Metabolic Disruptions and Non-Communicable Disease Risks Associated with Long-Term Particulate Matter Exposure in Northern Thailand: An NMR-Based Metabolomics Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemical Reagents
2.2. Participants and Study Design
2.3. Preparation of Blood Samples
2.4. 1H-NMR Spectroscopy
2.5. Metabolite Identification and Spectral Analysis
2.6. Data Processing
2.7. Statistical Analysis
2.8. Metabolomics Data Analysis
3. Results
3.1. Participant Demographic Information
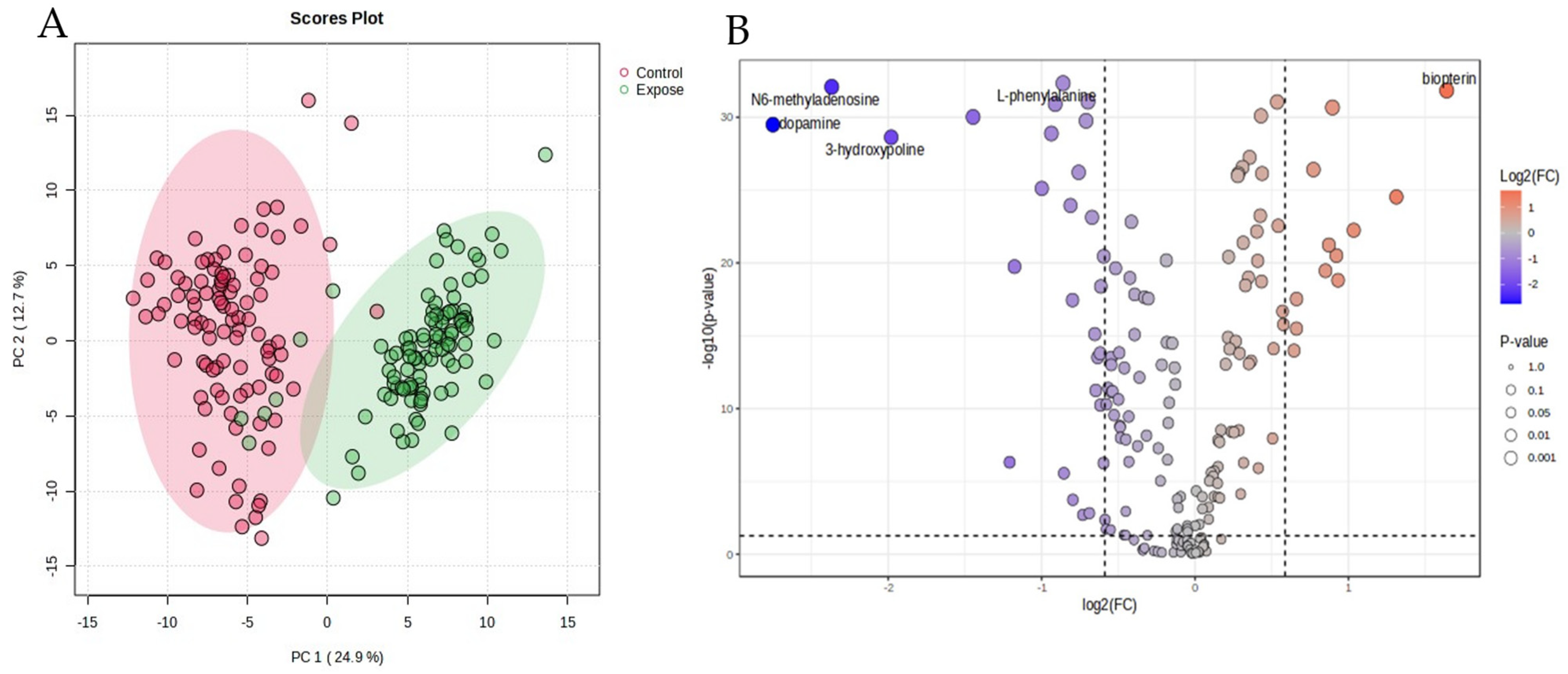
3.2. Analysis of Different Metabolites Between the Particulate Matter Exposure and the Control Group
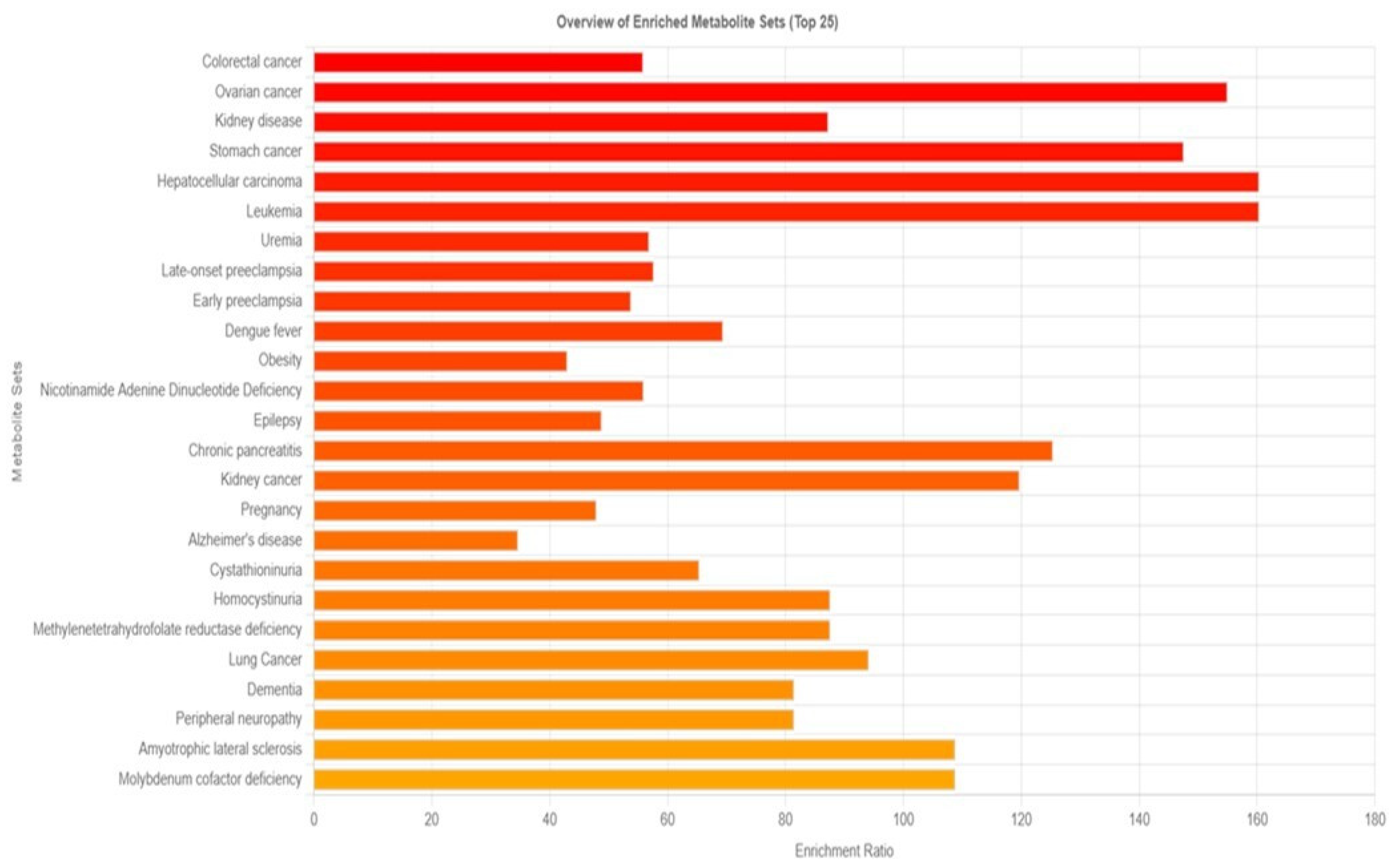
3.3. Analysis of Long-Term PM2.5 Exposure-Related Diseases
3.4. Impact of Particulate Exposure to Metabolic Pathways
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xing, Y.F.; Xu, Y.H.; Shi, M.H.; Lian, Y.X. The impact of PM2.5 on the human respiratory system. J. Thorac. Dis. 2016, 8, E69–E74. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.; Yang, Q.; Liu, G. Rare earth elements unintentionally released from global industrial activities. J. Hazard. Mater. 2024, 480, 136146. [Google Scholar] [CrossRef] [PubMed]
- Chansuebsri, S.; Kraisitnitikul, P.; Wiriya, W.; Chantara, S. Fresh and aged PM2.5 and their ion composition in rural and urban atmospheres of Northern Thailand in relation to source identification. Chemosphere 2022, 286, 131803. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Chen, D.; Zhao, C.; Kwan, M.P.; Cai, J.; Zhuang, Y.; Zhao, B.; Wang, X.; Chen, B.; Yang, J.; et al. Influence of meteorological conditions on PM2.5 concentrations across China: A review of methodology and mechanism. Environ. Int. 2020, 139, 105558. [Google Scholar] [CrossRef]
- Wang, J.; Lin, L.; Huang, J.; Zhang, J.; Duan, J.; Guo, X.; Wu, S.; Sun, Z. Impact of PM2.5 exposure on plasma metabolome in healthy adults during air pollution waves: A randomized, crossover trial. J. Hazard. Mater. 2022, 436, 129180. [Google Scholar] [CrossRef]
- Haulica, I.; Capalna, S.; Badescu, A.; Picioreanu, A.; Topoliceanu, F. Contributions to the study of the metabolic basis of audiogenic convulsions. Fiziol. Norm. Patol. 1966, 12, 223–227. [Google Scholar]
- Cohen, A.J.; Brauer, M.; Burnett, R.; Anderson, H.R.; Frostad, J.; Estep, K.; Balakrishnan, K.; Brunekreef, B.; Dandona, L.; Dandona, R.; et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: An analysis of data from the Global Burden of Diseases Study 2015. Lancet 2017, 389, 1907–1918. [Google Scholar] [CrossRef]
