. [Google Scholar] [CrossRef]
- Marquardt, S.; Solanki, M.; Spitschak, A.; Vera, J.; Pützer, B.M. Emerging functional markers for cancer stem cell-based therapies: Understanding signaling networks for targeting metastasis. Semin. Cancer Biol. 2018, 53, 90–109. [Google Scholar] [CrossRef]
- Alla, V.; Engelmann, D.; Niemetz, A.; Pahnke, J.; Schmidt, A.; Kunz, M.; Emmrich, S.; Steder, M.; Koczan, D.; Pützer, B.M. E2F1 in melanoma progression and metastasis. J. Natl. Cancer Inst. 2010, 102, 127–133. [Google Scholar] [CrossRef]
- Pützer, B.M.; Engelmann, D. E2F1 apoptosis counterattacked: Evil strikes back. Trends Mol. Med. 2013, 19, 89–98. [Google Scholar] [CrossRef]
- Khan, F.M.; Marquardt, S.; Gupta, S.K.; Knoll, S.; Schmitz, U.; Spitschak, A.; Engelmann, D.; Vera, J.; Wolkenhauer, O.; Pützer, B.M. Unraveling a tumor type-specific regulatory core underlying E2F1-mediated epithelial-mesenchymal transition to predict receptor protein signatures. Nat. Commun. 2017, 8, 198. [Google Scholar] [CrossRef]
- Goody, D.; Gupta, S.K.; Engelmann, D.; Spitschak, A.; Marquardt, S.; Mikkat, S.; Meier, C.; Hauser, C.; Gundlach, J.-P.; Egberts, J.-H.; et al. Drug Repositioning Inferred from E2F1-Coregulator Interactions Studies for the Prevention and Treatment of Metastatic Cancers. Theranostics 2019, 9, 1490–1509. [Google Scholar] [CrossRef]
- Kumagai, S.; Togashi, Y.; Kamada, T.; Sugiyama, E.; Nishinakamura, H.; Takeuchi, Y.; Vitaly, K.; Itahashi, K.; Maeda, Y.; Matsui, S.; et al. The PD-1 expression balance between effector and regulatory T cells predicts the clinical efficacy of PD-1 blockade therapies. Nat. Immunol. 2020, 21, 1346–1358. [Google Scholar] [CrossRef] [PubMed]
- Capone, M.; Fratangelo, F.; Giannarelli, D.; Sorrentino, C.; Turiello, R.; Zanotta, S.; Galati, D.; Madonna, G.; Tuffanelli, M.; Scarpato, L.; et al. Frequency of circulating CD8+CD73+T cells is associated with survival in nivolumab-treated melanoma patients. J. Transl. Med. 2020, 18, 121. [Google Scholar] [CrossRef] [PubMed]
- Araujo, B.; de Lima, V.; Hansen, M.; Spanggaard, I.; Rohrberg, K.; Reker Hadrup, S.; Lassen, U.; Svane, I.M. Immune Cell Profiling of Peripheral Blood as Signature for Response During Checkpoint Inhibition Across Cancer Types. Front. Oncol. 2021, 11, 558248. [Google Scholar] [CrossRef]
- Lei, Y.; Li, X.; Huang, Q.; Zheng, X.; Liu, M. Progress and Challenges of Predictive Biomarkers for Immune Checkpoint Blockade. Front. Oncol. 2021, 11, 617335. [Google Scholar] [CrossRef]
- Edwards, J.M.; Andrews, M.C.; Burridge, H.; Smith, R.; Owens, C.; Edinger, M.; Pilkington, K.; Desfrancois, J.; Shackleton, M.; Senthi, S.; et al. Design, optimisation and standardisation of a high-dimensional spectral flow cytometry workflow assessing T-cell immunophenotype in patients with melanoma. Clin. Transl. Immunol. 2023, 12, e1466. [Google Scholar] [CrossRef]
- Davis, A.A.; Patel, V.G. The role of PD-L1 expression as a predictive biomarker: An analysis of all US Food and Drug Administration (FDA) approvals of immune checkpoint inhibitors. J. Immunother. Cancer 2019, 7, 278. [Google Scholar] [CrossRef] [PubMed]
- Ricciuti, B.; Wang, X.; Alessi, J.V.; Rizvi, H.; Mahadevan, N.R.; Li, Y.Y.; Polio, A.; Lindsay, J.; Umeton, R.; Sinha, R.; et al. Association of High Tumor Mutation Burden in Non-Small Cell Lung Cancers with Increased Immune Infiltration and Improved Clinical Outcomes of PD-L1 Blockade Across PD-L1 Expression Levels. JAMA Oncol. 2022, 8, 1160–1168. [Google Scholar] [CrossRef]
