Serum Levels of Irisin Are Positively Associated with Improved Cardiac Function in Patients with Heart Failure with Reduced Ejection Fraction
Abstract
1. Introduction
2. Materials and Methods
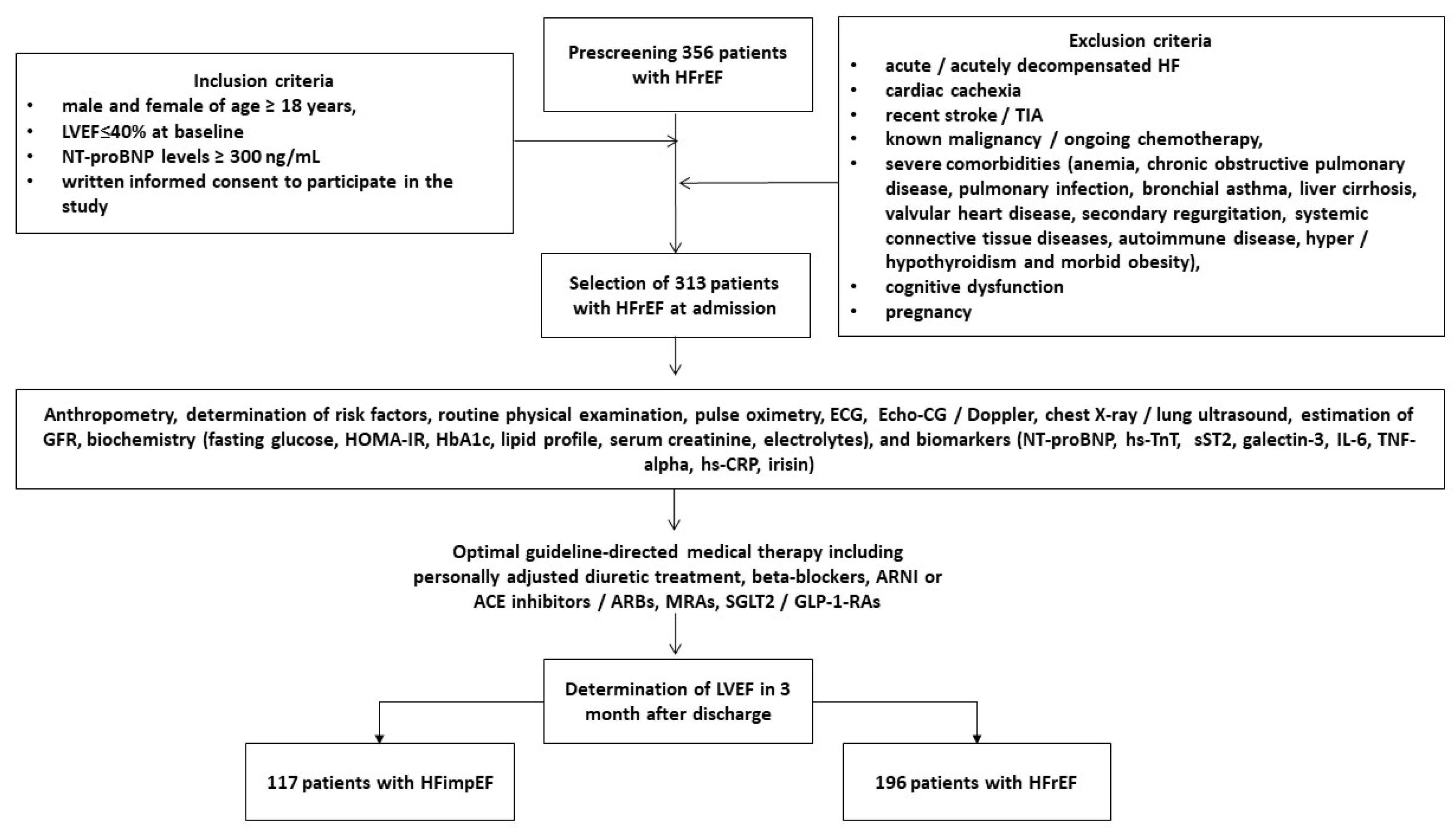
2.1. Patient Population and Structure of the Study
2.2. The Evaluation of Participants’ Demographics, Anthropometry Parameters, and Concomitant Diseases/Conditions
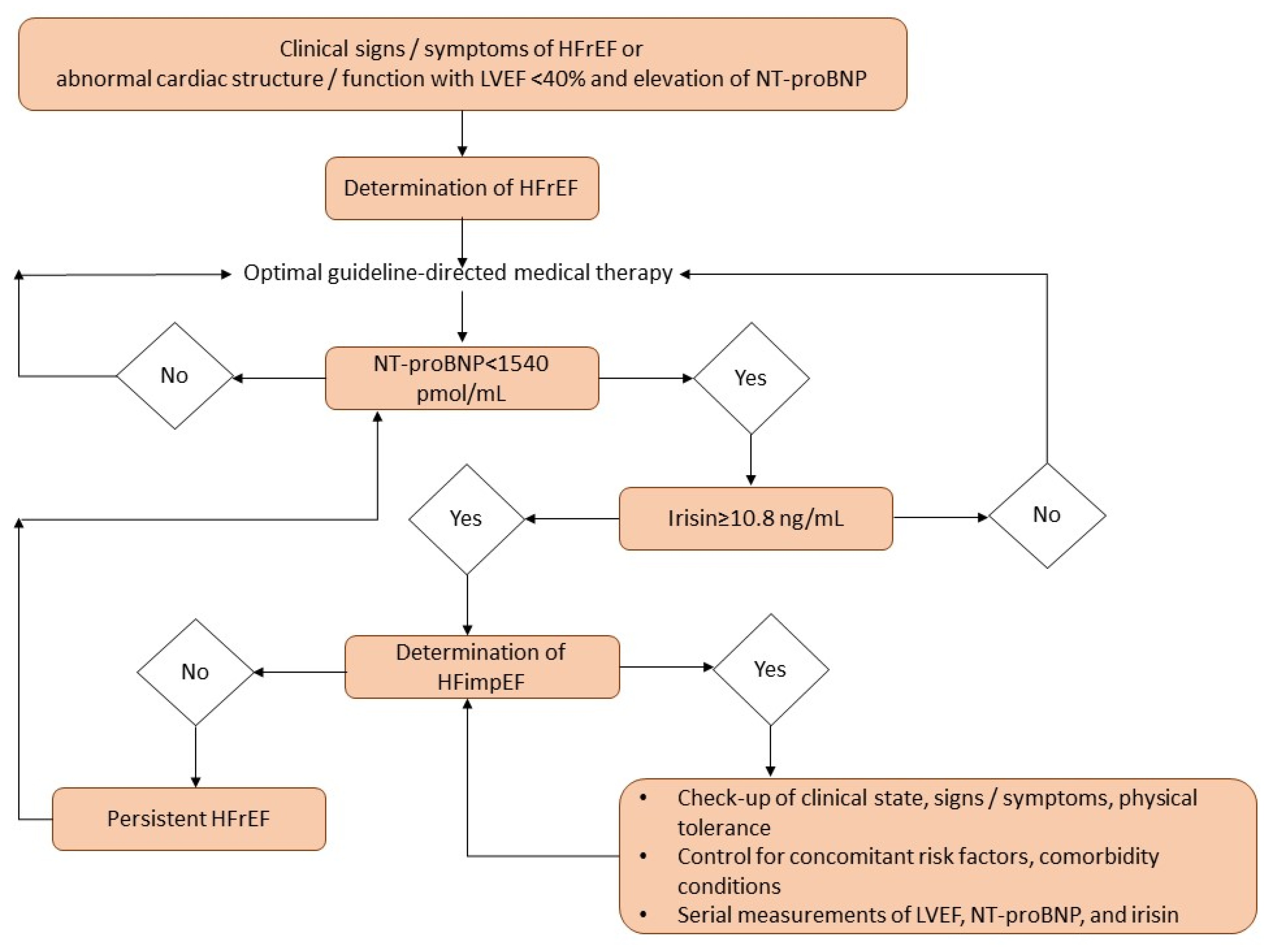
2.3. Determination of HFimpEF
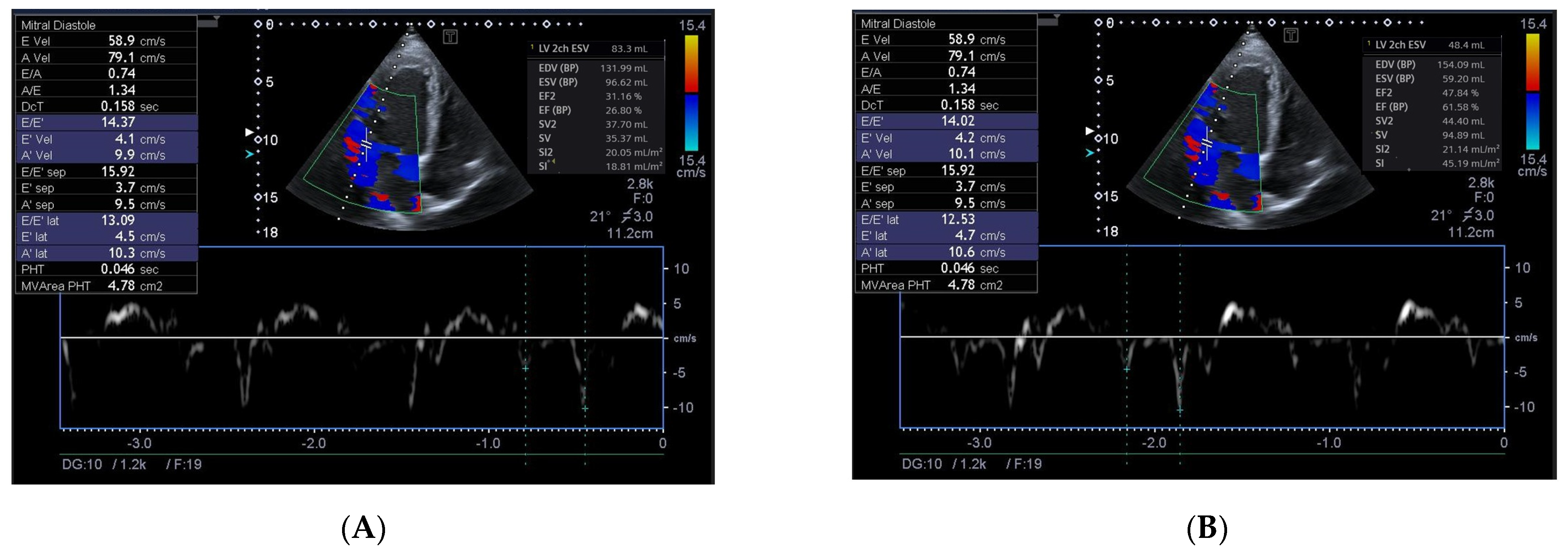
2.4. Echocardiography Examination
2.5. Glomerular Filtration Rate and Insulin Resistance Determination
2.6. Circulating Biomarker Evaluation
2.7. Statistics
3. Results
3.1. Clinical Characteristics, Echocardiographic Parameters and Laboratory Findings
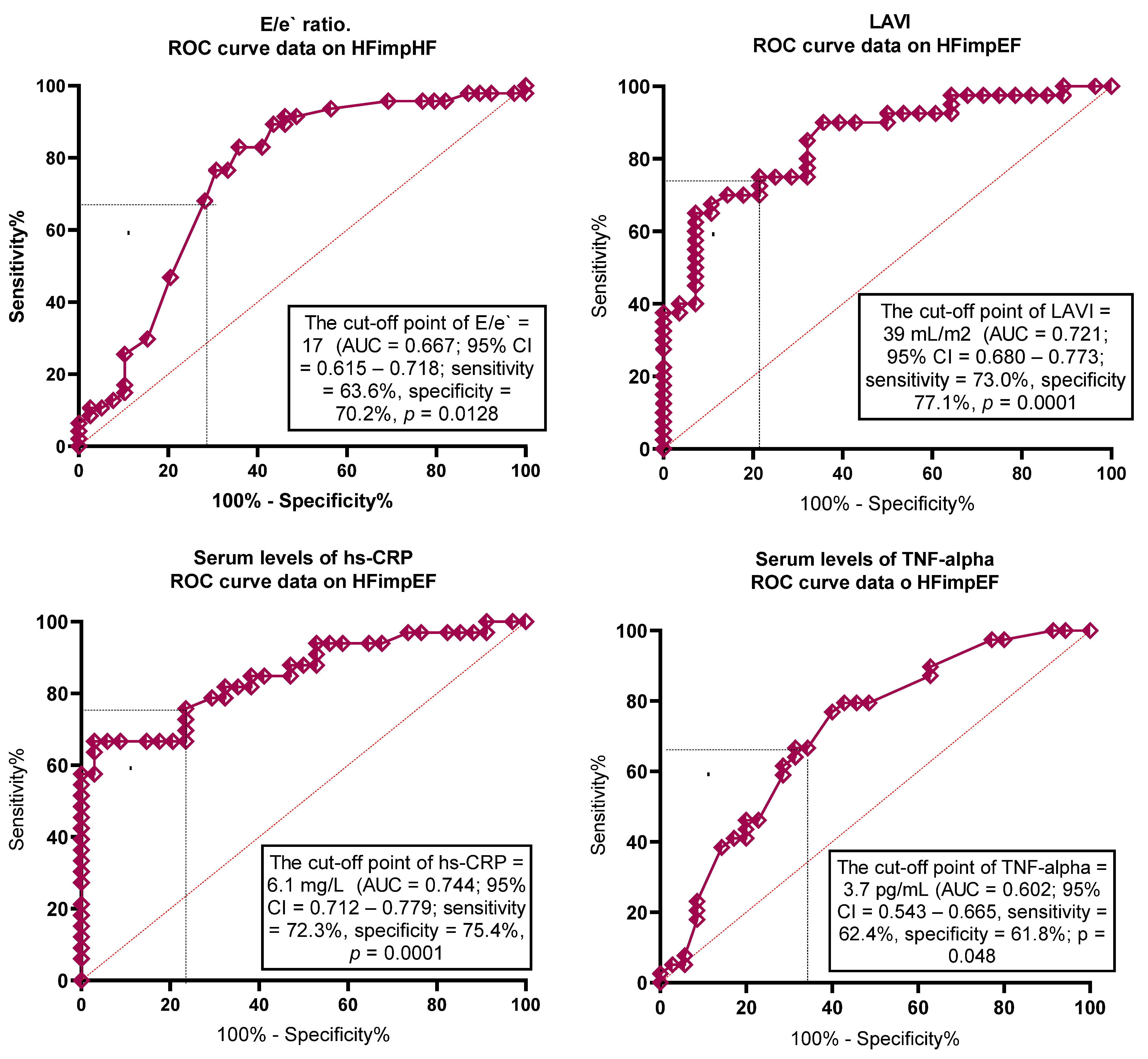
3.2. The Optimal Cut-Offs for Possible Predictors of HFimpEF
3.3. Predictive Factors for HFimpEF: Univariate Logistic Regression and Backward Stepwise Multivariate Logistic Regression Models
3.4. Prediction Model Comparison
4. Discussion
5. Clinical Implications
6. Study Limitations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Savarese, G.; Becher, P.M.; Lund, L.H.; Seferovic, P.; Rosano, G.M.C.; Coats, A.J.S. Global burden of heart failure: A comprehensive and updated review of epidemiology. Cardiovasc. Res. 2023, 118, 3272–3287, Erratum in Cardiovasc. Res. 2023, 119, 1453. https://doi.org/10.1093/cvr/cvad026. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, B.; Ahmad, T.; Alexander, K.M.; Baker, W.L.; Bosak, K.; Breathett, K.; Fonarow, G.C.; Heidenreich, P.; Ho, J.E.; Hsich, E.; et al. Heart Failure Epidemiology and Outcomes Statistics: A Report of the Heart Failure Society of America. J. Card. Fail. 2023, 29, 1412–1451. [Google Scholar] [CrossRef]
- Emmons-Bell, S.; Johnson, C.; Roth, G. Prevalence, incidence and survival of heart failure: A systematic review. Heart 2022, 108, 1351–1360. [Google Scholar] [CrossRef]
- Denfeld, Q.E.; Winters-Stone, K.; Mudd, J.O.; Gelow, J.M.; Kurdi, S.; Lee, C.S. The prevalence of frailty in heart failure: A systematic review and meta-analysis. Int. J. Cardiol. 2017, 236, 283–289. [Google Scholar] [CrossRef]
- Shah, K.S.; Xu, H.; Matsouaka, R.A.; Bhatt, D.L.; Heidenreich, P.A.; Hernandez, A.F.; Devore, A.D.; Yancy, C.W.; Fonarow, G.C. Heart Failure with Preserved, Borderline, and Reduced Ejection Fraction: 5-Year Outcomes. J. Am. Coll. Cardiol. 2017, 70, 2476–2486. [Google Scholar] [CrossRef]
- Chioncel, O.; Lainscak, M.; Seferovic, P.M.; Anker, S.D.; Crespo-Leiro, M.G.; Harjola, V.P.; Parissis, J.; Laroche, C.; Piepoli, M.F.; Fonseca, C.; et al. Epidemiology and one-year outcomes in patients with chronic heart failure and preserved, mid-range and reduced ejection fraction: An analysis of the ESC Heart Failure Long-Term Registry. Eur. J. Heart Fail. 2017, 19, 1574–1585. [Google Scholar] [CrossRef]
