Rhythms in Remodeling: Posttranslational Regulation of Bone by the Circadian Clock
Abstract
1. Introduction
2. Circadian Clock Mechanisms in Bone
3. Overview of Posttranslational Modifications
4. Posttranslational Regulation of Bone Through the Circadian Timing System
4.1. Phosphorylation’s Role in Circadian Rhythms and Bone Regulation
4.2. Acetylation and Deacetylation in Circadian and Bone Regulation
4.3. Ubiquitination in Circadian and Bone Regulation
4.4. Sumoylation in Circadian and Bone Regulation
4.5. Links Between Posttranslational Modifications Induced by Circadian Clock Dysfunction and Bone Disorders
4.6. Future Directions and Clinical Implications
5. Conclusions
Funding
Conflicts of Interest
Abbreviations
| CLOCK | Circadian locomotor output cycles kaput |
| BMAL1 | Basic helix-loop-helix ARNT-like protein 1 |
| PER | Period |
| CRY | Cryptochrome |
| PTMs | Posttranslational modifications |
| SCN | Suprachiasmatic nucleus |
| RANKL | Receptor activator of nuclear factor kappa-B ligand |
| CK1 | Casein kinase 1 |
| SIRT1 | Sirtuin 1 |
| AMPK | AMP-activated protein kinase |
| NF-κB | Nuclear factor kappa B subunit 1 |
| SMAD1 | Mothers against decapentaplegic homolog 1 |
| RUNX2 | Runt-related transcription factor 2 |
| GSK-3β | Glycogen synthase kinase 3 beta |
| p300/CBP | CREBBP |
| HATs | Histone acetyltransferases |
| ALP | Alkaline phosphatases |
| COL1A1 | Collagen, type I |
| OPG | Osteoprotegerin |
| UPS | Ubiquitin–proteasome system |
| β-TrCP | beta-transducin repeat-containing protein |
| FBXL3 | F-box and leucine rich repeat protein 3 |
| SMURF1 | Smad ubiquitination regulation factor 1 |
| TRAF6 | TNF receptor-associated factor 6 |
| DUBs | Deubiquitinating enzymes |
| CYLD | Cylindromatosis |
| NFATc1 | TF nuclear factor of activated T cells 1 |
| C/EBPβ | CCAAT/enhancer-binding protein beta |
| FGFR3 | Fibroblast growth factor receptor 3 |
| MAPK | Mitogen activated protein kinase |
| RA | Rheumatoid arthritis |
| AS | Ankylosing spondylitis |
References
- Chaput, J.P.; McHill, A.W.; Cox, R.C.; Broussard, J.L.; Dutil, C.; da Costa, B.G.G.; Sampasa-Kanyinga, H.; Wright, K.P., Jr. The role of insufficient sleep and circadian misalignment in obesity. Nat. Rev. Endocrinol. 2023, 19, 82–97. [Google Scholar] [CrossRef] [PubMed]
- Patke, A.; Young, M.W.; Axelrod, S. Molecular mechanisms and physiological importance of circadian rhythms. Nat. Rev. Mol. Cell Biol. 2020, 21, 67–84. [Google Scholar] [CrossRef]
- Ayyar, V.S.; Sukumaran, S. Circadian rhythms: Influence on physiology, pharmacology, and therapeutic interventions. J. Pharmacokinet. Pharmacodyn. 2021, 48, 321–338. [Google Scholar] [CrossRef] [PubMed]
- Fagiani, F.; Di Marino, D.; Romagnoli, A.; Travelli, C.; Voltan, D.; Di Cesare Mannelli, L.; Racchi, M.; Govoni, S.; Lanni, C. Molecular regulations of circadian rhythm and implications for physiology and diseases. Signal Transduct. Target. Ther. 2022, 7, 41. [Google Scholar] [CrossRef]
- Bouvard, B.; Mabilleau, G. Gut hormones and bone homeostasis: Potential therapeutic implications. Nat. Rev. Endocrinol. 2024, 20, 553–564. [Google Scholar] [CrossRef] [PubMed]
- Kawai, M.; Rosen, C.J. PPARgamma: A circadian transcription factor in adipogenesis and osteogenesis. Nat. Rev. Endocrinol. 2010, 6, 629–636. [Google Scholar] [CrossRef]
- de Crombrugghe, B. Osteoblasts clock in for their day job. Cell 2005, 122, 651–653. [Google Scholar] [CrossRef][Green Version]