- Zhou, K.I.; Peterson, B.; Serritella, A.; Thomas, J.; Reizine, N.; Moya, S.; Tan, C.; Wang, Y.; Catenacci, D.V.T. Spatial and Temporal Heterogeneity of PD-L1 Expression and Tumor Mutational Burden in Gastroesophageal Adenocarcinoma at Baseline Diagnosis and after Chemotherapy. Clin. Cancer Res. 2020, 26, 6453–6463. [Google Scholar] [CrossRef]
- Song, P.; Guo, L.; Li, W.; Zhang, F.; Ying, J.; Gao, S. Clinicopathologic Correlation with Expression of PD-L1 on Both Tumor Cells and Tumor-infiltrating Immune Cells in Patients with Non-Small Cell Lung Cancer. J. Immunother. 2019, 42, 23–28. [Google Scholar] [CrossRef]
- Dreyer, F.S.; Cantone, M.; Eberhardt, M.; Jaitly, T.; Walter, L.; Wittmann, J.; Gupta, S.K.; Khan, F.M.; Wolkenhauer, O.; Pützer, B.M.; et al. A web platform for the network analysis of high-throughput data in melanoma and its use to investigate mechanisms of resistance to anti-PD1 immunotherapy. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 2315–2328. [Google Scholar] [CrossRef]
- Casey, S.C.; Tong, L.; Li, Y.; Do, R.; Walz, S.; Fitzgerald, K.N.; Gouw, A.M.; Baylot, V.; Gütgemann, I.; Eilers, M.; et al. MYC regulates the antitumor immune response through CD47 and PD-L1. Science 2016, 352, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Zu, H.; Chen, X. Epigenetics behind CD8+ T cell activation and exhaustion. Genes Immun. 2024, 25, 525–540. [Google Scholar] [CrossRef] [PubMed]
- Koh, C.-H.; Lee, S.; Kwak, M.; Kim, B.-S.; Chung, Y. CD8 T-cell subsets: Heterogeneity, functions, and therapeutic potential. Exp. Mol. Med. 2023, 55, 2287–2299. [Google Scholar] [CrossRef]
- Casalegno Garduño, R.; Däbritz, J. New Insights on CD8+ T Cells in Inflammatory Bowel Disease and Therapeutic Approaches. Front. Immunol. 2021, 12, 738762. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Tsuchihashi, K.; Ueno, S.; Uehara, K.; Taguchi, R.; Ito, M.; Isobe, T.; Imajima, T.; Kitazono, T.; Tanoue, K.; et al. Efficacy of pembrolizumab in microsatellite-stable, tumor mutational burden-high metastatic colorectal cancer: Genomic signatures and clinical outcomes. ESMO Open 2025, 10, 104108. [Google Scholar] [CrossRef]
- Huang, A.C.; Postow, M.A.; Orlowski, R.J.; Mick, R.; Bengsch, B.; Manne, S.; Xu, W.; Harmon, S.; Giles, J.R.; Wenz, B.; et al. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature 2017, 545, 60–65. [Google Scholar] [CrossRef]
- Paijens, S.T.; Vledder, A.; de Bruyn, M.; Nijman, H.W. Tumor-infiltrating lymphocytes in the immunotherapy era. Cell. Mol. Immunol. 2021, 18, 842–859. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.-T.; Schlom, J.; Donahue, R.N. Blood-based biomarkers in patients with non-small cell lung cancer treated with immune checkpoint blockade. J. Exp. Clin. Cancer Res. 2024, 43, 82. [Google Scholar] [CrossRef]
- Tumeh, P.C.; Harview, C.L.; Yearley, J.H.; Shintaku, I.P.; Taylor, E.J.M.; Robert, L.; Chmielowski, B.; Spasic, M.; Henry, G.; Ciobanu, V.; et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014, 515, 568–571. [Google Scholar] [CrossRef]
- Geng, Y.; Shao, Y.; He, W.; Hu, W.; Xu, Y.; Chen, J.; Wu, C.; Jiang, J. Prognostic Role of Tumor-Infiltrating Lymphocytes in Lung Cancer: A Meta-Analysis. Cell. Physiol. Biochem. 2015, 37, 1560–1571. [Google Scholar] [CrossRef]
- Chen, P.-L.; Roh, W.; Reuben, A.; Cooper, Z.A.; Spencer, C.N.; Prieto, P.A.; Miller, J.P.; Bassett, R.L.; Gopalakrishnan, V.; Wani, K.; et al. Analysis of Immune Signatures in Longitudinal Tumor Samples Yields Insight into Biomarkers of Response and Mechanisms of Resistance to Immune Checkpoint Blockade. Cancer Discov. 2016, 6, 827–837. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Li, C.; Cai, X.; Xie, Z.; Zhou, L.; Cheng, B.; Zhong, R.; Xiong, S.; Li, J.; Chen, Z.; et al. The association between CD8+ tumor-infiltrating lymphocytes and the clinical outcome of cancer immunotherapy: A systematic review and meta-analysis. EClinicalMedicine 2021, 41, 101134. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Hu, X.; Feng, K.; Gao, R.; Xue, Z.; Zhang, S.; Zhang, Y.; Corse, E.; Hu, Y.; Han, W.; et al. Temporal single-cell tracing reveals clonal revival and expansion of precursor exhausted T cells during anti-PD-1 therapy in lung cancer. Nat. Cancer 2022, 3, 108–121. [Google Scholar] [CrossRef] [PubMed]