- McDowell, K.; Kondo, T.; Talebi, A.; Teh, K.; Bachus, E.; de Boer, R.A.; Campbell, R.T.; Claggett, B.; Desai, A.S.; Docherty, K.F.; et al. Prognostic Models for Mortality and Morbidity in Heart Failure with Preserved Ejection Fraction. JAMA Cardiol. 2024, 9, 457–465, Erratum in JAMA Cardiol. 2024, 9, 861. https://doi.org/10.1001/jamacardio.2024.2464. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pensa, A.V.; Khan, S.S.; Shah, R.V.; Wilcox, J.E. Heart failure with improved ejection fraction: Beyond diagnosis to trajectory analysis. Prog. Cardiovasc. Dis. 2024, 82, 102–112. [Google Scholar] [CrossRef]
- Pabon, M.A.; Vaduganathan, M.; Claggett, B.L.; Chatur, S.; Siqueira, S.; Marti-Castellote, P.; de Boer, R.A.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; et al. In-hospital course of patients with heart failure with improved ejection fraction in the DELIVER trial. Eur. J. Heart Fail. 2024, 26, 2532–2540. [Google Scholar] [CrossRef] [PubMed]
- Park, C.S.; Park, J.J.; Mebazaa, A.; Oh, I.Y.; Park, H.A.; Cho, H.J.; Lee, H.Y.; Kim, K.H.; Yoo, B.S.; Kang, S.M.; et al. Characteristics, Outcomes, and Treatment of Heart Failure with Improved Ejection Fraction. J. Am. Heart Assoc. 2019, 8, e011077. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726, Erratum in Eur. Heart J. 2021, 42, 4901. https://doi.org/10.1093/eurheartj/ehab670. [Google Scholar] [CrossRef]
- Licordari, R.; Correale, M.; Bonanno, S.; Beltrami, M.; Ciccarelli, M.; Micari, A.; Palazzuoli, A.; Dattilo, G. Beyond Natriuretic Peptides: Unveiling the Power of Emerging Biomarkers in Heart Failure. Biomolecules 2024, 14, 309. [Google Scholar] [CrossRef]
- Pascual-Figal, D.A.; Hernández-Vicente, A.; Pastor-Pérez, F.; Martínez-Sellés, M.; Solé-González, E.; Alvarez-García, J.; García-Pavía, P.; Varela-Román, A.; Sánchez, P.L.; Delgado, J.F.; et al. N-terminal pro-B-type natriuretic peptide post-discharge monitoring in the management of patients with heart failure and preserved ejection fraction—A randomized trial: The NICE study. Eur. J. Heart Fail. 2024, 26, 776–784. [Google Scholar] [CrossRef]
- Mueller, C.; McDonald, K.; de Boer, R.A.; Maisel, A.; Cleland, J.G.F.; Kozhuharov, N.; Coats, A.J.S.; Metra, M.; Mebazaa, A.; Ruschitzka, F.; et al. Heart Failure Association of the European Society of Cardiology practical guidance on the use of natriuretic peptide concentrations. Eur. J. Heart Fail. 2019, 21, 715–731. [Google Scholar] [CrossRef]
- Sciatti, E.; Merlo, A.; Scangiuzzi, C.; Limonta, R.; Gori, M.; D’Elia, E.; Aimo, A.; Vergaro, G.; Emdin, M.; Senni, M. Prognostic Value of sST2 in Heart Failure. J. Clin. Med. 2023, 12, 3970. [Google Scholar] [CrossRef]
- Girerd, N.; Cleland, J.; Anker, S.D.; Byra, W.; Lam, C.S.P.; Lapolice, D.; Mehra, M.R.; van Veldhuisen, D.J.; Bresso, E.; Lamiral, Z.; et al. Inflammation and remodeling pathways and risk of cardiovascular events in patients with ischemic heart failure and reduced ejection fraction. Sci. Rep. 2022, 12, 8574. [Google Scholar] [CrossRef]
- Pellicori, P.; Zhang, J.; Cuthbert, J.; Urbinati, A.; Shah, P.; Kazmi, S.; Clark, A.L.; Cleland, J.G.F. High-sensitivity C-reactive protein in chronic heart failure: Patient characteristics, phenotypes, and mode of death. Cardiovasc. Res. 2020, 116, 91–100. [Google Scholar] [CrossRef]
- Markousis-Mavrogenis, G.; Tromp, J.; Ouwerkerk, W.; Devalaraja, M.; Anker, S.D.; Cleland, J.G.; Dickstein, K.; Filippatos, G.S.; van der Harst, P.; Lang, C.C.; et al. The clinical significance of interleukin-6 in heart failure: Results from the BIOSTAT-CHF study. Eur. J. Heart Fail. 2019, 21, 965–973. [Google Scholar] [CrossRef]
