Taiwan Green Propolis Nanoparticles Induce Antiproliferation and Apoptosis in Oral Cancer Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Acquisition and Extraction of TGP and Fruit Peel of Macaranga tanarius
2.2. Compound Analysis of Taiwan Green Propolis
2.3. MTT Assay of Cell Viability
2.4. Cell Cycle Assay Using Flow Cytometry
2.5. Apoptosis Marker Protein Extraction
2.6. Protein Quantification
2.7. Acrylamide Protein Electrophoresis and Transfer
2.8. Nanoprocessing of Taiwan Green Propolis Using Zein
2.9. Estimating Encapsulation Efficiency of Nanoparticles
2.10. Statistical Analysis
3. Results
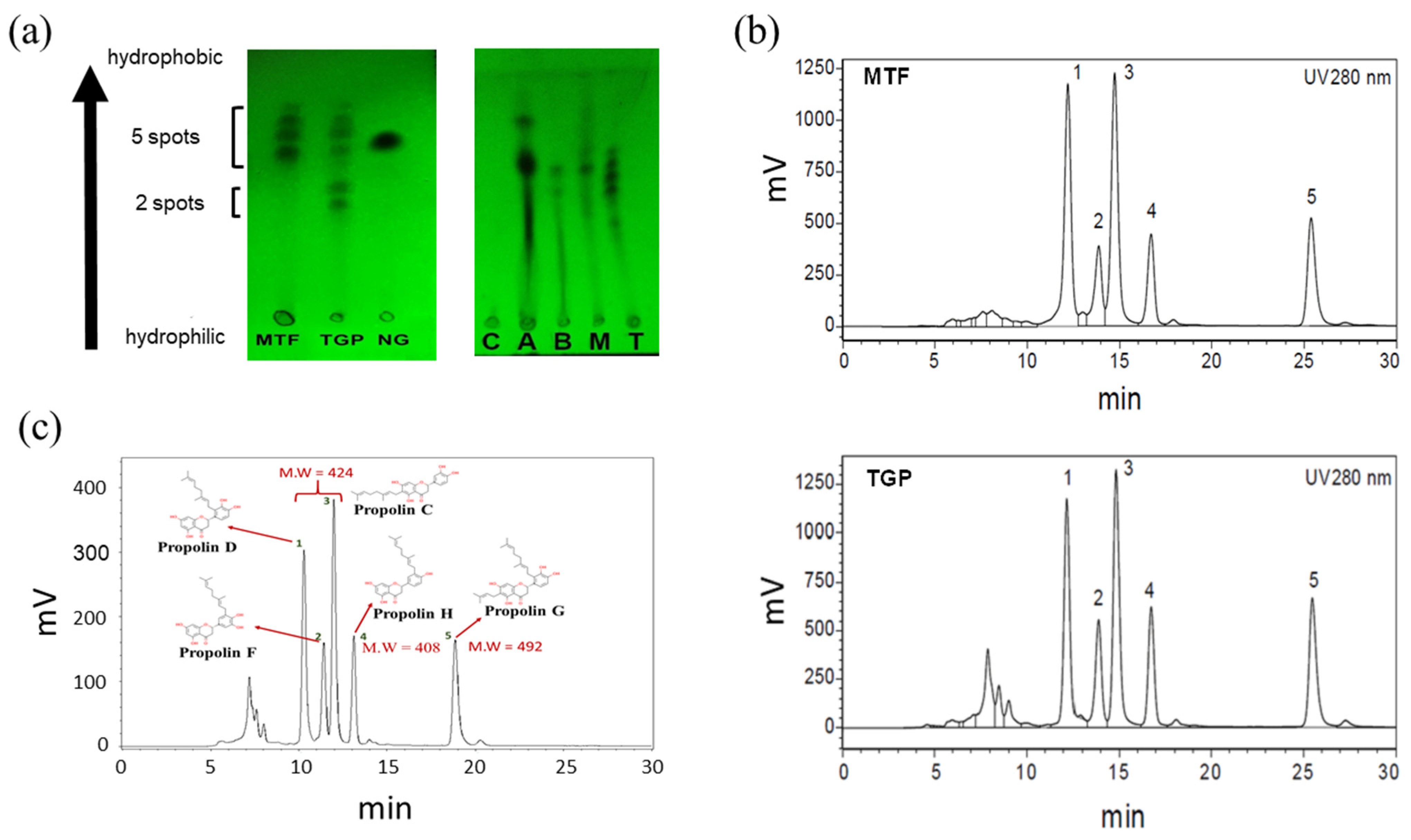
3.1. TLC Analysis Revealed Similar Compositions in MTF and TGP
3.2. HPLC Analysis of TGP and MTF
3.3. Establishment of LC/MS Spectra for TGP Extract
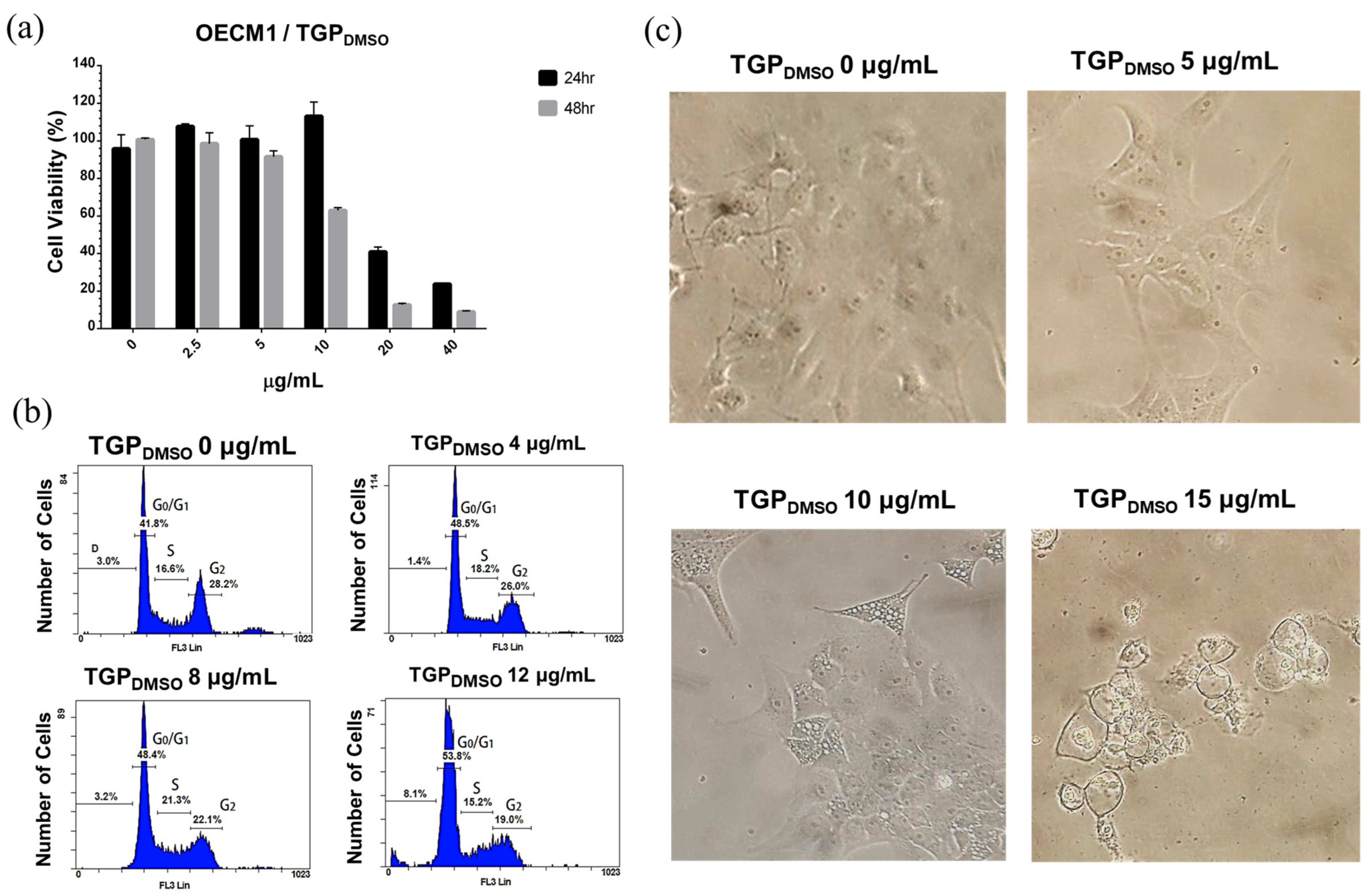
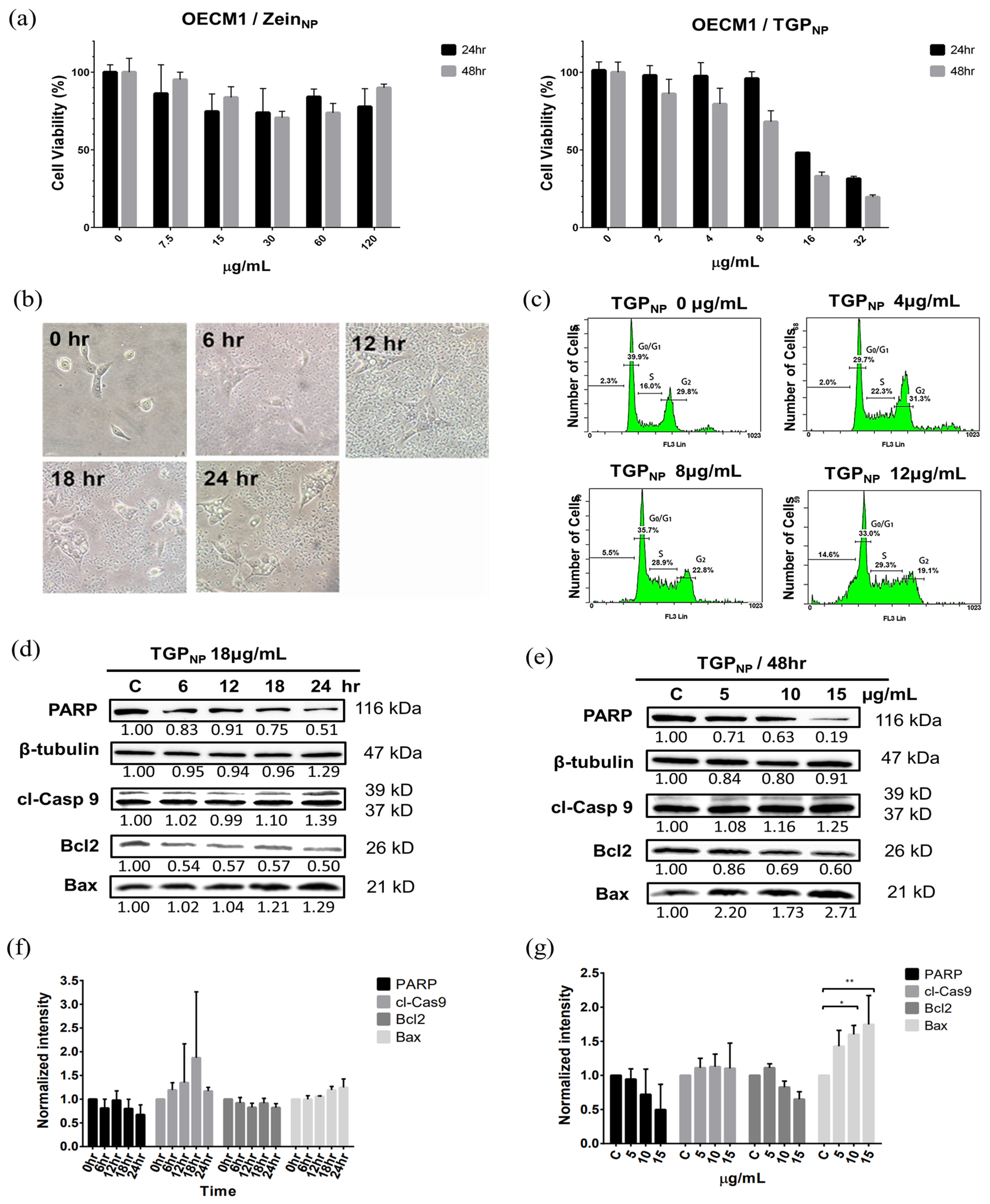
3.4. Cell Viability Characterisation After TGPDMSO Treatment Using MTT
3.5. Cell Cycle Analysis of OECM1 Cells in TGPDMSO-Containing Culture Medium
3.6. TGPDMSO Induces Apoptosis and Cell Morphology Changes
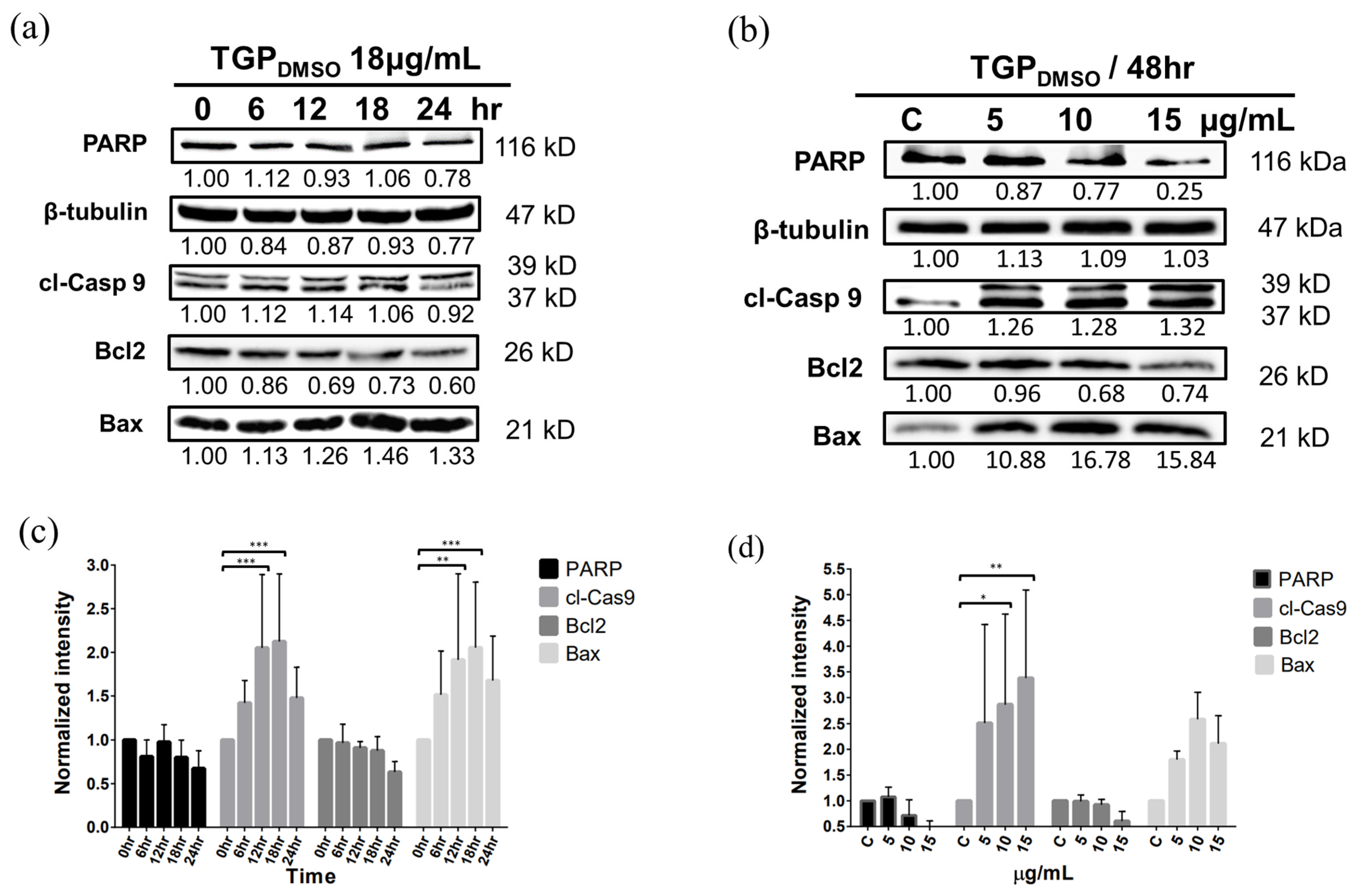
3.7. Western Blotting Demonstrates That TGPDMSO Facilitates Apoptosis in OECM1 Cells
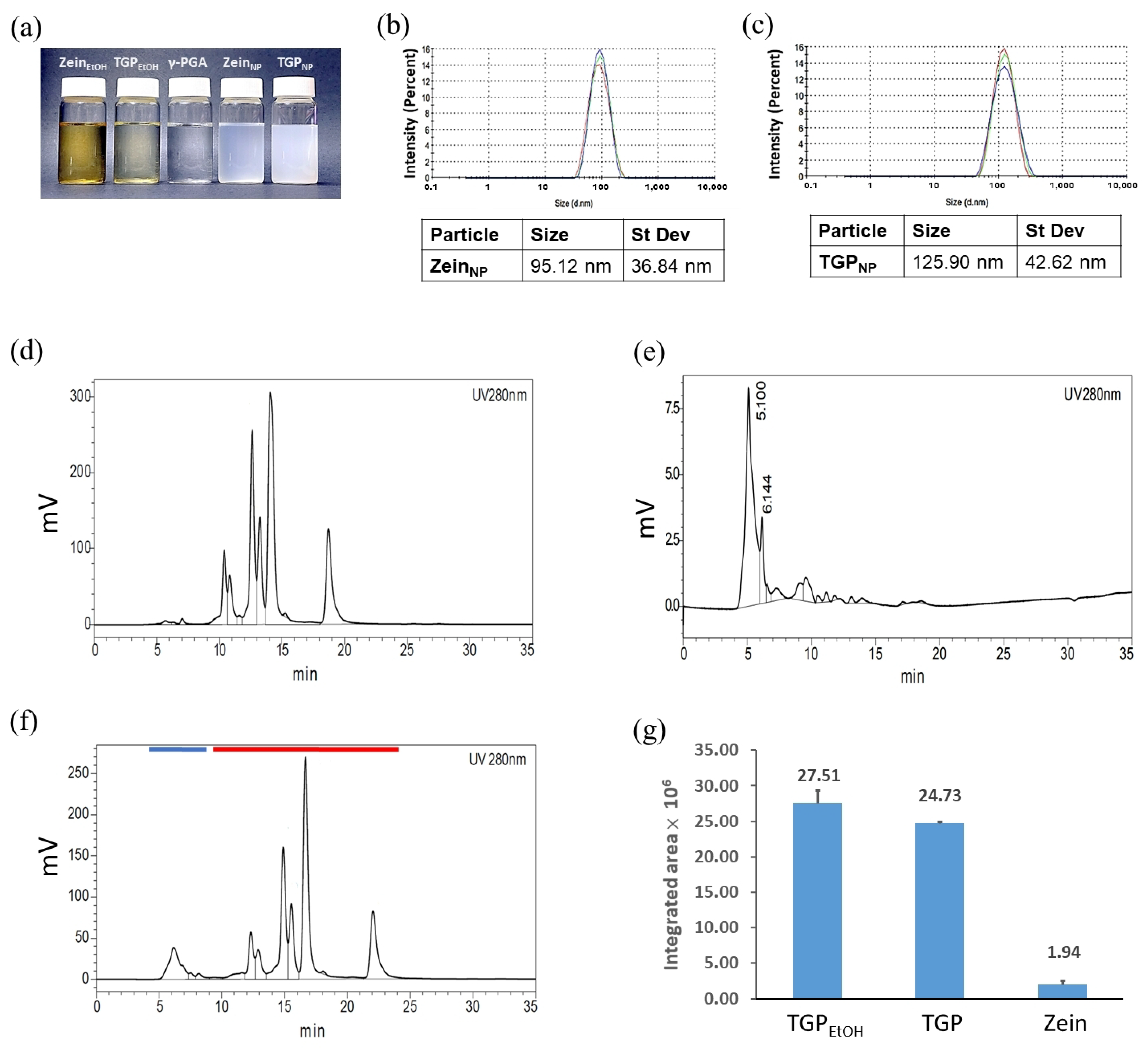
3.8. Preparation and Analysis of Nanoencapsulated TGPNP
3.9. TGPNP Retain Cytotoxicity Comparable to OECM1
3.10. Apoptosis and Morphological Changes in OECM1 Cells After Uptake of TGPNP
3.11. TGPNP Arrested the Cell Cycle and Induced the Apoptotic Protein Expression of OECM1 Cells
4. Discussion
4.1. The Ingredients of Taiwan Green Propolis Are Unique Due to the Habits of Bees and Taiwan’s Geographical Environment
4.2. Rapid Testing of TGP Source Through TLC
4.3. TGP Crude Extracts and Purified Products Exhibit the Same Cytotoxicity for Cancer Cells
4.4. TGPNP Can Achieve Anticancer Efficacy Comparable to DMSO-Dissolved TGP
4.5. TGPNP Demonstrate Excellent Safety and Multiple Advantages
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BGP | Brazilian green propolis |
| BSA | Bovine serum albumin |