- Cillo, A.R.; Cardello, C.; Shan, F.; Karapetyan, L.; Kunning, S.; Sander, C.; Rush, E.; Karunamurthy, A.; Massa, R.C.; Rohatgi, A.; et al. Blockade of LAG-3 and PD-1 leads to co-expression of cytotoxic and exhaustion gene modules in CD8+ T cells to promote antitumor immunity. Cell 2024, 187, 4373–4388.e15. [Google Scholar] [CrossRef]
- Guan, X.; Hu, R.; Choi, Y.; Srivats, S.; Nabet, B.Y.; Silva, J.; McGinnis, L.; Hendricks, R.; Nutsch, K.; Banta, K.L.; et al. Anti-TIGIT antibody improves PD-L1 blockade through myeloid and Treg cells. Nature 2024, 627, 646–655. [Google Scholar] [CrossRef]
- Tawbi, H.A.; Schadendorf, D.; Lipson, E.J.; Ascierto, P.A.; Matamala, L.; Gutiérrez, E.C.; Rutkowski, P.; Gogas, H.J.; Lao, C.D.; de Menezes, J.J.; et al. Relatlimab and Nivolumab versus Nivolumab in Untreated Advanced Melanoma. N. Engl. J. Med. 2022, 386, 24–34. [Google Scholar] [CrossRef]
- Long, G.V.; Stephen Hodi, F.; Lipson, E.J.; Schadendorf, D.; Ascierto, P.A.; Matamala, L.; Salman, P.; Castillo Gutiérrez, E.; Rutkowski, P.; Gogas, H.J.; et al. Overall Survival and Response with Nivolumab and Relatlimab in Advanced Melanoma. N. Engl. J. Med. Evid. 2023, 2, EVIDoa2200239. [Google Scholar] [CrossRef]
- Tawbi, H.A.; Hodi, F.S.; Lipson, E.J.; Schadendorf, D.; Ascierto, P.A.; Matamala, L.; Castillo Gutiérrez, E.; Rutkowski, P.; Gogas, H.; Lao, C.D.; et al. Three-Year Overall Survival with Nivolumab Plus Relatlimab in Advanced Melanoma from RELATIVITY-047. J. Clin. Oncol. 2024, JCO2401124. [Google Scholar] [CrossRef]
- Li, H.; Zandberg, D.P.; Kulkarni, A.; Chiosea, S.I.; Santos, P.M.; Isett, B.R.; Joy, M.; Sica, G.L.; Contrera, K.J.; Tatsuoka, C.M.; et al. Distinct CD8+ T cell dynamics associate with response to neoadjuvant cancer immunotherapies. Cancer Cell 2025. [Google Scholar] [CrossRef]
- Andrews, L.P.; Butler, S.C.; Cui, J.; Cillo, A.R.; Cardello, C.; Liu, C.; Brunazzi, E.A.; Baessler, A.; Xie, B.; Kunning, S.R.; et al. LAG-3 and PD-1 synergize on CD8+ T cells to drive T cell exhaustion and hinder autocrine IFN-γ-dependent anti-tumor immunity. Cell 2024, 187, 4355–4372.e22. [Google Scholar] [CrossRef]
- Cho, B.C.; Abreu, D.R.; Hussein, M.; Cobo, M.; Patel, A.J.; Secen, N.; Lee, K.H.; Massuti, B.; Hiret, S.; Yang, J.C.H.; et al. Tiragolumab plus atezolizumab versus placebo plus atezolizumab as a first-line treatment for PD-L1-selected non-small-cell lung cancer (CITYSCAPE): Primary and follow-up analyses of a randomised, double-blind, phase 2 study. Lancet Oncol. 2022, 23, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Maurice-Dror, C.; Lee, D.H.; Kim, D.-W.; Nagrial, A.; Voskoboynik, M.; Chung, H.C.; Mileham, K.; Vaishampayan, U.; Rasco, D.; et al. First-in-human phase 1 study of the anti-TIGIT antibody vibostolimab as monotherapy or with pembrolizumab for advanced solid tumors, including non-small-cell lung cancer. Ann. Oncol. 2022, 33, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.; Tian, W.; Wang, Z.; Zhang, J.; Zhou, R. Co-inhibition of TIGIT and PD-1/PD-L1 in Cancer Immunotherapy: Mechanisms and Clinical Trials. Mol. Cancer 2023, 22, 93. [Google Scholar] [CrossRef] [PubMed]
