Protective Effects of Hydrogen-Rich Saline Against Hemorrhagic Shock in Rats via an Endothelial Glycocalyx Pathway
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Surgical Preparation
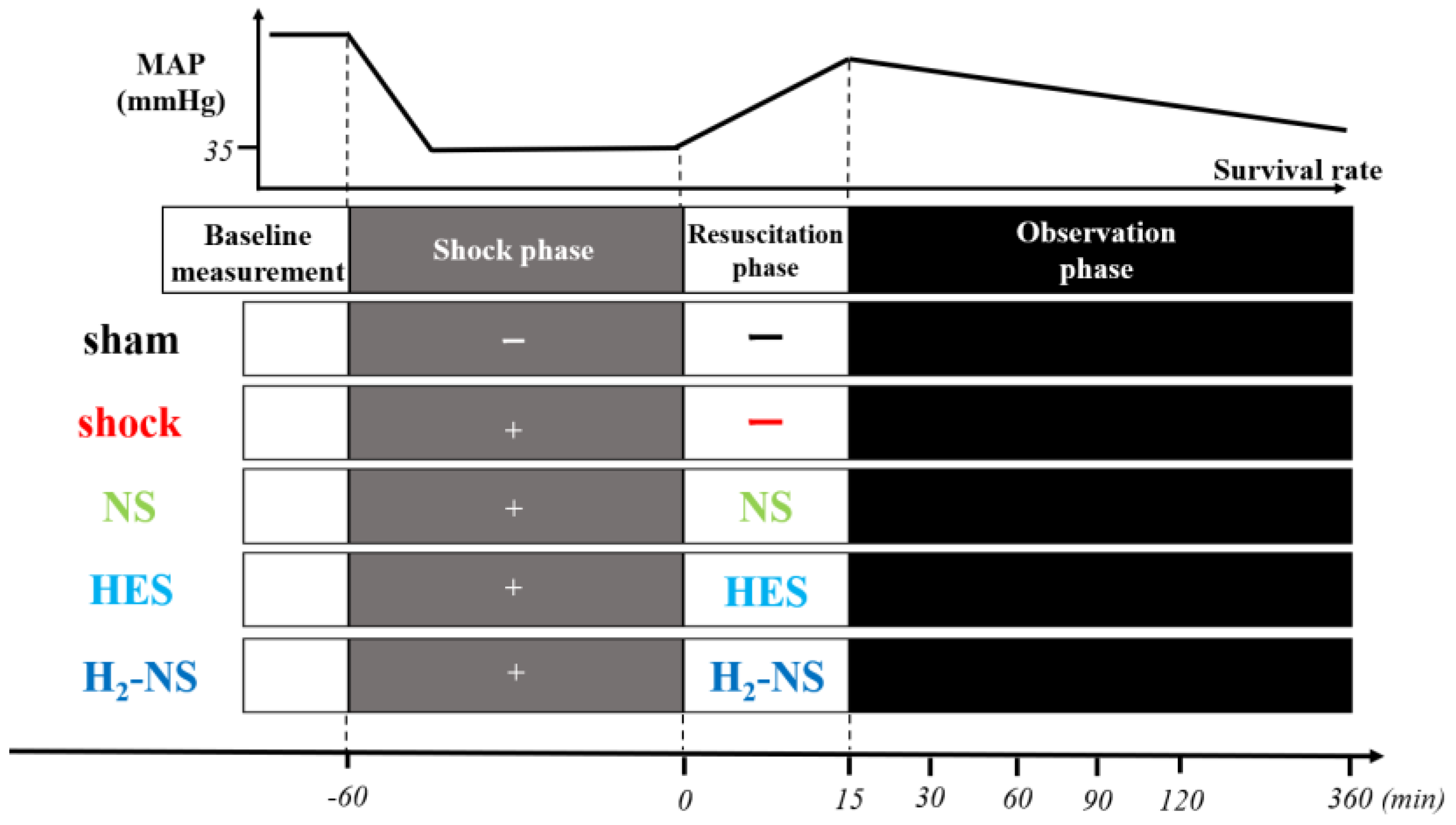
2.2. Study Protocol
2.3. H2-Rich Saline
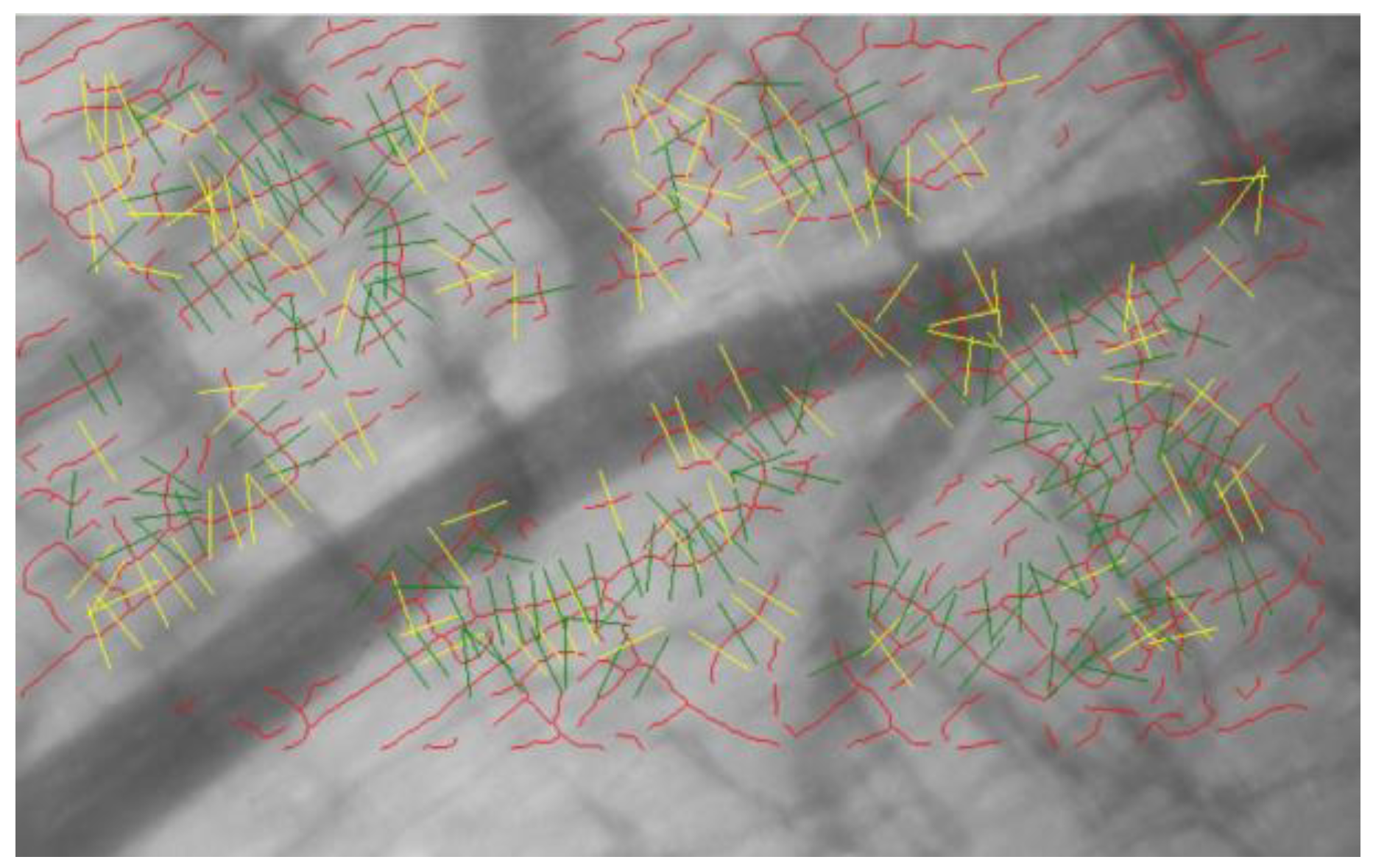
2.4. Glycocalyx Analysis
2.5. Cardiac Contraction
2.6. Blood Analysis
2.7. Reactive Oxygen Species
2.8. Histopathological Analysis
2.9. Survival Rate
2.10. Statistical Analysis
3. Results
3.1. Characteristics of Rats
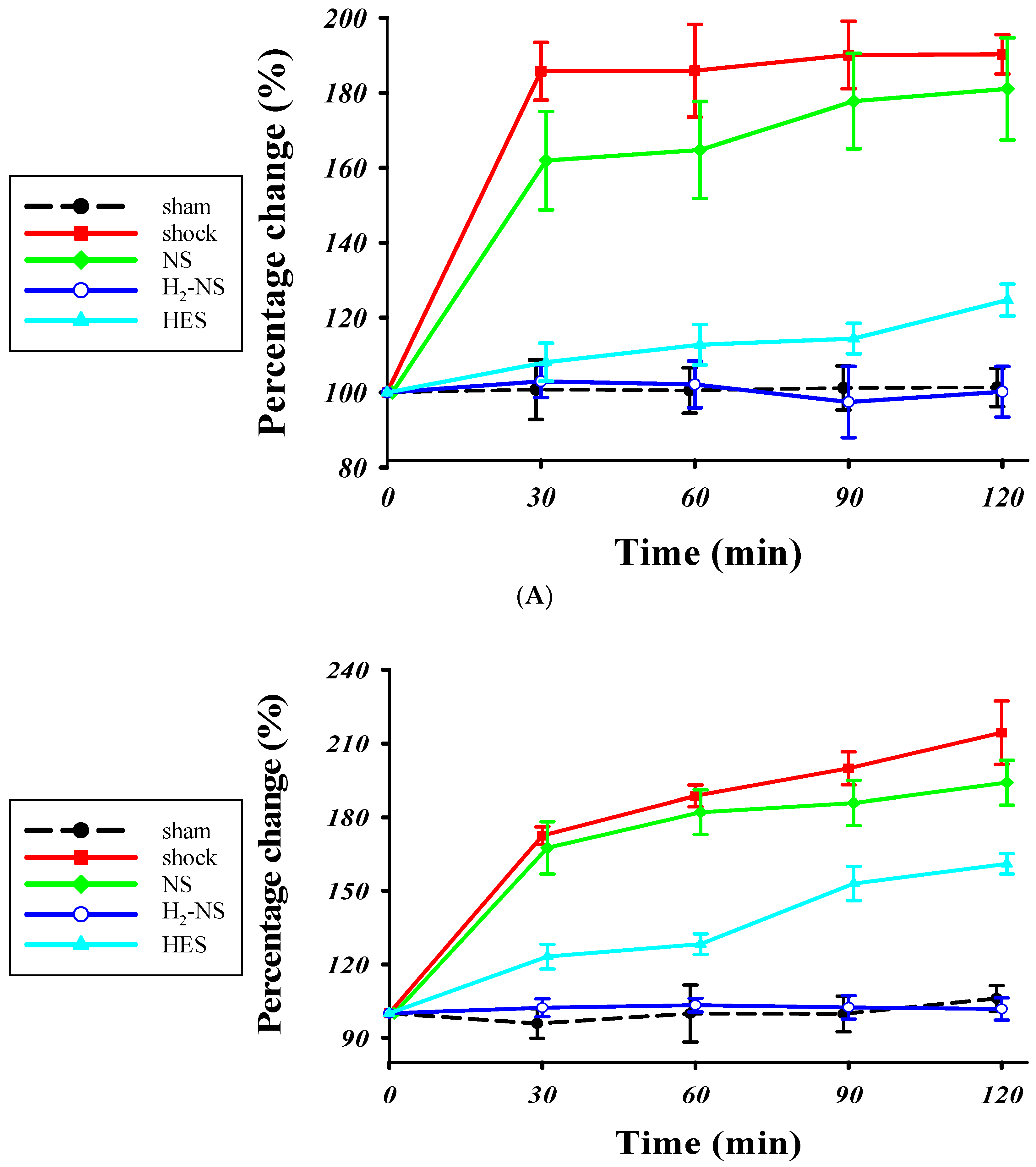
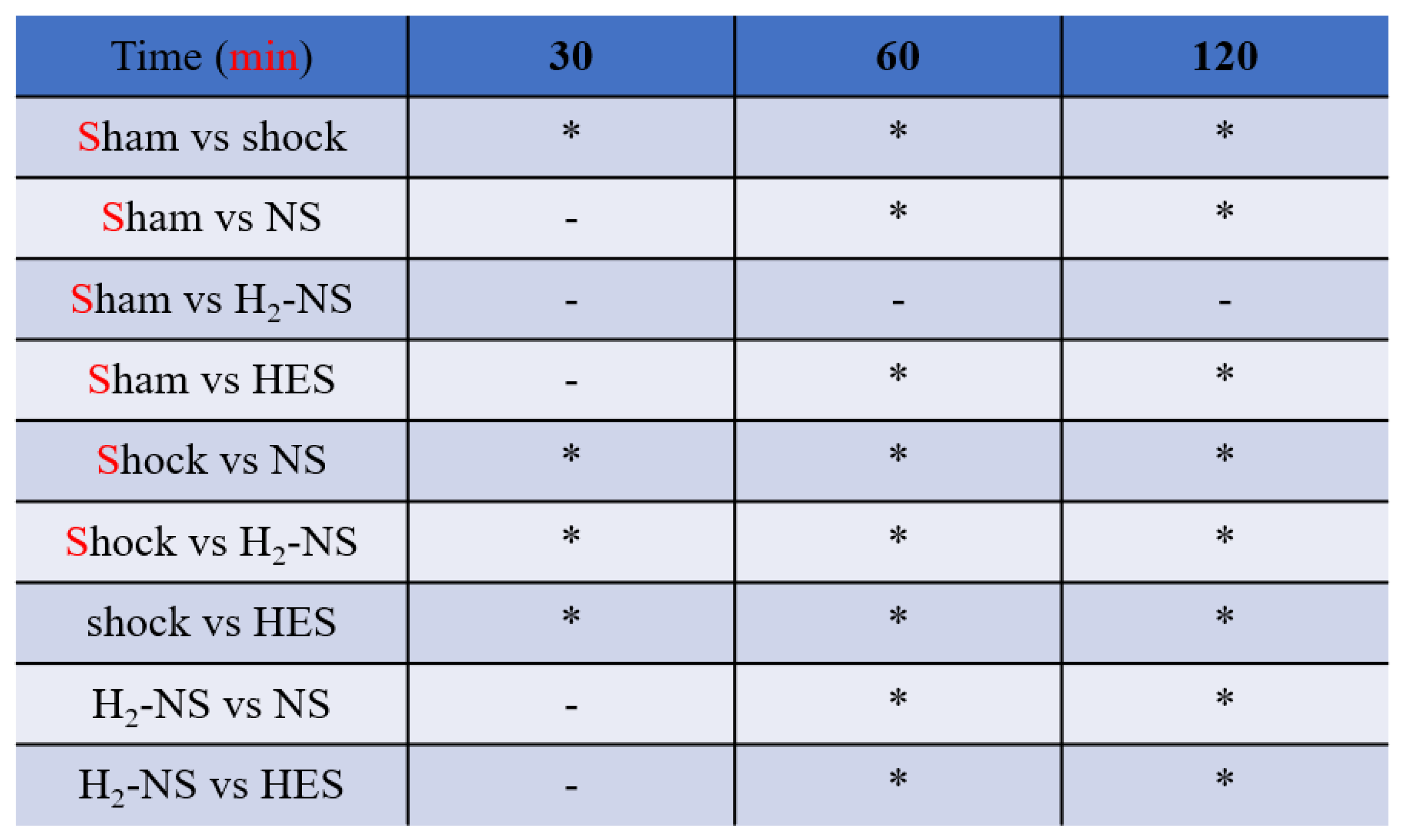
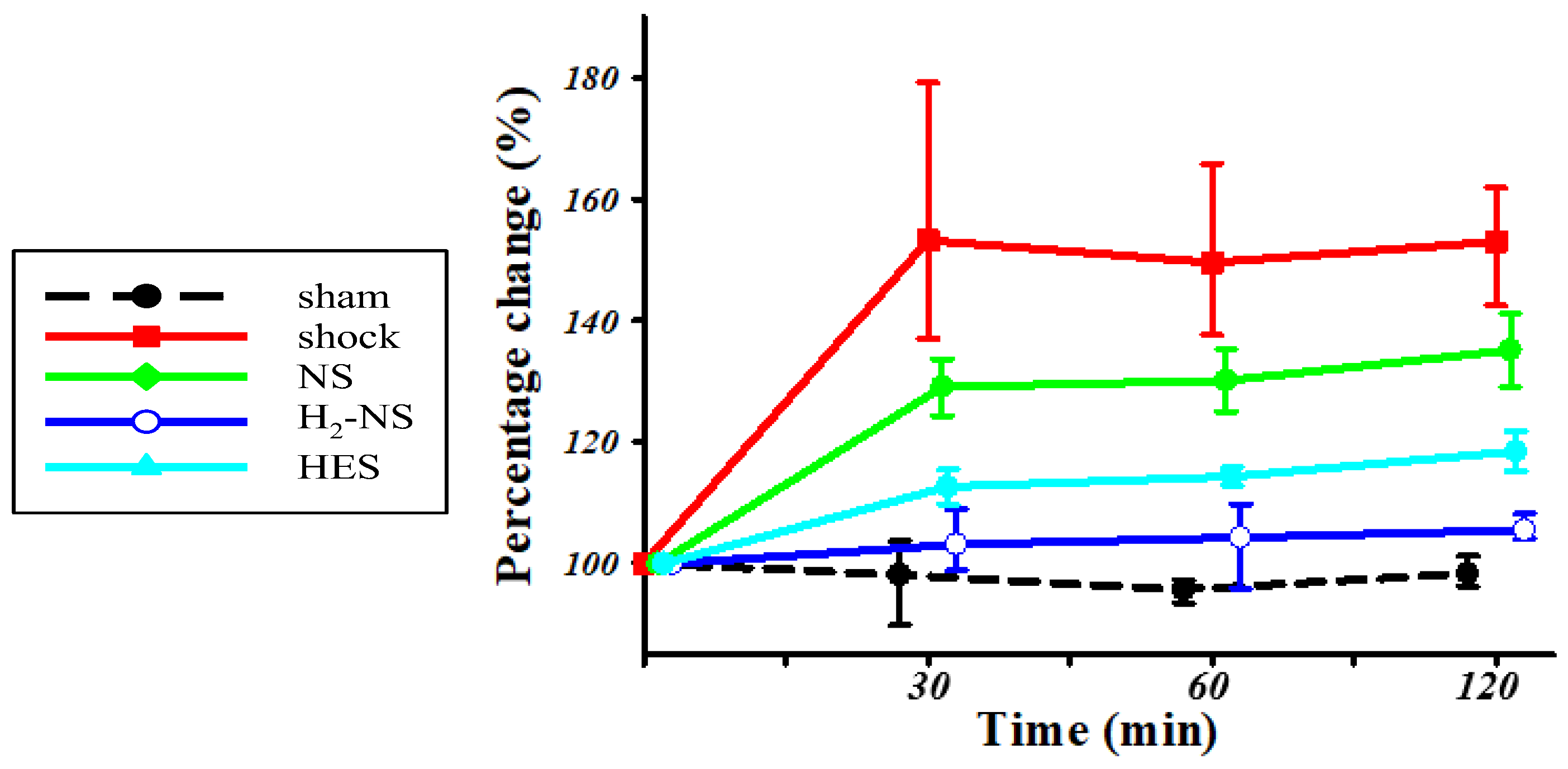
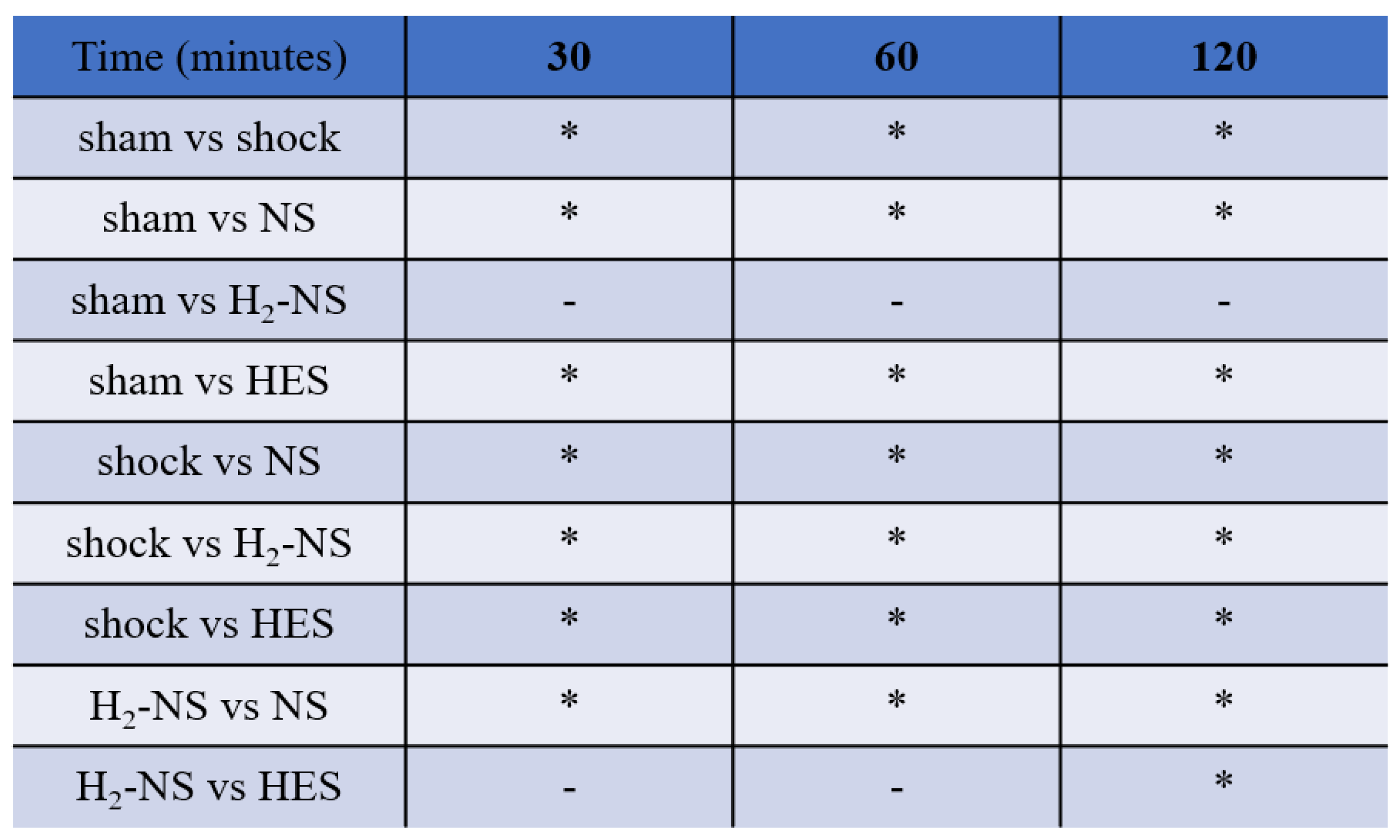
3.2. Effect of H2 on Glycocalyx Shedding
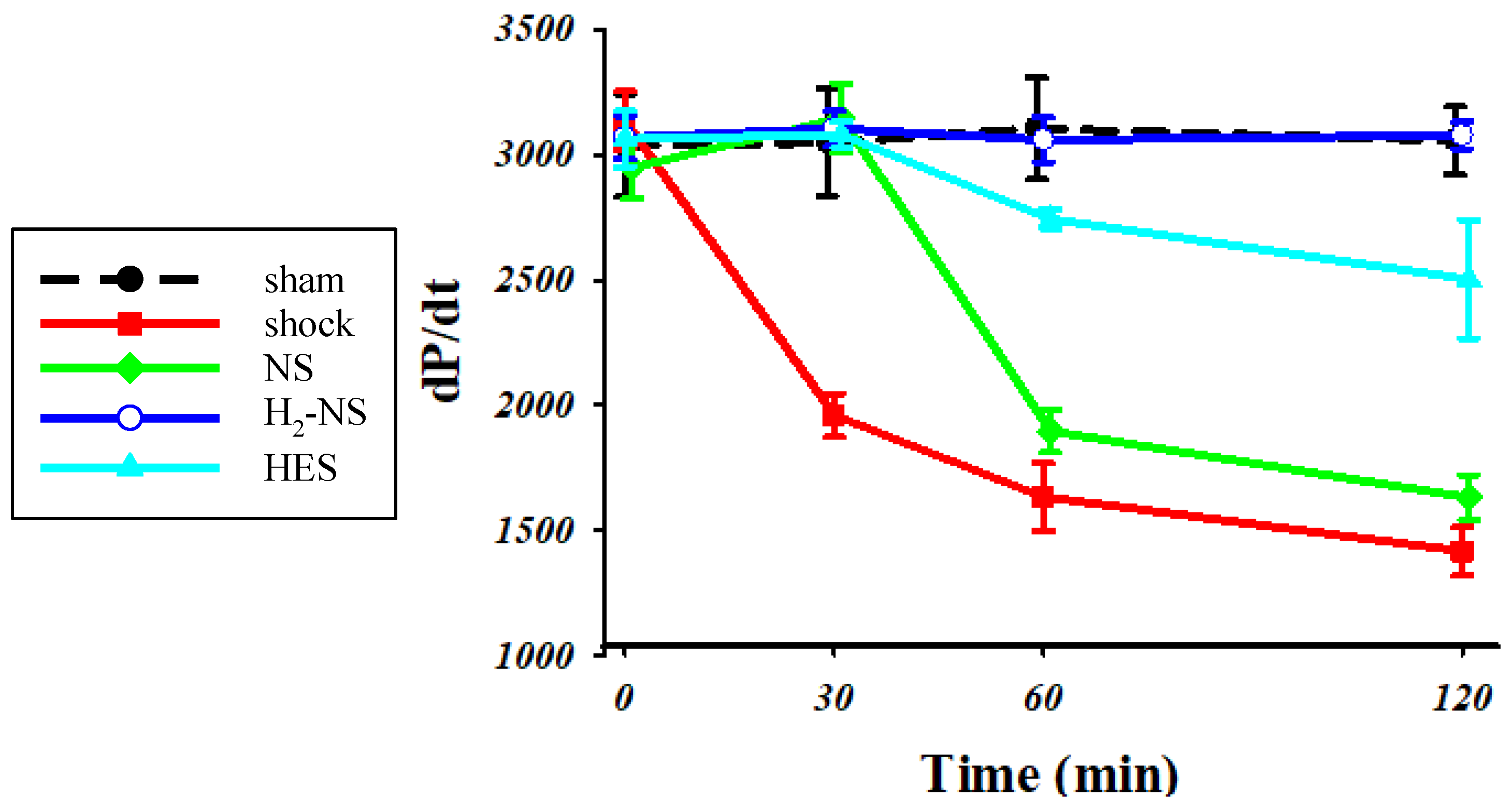
3.3. Effect of H2 on Cardiac Contraction
3.4. Effect of H2 on ROS Levels
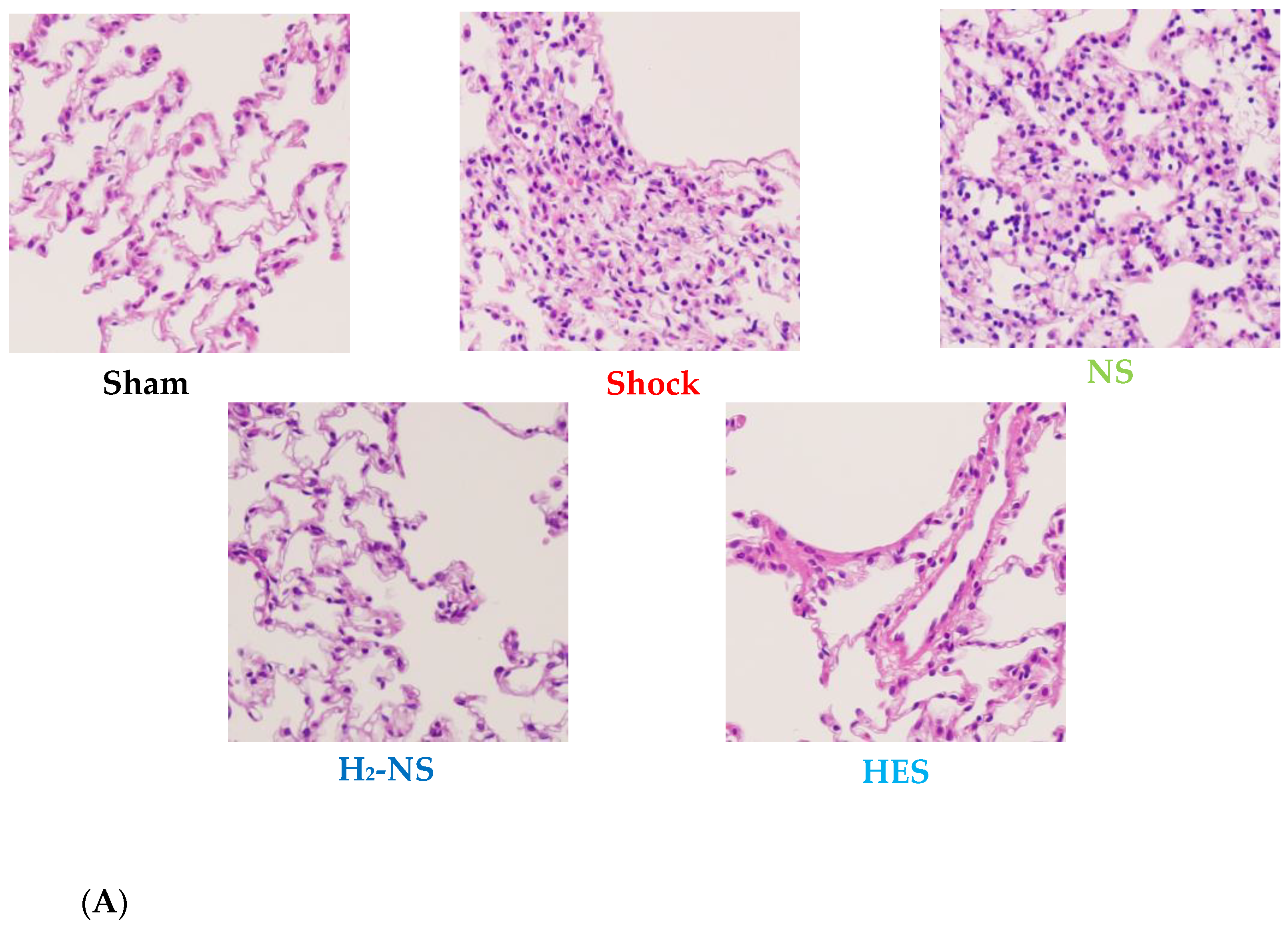
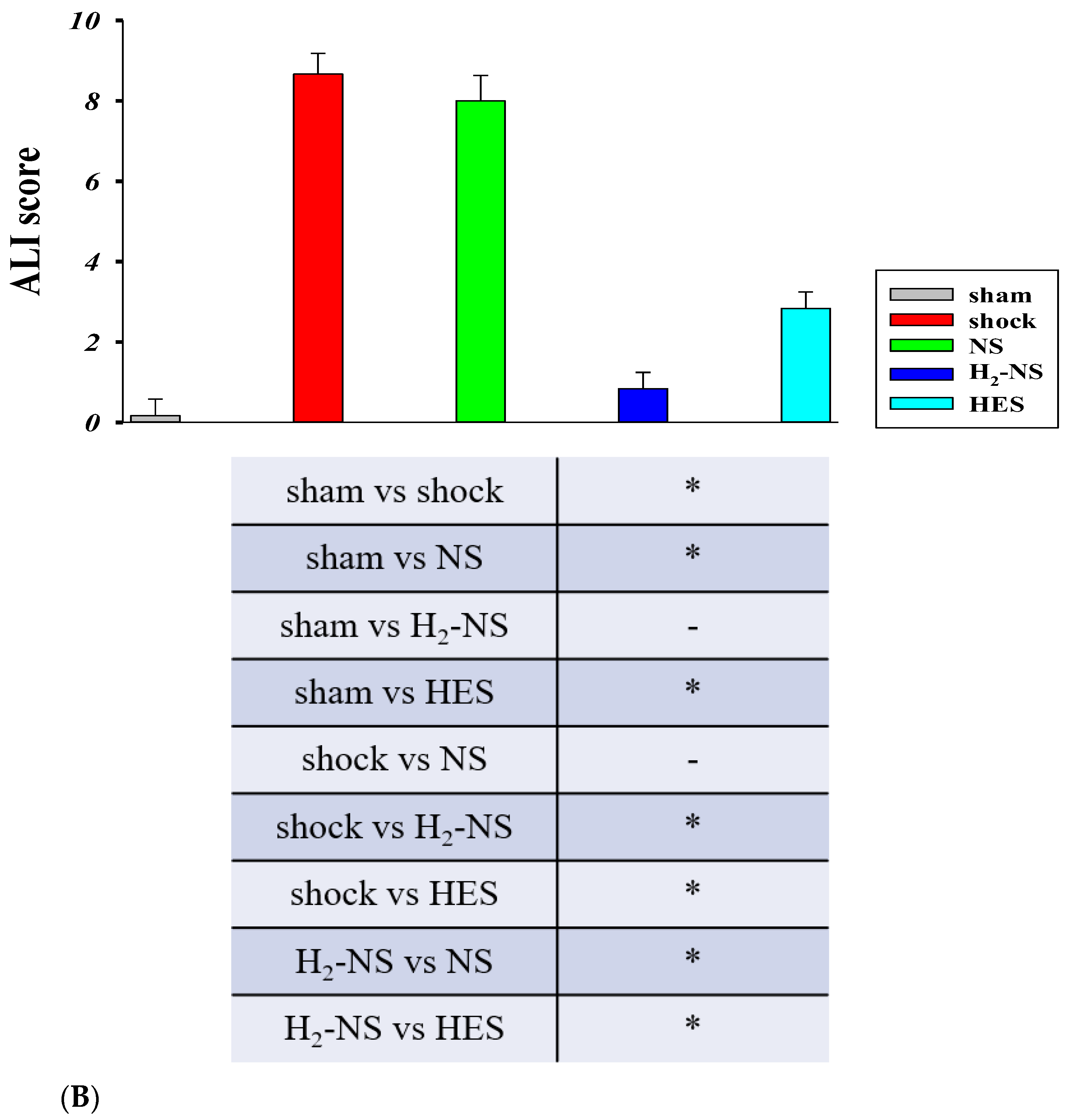
3.5. Effect of H2 on Lung Histopathology
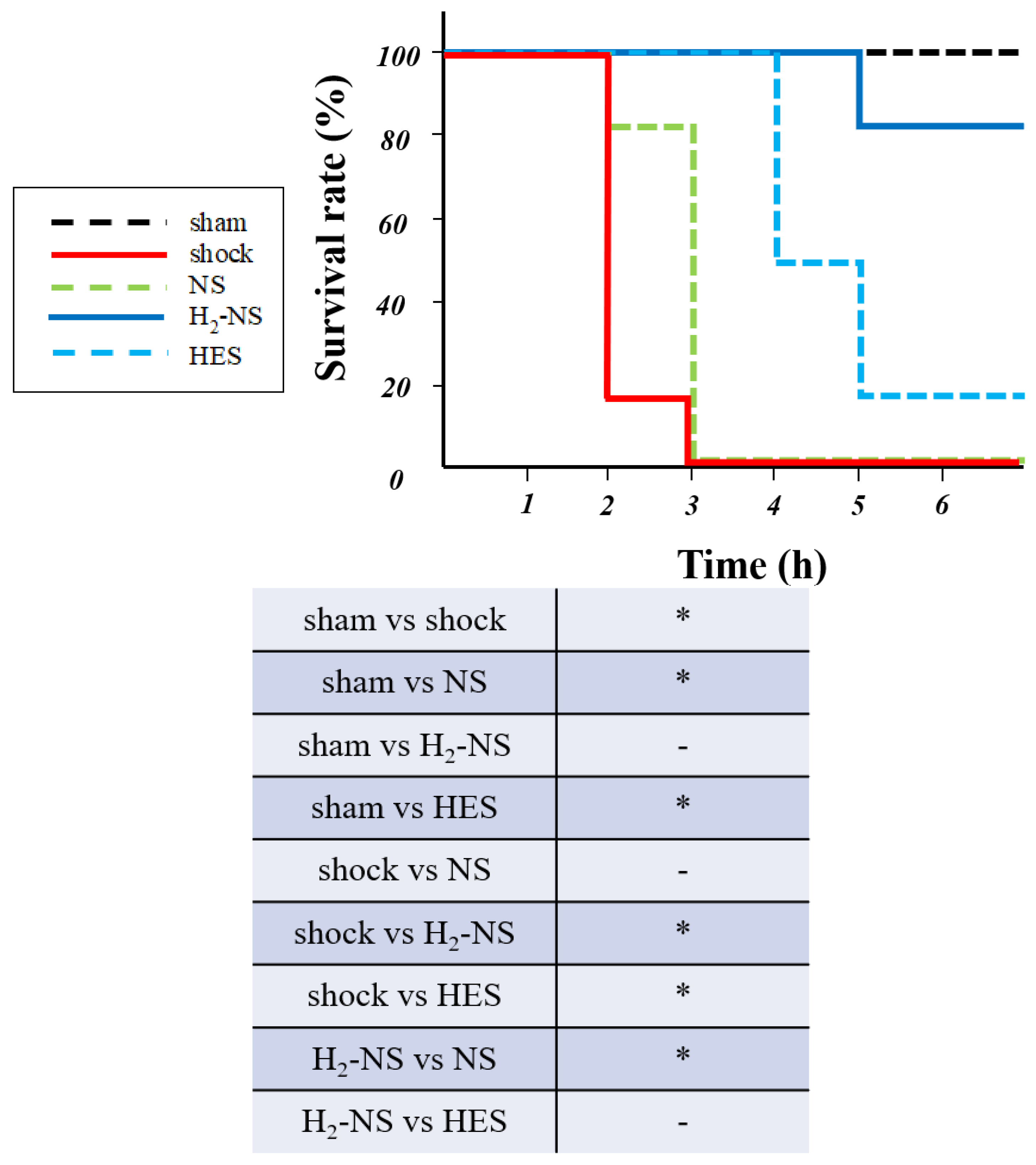
3.6. Effect of H2 on Survival Rate
3.7. Blood Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALI | acute lung injury |
| ANOVA | analysis of variance |
| BUN | blood urine nitrogen |
| d-ROMS | derivatives-reactive oxygen metabolites |
| H2 | hydrogen |
| HES | hydroxyethyl starch |
| MAP | mean arterial pressure |
| NS | normal saline |
| PBR | perfusion boundary region |
| ROS | reactive oxygen species |
References
- Cannon, J.W. Hemorrhagic Shock. N. Engl. J. Med. 2018, 378, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.; Holcomb, J.B. Optimal Fluid Therapy for Traumatic Hemorrhagic Shock. Crit. Care Clin. 2017, 33, 15–36. [Google Scholar] [CrossRef] [PubMed]