| DLS | Dynamic light scattering |
| DMSO | Dimethyl sulfoxide |
| EE | Encapsulation efficiency |
| EtOH | Ethanol |
| γ-PGA | Gamma-polyglutamic acid |
| HEMe | n-Hexane/ethyl acetate/methanol, 6:3:1 |
| LC/MS | Liquid chromatography/mass spectrometry |
| MDR | Multidrug resistance |
| MRP | Multidrug resistance protein |
| MTF | Fruit peel of Macaranga tanarius |
| MTT | 3-[4,5-Dimethylthiazol-2-yl]-2,5 diphenyl tetrazolium bromide |
| PBS | Phosphate-buffered saline |
| PI | Propidium iodide |
| RIPA | Radioimmunoprecipitation assay buffer |
| TGP | Taiwan green propolis |
| TGPDMSO | TGP in DMSO |
| TGPEtOH | TGP in 80% ethanol stock solution |
| TGPNP | TGP nanoparticles |
| TLC | Thin-layer chromatography |
References
- Khalifa, S.A.M.; Elshafiey, E.H.; Shetaia, A.A.; El-Wahed, A.A.A.; Algethami, A.F.; Musharraf, S.G.; AlAjmi, M.F.; Zhao, C.; Masry, S.H.D.; Abdel-Daim, M.M.; et al. Overview of Bee Pollination and Its Economic Value for Crop Production. Insects 2021, 12, 688. [Google Scholar] [CrossRef] [PubMed]
- Giampieri, F.; Quiles, J.L.; Cianciosi, D.; Forbes-Hernandez, T.Y.; Orantes-Bermejo, F.J.; Alvarez-Suarez, J.M.; Battino, M. Bee Products: An Emblematic Example of Underutilized Sources of Bioactive Compounds. J. Agric. Food Chem. 2022, 70, 6833–6848. [Google Scholar] [CrossRef] [PubMed]
- Arung, E.T.; Ramadhan, R.; Khairunnisa, B.; Amen, Y.; Matsumoto, M.; Nagata, M.; Kusuma, I.W.; Paramita, S.; Sukemi; Yadi; et al. Cytotoxicity effect of honey, bee pollen, and propolis from seven stingless bees in some cancer cell lines. Saudi J. Biol. Sci. 2021, 28, 7182–7189. [Google Scholar] [CrossRef] [PubMed]
- Sung, S.H.; Choi, G.H.; Lee, N.W.; Shin, B.C. External Use of Propolis for Oral, Skin, and Genital Diseases: A Systematic Review and Meta-Analysis. Evid.-Based Complement. Altern. Med. 2017, 2017, 8025752. [Google Scholar] [CrossRef]
- Hossain, R.; Quispe, C.; Khan, R.A.; Saikat, A.S.M.; Ray, P.; Ongalbek, D.; Yeskaliyeva, B.; Jain, D.; Smeriglio, A.; Trombetta, D.; et al. Propolis: An update on its chemistry and pharmacological applications. Chin. Med. 2022, 17, 100. [Google Scholar] [CrossRef]