- Feng, S.; Gao, D.; Liao, F.; Zhou, F.; Wang, X. The health effects of ambient PM2.5 and potential mechanisms. Ecotoxicol. Environ. Saf. 2016, 128, 67–74. [Google Scholar] [CrossRef]
- Li, T.; Yu, Y.; Sun, Z.; Duan, J. A comprehensive understanding of ambient particulate matter and its components on the adverse health effects based from epidemiological and laboratory evidence. Part. Fibre Toxicol. 2022, 19, 67. [Google Scholar] [CrossRef]
- Yitshak-Sade, M.; Bobb, J.F.; Schwartz, J.D.; Kloog, I.; Zanobetti, A. The association between short and long-term exposure to PM2.5 and temperature and hospital admissions in New England and the synergistic effect of the short-term exposures. Sci. Total Environ. 2018, 639, 868–875. [Google Scholar] [CrossRef]
- Kloog, I.; Ridgway, B.; Koutrakis, P.; Coull, B.A.; Schwartz, J.D. Long- and short-term exposure to PM2.5 and mortality: Using novel exposure models. Epidemiology 2013, 24, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Cai, Y.; Yao, H.; Lin, C.; Xie, Y.; Tang, S.; Zhang, A. Small molecule metabolites: Discovery of biomarkers and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 132. [Google Scholar] [CrossRef] [PubMed]
- Nassan, F.L.; Wang, C.; Kelly, R.S.; Lasky-Su, J.A.; Vokonas, P.S.; Koutrakis, P.; Schwartz, J.D. Ambient PM2.5 species and ultrafine particle exposure and their differential metabolomic signatures. Environ. Int. 2021, 151, 106447. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Goodrich, J.; Walker, D.I.; Lin, Y.; Lurmann, F.; Qiu, C.; Jones, D.P.; Gilliland, F.; Chazi, L.; Chen, Z. Metabolic pathways altered by air pollutant exposure in association with lipid profiles in young adults. Environ. Pollut. 2023, 327, 121522. [Google Scholar] [CrossRef]
- Zhang, C.; Ma, T.; Liu, C.; Ma, D.; Wang, J.; Liu, M.; Ran, J.; Wang, X.; Deng, X. PM2.5 induced liver lipid metabolic disorders in C57BL/6J mice. Front. Endocrinol. 2023, 14, 1212291. [Google Scholar] [CrossRef]
- Yan, R.; Ji, S.; Ku, T.; Sang, N. Cross-Omics Analyses Reveal the Effects of Ambient PM2.5 Exposure on Hepatic Metabolism in Female Mice. Toxics 2024, 12, 587. [Google Scholar] [CrossRef]
- Song, X.; Liu, J.; Geng, N.; Shan, Y.; Zhang, B.; Zhao, B.; Ni, Y.; Liang, Z.; Chen, J.; Zhang, L.; et al. Multi-omics analysis to reveal disorders of cell metabolism and integrin signaling pathways induced by PM2.5. J. Hazard. Mater. 2022, 424, 127573. [Google Scholar] [CrossRef]