- Joller, N.; Anderson, A.C.; Kuchroo, V.K. LAG-3, TIM-3, and TIGIT: Distinct functions in immune regulation. Immunity 2024, 57, 206–222. [Google Scholar] [CrossRef]
- Sauer, N.; Janicka, N.; Szlasa, W.; Skinderowicz, B.; Kołodzińska, K.; Dwernicka, W.; Oślizło, M.; Kulbacka, J.; Novickij, V.; Karłowicz-Bodalska, K. TIM-3 as a promising target for cancer immunotherapy in a wide range of tumors. Cancer Immunol. Immunother. 2023, 72, 3405–3425. [Google Scholar] [CrossRef]
- Huuhtanen, J.; Kasanen, H.; Peltola, K.; Lönnberg, T.; Glumoff, V.; Brück, O.; Dufva, O.; Peltonen, K.; Vikkula, J.; Jokinen, E.; et al. Single-cell characterization of anti–LAG-3 and anti–PD-1 combination treatment in patients with melanoma. J. Clin. Investig. 2023, 133. [Google Scholar] [CrossRef]
- Battin, C.; Kaufmann, G.; Leitner, J.; Tobias, J.; Wiedermann, U.; Rölle, A.; Meyer, M.; Momburg, F.; Steinberger, P. NKG2A-checkpoint inhibition and its blockade critically depends on peptides presented by its ligand HLA-E. Immunology 2022, 166, 507–521. [Google Scholar] [CrossRef]
- Wistuba-Hamprecht, K.; Martens, A.; Heubach, F.; Romano, E.; Geukes Foppen, M.; Yuan, J.; Postow, M.; Wong, P.; Mallardo, D.; Schilling, B.; et al. Peripheral CD8 effector-memory type 1 T-cells correlate with outcome in ipilimumab-treated stage IV melanoma patients. Eur. J. Cancer 2017, 73, 61–70. [Google Scholar] [CrossRef]
- Geng, R.; Tang, H.; You, T.; Xu, X.; Li, S.; Li, Z.; Liu, Y.; Qiu, W.; Zhou, N.; Li, N.; et al. Peripheral CD8+CD28+ T lymphocytes predict the efficacy and safety of PD-1/PD-L1 inhibitors in cancer patients. Front. Immunol. 2023, 14, 1125876. [Google Scholar] [CrossRef]
- Franciszkiewicz, K.; Boissonnas, A.; Boutet, M.; Combadière, C.; Mami-Chouaib, F. Role of chemokines and chemokine receptors in shaping the effector phase of the antitumor immune response. Cancer Res. 2012, 72, 6325–6332. [Google Scholar] [CrossRef]
- Yamauchi, T.; Hoki, T.; Oba, T.; Jain, V.; Chen, H.; Attwood, K.; Battaglia, S.; George, S.; Chatta, G.; Puzanov, I.; et al. T-cell CX3CR1 expression as a dynamic blood-based biomarker of response to immune checkpoint inhibitors. Nat. Commun. 2021, 12, 1402. [Google Scholar] [CrossRef]
- Abdelfatah, E.; Long, M.D.; Kajihara, R.; Oba, T.; Yamauchi, T.; Chen, H.; Sarkar, J.; Attwood, K.; Matsuzaki, J.; Segal, B.H.; et al. Predictive and Prognostic Implications of Circulating CX3CR1+ CD8+ T Cells in Non-Small Cell Lung Cancer Patients Treated with Chemo-Immunotherapy. Cancer Res. Commun. 2023, 3, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Sun, Z.; Chen, L. Memory T cells: Strategies for optimizing tumor immunotherapy. Protein Cell 2020, 11, 549–564. [Google Scholar] [CrossRef]
- Benichou, G.; Gonzalez, B.; Marino, J.; Ayasoufi, K.; Valujskikh, A. Role of Memory T Cells in Allograft Rejection and Tolerance. Front. Immunol. 2017, 8, 170. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Cai, C.; Samir, J.; Palgen, J.-L.; Keoshkerian, E.; Li, H.; Bull, R.A.; Luciani, F.; An, H.; Lloyd, A.R. Human CD8 T-stem cell memory subsets phenotypic and functional characterization are defined by expression of CD122 or CXCR3. Eur. J. Immunol. 2021, 51, 1732–1747. [Google Scholar] [CrossRef]
- Tietze, J.K.; Angelova, D.; Heppt, M.V.; Reinholz, M.; Murphy, W.J.; Spannagl, M.; Ruzicka, T.; Berking, C. The proportion of circulating CD45RO+CD8+ memory T cells is correlated with clinical response in melanoma patients treated with ipilimumab. Eur. J. Cancer 2017, 75, 268–279. [Google Scholar] [CrossRef] [PubMed]
- De Coaña, Y.P.; Wolodarski, M.; Poschke, I.; Yoshimoto, Y.; Yang, Y.; Nyström, M.; Edbäck, U.; Brage, S.E.; Lundqvist, A.; Masucci, G.V.; et al. Ipilimumab treatment decreases monocytic MDSCs and increases CD8 effector memory T cells in long-term survivors with advanced melanoma. Oncotarget 2017, 8, 21539–21553. [Google Scholar] [CrossRef]