- Bankova, V.S.; de Castro, S.L.; Marcucci, M.C. Propolis: Recent advances in chemistry and plant origin. Apidologie 2000, 31, 3–15. [Google Scholar] [CrossRef]
- Singh, S.; Awasthi, M.; Pandey, V.P.; Dwivedi, U.N. Natural Products as Anticancerous Therapeutic Molecules with Special Reference to Enzymatic Targets Topoisomerase, COX, LOX and Aromatase. Curr. Protein Pept. Sci. 2018, 19, 238–274. [Google Scholar] [CrossRef]
- Salatino, A.; Salatino, M.L.F.; Negri, G. How diverse is the chemistry and plant origin of Brazilian propolis? Apidologie 2021, 52, 1075–1097. [Google Scholar] [CrossRef]
- Samadi, N.; Mozaffari-Khosravi, H.; Rahmanian, M.; Askarishahi, M. Effects of bee propolis supplementation on glycemic control, lipid profile and insulin resistance indices in patients with type 2 diabetes: A randomized, double-blind clinical trial. J. Integr. Med. 2017, 15, 124–134. [Google Scholar] [CrossRef]
- Cikman, O.; Bulut, A.; Taysi, S. Protective effect of propolis in protecting against radiation-induced oxidative stress in the liver as a distant organ. Sci. Rep. 2024, 14, 21766. [Google Scholar] [CrossRef]
- Park, Y.K.; Paredes-Guzman, J.F.; Aguiar, C.L.; Alencar, S.M.; Fujiwara, F.Y. Chemical Constituents in Baccharis dracunculifolia as the Main Botanical Origin of Southeastern Brazilian Propolis. J. Agric. Food Chem. 2004, 52, 1100–1103. [Google Scholar] [CrossRef]
- Kumazawa, S.; Nakamura, J.; Murase, M.; Miyagawa, M.; Ahn, M.R.; Fukumoto, S. Plant origin of Okinawan propolis: Honeybee behavior observation and phytochemical analysis. Naturwissenschaften 2008, 95, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Kumazawa, S.; Ueda, R.; Hamasaka, T.; Fukumoto, S.; Fujimoto, T.; Nakayama, T. Antioxidant prenylated flavonoids from propolis collected in Okinawa, Japan. J. Agric. Food Chem. 2007, 55, 7722–7725. [Google Scholar] [CrossRef]