- Sun, J.; Peng, S.; Li, Z.; Liu, F.; Wu, C.; Lu, Y.; Xiang, H. Association of Short-Term Exposure to PM2.5 with Blood Lipids and the Modification Effects of Insulin Resistance: A Panel Study in Wuhan. Toxics 2022, 10, 663. [Google Scholar] [CrossRef]
- Feng, S.; Huang, F.; Zhang, Y.; Feng, Y.; Zhang, Y.; Cao, Y.; Wang, X. The pathophysiological and molecular mechanisms of atmospheric PM2.5 affecting cardiovascular health: A review. Ecotoxicol. Environ. Saf. 2023, 249, 114444. [Google Scholar] [CrossRef]
- Chanda, F.; Lin, K.X.; Chaurembo, A.I.; Huang, J.Y.; Zhang, H.J.; Deng, W.H.; Xu, Y.J.; Li, Y.; Fu, L.D.; Cui, H.D.; et al. PM2.5-mediated cardiovascular disease in aging: Cardiometabolic risks, molecular mechanisms and potential interventions. Sci. Total Environ. 2024, 954, 176255. [Google Scholar] [CrossRef]
- Shi, F.; Zhang, Z.; Wang, J.; Wang, Y.; Deng, J.; Zeng, Y.; Zou, P.; Ling, X.; Han, F.; Liu, J.; et al. Analysis by Metabolomics and Transcriptomics for the Energy Metabolism Disorder and the Aryl Hydrocarbon Receptor Activation in Male Reproduction of Mice and GC-2spd Cells Exposed to PM2.5. Front. Endocrinol. 2021, 12, 807374. [Google Scholar] [CrossRef] [PubMed]
- Song, T.; Wang, S.; Zhang, Y.; Song, J.; Liu, F.; Fu, P.; Shiraiwa, M.; Xie, Z.; Yue, D.; Zhong, L.; et al. Proteins and Amino Acids in Fine Particulate Matter in Rural Guangzhou, Southern China: Seasonal Cycles, Sources, and Atmospheric Processes. Environ. Sci. Technol. 2017, 51, 6773–6781. [Google Scholar] [CrossRef] [PubMed]
- Weng, L.; Quinlivan, E.; Gong, Y.; Beitelshees, A.L.; Shahin, M.H.; Turner, S.T.; Chapman, A.B.; Gums, J.G.; Johnson, J.A.; Frye, R.F.; et al. Association of branched and aromatic amino acids levels with metabolic syndrome and impaired fasting glucose in hypertensive patients. Metab. Syndr. Relat. Disord. 2015, 13, 195–202. [Google Scholar] [CrossRef]
- Li, J.; Hu, Y.; Liu, L.; Wang, Q.; Zeng, J.; Chen, C. PM2.5 exposure perturbs lung microbiome and its metabolic profile in mice. Sci. Total Environ. 2020, 721, 137432. [Google Scholar] [CrossRef]
- Zheng, X.Y.; Tang, S.L.; Liu, T.; Wang, Y.; Xu, X.J.; Xiao, N.; Li, C.; Xu, Y.J.; He, Z.X.; Ma, S.L.; et al. Effects of long-term PM2.5 exposure on metabolic syndrome among adults and elderly in Guangdong, China. Environ. Health 2022, 21, 84. [Google Scholar] [CrossRef]