- Raggatt, L.J.; Partridge, N.C. Cellular and molecular mechanisms of bone remodeling. J. Biol. Chem. 2010, 285, 25103–25108. [Google Scholar] [CrossRef]
- Dirckx, N.; Moorer, M.C.; Clemens, T.L.; Riddle, R.C. The role of osteoblasts in energy homeostasis. Nat. Rev. Endocrinol. 2019, 15, 651–665. [Google Scholar] [CrossRef]
- Salazar, V.S.; Gamer, L.W.; Rosen, V. BMP signalling in skeletal development, disease and repair. Nat. Rev. Endocrinol. 2016, 12, 203–221. [Google Scholar] [CrossRef]
- Chotiyarnwong, P.; McCloskey, E.V. Pathogenesis of glucocorticoid-induced osteoporosis and options for treatment. Nat. Rev. Endocrinol. 2020, 16, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.; Zhou, X.; Tang, Q.; Yin, Y.; Feng, G.; Li, S.; Chen, L. Circadian rhythms affect bone reconstruction by regulating bone energy metabolism. J. Transl. Med. 2021, 19, 410. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Hua, B.; Yang, Y.; Xu, L.; Cai, T.; Sun, N.; Yan, Z.; Lu, C.; Qian, R. The Circadian Gene Clock Regulates Bone Formation Via PDIA3. J. Bone Miner. Res. 2017, 32, 861–871. [Google Scholar] [CrossRef] [PubMed]
- Kikyo, N. Circadian Regulation of Bone Remodeling. Int. J. Mol. Sci. 2024, 25, 4717. [Google Scholar] [CrossRef]
- Yang, N.; Meng, Q.J. Circadian Clocks in Articular Cartilage and Bone: A Compass in the Sea of Matrices. J. Biol. Rhythms 2016, 31, 415–427. [Google Scholar] [CrossRef]
- Richards, J.; Gumz, M.L. Advances in understanding the peripheral circadian clocks. FASEB J. 2012, 26, 3602–3613. [Google Scholar] [CrossRef]
- Xu, C.; Ochi, H.; Fukuda, T.; Sato, S.; Sunamura, S.; Takarada, T.; Hinoi, E.; Okawa, A.; Takeda, S. Circadian Clock Regulates Bone Resorption in Mice. J. Bone Miner. Res. 2016, 31, 1344–1355. [Google Scholar] [CrossRef]
- Mehra, A.; Baker, C.L.; Loros, J.J.; Dunlap, J.C. Post-translational modifications in circadian rhythms. Trends Biochem. Sci. 2009, 34, 483–490. [Google Scholar] [CrossRef]
- Yan, J.; Kim, Y.J.; Somers, D.E. Post-Translational Mechanisms of Plant Circadian Regulation. Genes 2021, 12, 325. [Google Scholar] [CrossRef]
- Li, W.; Li, F.; Zhang, X.; Lin, H.K.; Xu, C. Insights into the post-translational modification and its emerging role in shaping the tumor microenvironment. Signal Transduct. Target. Ther. 2021, 6, 422. [Google Scholar] [CrossRef]
- Wu, X.; Xu, M.; Geng, M.; Chen, S.; Little, P.J.; Xu, S.; Weng, J. Targeting protein modifications in metabolic diseases: Molecular mechanisms and targeted therapies. Signal Transduct. Target. Ther. 2023, 8, 220. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, S.; Tao, Y. Regulating tumor suppressor genes: Post-translational modifications. Signal Transduct. Target. Ther. 2020, 5, 90. [Google Scholar] [CrossRef] [PubMed]
- Duszka, K.; Wahli, W. Peroxisome Proliferator-Activated Receptors as Molecular Links Between Caloric Restriction and Circadian Rhythm. Nutrients 2020, 12, 3476. [Google Scholar] [CrossRef]
- Chen, L.C.; Hsieh, Y.L.; Tan, G.Y.T.; Kuo, T.Y.; Chou, Y.C.; Hsu, P.H.; Hwang-Verslues, W.W. Differential effects of SUMO1 and SUMO2 on circadian protein PER2 stability and function. Sci. Rep. 2021, 11, 14431. [Google Scholar] [CrossRef]
- Stojkovic, K.; Wing, S.S.; Cermakian, N. A central role for ubiquitination within a circadian clock protein modification code. Front. Mol. Neurosci. 2014, 7, 69. [Google Scholar] [CrossRef]