- Kumazawa, S.; Murase, M.; Momose, N.; Fukumoto, S. Analysis of antioxidant prenylflavonoids in different parts of Macaranga tanarius, the plant origin of Okinawan propolis. Asian Pac. J. Trop. Med. 2014, 7, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.T.; Chien, Y.H.; Yu, Y.H.; Chen, Y.W. Extraction and Analysis of Taiwanese Green Propolis. J. Vis. Exp. 2019, 143, e58743. [Google Scholar]
- Shahinozzaman, M.; Obanda, D.N.; Tawata, S. Chemical composition and pharmacological properties of Macaranga-type Pacific propolis: A review. Phytother. Res. 2020, 35, 207–222. [Google Scholar] [CrossRef]
- Iriti, M.; Varoni, E.M. Chemopreventive potential of flavonoids in oral squamous cell carcinoma in human studies. Nutrients 2013, 5, 2564–2576. [Google Scholar] [CrossRef]
- Pakravan, F.; Abbasi, F.; Garshasbi, M.A.; Isfahani, M.N. Relationship between oral cancer stage and elapsed time from the onset of signs and symptoms to diagnosis and treatment. Cancer Treat. Res. Commun. 2021, 28, 100428. [Google Scholar] [CrossRef]
- Liu, C.; Wang, M.; Zhang, H.; Li, C.; Zhang, T.; Liu, H.; Zhu, S.; Chen, J. Tumor microenvironment and immunotherapy of oral cancer. Eur. J. Med. Res. 2022, 27, 198. [Google Scholar] [CrossRef]
- Clark, H.; Knapik, L.O.; Zhang, Z.; Wu, X.; Naik, M.T.; Oulhen, N.; Wessel, G.M.; Brayboy, L.M. Dysfunctional MDR-1 disrupts mitochondrial homeostasis in the oocyte and ovary. Sci. Rep. 2019, 9, 9616. [Google Scholar] [CrossRef]
- Albermann, N.; Schmitz-Winnenthal, F.H.; Z’Graggen, K.; Volk, C.; Hoffmann, M.M.; Haefeli, W.E.; Weiss, J. Expression of the drug transporters MDR1/ABCB1, MRP1/ABCC1, MRP2/ABCC2, BCRP/ABCG2, and PXR in peripheral blood mononuclear cells and their relationship with the expression in intestine and liver. Biochem. Pharmacol. 2005, 70, 949–958. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Graeser, R.; Kratz, F.; Geckeler, K.E. Paclitaxel-Loaded Polymer Nanoparticles for the Reversal of Multidrug Resistance in Breast Cancer Cells. Adv. Funct. Mater. 2011, 21, 4211–4218. [Google Scholar] [CrossRef]