- Verbrugge, F.H.; Omote, K.; Reddy, Y.N.V.; Sorimachi, H.; Obokata, M.; Borlaug, B.A. Heart failure with preserved ejection fraction in patients with normal natriuretic peptide levels is associated with increased morbidity and mortality. Eur. Heart J. 2022, 43, 1941–1951. [Google Scholar] [CrossRef]
- Chen, Y.Y.; Liang, L.; Tian, P.C.; Feng, J.Y.; Huang, L.Y.; Huang, B.P.; Zhao, X.M.; Wu, Y.H.; Wang, J.; Guan, J.Y.; et al. Clinical characteristics and outcomes of patients hospitalized with heart failure with preserved ejection fraction and low NT-proBNP levels. Medicine 2023, 102, e36351. [Google Scholar] [CrossRef]
- Murphy, S.P.; Kakkar, R.; McCarthy, C.P.; Januzzi, J.L., Jr. Inflammation in Heart Failure: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2020, 75, 1324–1340. [Google Scholar] [CrossRef]
- Cocco, G.; Jerie, P.; Amiet, P.; Pandolfi, S. Inflammation in Heart Failure: Known knowns and unknown unknowns. Expert Opin. Pharmacother. 2017, 18, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Berezin, A.E.; Berezin, A.A. Point-of-care heart failure platform: Where are we now and where are we going to? Expert Rev. Cardiovasc. Ther. 2022, 20, 419–429. [Google Scholar] [CrossRef] [PubMed]
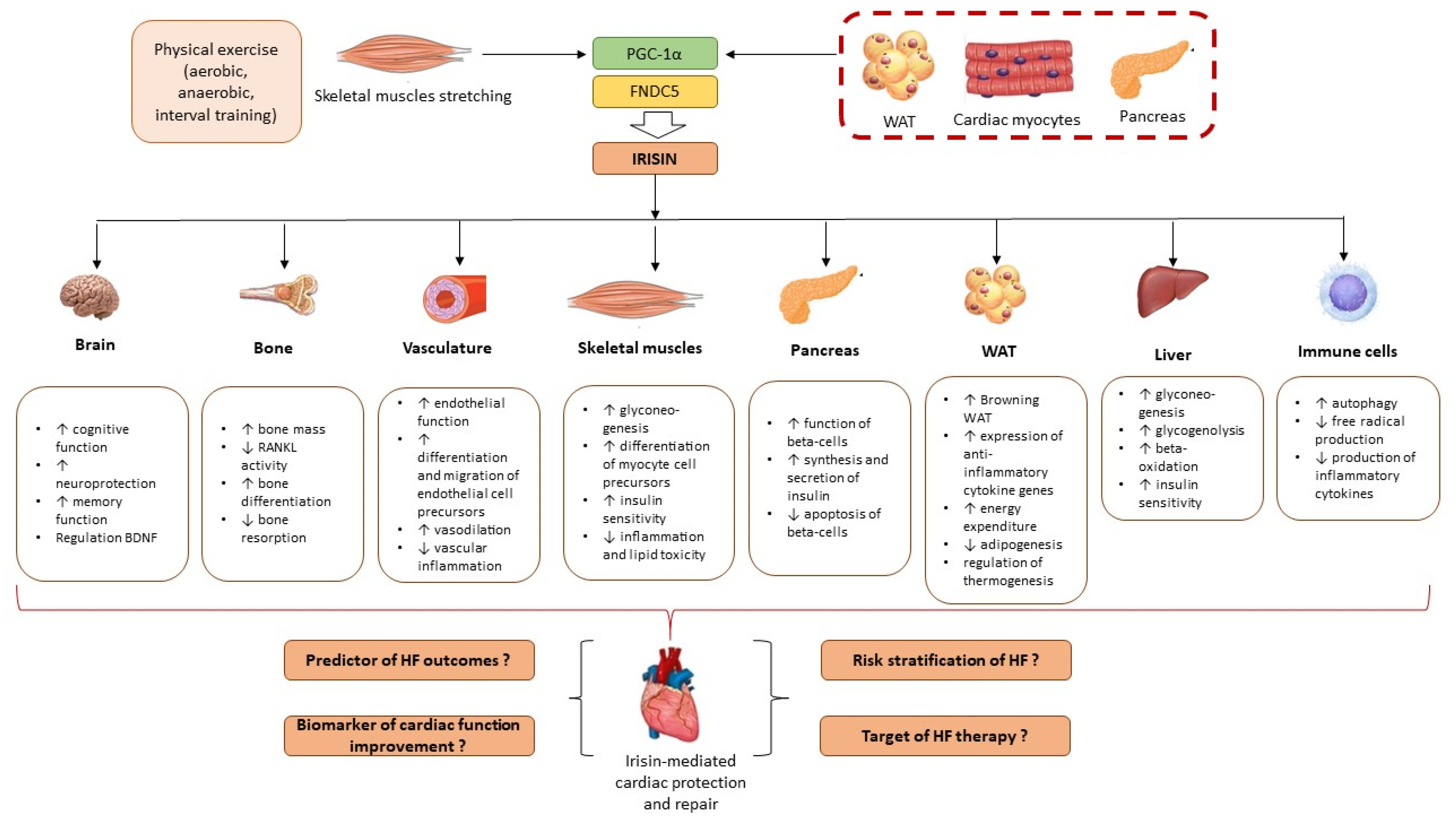
- Liu, S.; Cui, F.; Ning, K.; Wang, Z.; Fu, P.; Wang, D.; Xu, H. Role of irisin in physiology and pathology. Front. Endocrinol. 2022, 13, 962968. [Google Scholar] [CrossRef]
- De Sousa, R.A.L. Exercise-produced irisin effects on brain-related pathological conditions. Metab. Brain Dis. 2024, 39, 1679–1687. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Hua, Y.; Li, Q.; Zhu, W.; Pan, Y.; Yang, Y.; Li, X.; Wu, M.; Wang, J.; Gan, X. FNDC5/irisin facilitates muscle-adipose-bone connectivity through ubiquitination-dependent activation of runt-related transcriptional factors RUNX1/2. J. Biol. Chem. 2022, 298, 101679. [Google Scholar] [CrossRef]
- Hofmann, T.; Elbelt, U.; Stengel, A. Irisin as a muscle-derived hormone stimulating thermogenesis--a critical update. Peptides 2014, 54, 89–100. [Google Scholar] [CrossRef]
- Tomasello, L.; Pitrone, M.; Guarnotta, V.; Giordano, C.; Pizzolanti, G. Irisin: A Possible Marker of Adipose Tissue Dysfunction in Obesity. Int. J. Mol. Sci. 2023, 24, 12082. [Google Scholar] [CrossRef]
- Ho, M.Y.; Wang, C.Y. Role of Irisin in Myocardial Infarction, Heart Failure, and Cardiac Hypertrophy. Cells 2021, 10, 2103. [Google Scholar] [CrossRef]
- Tang, Y.J.; Zhang, Z.; Yan, T.; Chen, K.; Xu, G.F.; Xiong, S.Q.; Wu, D.Q.; Chen, J.; Jose, P.A.; Zeng, C.Y.; et al. Irisin attenuates type 1 diabetic cardiomyopathy by anti-ferroptosis via SIRT1-mediated deacetylation of p53. Cardiovasc. Diabetol. 2024, 23, 116. [Google Scholar] [CrossRef]
- Zhang, X.; Hu, C.; Kong, C.Y.; Song, P.; Wu, H.M.; Xu, S.C.; Yuan, Y.P.; Deng, W.; Ma, Z.G.; Tang, Q.-Z. FNDC5 alleviates oxidative stress and cardiomyocyte apoptosis in doxorubicin-induced cardiotoxicity via activating AKT. Cell Death Differ. 2020, 27, 540–555. [Google Scholar] [CrossRef] [PubMed]