- Sade-Feldman, M.; Yizhak, K.; Bjorgaard, S.L.; Ray, J.P.; de Boer, C.G.; Jenkins, R.W.; Lieb, D.J.; Chen, J.H.; Frederick, D.T.; Barzily-Rokni, M.; et al. Defining T Cell States Associated with Response to Checkpoint Immunotherapy in Melanoma. Cell 2019, 176, 404. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, A.; Yang, Y.; Xia, Y.; Li, W.; Liu, Y.; Zhang, J.; Cui, Q.; Wang, D.; Liu, X.; et al. Clinical predictive value of naïve and memory T cells in advanced NSCLC. Front. Immunol. 2022, 13, 996348. [Google Scholar] [CrossRef]
- Ren, S.; Lan, T.; Wu, F.; Chen, S.; Jiang, X.; Huo, C.; Li, Z.; Xie, S.; Wu, D.; Wang, R.; et al. Intratumoral CD103+ CD8+ T cells predict response to neoadjuvant chemoimmunotherapy in advanced head and neck squamous cell carcinoma. Cancer Commun. 2023, 43, 1143–1163. [Google Scholar] [CrossRef]
- Edwards, J.; Wilmott, J.S.; Madore, J.; Gide, T.N.; Quek, C.; Tasker, A.; Ferguson, A.; Chen, J.; Hewavisenti, R.; Hersey, P.; et al. CD103+ Tumor-Resident CD8+ T Cells Are Associated with Improved Survival in Immunotherapy-Naïve Melanoma Patients and Expand Significantly During Anti-PD-1 Treatment. Clin. Cancer Res. 2018, 24, 3036–3045. [Google Scholar] [CrossRef]
- Baessler, A.; Vignali, D.A.A. T Cell Exhaustion. Annu. Rev. Immunol. 2024, 42, 179–206. [Google Scholar] [CrossRef] [PubMed]
- Blank, C.U.; Haining, W.N.; Held, W.; Hogan, P.G.; Kallies, A.; Lugli, E.; Lynn, R.C.; Philip, M.; Rao, A.; Restifo, N.P.; et al. Defining “T cell exhaustion”. Nat. Rev. Immunol. 2019, 19, 665–674. [Google Scholar] [CrossRef]
- Zhang, J.; Ren, Z.; Hu, Y.; Shang, S.; Wang, R.; Ma, J.; Zhang, Z.; Wu, M.; Wang, F.; Yu, J.; et al. High HPK1+PD-1+TIM-3+CD8+ T cells infiltration predicts poor prognosis to immunotherapy in NSCLC patients. Int. Immunopharmacol. 2024, 127, 111363. [Google Scholar] [CrossRef] [PubMed]
- Zander, R.; Cui, W. Exhausted CD8+ T cells face a developmental fork in the road. Trends Immunol. 2023, 44, 276–286. [Google Scholar] [CrossRef]
- Daud, A.I.; Loo, K.; Pauli, M.L.; Sanchez-Rodriguez, R.; Sandoval, P.M.; Taravati, K.; Tsai, K.; Nosrati, A.; Nardo, L.; Alvarado, M.D.; et al. Tumor immune profiling predicts response to anti-PD-1 therapy in human melanoma. J. Clin. Investig. 2016, 126, 3447–3452. [Google Scholar] [CrossRef] [PubMed]
- Kansy, B.A.; Concha-Benavente, F.; Srivastava, R.M.; Jie, H.-B.; Shayan, G.; Lei, Y.; Moskovitz, J.; Moy, J.; Li, J.; Brandau, S.; et al. PD-1 Status in CD8+ T Cells Associates with Survival and Anti-PD-1 Therapeutic Outcomes in Head and Neck Cancer. Cancer Res. 2017, 77, 6353–6364. [Google Scholar] [CrossRef]
- Mazzaschi, G.; Minari, R.; Zecca, A.; Cavazzoni, A.; Ferri, V.; Mori, C.; Squadrilli, A.; Bordi, P.; Buti, S.; Bersanelli, M.; et al. Soluble PD-L1 and Circulating CD8+PD-1+ and NK Cells Enclose a Prognostic and Predictive Immune Effector Score in Immunotherapy Treated NSCLC patients. Lung Cancer 2020, 148, 1–11. [Google Scholar] [CrossRef]
- Yeong, J.; Suteja, L.; Simoni, Y.; Lau, K.W.; Tan, A.C.; Li, H.H.; Lim, S.; Loh, J.H.; Wee, F.Y.T.; Nerurkar, S.N.; et al. Intratumoral CD39+CD8+ T Cells Predict Response to Programmed Cell Death Protein-1 or Programmed Death Ligand-1 Blockade in Patients With NSCLC. J. Thorac. Oncol. 2021, 16, 1349–1358. [Google Scholar] [CrossRef]
- Liston, A.; Aloulou, M. A fresh look at a neglected regulatory lineage: CD8+Foxp3+ Regulatory T cells. Immunol. Lett. 2022, 247, 22–26. [Google Scholar] [CrossRef]