- Straat, M.; Müller, M.C.; Meijers, J.C.; Arbous, M.S.; Spoelstra-de Man, A.M.; Beurskens, C.J.; Vroom, M.B.; Juffermans, N.P. Effect of transfusion of fresh frozen plasma on parameters of endothelial condition and inflammatory status in non-bleeding critically ill patients: A prospective substudy of a randomized trial. Crit. Care 2015, 19, 163. [Google Scholar] [CrossRef] [PubMed]
- Jacob, M.; Bruegger, D.; Rehm, M.; Stoeckelhuber, M.; Welsch, U.; Conzen, P.; Becker, B.F. The endothelial glycocalyx affords compatibility of Starling’s principle and high cardiac interstitial albumin levels. Cardiovasc. Res. 2007, 73, 575–586. [Google Scholar] [CrossRef]
- Delaney, M.; Wendel, S.; Bercovitz, R.S.; Cid, J.; Cohn, C.; Dunbar, N.M.; Apelseth, T.O.; Popovsky, M.; Stanworth, S.J.; Tinmouth, A.; et al. Transfusion reactions: Prevention, diagnosis, and treatment. Lancet 2016, 388, 2825–2836. [Google Scholar] [CrossRef]
- Chipman, A.M.; Jenne, C.; Wu, F.; Kozar, R.A. Contemporary resuscitation of hemorrhagic shock: What will the future hold? Am. J. Surg. 2020, 220, 580–588. [Google Scholar] [CrossRef]
- Milford, E.M.; Reade, M.C. Resuscitation Fluid Choices to Preserve the Endothelial Glycocalyx. Crit. Care 2019, 23, 77. [Google Scholar] [CrossRef]
- Alphonsus, C.S.; Rodseth, R.N. The endothelial glycocalyx: A review of the vascular barrier. Anaesthesia 2014, 69, 777–784. [Google Scholar] [CrossRef]
- Becker, B.F.; Chappell, D.; Bruegger, D.; Annecke, T.; Jacob, M. Therapeutic strategies targeting the endothelial glycocalyx: Acute deficits, but great potential. Cardiovasc. Res. 2010, 87, 300–310. [Google Scholar] [CrossRef]