- Bezerra, F.W.F.; Silva, J.M.E.; Fontanari, G.G.; Oliveira, J.A.R.; Rai, M.; Chiste, R.C.; Martins, L. Sustainable Applications of Nanopropolis to Combat Foodborne Illnesses. Molecules 2023, 28, 6785. [Google Scholar] [CrossRef]
- Ganesan, P.; Ramalingam, P.; Karthivashan, G.; Ko, Y.T.; Choi, D.K. Recent developments in solid lipid nanoparticle and surface-modified solid lipid nanoparticle delivery systems for oral delivery of phyto-bioactive compounds in various chronic diseases. Int. J. Nanomed. 2018, 13, 1569–1583. [Google Scholar] [CrossRef]
- Guo, S.B.; Meng, Y.; Lin, L.; Zhou, Z.Z.; Li, H.L.; Tian, X.P.; Huang, W.J. Artificial intelligence alphafold model for molecular biology and drug discovery: A machine-learning-driven informatics investigation. Mol. Cancer 2024, 23, 223. [Google Scholar] [CrossRef]
- Guo, S.B.; Hu, L.S.; Huang, W.J.; Zhou, Z.Z.; Luo, H.Y.; Tian, X.P. Comparative investigation of neoadjuvant immunotherapy versus adjuvant immunotherapy in perioperative patients with cancer: A global-scale, cross-sectional, and large-sample informatics study. Int. J. Surg. 2024, 110, 4660–4671. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Martinez, N.L.; Cadena-Galeana, A.D.; Villanueva-Sanchez, F.G.; Perez-Cornejo, N.; Avelar-Juarez, K.M.; Ramos-Baena, J.D.; Cruz-Monroy, E.A.; Vazquez-Zuniga, U.; Garcia-Contreras, R. Efficacy of Antineoplastic Nanocarriers on 3D Oral Cancer Spheroids. Vivo 2023, 37, 1658–1665. [Google Scholar] [CrossRef]
- Errico, C.; Bartoli, C.; Chiellini, F.; Chiellini, E. Poly(hydroxyalkanoates)-based polymeric nanoparticles for drug delivery. J. Biomed. Biotechnol. 2009, 2009, 571702. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Xia, Q.; Zhang, B.; Wang, Q.; Huang, Q. Understanding the dissolution of alpha-zein in aqueous ethanol and acetic acid solutions. J. Phys. Chem. B 2012, 116, 12057–12064. [Google Scholar] [CrossRef]
- Zhang, H.; van Os, W.L.; Tian, X.; Zu, G.; Ribovski, L.; Bron, R.; Bussmann, J.; Kros, A.; Liu, Y.; Zuhorn, I.S. Development of curcumin-loaded zein nanoparticles for transport across the blood-brain barrier and inhibition of glioblastoma cell growth. Biomater. Sci. 2021, 9, 7092–7103. [Google Scholar] [CrossRef]