- Dutta, A.; Chavalparit, O. Unmasking the veil of PM2.5 pollution: A comprehensive analysis of health effects, economic losses, and environmental implications in North Indian States. Ecotoxicol. Environ. Saf. 2025, 292, 117922. [Google Scholar] [CrossRef]
- Nan, N.; Yan, Z.; Zhang, Y.; Chen, R.; Qin, G.; Sang, N. Overview of PM2.5 and health outcomes: Focusing on components, sources, and pollutant mixture co-exposure. Chemosphere 2023, 323, 138181. [Google Scholar] [CrossRef]
- Thangavel, P.; Park, D.; Lee, Y.C. Recent Insights into Particulate Matter (PM2.5)-Mediated Toxicity in Humans: An Overview. Int. J. Environ. Res. Public Health 2022, 19, 7511. [Google Scholar] [CrossRef]
- Somtua, P.; Jaikang, C.; Konguthaithip, G.; Intui, K.; Watcharakhom, S.; O’Brien, T.E.; Amornlertwatana, Y. Postmortem Alteration of Purine Metabolism in Coronary Artery Disease. Metabolites 2023, 13, 1135. [Google Scholar] [CrossRef]
- Lim, E.Y.; Kim, G.D. Particulate Matter-Induced Emerging Health Effects Associated with Oxidative Stress and Inflammation. Antioxidants 2024, 13, 1256. [Google Scholar] [CrossRef]
- Jarernwong, K.; Gheewala, S.H.; Sampattagul, S. Health impact related to ambient particulate matter exposure as a spatial health risk map case study in Chiang Mai, Thailand. Atmosphere 2023, 14, 261. [Google Scholar] [CrossRef]
- Moran, J.; NaSuwan, C.; Poocharoen, O.-O. The haze problem in Northern Thailand and policies to combat it: A review. Environ. Sci. Policy 2019, 97, 1–15. [Google Scholar] [CrossRef]
- Rajagopalan, S.; Park, B.; Palanivel, R.; Vinayachandran, V.; Deiuliis, J.A.; Gangwar, R.S.; Das, L.; Yin, J.; Choi, Y.; Al-Kindi, S.; et al. Metabolic effects of air pollution exposure and reversibility. J. Clin. Investig. 2020, 130, 6034–6040. [Google Scholar] [CrossRef]
- Geng, N.; Ren, X.; Gong, Y.; Zhang, H.; Wang, F.; Xing, L.; Cao, R.; Xu, J.; Gao, Y.; Giesy, J.P.; et al. Integration of metabolomics and transcriptomics reveals short-chain chlorinated paraffin-induced hepatotoxicity in male Sprague-Dawley rat. Environ. Int. 2019, 133, 105231. [Google Scholar] [CrossRef]
- An, Z.; Liu, G.; Shen, L.; Qi, Y.; Hu, Q.; Song, J.; Li, J.; Du, J.; Bai, Y.; Wu, W. Mitochondrial dysfunction induced by ambient fine particulate matter and potential mechanisms. Environ. Res. 2024, 262, 119930. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, M.; Li, Z.; Yue, J.; Xu, M.; Zhang, Y.; Yung, K.K.L.; Li, R. Fine particulate matter induces mitochondrial dysfunction and oxidative stress in human SH-SY5Y cells. Chemosphere 2019, 218, 577–588. [Google Scholar] [CrossRef]