- Timperi, E.; Barnaba, V. CD39 Regulation and Functions in T Cells. Int. J. Mol. Sci. 2021, 22, 8068. [Google Scholar] [CrossRef] [PubMed]
- Koh, J.; Kim, Y.; Lee, K.Y.; Hur, J.Y.; Kim, M.S.; Kim, B.; Cho, H.J.; Lee, Y.C.; Bae, Y.H.; Ku, B.M.; et al. MDSC subtypes and CD39 expression on CD8+ T cells predict the efficacy of anti-PD-1 immunotherapy in patients with advanced NSCLC. Eur. J. Immunol. 2020, 50, 1810–1819. [Google Scholar] [CrossRef]
- Briceño, P.; Rivas-Yañez, E.; Rosemblatt, M.V.; Parra-Tello, B.; Farías, P.; Vargas, L.; Simon, V.; Cárdenas, C.; Lladser, A.; Salazar-Onfray, F.; et al. CD73 Ectonucleotidase Restrains CD8+ T Cell Metabolic Fitness and Anti-tumoral Activity. Front. Cell Dev. Biol. 2021, 9, 638037. [Google Scholar] [CrossRef] [PubMed]
- Da, M.; Chen, L.; Enk, A.; Ring, S.; Mahnke, K. The Multifaceted Actions of CD73 During Development and Suppressive Actions of Regulatory T Cells. Front. Immunol. 2022, 13, 914799. [Google Scholar] [CrossRef]
- Geels, S.N.; Moshensky, A.; Sousa, R.S.; Murat, C.; Bustos, M.A.; Walker, B.L.; Singh, R.; Harbour, S.N.; Gutierrez, G.; Hwang, M.; et al. Interruption of the intratumor CD8+ T cell:Treg crosstalk improves the efficacy of PD-1 immunotherapy. Cancer Cell 2024, 42, 1051–1066.e7. [Google Scholar] [CrossRef] [PubMed]
- van Gulijk, M.; van Krimpen, A.; Schetters, S.; Eterman, M.; van Elsas, M.; Mankor, J.; Klaase, L.; de Bruijn, M.; van Nimwegen, M.; van Tienhoven, T.; et al. PD-L1 checkpoint blockade promotes regulatory T cell activity that underlies therapy resistance. Sci. Immunol. 2023, 8, eabn6173. [Google Scholar] [CrossRef]
- Ottonello, S.; Genova, C.; Cossu, I.; Fontana, V.; Rijavec, E.; Rossi, G.; Biello, F.; Dal Bello, M.G.; Tagliamento, M.; Alama, A.; et al. Association Between Response to Nivolumab Treatment and Peripheral Blood Lymphocyte Subsets in Patients with Non-small Cell Lung Cancer. Front. Immunol. 2020, 11, 125. [Google Scholar] [CrossRef]
- Rogado, J.; Pozo, F.; Troule, K.; Sánchez-Torres, J.M.; Romero-Laorden, N.; Mondejar, R.; Donnay, O.; Ballesteros, A.; Pacheco-Barcia, V.; Aspa, J.; et al. Peripheral Blood Mononuclear Cells Predict Therapeutic Efficacy of Immunotherapy in NSCLC. Cancers 2022, 14, 2898. [Google Scholar] [CrossRef]
- Nabet, B.Y.; Esfahani, M.S.; Moding, E.J.; Hamilton, E.G.; Chabon, J.J.; Rizvi, H.; Steen, C.B.; Chaudhuri, A.A.; Liu, C.L.; Hui, A.B.; et al. Noninvasive Early Identification of Therapeutic Benefit from Immune Checkpoint Inhibition. Cell 2020, 183, 363–376.e13. [Google Scholar] [CrossRef]
- Adams, S.; Diamond, J.R.; Hamilton, E.; Pohlmann, P.R.; Tolaney, S.M.; Chang, C.-W.; Zhang, W.; Iizuka, K.; Foster, P.G.; Molinero, L.; et al. Atezolizumab Plus nab-Paclitaxel in the Treatment of Metastatic Triple-Negative Breast Cancer With 2-Year Survival Follow-up: A Phase 1b Clinical Trial. JAMA Oncol. 2019, 5, 334–342. [Google Scholar] [CrossRef]
- Kist de Ruijter, L.; van de Donk, P.P.; Hooiveld-Noeken, J.S.; Giesen, D.; Elias, S.G.; Lub-de Hooge, M.N.; Oosting, S.F.; Jalving, M.; Timens, W.; Brouwers, A.H.; et al. Whole-body CD8+ T cell visualization before and during cancer immunotherapy: A phase 1/2 trial. Nat. Med. 2022, 28, 2601–2610. [Google Scholar] [CrossRef] [PubMed]
- Kok, I.C.; Hooiveld, J.S.; van de Donk, P.P.; Giesen, D.; van der Veen, E.L.; Lub-de Hooge, M.N.; Brouwers, A.H.; Hiltermann, T.J.N.; van der Wekken, A.J.; Hijmering-Kappelle, L.B.M.; et al. 89Zr-pembrolizumab imaging as a non-invasive approach to assess clinical response to PD-1 blockade in cancer. Ann. Oncol. 2022, 33, 80–88. [Google Scholar] [CrossRef]