- Li, R.L.; Wu, S.S.; Wu, Y.; Wang, X.X.; Chen, H.Y.; Xin, J.J.; Li, H.; Lan, J.; Xue, K.Y.; Li, X.; et al. Irisin alleviates pressure overload-induced cardiac hypertrophy by inducing protective autophagy via mTOR-independent activation of the AMPK-ULK1 pathway. J. Mol. Cell Cardiol. 2018, 121, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Zhang, B.; Wang, X. Lower irisin levels in coronary artery disease: A meta-analysis. Minerva Endocrinol. 2020, 45, 61–69. [Google Scholar] [CrossRef]
- Ozturk, D.; Melekoglu, A.; Altinbilek, E.; Calik, M.; Kosem, A.; Kilci, H.; Misirlioglu, N.F.; Uzun, H. Association Between Serum Irisin Levels and ST-Segment Elevation Myocardial Infarction. Int. J. Gen. Med. 2023, 16, 1355–1362. [Google Scholar] [CrossRef]
- Hou, Q.; Song, R.; Zhao, X.; Yang, C.; Feng, Y. Lower circulating irisin levels in type 2 diabetes mellitus patients with chronic complications: A meta-analysis. Heliyon 2023, 9, e21859. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.X.; Fan, Z.J.; Wu, L.Y.; Wang, S.Y.; Zhang, J.L.; Dong, X.T.; Zhang, A.H. Serum irisin levels are negatively associated with blood pressure in dialysis patients. Hypertens. Res. 2023, 46, 2738–2745. [Google Scholar] [CrossRef]
- Yildiz Kopuz, T.N.; Dagdeviren, M.; Fisunoglu, M. Serum irisin levels in newly diagnosed type-II diabetic patients: No association with the overall diet quality but strong association with fruit intake. Clin. Nutr. ESPEN 2022, 49, 357–364. [Google Scholar] [CrossRef]
- Shen, X.; Chen, Y.; Zhang, J.; Yang, M.; Huang, L.; Luo, J.; Xu, L. The association between circulating irisin levels and osteoporosis in women: A systematic review and meta-analysis of observational studies. Front. Endocrinol. 2024, 15, 1388717. [Google Scholar] [CrossRef]
- Berezin, A.E.; Berezin, A.A. Biomarkers in Heart Failure: From Research to Clinical Practice. Ann. Lab. Med. 2023, 43, 225–236. [Google Scholar] [CrossRef]
- Mitchell, C.; Rahko, P.S.; Blauwet, L.A.; Canaday, B.; Finstuen, J.A.; Foster, M.C.; Horton, K.; Ogunyankin, K.O.; Palma, R.A.; Velazquez, E.J. Guidelines for Performing a Comprehensive Transthoracic Echocardiographic Examination in Adults: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2018, 32, 1–64. [Google Scholar] [CrossRef]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A New Equation to Estimate Glomerular Filtration Rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Ling, Y.; Guo, W.; Li, Q.; Yu, S.; Huang, H.; Zhang, R.; Gong, Z.; Liu, J.; Mo, L.; et al. Prevalence and Prognosis of HFimpEF Developed from Patients With Heart Failure With Reduced Ejection Fraction: Systematic Review and Meta-Analysis. Front. Cardiovasc. Med. 2021, 8, 757596. [Google Scholar] [CrossRef]
- Wu, N.; Lang, X.; Zhang, Y.; Zhao, B.; Zhang, Y. Predictors and Prognostic Factors of Heart Failure with Improved Ejection Fraction. Rev. Cardiovasc. Med. 2024, 25, 280. [Google Scholar] [CrossRef] [PubMed]
- Ho, L.T.; Juang, J.J.; Chen, Y.H.; Chen, Y.S.; Hsu, R.B.; Huang, C.C.; Lee, C.M.; Chien, K.L. Predictors of Left Ventricular Ejection Fraction Improvement in Patients with Early-Stage Heart Failure with Reduced Ejection Fraction. Acta Cardiol. Sin. 2023, 39, 854–861. [Google Scholar] [CrossRef]