- Stechschulte, L.A.; Czernik, P.J.; Rotter, Z.C.; Tausif, F.N.; Corzo, C.A.; Marciano, D.P.; Asteian, A.; Zheng, J.; Bruning, J.B.; Kamenecka, T.M.; et al. PPARG Post-translational Modifications Regulate Bone Formation and Bone Resorption. EBioMedicine 2016, 10, 174–184. [Google Scholar] [CrossRef]
- de Assis, L.V.M.; Oster, H. The circadian clock and metabolic homeostasis: Entangled networks. Cell Mol. Life Sci. 2021, 78, 4563–4587. [Google Scholar] [CrossRef]
- Astiz, M.; Heyde, I.; Oster, H. Mechanisms of Communication in the Mammalian Circadian Timing System. Int. J. Mol. Sci. 2019, 20, 343. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, J.S. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 2017, 18, 164–179. [Google Scholar] [CrossRef]
- Morris, H.; Goncalves, C.F.; Dudek, M.; Hoyland, J.; Meng, Q.J. Tissue physiology revolving around the clock: Circadian rhythms as exemplified by the intervertebral disc. Ann. Rheum. Dis. 2021, 80, 828–839. [Google Scholar] [CrossRef]
- Tsang, K.; Liu, H.; Yang, Y.; Charles, J.F.; Ermann, J. Defective circadian control in mesenchymal cells reduces adult bone mass in mice by promoting osteoclast function. Bone 2019, 121, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Takarada, T.; Xu, C.; Ochi, H.; Nakazato, R.; Yamada, D.; Nakamura, S.; Kodama, A.; Shimba, S.; Mieda, M.; Fukasawa, K.; et al. Bone Resorption Is Regulated by Circadian Clock in Osteoblasts. J. Bone Miner. Res. 2017, 32, 872–881. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Patel, M.S.; Bradley, A.; Wagner, E.F.; Karsenty, G. The molecular clock mediates leptin-regulated bone formation. Cell 2005, 122, 803–815. [Google Scholar] [CrossRef]
- Swanson, C.M.; Kohrt, W.M.; Buxton, O.M.; Everson, C.A.; Wright, K.P., Jr.; Orwoll, E.S.; Shea, S.A. The importance of the circadian system & sleep for bone health. Metabolism 2018, 84, 28–43. [Google Scholar] [CrossRef] [PubMed]
- Fujihara, Y.; Kondo, H.; Noguchi, T.; Togari, A. Glucocorticoids mediate circadian timing in peripheral osteoclasts resulting in the circadian expression rhythm of osteoclast-related genes. Bone 2014, 61, 1–9. [Google Scholar] [CrossRef]
- Silva, B.C.; Bilezikian, J.P. Parathyroid hormone: Anabolic and catabolic actions on the skeleton. Curr. Opin. Pharmacol. 2015, 22, 41–50. [Google Scholar] [CrossRef]
- Lee, J.M.; Hammaren, H.M.; Savitski, M.M.; Baek, S.H. Control of protein stability by post-translational modifications. Nat. Commun. 2023, 14, 201. [Google Scholar] [CrossRef]
- Wang, Y.C.; Peterson, S.E.; Loring, J.F. Protein post-translational modifications and regulation of pluripotency in human stem cells. Cell Res. 2014, 24, 143–160. [Google Scholar] [CrossRef]
- Beltrao, P.; Bork, P.; Krogan, N.J.; van Noort, V. Evolution and functional cross-talk of protein post-translational modifications. Mol. Syst. Biol. 2013, 9, 714. [Google Scholar] [CrossRef]
- Tootle, T.L.; Rebay, I. Post-translational modifications influence transcription factor activity: A view from the ETS superfamily. Bioessays 2005, 27, 285–298. [Google Scholar] [CrossRef]
- Keverne, E.B.; Pfaff, D.W.; Tabansky, I. Epigenetic changes in the developing brain: Effects on behavior. Proc. Natl. Acad. Sci. USA 2015, 112, 6789–6795. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Hu, J.; Wu, S.; Fleishman, J.S.; Li, Y.; Xu, Y.; Zou, W.; Wang, J.; Feng, Y.; Chen, J.; et al. Targeting epigenetic and posttranslational modifications regulating ferroptosis for the treatment of diseases. Signal Transduct. Target. Ther. 2023, 8, 449. [Google Scholar] [CrossRef]