- Niemeijer, A.-L.N.; Oprea-Lager, D.E.; Huisman, M.C.; Hoekstra, O.S.; Boellaard, R.; de Wit-van der Veen, B.J.; Bahce, I.; Vugts, D.J.; van Dongen, G.A.M.S.; Thunnissen, E.; et al. Study of 89Zr-Pembrolizumab PET/CT in Patients with Advanced-Stage Non-Small Cell Lung Cancer. J. Nucl. Med. 2022, 63, 362–367. [Google Scholar] [CrossRef] [PubMed]
- McGrail, D.J.; Pilié, P.G.; Rashid, N.U.; Voorwerk, L.; Slagter, M.; Kok, M.; Jonasch, E.; Khasraw, M.; Heimberger, A.B.; Lim, B.; et al. High tumor mutation burden fails to predict immune checkpoint blockade response across all cancer types. Ann. Oncol. 2021, 32, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Han, T.; Wang, X.; Wang, Y.; Yang, R.; Yang, Q. Development of a CD8+ T cell associated signature for predicting the prognosis and immunological characteristics of gastric cancer by integrating single-cell and bulk RNA-sequencing. Sci. Rep. 2024, 14, 4524. [Google Scholar] [CrossRef]
- Ghiringhelli, F.; Bibeau, F.; Greillier, L.; Fumet, J.-D.; Ilie, A.; Monville, F.; Laugé, C.; Catteau, A.; Boquet, I.; Majdi, A.; et al. Immunoscore immune checkpoint using spatial quantitative analysis of CD8 and PD-L1 markers is predictive of the efficacy of anti- PD1/PD-L1 immunotherapy in non-small cell lung cancer. EBioMedicine 2023, 92, 104633. [Google Scholar] [CrossRef]
- Paul, M.S.; Ohashi, P.S. The Roles of CD8+ T Cell Subsets in Antitumor Immunity. Trends Cell Biol. 2020, 30, 695–704. [Google Scholar] [CrossRef]
- Liu, J.; Liu, D.; Hu, G.; Wang, J.; Chen, D.; Song, C.; Cai, Y.; Zhai, C.; Xu, W. Circulating memory PD-1+CD8+ T cells and PD-1+CD8+T/PD-1+CD4+T cell ratio predict response and outcome to immunotherapy in advanced gastric cancer patients. Cancer Cell Int. 2023, 23, 274. [Google Scholar] [CrossRef]
- Simoni, Y.; Becht, E.; Fehlings, M.; Loh, C.Y.; Koo, S.-L.; Teng, K.W.W.; Yeong, J.P.S.; Nahar, R.; Zhang, T.; Kared, H.; et al. Bystander CD8+ T cells are abundant and phenotypically distinct in human tumour infiltrates. Nature 2018, 557, 575–579. [Google Scholar] [CrossRef]
- Wu, T.D.; Madireddi, S.; de Almeida, P.E.; Banchereau, R.; Chen, Y.-J.J.; Chitre, A.S.; Chiang, E.Y.; Iftikhar, H.; O’Gorman, W.E.; Au-Yeung, A.; et al. Peripheral T cell expansion predicts tumour infiltration and clinical response. Nature 2020, 579, 274–278. [Google Scholar] [CrossRef]
- Zhang, J.; Ji, Z.; Caushi, J.X.; El Asmar, M.; Anagnostou, V.; Cottrell, T.R.; Chan, H.Y.; Suri, P.; Guo, H.; Merghoub, T.; et al. Compartmental Analysis of T-cell Clonal Dynamics as a Function of Pathologic Response to Neoadjuvant PD-1 Blockade in Resectable Non-Small Cell Lung Cancer. Clin. Cancer Res. 2020, 26, 1327–1337. [Google Scholar] [CrossRef] [PubMed]
- Puig-Saus, C.; Sennino, B.; Peng, S.; Wang, C.L.; Pan, Z.; Yuen, B.; Purandare, B.; An, D.; Quach, B.B.; Nguyen, D.; et al. Neoantigen-targeted CD8+ T cell responses with PD-1 blockade therapy. Nature 2023, 615, 697–704. [Google Scholar] [CrossRef]
- Yost, K.E.; Satpathy, A.T.; Wells, D.K.; Qi, Y.; Wang, C.; Kageyama, R.; McNamara, K.L.; Granja, J.M.; Sarin, K.Y.; Brown, R.A.; et al. Clonal replacement of tumor-specific T cells following PD-1 blockade. Nat. Med. 2019, 25, 1251–1259. [Google Scholar] [CrossRef]
- Bensch, F.; van der Veen, E.L.; Lub-de Hooge, M.N.; Jorritsma-Smit, A.; Boellaard, R.; Kok, I.C.; Oosting, S.F.; Schröder, C.P.; Hiltermann, T.J.N.; van der Wekken, A.J.; et al. 89Zr-atezolizumab imaging as a non-invasive approach to assess clinical response to PD-L1 blockade in cancer. Nat. Med. 2018, 24, 1852–1858. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Liu, D.; Li, L. PD-1/PD-L1 pathway: Current researches in cancer. Am. J. Cancer Res. 2020, 10, 727–742. [Google Scholar]