- Kozar, R.A.; Peng, Z.; Zhang, R.; Holcomb, J.B.; Pati, S.; Park, P.; Ko, T.C.; Paredes, A. Plasma restoration of endothelial glycocalyx in a rodent model of hemorrhagic shock. Anesth. Analg. 2011, 112, 1289–1295. [Google Scholar] [CrossRef]
- Shen, M.; Zhang, H.; Yu, C.; Wang, F.; Sun, X. A review of experimental studies of hydrogen as a new therapeutic agent in emergency and critical care medicine. Med. Gas Res. 2014, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- TaTamura, T.; Sano, M.; Matsuoka, T.; Yoshizawa, J.; Yamamoto, R.; Katsumata, Y.; Endo, J.; Homma, K.; Kajimura, M.; Suzuki, M.; et al. Hydrogen Gas Inhalation Attenuates Endothelial Glycocalyx Damage and Stabilizes Hemodynamics in a Rat Hemorrhagic Shock Model. Shock 2020, 54, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Mimuro, S.; Katoh, T.; Kurita, T.; Truong, S.K.; Kobayashi, K.; Makino, H.; Doi, M.; Nakajima, Y. 1.2% Hydrogen gas inhalation protects the endothelial glycocalyx during hemorrhagic shock: A prospective laboratory study in rats. J. Anesth. 2020, 34, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Hirano, M.; Sugai, K.; Fujisawa, M.; Kobayashi, E.; Katsumata, Y.; Hakamata, Y.; Sano, M. Pharmacokinetics of hydrogen administered intraperitoneally as hydrogen-rich saline and its effect on ischemic neuronal cell death in the brain in gerbils. PLoS ONE 2022, 17, e0279410. [Google Scholar] [CrossRef]
- Cornelli, U.; Terranova, R.; Luca, S.; Cornelli, M.; Alberti, A. Bioavailability and antioxidant activity of some food supplements in men and women using the D-Roms test as a marker of oxidative stress. J. Nutr. 2001, 131, 3208–3211. [Google Scholar] [CrossRef]