- Vardeny, O.; Desai, A.S.; Jhund, P.S.; Fang, J.C.; Claggett, B.; de Boer, R.A.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.P.; et al. Dapagliflozin and Mode of Death in Heart Failure with Improved Ejection Fraction: A Post Hoc Analysis of the DELIVER Trial. JAMA Cardiol. 2024, 9, 283–289. [Google Scholar] [CrossRef]
- Bozkurt, B.; Coats, A.J.S.; Tsutsui, H.; Abdelhamid, C.M.; Adamopoulos, S.; Albert, N.; Anker, S.D.; Atherton, J.; Böhm, M.; Butler, J.; et al. Universal definition and classification of heart failure: A report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure: Endorsed by the Canadian Heart Failure Society, Heart Failure Association of India, Cardiac Society of Australia and New Zealand, and Chinese Heart Failure Association. Eur. J. Heart Fail. 2021, 23, 352–380. [Google Scholar] [CrossRef]
- Huang, W.; Nurhafizah, A.; Frederich, A.; Khairunnisa, A.R.; Kezia, C.; Fathoni, M.I.; Samban, S.; Flindy, S. Risk and Protective Factors of Poor Clinical Outcomes in Heart Failure with Improved Ejection Fraction Population: A Systematic Review and Meta-Analysis. Curr. Cardiol. Rep. 2025, 27, 4. [Google Scholar] [CrossRef]
- Pintér, A.; Behon, A.; Veres, B.; Merkel, E.D.; Schwertner, W.R.; Kuthi, L.K.; Masszi, R.; Lakatos, B.K.; Kovács, A.; Becker, D.; et al. The Prognostic Value of Anemia in Patients with Preserved, Mildly Reduced and Recovered Ejection Fraction. Diagnostics 2022, 12, 517. [Google Scholar] [CrossRef]
- Kondo, T.; Campbell, R.; Jhund, P.S.; Anand, I.S.; Carson, P.E.; Lam, C.S.P.; Shah, S.J.; Vaduganathan, M.; Zannad, F.; Zile, M.R.; et al. Low Natriuretic Peptide Levels and Outcomes in Patients with Heart Failure and Preserved Ejection Fraction. JACC Heart Fail. 2024, 12, 1442–1455. [Google Scholar] [CrossRef]
- Berezina, T.A.; Berezin, O.O.; Novikov, E.V.; Lichtenauer, M.; Berezin, A.E. Irisin Predicts Poor Clinical Outcomes in Patients with Heart Failure with Preserved Ejection Fraction and Low Levels of N-Terminal Pro-B-Type Natriuretic Peptide. Biomolecules 2024, 14, 1615. [Google Scholar] [CrossRef]
- Li, J.; Xie, S.; Guo, L.; Jiang, J.; Chen, H. Irisin: Linking metabolism with heart failure. Am. J. Transl. Res. 2020, 12, 6003–6014. [Google Scholar] [PubMed] [PubMed Central]
- Wang, Z.; Chen, K.; Han, Y.; Zhu, H.; Zhou, X.; Tan, T.; Zeng, J.; Zhang, J.; Liu, Y.; Li, Y.; et al. Irisin Protects Heart Against Ischemia-Reperfusion Injury Through a SOD2-Dependent Mitochondria Mechanism. J. Cardiovasc. Pharmacol. 2018, 72, 259–269. [Google Scholar] [CrossRef]
- Silvestrini, A.; Bruno, C.; Vergani, E.; Venuti, A.; Favuzzi, A.M.R.; Guidi, F.; Nicolotti, N.; Meucci, E.; Mordente, A.; Mancini, A. Circulating irisin levels in heart failure with preserved or reduced ejection fraction: A pilot study. PLoS ONE 2019, 14, e0210320. [Google Scholar] [CrossRef]
- Berezin, A.A.; Lichtenauer, M.; Boxhammer, E.; Stöhr, E.; Berezin, A.E. Discriminative Value of Serum Irisin in Prediction of Heart Failure with Different Phenotypes among Patients with Type 2 Diabetes Mellitus. Cells 2022, 11, 2794. [Google Scholar] [CrossRef]