- Miao, X.; Li, W.; Niu, B.; Li, J.; Sun, J.; Qin, M.; Zhou, Z. Mitochondrial dysfunction in endothelial cells induced by airborne fine particulate matter (<2.5 mum). J. Appl. Toxicol. 2019, 39, 1424–1432. [Google Scholar] [CrossRef]
- Jin, X.; Xue, B.; Zhou, Q.; Su, R.; Li, Z. Mitochondrial damage mediated by ROS incurs bronchial epithelial cell apoptosis upon ambient PM2.5 exposure. J. Toxicol. Sci. 2018, 43, 101–111. [Google Scholar] [CrossRef]
- Redmann, M.; Benavides, G.A.; Wani, W.Y.; Berryhill, T.F.; Ouyang, X.; Johnson, M.S.; Ravi, S.; Mitra, K.; Barnes, S.; Darley-Usmar, V.M.; et al. Methods for assessing mitochondrial quality control mechanisms and cellular consequences in cell culture. Redox Biol. 2018, 17, 59–69. [Google Scholar] [CrossRef]
- Xu, X.; Liu, C.; Xu, Z.; Tzan, K.; Zhong, M.; Wang, A.; Lippmann, M.; Chen, L.C.; Rajagopalan, S.; Sun, Q. Long-term exposure to ambient fine particulate pollution induces insulin resistance and mitochondrial alteration in adipose tissue. Toxicol. Sci. 2011, 124, 88–98. [Google Scholar] [CrossRef]
- Morris, R.H.; Counsell, S.J.; McGonnell, I.M.; Thornton, C. Exposure to urban particulate matter (UPM) impairs mitochondrial dynamics in BV2 cells, triggering a mitochondrial biogenesis response. J. Physiol. 2024, 602, 2737–2750. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.H.; Li, S.Q.; Zou, G.A.; Yu, C.Y.; Sun, Y.G.; Zhang, H.W.; Gu, Y.; Zou, Z.M. Urinary metabonomics study of anti-depressive effect of Chaihu-Shu-Gan-San on an experimental model of depression induced by chronic variable stress in rats. J. Pharm. Biomed. Anal. 2011, 55, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, D.; Tankiewicz, A.; Buczko, W. Kynurenine and its metabolites in the rat with experimental renal insufficiency. J. Physiol. Pharmacol. 2001, 52, 755–766. [Google Scholar]
- Pawlak, D.; Tankiewicz, A.; Matys, T.; Buczko, W. Peripheral distribution of kynurenine metabolites and activity of kynurenine pathway enzymes in renal failure. J. Physiol. Pharmacol. 2003, 54, 175–189. [Google Scholar]
- Fu, P.; Li, R.; Sze, S.C.W.; Yung, K.K.L. Associations between fine particulate matter and colorectal cancer: A systematic review and meta-analysis. Rev. Environ. Health 2024, 39, 447–457. [Google Scholar] [CrossRef]
- Jenwitheesuk, K.; Peansukwech, U.; Jenwitheesuk, K. Accumulated ambient air pollution and colon cancer incidence in Thailand. Sci. Rep. 2020, 10, 17765. [Google Scholar] [CrossRef]