- Tsuchiya, M.; Sato, E.F.; Inoue, M.; Asada, A. Open abdominal surgery increases intraoperative oxidative stress: Can it be prevented? Anesth. Analg. 2008, 107, 1946–1952. [Google Scholar] [CrossRef]
- Uekusa, H.; Miyazaki, C.; Kondo, K.; Harada, N.; Nomoto, J.; Sugo, N.; Nemoto, M. Hydroperoxide in internal jugular venous blood reflects occurrence of subarachnoid hemorrhage-induced delayed cerebral vasospasm. J. Stroke Cerebrovasc. Dis. Off. J. Natl. Stroke Assoc. 2014, 23, 2217–2224. [Google Scholar] [CrossRef]
- Fukui, T.; Yamauchi, K.; Maruyama, M.; Yasuda, T.; Kohno, M.; Abe, Y. Significance of measuring oxidative stress in lifestyle-related diseases from the viewpoint of correlation between d-ROMs and BAP in Japanese subjects. Hypertens. Res. 2011, 34, 1041–1045. [Google Scholar] [CrossRef]
- Smith, K.M.; Mrozek, J.D.; Simonton, S.C.; Bing, D.R.; Meyers, P.A.; Connett, J.E.; Mammel, M.C. Prolonged partial liquid ventilation using conventional and high-frequency ventilatory techniques: Gas exchange and lung pathology in an animal model of respiratory distress syndrome. Crit. Care Med. 1997, 25, 1888–1897. [Google Scholar] [CrossRef]
- Merryman, W.D.; Youn, I.; Lukoff, H.D.; Krueger, P.M.; Guilak, F.; Hopkins, R.A.; Sacks, M.S. Correlation between heart valve interstitial cell stiffness and transvalvular pressure: Implications for collagen biosynthesis. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H224–H231. [Google Scholar] [CrossRef]