- Santos, A.L.; Lindner, A.B. Protein Posttranslational Modifications: Roles in Aging and Age-Related Disease. Oxid. Med. Cell Longev. 2017, 2017, 5716409. [Google Scholar] [CrossRef]
- Creecy, A.; Brown, K.L.; Rose, K.L.; Voziyan, P.; Nyman, J.S. Post-translational modifications in collagen type I of bone in a mouse model of aging. Bone 2021, 143, 115763. [Google Scholar] [CrossRef] [PubMed]
- Philpott, J.M.; Freeberg, A.M.; Park, J.; Lee, K.; Ricci, C.G.; Hunt, S.R.; Narasimamurthy, R.; Segal, D.H.; Robles, R.; Cai, Y.; et al. PERIOD phosphorylation leads to feedback inhibition of CK1 activity to control circadian period. Mol. Cell 2023, 83, 1677–1692 e1678. [Google Scholar] [CrossRef] [PubMed]
- Nakahata, Y.; Kaluzova, M.; Grimaldi, B.; Sahar, S.; Hirayama, J.; Chen, D.; Guarente, L.P.; Sassone-Corsi, P. The NAD+-dependent deacetylase SIRT1 modulates CLOCK-mediated chromatin remodeling and circadian control. Cell 2008, 134, 329–340. [Google Scholar] [CrossRef]
- Yuan, G.; Xu, L.; Cai, T.; Hua, B.; Sun, N.; Yan, Z.; Lu, C.; Qian, R. Clock mutant promotes osteoarthritis by inhibiting the acetylation of NFkappaB. Osteoarthr. Cartil. 2019, 27, 922–931. [Google Scholar] [CrossRef]
- Abdalla, O.; Mascarenhas, B.; Cheng, H.M. Death of a Protein: The Role of E3 Ubiquitin Ligases in Circadian Rhythms of Mice and Flies. Int. J. Mol. Sci. 2022, 23, 10569. [Google Scholar] [CrossRef]
- Liu, H.; Craig, S.E.L.; Molchanov, V.; Floramo, J.S.; Zhao, Y.; Yang, T. SUMOylation in Skeletal Development, Homeostasis, and Disease. Cells 2022, 11, 2710. [Google Scholar] [CrossRef]
- Katagiri, T.; Takahashi, N. Regulatory mechanisms of osteoblast and osteoclast differentiation. Oral. Dis. 2002, 8, 147–159. [Google Scholar] [CrossRef]
- Nishi, H.; Hashimoto, K.; Panchenko, A.R. Phosphorylation in protein-protein binding: Effect on stability and function. Structure 2011, 19, 1807–1815. [Google Scholar] [CrossRef]
- Marzoll, D.; Serrano, F.E.; Shostak, A.; Schunke, C.; Diernfellner, A.C.R.; Brunner, M. Casein kinase 1 and disordered clock proteins form functionally equivalent, phospho-based circadian modules in fungi and mammals. Proc. Natl. Acad. Sci. USA 2022, 119, e2118286119. [Google Scholar] [CrossRef] [PubMed]
- Etchegaray, J.P.; Machida, K.K.; Noton, E.; Constance, C.M.; Dallmann, R.; Di Napoli, M.N.; DeBruyne, J.P.; Lambert, C.M.; Yu, E.A.; Reppert, S.M.; et al. Casein kinase 1 delta regulates the pace of the mammalian circadian clock. Mol. Cell Biol. 2009, 29, 3853–3866. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Mulati, M.; Saito, M.; Numata, H.; Kobayashi, Y.; Ochi, H.; Sato, S.; Kaldis, P.; Okawa, A.; Inose, H. Loss of cyclin-dependent kinase 1 impairs bone formation, but does not affect the bone-anabolic effects of parathyroid hormone. J. Biol. Chem. 2018, 293, 19387–19399. [Google Scholar] [CrossRef]
- Lee, Y.; Kim, E.K. AMP-activated protein kinase as a key molecular link between metabolism and clockwork. Exp. Mol. Med. 2013, 45, e33. [Google Scholar] [CrossRef] [PubMed]
- Jordan, S.D.; Lamia, K.A. AMPK at the crossroads of circadian clocks and metabolism. Mol. Cell Endocrinol. 2013, 366, 163–169. [Google Scholar] [CrossRef]