- Berezin, A.A.; Fushtey, I.M.; Pavlov, S.V.; Berezin, A.E. Predictive value of serum irisin for chronic heart failure in patients with type 2 diabetes mellitus. Mol. Biomed. 2022, 3, 34. [Google Scholar] [CrossRef]
- Abubakar, M.; Irfan, U.; Abdelkhalek, A.; Javed, I.; Khokhar, M.I.; Shakil, F.; Raza, S.; Salim, S.S.; Altaf, M.M.; Habib, R.; et al. Comprehensive Quality Analysis of Conventional and Novel Biomarkers in Diagnosing and Predicting Prognosis of Coronary Artery Disease, Acute Coronary Syndrome, and Heart Failure, a Comprehensive Literature Review. J. Cardiovasc. Transl. Res. 2024, 17, 1258–1285. [Google Scholar] [CrossRef]
| Variables | Entire HF Patient Cohort (n = 313) | Patients with HFimpEF (n = 117) | Patients with HFrEF (n = 196) | p Value Between Cohorts |
|---|---|---|---|---|
| Demographics and anthropometry parameters | ||||
| Age, year | 69 (61–78) | 67 (60–75) | 70 (62–81) | 0.146 |
| Male gender, n (%) | 184 (58.9) | 68 (58.1) | 116 (59.2) | 0.146 |
| BMI, kg/m2 | 26.2 ± 4.26 | 25.3 ±3.88 | 26.9 ± 3.97 | 0.272 |
| Waist circumference, cm | 101 ± 7 | 99 ± 5 | 101 ± 8 | 0.690 |
| Medical history | ||||
| Smoking history, n (%) | 135 (43.1) | 48 (41.0) | 87 (44.4) | 0.642 |
| Abdominal obesity, n (%) | 75 (24.0) | 27 (23.1) | 48 (24.5) | 0.475 |
| Dyslipidemia, n (%) | 234 (74.8) | 86 (73.5) | 148 (75.5) | 0.344 |
| Hypertension, n (%) | 176 (56.2) | 66 (56.4) | 110 (56.1) | 0.871 |
| Stable CAD, n (%) | 162 (51.8) | 57 (48.7) | 105 (53.6) | 0.046 |
| Dilated cardiomyopathy, n (%) | 57 (18.2) | 20 (17.1) | 37 (18.9) | 0.242 |
| Atrial fibrillation, n (%) | 93 (29.7) | 28 (23.9) | 65 (33.2) | 0.048 |
| LVH, n (%) | 217 (69.3) | 81 (69.2) | 136 (69.4) | 0.844 |
| CKD 1–3 grades, n (%) | 68 (21.7) | 22 (18.8) | 46 (23.5) | 0.044 |
| T2DM, n (%) | 102 (32.6) | 38 (32.5) | 64 (32.7) | 0.526 |
| PCI history, n (%) | 97 (31.0) | 42 (35.9) | 55 (28.1) | 0.048 |
| NYHA functional class, n (%) | ||||
| II | 72 (23.0) | 29 (24.8) | 43 (21.9) | 0.142 |
| III | 184 (58.9) | 69 (59.0) | 115 (58.7) | 0.416 |
| IV | 57 (18.1) | 19 (16.2) | 38 (19.4) | 0.144 |
| Hemodynamic and echocardiographic parameters | ||||
| Systolic BP, mm Hg | 127 ± 8 | 129 ± 8 | 126 ± 9 | 0.395 |
| Diastolic BP, mm Hg | 68 ± 9 | 69 ± 7 | 68 ± 7 | 0.462 |
| LVEDV, mL | 176 (154–197) | 178 (155–201) | 173 (149–193) | 0.274 |
| LVESV, mL | 103 (98–106) | 99 (95–103) | 110 (97–119) | 0.022 |
| LVEF, % | 41 (34–51) | 44 (40–47) | 37 (33–39) | 0.024 |
| LVMI, g/m2 | 148 ± 22 | 147 ± 19 | 155 ± 20 | 0.226 |
| LAVI, mL/m2 | 44 (35–54) | 42 (36–49) | 47 (40–53) | 0.046 |
| TAPSE, mm | 20 (15–26) | 19 (14–24) | 22 (15–27) | 0.611 |
| E/e`, unit | 17 ± 7 | 16 ± 4 | 17 ± 5 | 0.355 |
| Laboratory findings | ||||
| eGFR, mL/min/1.73 m2 | 64 ± 19 | 65 ± 15 | 61 ± 13 | 0.331 |
| K, mmol/L | 4.1 (3.3–5.3) | 4.3 (3.4–5.5) | 4.0 (3.1–5.10) | 0.124 |
| Na, mmol/L | 139 (128–146) | 139 (125–149) | 137 (127–145) | 0.846 |
| HOMA-IR, units | 5.11 ± 2.33 | 5.05 ± 2.23 | 5.19± 2.25 | 0.658 |
| Glucose, mmol/L | 4.68 ± 0.57 | 4.59 ± 0.52 | 4.70 ± 0.51 | 0.681 |
| HbA1c, % | 5.10 ± 1.99 | 5.07 ± 1.65 | 5.11± 1.57 | 0.560 |
| Hemoglobin, g/L | 13.9 (12.6–15.1) | 13.8 (12.5–14.7) | 14.0 (12.6–15.3) | 0.674 |