- Guerci, P.; Ergin, B.; Uz, Z.; Ince, Y.; Westphal, M.; Heger, M.; Ince, C. Glycocalyx Degradation Is Independent of Vascular Barrier Permeability Increase in Nontraumatic Hemorrhagic Shock in Rats. Anesth. Analg. 2019, 129, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Rovas, A.; Lukasz, A.H.; Vink, H.; Urban, M.; Sackarnd, J.; Pavenstädt, H.; Kümpers, P. Bedside analysis of the sublingual microvascular glycocalyx in the emergency room and intensive care unit—The GlycoNurse study. Scand. J. Trauma Resusc. Emerg. Med. 2018, 26, 16. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Chen-Yoshikawa, T.F.; Saito, M.; Tanaka, S.; Miyamoto, E.; Ohata, K.; Kondo, T.; Motoyama, H.; Hijiya, K.; Aoyama, A.; et al. Immersing lungs in hydrogen-rich saline attenuates lung ischaemia-reperfusion injury. Eur. J. Cardiothorac. Surg. 2017, 51, 442–448. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Meng, X.; Xu, H.; Dang, Y.; Fan, Y.; Lv, M.; Sang, H.; Xu, L. Hyperoxygenated Hydrogen-Rich Solution Suppresses Lung Injury Induced by Hemorrhagic Shock in Rats. J. Surg. Res. 2019, 239, 103–114. [Google Scholar] [CrossRef]
- Chelazzi, C.; Villa, G.; Mancinelli, P.; De Gaudio, A.R.; Adembri, C. Glycocalyx and sepsis-induced alterations in vascular permeability. Crit. Care 2015, 19, 26. [Google Scholar] [CrossRef]
- Rovas, A.; Seidel, L.M.; Vink, H.; Pohlkötter, T.; Pavenstädt, H.; Ertmer, C.; Hessler, M.; Kümpers, P. Association of sublingual microcirculation parameters and endothelial glycocalyx dimensions in resuscitated sepsis. Crit. Care 2019, 23, 260. [Google Scholar] [CrossRef]