- Jeyabalan, J.; Shah, M.; Viollet, B.; Chenu, C. AMP-activated protein kinase pathway and bone metabolism. J. Endocrinol. 2012, 212, 277–290. [Google Scholar] [CrossRef]
- Guo, Q.; Jin, Y.; Chen, X.; Ye, X.; Shen, X.; Lin, M.; Zeng, C.; Zhou, T.; Zhang, J. NF-kappaB in biology and targeted therapy: New insights and translational implications. Signal Transduct. Target. Ther. 2024, 9, 53. [Google Scholar] [CrossRef]
- Feng, G.; Zhao, J.; Peng, J.; Luo, B.; Zhang, J.; Chen, L.; Xu, Z. Circadian clock-A promising scientific target in oral science. Front. Physiol. 2022, 13, 1031519. [Google Scholar] [CrossRef]
- Gao, W.; Li, R.; Ye, M.; Zhang, L.; Zheng, J.; Yang, Y.; Wei, X.; Zhao, Q. The circadian clock has roles in mesenchymal stem cell fate decision. Stem Cell Res. Ther. 2022, 13, 200. [Google Scholar] [CrossRef]
- Li, T.; Zhang, S.; Yang, Y.; Zhang, L.; Yuan, Y.; Zou, J. Co-regulation of circadian clock genes and microRNAs in bone metabolism. J. Zhejiang Univ. Sci. B 2022, 23, 529–546. [Google Scholar] [CrossRef]
- Baron, R.; Rawadi, G. Targeting the Wnt/beta-catenin pathway to regulate bone formation in the adult skeleton. Endocrinology 2007, 148, 2635–2643. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Meng, H.; Xu, M.; Gao, X.; Sun, X.; Jin, X.; Sun, H. BMAL1 regulates osteoblast differentiation through mTOR/GSK3beta/beta-catenin pathway. J. Mol. Endocrinol. 2023, 70, e220181. [Google Scholar] [CrossRef]
- Zheng, J.; Zhang, L.; Tan, Z.; Zhao, Q.; Wei, X.; Yang, Y.; Li, R. Bmal1- and Per2-mediated regulation of the osteogenic differentiation and proliferation of mouse bone marrow mesenchymal stem cells by modulating the Wnt/beta-catenin pathway. Mol. Biol. Rep. 2022, 49, 4485–4501. [Google Scholar] [CrossRef]
- Qian, Z.; Zhang, Y.; Kang, X.; Li, H.; Zhang, Y.; Jin, X.; Gao, X.; Xu, M.; Ma, Z.; Zhao, L.; et al. Postnatal Conditional Deletion of Bmal1 in Osteoblasts Enhances Trabecular Bone Formation Via Increased BMP2 Signals. J. Bone Miner. Res. 2020, 35, 1481–1493. [Google Scholar] [CrossRef]
- Huang, W.; Yang, S.; Shao, J.; Li, Y.P. Signaling and transcriptional regulation in osteoblast commitment and differentiation. Front. Biosci. 2007, 12, 3068–3092. [Google Scholar] [CrossRef] [PubMed]
- Reale, M.E.; Webb, I.C.; Wang, X.; Baltazar, R.M.; Coolen, L.M.; Lehman, M.N. The transcription factor Runx2 is under circadian control in the suprachiasmatic nucleus and functions in the control of rhythmic behavior. PLoS ONE 2013, 8, e54317. [Google Scholar] [CrossRef] [PubMed]
- Xia, C.; Tao, Y.; Li, M.; Che, T.; Qu, J. Protein acetylation and deacetylation: An important regulatory modification in gene transcription (Review). Exp. Ther. Med. 2020, 20, 2923–2940. [Google Scholar] [CrossRef]
- Qin, Y.; Chen, Z.H.; Wu, J.J.; Zhang, Z.Y.; Yuan, Z.D.; Guo, D.Y.; Chen, M.N.; Li, X.; Yuan, F.L. Circadian clock genes as promising therapeutic targets for bone loss. Biomed. Pharmacother. 2023, 157, 114019. [Google Scholar] [CrossRef]
- Tian, Q.; Gao, S.; Zhou, X.; Zheng, L.; Zhou, Y. Histone Acetylation in the Epigenetic Regulation of Bone Metabolism and Related Diseases. Stem Cells Int. 2021, 2021, 8043346. [Google Scholar] [CrossRef]