| Hematocrit, % | 38 (34–42) | 38 (35–40) | 39 (35–43) | 0.644 |
| Creatinine, µmol/L | 104 ± 10 | 97 ± 11 | 106 ± 9 | 0.128 |
| Serum uric acid, µmol/L | 359 ± 85 | 352 ± 80 | 360 ± 88 | 0.672 |
| TC, mmol/L | 5.69 ± 0.60 | 5.61 ± 0.68 | 5.73 ± 0.66 | 0.654 |
| HDL-C, mmol/L | 1.02 ± 0.10 | 1.03 ± 0.09 | 1.02 ± 0.10 | 0.748 |
| LDL-C, mmol/L | 3.60± 0.20 | 3.50 ± 0.18 | 3.60± 0.20 | 0.786 |
| Triglycerides, mmol/L | 2.34 ± 0.37 | 2.30 ± 0.29 | 2.41 ± 0.27 | 0.650 |
| hs-CRP, mg/L | 5.98 (2.24–9.70) | 5.52 (2.12–8.16) | 6.11 (2.80–10.56) | 0.860 |
| TNF-alpha, pg/mL | 3.68 (2.10–5.23) | 3.45 (2.03–4.94) | 3.81 (2.19–5.21) | 0.547 |
| IL-6, ng/mL | 2.91 (0.76–4.95) | 2.70 (0.67–4.82) | 3.20 (0.88–5.61) | 0.216 |
| cTnT, ng/mL | 0.036 (0.004–0.112) | 0.021 (0.001–0.110) | 0.048 (0.003–0.120) | 0.690 |
| NT-proBNP, pmol/mL | 1810 (980–2560) | 1330 (870–1580) | 2310 (1130–3580) | 0.044 |
| sST2, ng/mL | 29.40 (13.90–45.70) | 27.63 (11.17–41.80) | 31.90 (15.82–47.54) | 0.844 |
| Galectin-3, ng/mL | 27.5 (11.6–53.4) | 24.1 (9.8–41.5) | 32.7 (10.1–60.3) | 0.671 |
| Irisin, ng/mL | 5.75 (2.18–9.12) | 8.23 (4.26–13.50) | 4.37 (1.62–7.17) | 0.001 |
| Concomitant medications and devices | ||||
| ACE inhibitors, n (%) | 122 (39.0) | 43 (36.8) | 79 (40.3) | 0.519 |
| ARBs, n (%) | 39 (12.5) | 20 (17.1) | 19 (9.7) | 0.050 |
| ARNI, n (%) | 152 (48.7) | 54 (46.2) | 98 (50.0) | 0.538 |
| Beta-blockers, n (%) | 285 (91.1) | 105 (89.7) | 180 (91.8) | 0.351 |
| Ivabradine, n (%) | 32(10.2) | 10 (8.5) | 22 (11.2) | 0.271 |
| CCBs, n (%) | 35 (11.2) | 11 (9.4) | 24 (12.2) | 0.164 |
| MRA, n (%) | 231 (73.8) | 86 (73.5) | 145 (74.0) | 0.834 |
| Diuretics, n (%) | 298 (98.2) | 112 (95.7) | 186 (94.9) | 0.877 |
| Antiplatelet agents, n (%) | 179 (57.2) | 69 (59.0) | 110 (56.1) | 0.048 |
| Anticoagulants, n (%) | 93 (29.7) | 28 (23.9) | 65 (33.2) | 0.048 |
| Metformin, n (%) | 97 (31.0) | 36 (30.8) | 61 (31.1) | 0.713 |
| SGLT2 inhibitors, n (%) | 227 (72.5) | 86 (73.5) | 141 (71.9) | 0.637 |
| GLP-1-RAs, n (%) | 34 (10.8) | 13 (11.1) | 21 (10.7) | 0.511 |
| Statins, n (%) | 234 (74.8) | 86 (73.5) | 148 (75.5) | 0.344 |
| RCT, n (%) | 22 (7.0) | 9 (7.7) | 13 (6.6) | 0.766 |
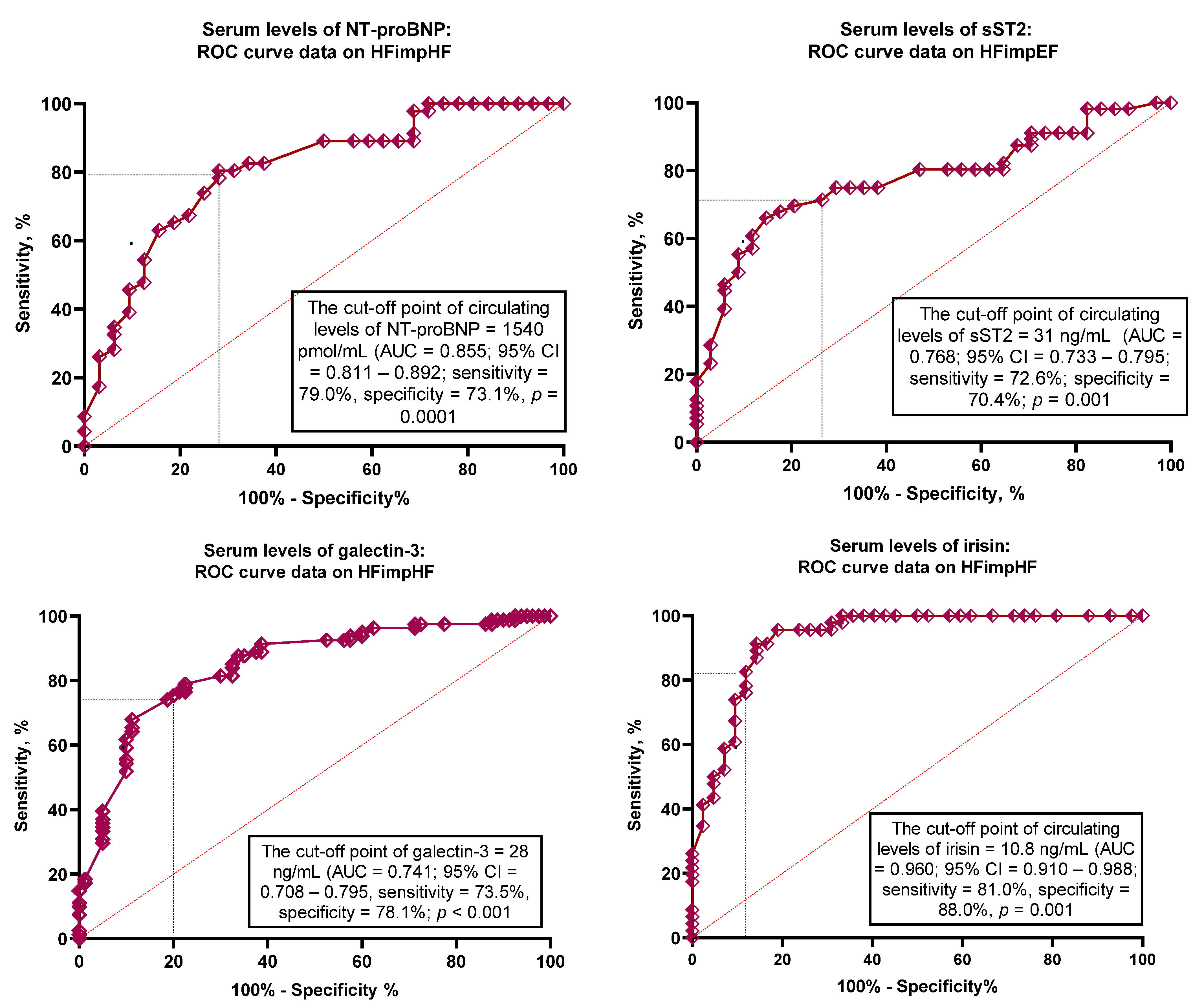
| Variables | AUC | 95% CI | p-Value | Cut-Offs | Se, % | Sp, % |
|---|---|---|---|---|---|---|
| LAVI | 0.721 | 0.680–0.773 | 0.001 | 39 mL/m2 | 73.9 | 77.1 |
| E/e` | 0.667 | 0.615–0.718 | 0.001 | 17 | 63.6 | 70.2 |
| hs-CRP | 0.744 | 0.712–0.779 | 0.001 | 6.1 mg/L | 72.3 | 75.4 |
| TNF-alpha | 0.602 | 0.543–0.665 | 0.048 | 3.7 pg/mL | 62.4 | 61.8 |
| NT-proBNP | 0.855 | 0.811–0.892 | 0.0001 | 1540 pmol/mL | 79.0 | 73.1 |
| sST2 | 0.768 | 0.733–0.795 | 0.001 | 31 ng/mL | 72.6 | 70.4 |
| Galectin-3 | 0.741 | 0.708–0.795 | 0.001 | 28 ng/mL | 73.5 | 78.1 |
| Irisin | 0.960 | 0.910–0.988 | 0.0001 | 10.8 ng/mL | 81.0 | 88.0 |
| Predictive Factors | Univariate Logistic Regression | Backward Stepwise Multivariate Logistic Regression | ||||
|---|---|---|---|---|---|---|
| Odds Ratio | 95% CI | p Value | Odds Ratio | 95% CI | p Value | |
| T2DM * | 0.97 | 0.91–1.02 | 0.212 | - | ||
| PCI history * | 0.95 | 0.89–1.13 | 0.437 | - | ||