- Yang, Y.; Zhu, Y.; Xi, X. Anti-inflammatory and antitumor action of hydrogen via reactive oxygen species. Oncol. Lett. 2018, 16, 2771–2776. [Google Scholar] [CrossRef]
- Kazuma, S.; Tokinaga, Y.; Kimizuka, M.; Azumaguchi, R.; Hamada, K.; Yamakage, M. Sevoflurane Promotes Regeneration of the Endothelial Glycocalyx by Upregulating Sialyltransferase. J. Surg. Res. 2019, 241, 40–47. [Google Scholar] [CrossRef]

| Sham | Shock | NS | HES | H2-NS | p Values | |
|---|---|---|---|---|---|---|
| Body weight (g) | 501.7 ± 14.7 | 498.3 ± 14.7 | 495.0 ± 10.5 | 496.7 ± 16.3 | 498.3 ± 18.3 | 0.941 |
| Blood loss (mL) | - | 10.0 ± 0.3 | 10.0 ± 0.3 | 10.0 ± 0.2 | 10.0 ± 0.3 | 0.938 |
| Resuscitation fluid volume (mL) | - | - | 31.7 ± 1.4 | 10.3 ± 0.7 | 9.9 ± 0.6 | <0.001 (H2-NS vs. NS) 0.781 (H2-NS vs. HES) |
| Sham | Shock | NS | HES | H2-NS | |
|---|---|---|---|---|---|
| Baseline | |||||
| pH | 7.40 ± 0.03 | 7.18 ± 0.03 | 7.28 ± 0.03 | 7.35 ± 0.03 | 7.37 ± 0.03 |
| PaCO2 (mmHg) | 33.7 ± 1.03 | 33.7 ± 1.75 | 34.0 ± 2.1 | 33.7 ± 1.5 | 33.0 ± 1.1 |
| PaO2 (mmHg) | 232.7 ± 11.38 | 233.0 ± 8.32 | 240.17 ± 8.33 | 234.5 ± 9.31 | 234.2 ± 7.89 |
| BE (mEq/L) | −1.1 ± 0.24 | −1.05 ± 0.33 | −1.17 ± 0.31 | −0.98 ± 0.25 | −1.0 ± 0.36 |
| Lac (mmol/L) | 1.75 ± 011 | 1.75 ± 0.13 | 1.7 ± 0.11 | 1.65 ± 0.12 | 1.8 ± 0.11 |
| Hb (g/dL) | 13.61 ± 0.48 | 13.63 ± 0.43 | 13.65 ± 0.54 | 13.67 ± 0.61 | 13.57 ± 0.55 |
| BUN (mg/dL) | 27.0 ± 1.27 | 27.5 ± 1.38 | 27.67 ± 1.21 | 27.17 ± 1.72 | 26.5 ± 1.05 |
| Cre (mg/dL) | 0.47 ± 0.1 | 0.43 ± 0.1 | 0.43 ± 0.1 | 0.48 ± 0.08 | 0.47 ± 0.1 |
| Sodium (mEq/L) | 138.3 ± 1.97 | 138.5 ± 2.07 | 138.5 ± 3.08 | 139.5 ± 3.27 | 138.85 ± 3.55 |