- Patel, A.; Hu, Y.; Tiwari, J.K.; Velikov, K.P. Synthesis and characterisation of zein–curcumin colloidal particles. Soft Matter 2010, 6, 6192–6199. [Google Scholar] [CrossRef]
- Lee, H.S.; Kang, N.W.; Kim, H.; Kim, D.H.; Chae, J.W.; Lee, W.; Song, G.Y.; Cho, C.W.; Kim, D.D.; Lee, J.Y. Chondroitin sulfate-hybridized zein nanoparticles for tumor-targeted delivery of docetaxel. Carbohydr. Polym. 2021, 253, 117187. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.W.; Ye, S.R.; Ting, C.; Yu, Y.H. Antibacterial activity of propolins from Taiwanese green propolis. J. Food Drug Anal. 2018, 26, 761–768. [Google Scholar] [CrossRef] [PubMed]
- Su, K.Y.; Hsieh, C.Y.; Chen, Y.W.; Chuang, C.T.; Chen, C.T.; Chen, Y.S. Taiwanese Green Propolis and Propolin G Protect the Liver from the Pathogenesis of Fibrosis via Eliminating TGF-beta-Induced Smad2/3 Phosphorylation. J. Agric. Food Chem. 2014, 62, 3192–3201. [Google Scholar] [CrossRef]
- Chen, C.N.; Weng, M.S.; Wu, C.L.; Lin, J.K. Comparison of Radical Scavenging Activity, Cytotoxic Effects and Apoptosis Induction in Human Melanoma Cells by Taiwanese Propolis from Different Sources. Evid.-Based Complement. Altern. Med. 2004, 1, 75–185. [Google Scholar] [CrossRef]
- Sakagami, S.F.; Yamane, S. Notes on taxonomy and nest architecture of the Taiwanese stingless bee Trigona (Lepidotrigona) ventralis hoozana. Bull. Fac. Educ. Ibaraki Univ. (Nat. Sci.) 1984, 33, 37–48. [Google Scholar]
- Chen, Y.W.; Wu, S.W.; Ho, K.K.; Lin, S.B.; Huang, C.Y.; Chen, C.N. Characterisation of Taiwanese propolis collected from different locations and seasons. J. Sci. Food Agric. 2007, 88, 412–419. [Google Scholar] [CrossRef]
- Moise, A.R.; Bobis, O. Baccharis dracunculifolia and Dalbergia ecastophyllum, Main Plant Sources for Bioactive Properties in Green and Red Brazilian Propolis. Plants 2020, 9, 1619. [Google Scholar] [CrossRef]
- Ye, Q.; Liu, K.; Shen, Q.; Li, Q.; Hao, J.; Han, F.; Jiang, R.W. Reversal of Multidrug Resistance in Cancer by Multi-Functional Flavonoids. Front. Oncol. 2019, 9, 487. [Google Scholar] [CrossRef]