| AF * | 0.95 | 0.91–0.98 | 0.010 | 0.95 | 0.90–0.98 | 0.010 |
| Stable CAD * | 1.02 | 0.94–1.17 | 0.380 | - | ||
| CKD stages 1–3 * | 0.93 | 0.87–0.99 | 0.047 | 0.95 | 0.89–1.01 | 0.177 |
| Dilated CMP * | 0.96 | 0.92–1.02 | 0.422 | - | ||
| LAVI < 39 mL/m2 vs. ≥39 mL/m2 | 1.32 | 1.15–1.56 | 0.001 | 1.23 | 1.11–1.39 | 0.001 |
| E/e` < 17 vs. ≥17 | 1.18 | 1.04–1.35 | 0.012 | 1.10 | 1.00–1.27 | 0.052 |
| hs-CRP < 6.1 mg/L vs. ≥6.1 mg/L | 1.12 | 1.06–1.20 | 0.018 | 1.09 | 1.00–1.20 | 0.120 |
| TNF-alpha < 3.7 bg/mL vs. ≥3.7 ng/mL | 1.06 | 1.01–1.12 | 0.044 | 1.05 | 0.99–1.10 | 0.206 |
| NT-proBNP < 1540 vs. ≥1540 pmol/mL | 1.56 | 1.12–2.15 | 0.001 | 1.47 | 1.11–2.12 | 0.001 |
| sST2 < 31 ng/mL vs. ≥31 ng/mL | 1.24 | 1.02–1.65 | 0.048 | 1.20 | 1.00–1.68 | 0.086 |
| Galectin-3 < 28 ng/mL vs. ≥28 ng/mL | 1.17 | 1.01–1.43 | 0.050 | 1.12 | 1.00–1.27 | 0.064 |
| Irisin ≥ 10.8 ng/mL vs. <10.8 ng/mL | 1.75 | 1.22–4.32 | 0.001 | 1.73 | 1.16–4.18 | 0.001 |
| Predictive Models | Dependent Variable: HFimpEF | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| AUC | Net Reclassification Improvement | Integrated Discrimination Indices | |||||||
| M | 95% CI | p Value | M | 95% CI | p Value | M | 95% CI | p Value | |
| Model 1 (NT-proBNP < 1540 pmol/mL) | 0.855 | 0.811–0.892 | - | Reference | - | Reference | - | ||
| Model 2 (presence of AF) | 0.820 | 0.715–0.944 | 0.427 | 0.10 | 0.06–0.15 | 0.388 | 0.11 | 0.05–0.17 | 0.481 |
| Model 3 (LAVI < 39 mL/m2) | 0.721 | 0.680–0.773 | 0.044 | 0.03 | 0.01–0.06 | 0.642 | 0.06 | 0.02–0.09 | 0.552 |
| Model 4 (irisin ≥ 10.8 ng/mL) | 0.960 | 0.910–0.988 | 0.001 | 0.36 | 0.24–0.49 | 0.001 | 0.44 | 0.38–0.52 | 0.001 |
| Model 1 + model 2 | 0.848 | 0.790–0.910 | 0.066 | 0.10 | 0.05–0.17 | 0.249 | 0.12 | 0.06–0.19 | 0.265 |
| Model 1 + model 3 | 0.851 | 0.810–0.912 | 0.270 | 0.09 | 0.03–0.15 | 0.338 | 0.11 | 0.03–0.17 | 0.286 |
| Model 1 + model 4 | 0.979 | 0.932–0.982 | 0.001 | 0.38 | 0.29–0.50 | 0.001 | 0.44 | 0.35–0.54 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berezin, A.E.; Berezina, T.A.; Novikov, E.V.; Berezin, O.O. Serum Levels of Irisin Are Positively Associated with Improved Cardiac Function in Patients with Heart Failure with Reduced Ejection Fraction. Biomedicines 2025, 13, 866. https://doi.org/10.3390/biomedicines13040866
Berezin AE, Berezina TA, Novikov EV, Berezin OO. Serum Levels of Irisin Are Positively Associated with Improved Cardiac Function in Patients with Heart Failure with Reduced Ejection Fraction. Biomedicines. 2025; 13(4):866. https://doi.org/10.3390/biomedicines13040866
Chicago/Turabian StyleBerezin, Alexander E., Tetiana A. Berezina, Evgen V. Novikov, and Oleksandr O. Berezin. 2025. "Serum Levels of Irisin Are Positively Associated with Improved Cardiac Function in Patients with Heart Failure with Reduced Ejection Fraction" Biomedicines 13, no. 4: 866. https://doi.org/10.3390/biomedicines13040866
APA StyleBerezin, A. E., Berezina, T. A., Novikov, E. V., & Berezin, O. O. (2025). Serum Levels of Irisin Are Positively Associated with Improved Cardiac Function in Patients with Heart Failure with Reduced Ejection Fraction. Biomedicines, 13(4), 866. https://doi.org/10.3390/biomedicines13040866