| Potassium (mEq/L) | 3.92 ± 0.29 | 3.77 ± 0.24 | 3.87 ± 0.25 | 3.88 ± 0.26 | 3.83 ± 0.25 |
| 30 min after resuscitation | |||||
| pH | 7.402 ± 0.04 | 7.18 ± 0.03 *† | 7.28 ± 0.02 *† | 7.35 ± 0.03 * | 7.37 ± 0.03 |
| PaCO2 (mmHg) | 33.7 ± 1.76 | 32.7 ± 1.37 | 32.0 ± 1.27 | 31.8 ± 0.75 | 32.8 ± 0.6 |
| PaO2 (mmHg) | 234.2 ± 5.56 | 96.3 ± 4.07 † | 87.6 ± 1.22 *† | 166.9 ± 6.50 | 230.9 ± 12.36 |
| BE (mEq/L) | −1.15 ± 0.39 | −13.63 ± 0.83 *† | −6.4 ± 0.4 * | −4.8 ± 0.37 | −3.77 ± 0.85 |
| Lac (mmol/L) | 1.73 ± 0.11 | 10.93 ± 0.69 *† | 8.08 ± 0.23 * | 4.05 ± 0.34 | 3.05 ± 0.17 |
| Hb (g/dL) | 13.65 ± 0.41 † | 6.97 ± 0.22 *† | 6.77 ± 0.22 *† | 9.2 ± 0.54 *† | 9.92 ± 0.12* |
| BUN (mg/dL) | 27.17 ± 1.72 | 32.67 ± 1.37 *† | 28.33 ± 1.5 | 27.83 ± 1.84 | 26.7 ± 0.82 |
| Cre (mg/dL) | 0.47 ± 0.12 | 2.27 ± 0.18 *† | 1.33 ± 0.14 *† | 1.38 ± 0.12 *† | 0.53 ± 0.1 |
| Sodium (mEq/L) | 139.7 ± 3.6 | 139.3 ± 2.8 | 140.0 ± 3.0 | 140.0 ± 3.3 | 138.5 ± 2.2 |
| Potassium (mEq/L) | 3.95 ± 0.24 | 4.4 ± 0.14 *† | 4.0 ± 0.3 | 3.87 ± 0.22 | 3.93 ± 0.21 |
| 120 min after resuscitation | |||||
| pH | 7.43 ± 0.03 | 7.22 ± 0.03 *† | 7.24 ± 0.02 *† | 7.36 ± 0.03 * | 7.39 ± 0.02 |
| PaCO2 (mmHg) | 32.0 ± 1.41 | 31.67 ± 1.86 | 32.5 ± 2.07 | 32.33 ± 1.75 | 32.5 ± 1.87 |
| PaO2 (mmHg) | 232.67 ± 11.38 | 95.48 ± 3.20 *† | 75.10 ± 2.80 *† | 168.10 ± 3.80 *† | 232.82 ± 8.22 |
| BE (mEq/L) | −1.70 ± 0.37 † | −16.13 ± 0.48 *† | −11.63 ± 0.86 *† | −6.40 ± 0.44 *† | −4.10 ± 0.76 * |
| Lac (mmol/L) | 1.72 ± 0.10 † | 12.67 ± 0.90 *† | 8.70 ± 0.48 *† | 5.77 ± 0.46 *† | 3.40 ± 0.33 * |
| Hb (g/dL) | 13.48 ± 0.46 | 6.83 ± 0.22 *† | 7.02 ± 0.50 *† | 9.82 ± 0.25 *† | 10.6 ± 0.41 * |