- Bartmanska, A.; Tronina, T.; Poplonski, J.; Milczarek, M.; Filip-Psurska, B.; Wietrzyk, J. Highly Cancer Selective Antiproliferative Activity of Natural Prenylated Flavonoids. Molecules 2018, 23, 2922. [Google Scholar] [CrossRef]
- Chen, C.N.; Wu, C.L.; Lin, J.K. Propolin C from propolis induces apoptosis through activating caspases, Bid and cytochrome c release in human melanoma cells. Biochem. Pharmacol. 2004, 67, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Forma, E.; Brys, M. Anticancer Activity of Propolis and Its Compounds. Nutrients 2021, 13, 2594. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.C.; Liu, S.Y.; Lin, M.H.; Kuo, Y.Y.; Liu, Y.C. The synergistic cytotoxicity of cisplatin and taxol in killing oral squamous cell carcinoma. Jpn. J. Clin. Oncol. 2004, 34, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Arruda, C.; Pena Ribeiro, V.; Oliveira Almeida, M.; Aldana Mejia, J.A.; Casoti, R.; Kenupp Bastos, J. Effect of light, oxygen and temperature on the stability of artepillin C and p-coumaric acid from Brazilian green propolis. J. Pharm. Biomed. Anal. 2020, 178, 112922. [Google Scholar] [CrossRef]
- Gonzalezl, M.; Gomez, M.I.; Tereschuk, M.L.; Molina, A. Thermal stability of propolis from Tucumán, Argentina. J. Apic. Res. 2009, 4, 270–278. [Google Scholar] [CrossRef]
- Fu, Q.T.; Zhong, X.Q.; Chen, M.Y.; Gu, J.Y.; Zhao, J.; Yu, D.H.; Tan, F. Luteolin-Loaded Nanoparticles for the Treatment of Melanoma. Int. J. Nanomed. 2023, 18, 2053–2068. [Google Scholar] [CrossRef]
- Chuacharoen, T.; Sabliov, C.M. Stability and controlled release of lutein loaded in zein nanoparticles with and without lecithin and pluronic F127 surfactants. Colloids Surf. A 2016, 503, 11–18. [Google Scholar] [CrossRef]
- Cai, X.; Ye, T.; Liu, C.; Lu, W.; Lu, M.; Zhang, J.; Wang, M.; Cao, P. Luteolin induced G2 phase cell cycle arrest and apoptosis on non-small cell lung cancer cells. Toxicol. Vitr. 2011, 25, 1385–1391. [Google Scholar] [CrossRef]