- Liu, X.Q.; Huang, J.; Song, C.; Zhang, T.L.; Liu, Y.P.; Yu, L. Neurodevelopmental toxicity induced by PM2.5 Exposure and its possible role in Neurodegenerative and mental disorders. Hum. Exp. Toxicol. 2023, 42, 9603271231191436. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, M. Corrigendum to “PM2.5 induces ferroptosis in human endothelial cells through iron overload and redox imbalance” [Environ. Pollut. 254 (2019) 112937]. Environ. Pollut. 2021, 276, 113640. [Google Scholar] [CrossRef]

| Parameters | Exposure (n = 99) | Control (n = 98) | p-Value |
|---|---|---|---|
| Sex: Female | 72 (72.7%) | 71 (72.4%) | 0.218 |
| Age (year) | 46.9 ± 12.3 | 41.7 ± 13.8 | 0.080 |
| Weight (kg) | 62.3 ± 12.1 | 64.8 ± 12.6 | 0.272 |
| Height (cm) | 159.1 ± 12.0 | 161.6 ± 8.6 | 0.971 |
| Body Mass Index, BMI (kg/m2) | 23.36 ± 4.0 | 24.5 ± 3.9 | 0.443 |
| Systolic Pressure (mmHg) | 130.1 ± 16.4 | 129.7 ± 22.9 | 0.200 |
| Diastolic Pressure (mmHg) | 81.2 ± 9.9 | 82.1 ± 14.5 | 0.397 |
| Heart Rate (beat/min) | 81.9 ± 11.7 | 82.1 ± 14.7 | 0.601 |
| Metabolites | Fold Change | log2 (FC) | −log10 (p) |
|---|---|---|---|
| Dopamine | 0.15 | −2.75 | 29.49 |
| N6-methyladenosine | 0.19 | −2.36 | 32.08 |
| 3-Hydroxyproline | 0.25 | −1.98 | 28.62 |
| 5-Carboxylcytosine | 0.37 | −1.44 | 30.02 |
| Cytidine | 0.43 | −1.20 | 6.31 |
| Betaine | 0.44 | −1.17 | 19.74 |
| 5-Hydroxylysine | 0.50 | −0.99 | 25.11 |
| 4-Hydroxyproline | 0.52 | −0.94 | 28.88 |
| L-Arginine | 0.53 | −0.91 | 30.91 |
| L-Phenylalanine | 0.55 | −0.86 | 32.33 |
| 1-Methylnicotinamide | 0.55 | −0.85 | 5.55 |
| 5-Formylcytosine | 0.57 | −0.81 | 23.94 |
| Ornithine | 0.57 | −0.79 | 17.44 |
| Dihydrofolate (7,8-dihydrofolate) | 0.58 | −0.79 | 3.73 |
| Homocysteine | 0.59 | −0.75 | 26.22 |
| 1-Methyladenosine | 0.60 | −0.73 | 2.96 |
| Pathways | Match Status | −log (p) | Holm p | Impact |
|---|---|---|---|---|
| Arginine biosynthesis | 7/14 | 70.40 | 1.96 × 10−69 | 0.48 |
| Alanine, aspartate, and glutamate metabolism | 11/28 | 64.49 | 1.57 × 10−63 | 0.65 |
| Glycosaminoglycan degradation | 1/23 | 58.83 | 6.95 × 10−58 | 0.0 |
| Pyruvate metabolism | 7/23 | 58.13 | 3.37 × 10−57 | 0.30 |
| Arginine and proline metabolism | 8/36 | 55.99 | 4.53 × 10−55 | 0.35 |
| Glycolysis/Gluconeogenesis | 5/26 | 54.84 | 6.31 × 10−54 | 0.23 |
| Cysteine and methionine metabolism | 8/33 | 53.97 | 4.56 × 10−53 | 0.53 |