| BUN (mg/dL) | 26.83 ± 1.47 | 32.0 ± 1.41 *† | 29.33 ± 2.07 * | 28.67 ± 0.52 | 27.83 ± 1.17 |
| Cre (mg/dL) | 0.52 ± 0.09 | 5.78 ± 0.28 *† | 2.4 ± 0.15 * | 2.45 ± 0.22 * | 0.85 ± 0.1 |
| Sodium (mEq/L) | 140.17 ± 3.19 | 141.5 ± 4.76 | 137.0 ± 1.41 | 140.33 ± 3.01 | 138.33 ± 3.27 |
| Potassium (mEq/L) | 3.88 ± 0.17 | 4.17 ± 0.18 * | 3.93 ± 0.1 | 3.88 ± 0.15 | 3.82 ± 0.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kimura, A.; Suehiro, K.; Yamada, T.; Shinta, Y.; Juri, T.; Fujimoto, Y.; Hirano, S.; Mori, T. Protective Effects of Hydrogen-Rich Saline Against Hemorrhagic Shock in Rats via an Endothelial Glycocalyx Pathway. Biomedicines 2025, 13, 833. https://doi.org/10.3390/biomedicines13040833
Kimura A, Suehiro K, Yamada T, Shinta Y, Juri T, Fujimoto Y, Hirano S, Mori T. Protective Effects of Hydrogen-Rich Saline Against Hemorrhagic Shock in Rats via an Endothelial Glycocalyx Pathway. Biomedicines. 2025; 13(4):833. https://doi.org/10.3390/biomedicines13040833
Chicago/Turabian StyleKimura, Aya, Koichi Suehiro, Tokuhiro Yamada, Yasuda Shinta, Takashi Juri, Yohei Fujimoto, Shinichi Hirano, and Takashi Mori. 2025. "Protective Effects of Hydrogen-Rich Saline Against Hemorrhagic Shock in Rats via an Endothelial Glycocalyx Pathway" Biomedicines 13, no. 4: 833. https://doi.org/10.3390/biomedicines13040833
APA StyleKimura, A., Suehiro, K., Yamada, T., Shinta, Y., Juri, T., Fujimoto, Y., Hirano, S., & Mori, T. (2025). Protective Effects of Hydrogen-Rich Saline Against Hemorrhagic Shock in Rats via an Endothelial Glycocalyx Pathway. Biomedicines, 13(4), 833. https://doi.org/10.3390/biomedicines13040833