- Lee, C.C.; Hsiao, C.Y.; Lee, S.C.; Huang, X.F.; Chang, K.F.; Lee, M.S.; Hsieh, M.C.; Tsai, N.M. Suppression of oral cancer by induction of cell cycle arrest and apoptosis using Juniperus communis extract. Biosci. Rep. 2020, 40, BSR20202083. [Google Scholar] [CrossRef]
- Abdel Hamid, H.M.; Darwish, Z.E.; Elsheikh, S.M.; Mourad, G.M.; Donia, H.M.; Afifi, M.M. Following cytotoxic nanoconjugates from injection to halting the cell cycle machinery and its therapeutic implications in oral cancer. BMC Cancer 2021, 21, 170. [Google Scholar] [CrossRef]
- Truong, K.D.; Nguyen, H.T.-L.; Nguyen, S.T. Comparative cytotoxic effects of methanol, ethanol and DMSO on human cancer cell lines. Biomed. Res. Ther. 2020, 7, 3855–3859. [Google Scholar]
- Essawy, M.M.; Mohamed, M.M.; Raslan, H.S.; Rafik, S.T.; Awaad, A.K.; Ramadan, O.R. The theranostic potentialities of bioavailable nanocurcumin in oral cancer management. BMC Complement. Med. Ther. 2022, 22, 309. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, W.-D.; Peng, S.-F.; Tsao, N.-W.; Wang, S.-Y.; Tzeng, S.-L.; Hu, N.-J. Taiwan Green Propolis Nanoparticles Induce Antiproliferation and Apoptosis in Oral Cancer Cells. Biomedicines 2025, 13, 921. https://doi.org/10.3390/biomedicines13040921
Huang W-D, Peng S-F, Tsao N-W, Wang S-Y, Tzeng S-L, Hu N-J. Taiwan Green Propolis Nanoparticles Induce Antiproliferation and Apoptosis in Oral Cancer Cells. Biomedicines. 2025; 13(4):921. https://doi.org/10.3390/biomedicines13040921
Chicago/Turabian StyleHuang, Wen-Da, Shu-Fen Peng, Nai-Wen Tsao, Sheng-Yang Wang, Shu-Ling Tzeng, and Nien-Jen Hu. 2025. "Taiwan Green Propolis Nanoparticles Induce Antiproliferation and Apoptosis in Oral Cancer Cells" Biomedicines 13, no. 4: 921. https://doi.org/10.3390/biomedicines13040921
APA StyleHuang, W.-D., Peng, S.-F., Tsao, N.-W., Wang, S.-Y., Tzeng, S.-L., & Hu, N.-J. (2025). Taiwan Green Propolis Nanoparticles Induce Antiproliferation and Apoptosis in Oral Cancer Cells. Biomedicines, 13(4), 921. https://doi.org/10.3390/biomedicines13040921