- Jensen, C.; Teng, Y. Is It Time to Start Transitioning From 2D to 3D Cell Culture? Front. Mol. Biosci. 2020, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Katt, M.E.; Placone, A.L.; Wong, A.D.; Xu, Z.S.; Searson, P.C. In Vitro Tumor Models: Advantages, Disadvantages, Variables, and Selecting the Right Platform. Front. Bioeng. Biotechnol. 2016, 4, 12. [Google Scholar] [CrossRef]
- Friedrich, J.; Seidel, C.; Ebner, R.; Kunz-Schughart, L.A. Spheroid-based drug screen: Considerations and practical approach. Nat. Protoc. 2009, 4, 309–324. [Google Scholar] [CrossRef]
- Ou, L.; Wang, H.; Huang, H.; Zhou, Z.; Lin, Q.; Guo, Y.; Mitchell, T.; Huang, A.C.; Karakousis, G.; Schuchter, L.; et al. Preclinical platforms to study therapeutic efficacy of human γδ T cells. Clin. Transl. Med. 2022, 12, e814. [Google Scholar] [CrossRef]
- Yoo, S.-K.; Fitzgerald, C.W.; Cho, B.A.; Fitzgerald, B.G.; Han, C.; Koh, E.S.; Pandey, A.; Sfreddo, H.; Crowley, F.; Korostin, M.R.; et al. Prediction of checkpoint inhibitor immunotherapy efficacy for cancer using routine blood tests and clinical data. Nat. Med. 2025, 31, 869–880. [Google Scholar] [CrossRef]
- Chang, T.-G.; Cao, Y.; Sfreddo, H.J.; Dhruba, S.R.; Lee, S.-H.; Valero, C.; Yoo, S.-K.; Chowell, D.; Morris, L.G.T.; Ruppin, E. LORIS robustly predicts patient outcomes with immune checkpoint blockade therapy using common clinical, pathologic and genomic features. Nat. Cancer 2024, 5, 1158–1175. [Google Scholar] [CrossRef] [PubMed]
- Chowell, D.; Yoo, S.-K.; Valero, C.; Pastore, A.; Krishna, C.; Lee, M.; Hoen, D.; Shi, H.; Kelly, D.W.; Patel, N.; et al. Improved prediction of immune checkpoint blockade efficacy across multiple cancer types. Nat. Biotechnol. 2022, 40, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Ascic, E.; Åkerström, F.; Sreekumar Nair, M.; Rosa, A.; Kurochkin, I.; Zimmermannova, O.; Catena, X.; Rotankova, N.; Veser, C.; Rudnik, M.; et al. In vivo dendritic cell reprogramming for cancer immunotherapy. Science 2024, 386, eadn9083. [Google Scholar] [CrossRef]
- Peng, L.; Sferruzza, G.; Yang, L.; Zhou, L.; Chen, S. CAR-T and CAR-NK as cellular cancer immunotherapy for solid tumors. Cell. Mol. Immunol. 2024, 21, 1089–1108. [Google Scholar] [CrossRef] [PubMed]
- Lin, P.; Lin, Y.; Mai, Z.; Zheng, Y.; Zheng, J.; Zhou, Z.; Zhao, X.; Cui, L. Targeting cancer with precision: Strategical insights into TCR-engineered T cell therapies. Theranostics 2025, 15, 300–323. [Google Scholar] [CrossRef]
- Birnboim-Perach, R.; Benhar, I. Using Combination therapy to overcome diverse challenges of Immune Checkpoint Inhibitors treatment. Int. J. Biol. Sci. 2024, 20, 3911–3922. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casalegno Garduño, R.; Spitschak, A.; Pannek, T.; Pützer, B.M. CD8+ T Cell Subsets as Biomarkers for Predicting Checkpoint Therapy Outcomes in Cancer Immunotherapy. Biomedicines 2025, 13, 930. https://doi.org/10.3390/biomedicines13040930
Casalegno Garduño R, Spitschak A, Pannek T, Pützer BM. CD8+ T Cell Subsets as Biomarkers for Predicting Checkpoint Therapy Outcomes in Cancer Immunotherapy. Biomedicines. 2025; 13(4):930. https://doi.org/10.3390/biomedicines13040930
Chicago/Turabian StyleCasalegno Garduño, Rosaely, Alf Spitschak, Tim Pannek, and Brigitte M. Pützer. 2025. "CD8+ T Cell Subsets as Biomarkers for Predicting Checkpoint Therapy Outcomes in Cancer Immunotherapy" Biomedicines 13, no. 4: 930. https://doi.org/10.3390/biomedicines13040930
APA StyleCasalegno Garduño, R., Spitschak, A., Pannek, T., & Pützer, B. M. (2025). CD8+ T Cell Subsets as Biomarkers for Predicting Checkpoint Therapy Outcomes in Cancer Immunotherapy. Biomedicines, 13(4), 930. https://doi.org/10.3390/biomedicines13040930