| Taurine and hypotaurine metabolism | 3/8 | 52.62 | 1.00 × 10−51 | 0.83 |
| Glyoxylate and dicarboxylate metabolism | 10/32 | 52.61 | 1.01 × 10−51 | 0.10 |
| Purine metabolism | 15/70 | 51.11 | 3.06 × 10−50 | 0.31 |
| Citrate cycle (TCA cycle) | 8/20 | 49.89 | 4.91 × 10−49 | 0.41 |
| Tyrosine metabolism | 10/42 | 49.95 | 1.22 × 10−48 | 0.41 |
| Phenylalanine, tyrosine, and tryptophan biosynthesis | 2/4 | 47.65 | 8.16 × 10−47 | 1.0 |
| Phenylalanine metabolism | 2/8 | 47.66 | 8.16 × 10−47 | 0.35 |
| Pantothenate and CoA biosynthesis | 4/20 | 45.91 | 4.22 × 10−45 | 0.0 |
| Glycine, serine, and threonine metabolism | 10/33 | 44.37 | 1.42 × 10−43 | 0.43 |
| Histidine metabolism | 4/16 | 40.48 | 1.08 × 10−39 | 0.22 |
| Thiamine metabolism | 1/7 | 40.43 | 1.18 × 10−39 | 0.0 |
| D-amino and metabolism | 2/15 | 39.62 | 7.35 × 10−39 | 0.0 |
| Glutathione metabolism | 7/28 | 39.46 | 1.03 × 10−38 | 0.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaikang, C.; Konguthaithip, G.; Amornlertwatana, Y.; Autsavapromporn, N.; Rattanachitthawat, S.; Liampongsabuddhi, N.; Monum, T. Metabolic Disruptions and Non-Communicable Disease Risks Associated with Long-Term Particulate Matter Exposure in Northern Thailand: An NMR-Based Metabolomics Study. Biomedicines 2025, 13, 742. https://doi.org/10.3390/biomedicines13030742
Jaikang C, Konguthaithip G, Amornlertwatana Y, Autsavapromporn N, Rattanachitthawat S, Liampongsabuddhi N, Monum T. Metabolic Disruptions and Non-Communicable Disease Risks Associated with Long-Term Particulate Matter Exposure in Northern Thailand: An NMR-Based Metabolomics Study. Biomedicines. 2025; 13(3):742. https://doi.org/10.3390/biomedicines13030742
Chicago/Turabian StyleJaikang, Churdsak, Giatgong Konguthaithip, Yutti Amornlertwatana, Narongchai Autsavapromporn, Sirichet Rattanachitthawat, Nitip Liampongsabuddhi, and Tawachai Monum. 2025. "Metabolic Disruptions and Non-Communicable Disease Risks Associated with Long-Term Particulate Matter Exposure in Northern Thailand: An NMR-Based Metabolomics Study" Biomedicines 13, no. 3: 742. https://doi.org/10.3390/biomedicines13030742
APA StyleJaikang, C., Konguthaithip, G., Amornlertwatana, Y., Autsavapromporn, N., Rattanachitthawat, S., Liampongsabuddhi, N., & Monum, T. (2025). Metabolic Disruptions and Non-Communicable Disease Risks Associated with Long-Term Particulate Matter Exposure in Northern Thailand: An NMR-Based Metabolomics Study. Biomedicines, 13(3), 742. https://doi.org/10.3390/biomedicines13030742