- Doi, M.; Hirayama, J.; Sassone-Corsi, P. Circadian regulator CLOCK is a histone acetyltransferase. Cell 2006, 125, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.M.; La Thangue, N.B. p300/CBP proteins: HATs for transcriptional bridges and scaffolds. J. Cell Sci. 2001, 114, 2363–2373. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, R.H.; Sadu, L.; Das, U.R.; Satishkumar, S.; Pranav Adithya, S.; Saranya, I.; Akshaya, R.L.; Selvamurugan, N. Role of p300, a histone acetyltransferase enzyme, in osteoblast differentiation. Differentiation 2022, 124, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Sack, M.N. Protein deacetylation by sirtuins: Delineating a post-translational regulatory program responsive to nutrient and redox stressors. Cell. Mol. Life Sci. 2010, 67, 3073–3087. [Google Scholar] [CrossRef]
- Asher, G.; Gatfield, D.; Stratmann, M.; Reinke, H.; Dibner, C.; Kreppel, F.; Mostoslavsky, R.; Alt, F.W.; Schibler, U. SIRT1 regulates circadian clock gene expression through PER2 deacetylation. Cell 2008, 134, 317–328. [Google Scholar] [CrossRef]
- Abe, T.; Sato, T.; Yoda, T.; Hoshi, K. The period circadian clock 2 gene responds to glucocorticoids and regulates osteogenic capacity. Regen. Ther. 2019, 11, 199–206. [Google Scholar] [CrossRef]
- Samsa, W.E.; Vasanji, A.; Midura, R.J.; Kondratov, R.V. Deficiency of circadian clock protein BMAL1 in mice results in a low bone mass phenotype. Bone 2016, 84, 194–203. [Google Scholar] [CrossRef]
- Masri, S.; Zocchi, L.; Katada, S.; Mora, E.; Sassone-Corsi, P. The circadian clock transcriptional complex: Metabolic feedback intersects with epigenetic control. Ann. N. Y. Acad. Sci. 2012, 1264, 103–109. [Google Scholar] [CrossRef]
- Jung-Hynes, B.; Ahmad, N. SIRT1 controls circadian clock circuitry and promotes cell survival: A connection with age-related neoplasms. FASEB J. 2009, 23, 2803–2809. [Google Scholar] [CrossRef]
- Chan, W.C.W.; Tan, Z.; To, M.K.T.; Chan, D. Regulation and Role of Transcription Factors in Osteogenesis. Int. J. Mol. Sci. 2021, 22, 5445. [Google Scholar] [CrossRef]
- Goncalves, C.F.; Meng, Q.J. Timing metabolism in cartilage and bone: Links between circadian clocks and tissue homeostasis. J. Endocrinol. 2019, 243, R29–R46. [Google Scholar] [CrossRef]
- Song, H.R.; Noh, Y.S. Rhythmic oscillation of histone acetylation and methylation at the Arabidopsis central clock loci. Mol. Cells 2012, 34, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Francis, M.; Pandya, M.; Gopinathan, G.; Lyu, H.; Ma, W.; Foyle, D.; Nares, S.; Luan, X. Histone Methylation Mechanisms Modulate the Inflammatory Response of Periodontal Ligament Progenitors. Stem Cells Dev. 2019, 28, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Jin, X.; Sun, X.; Ma, X.; Qin, Z.; Gao, X.; Kang, X.; Li, H.; Sun, H. SIRT1 maintains bone homeostasis by regulating osteoblast glycolysis through GOT1. Cell Mol. Life Sci. 2024, 81, 204. [Google Scholar] [CrossRef]
- Suresh, B.; Lee, J.; Kim, K.S.; Ramakrishna, S. The Importance of Ubiquitination and Deubiquitination in Cellular Reprogramming. Stem Cells Int. 2016, 2016, 6705927. [Google Scholar] [CrossRef]
- Crislip, G.R.; Johnston, J.G.; Douma, L.G.; Costello, H.M.; Juffre, A.; Boyd, K.; Li, W.; Maugans, C.C.; Gutierrez-Monreal, M.; Esser, K.A.; et al. Circadian Rhythm Effects on the Molecular Regulation of Physiological Systems. Compr. Physiol. 2021, 12, 2769–2798. [Google Scholar] [CrossRef] [PubMed]
- Ukita, Y.; Okumura, M.; Chihara, T. Ubiquitin proteasome system in circadian rhythm and sleep homeostasis: Lessons from Drosophila. Genes. Cells 2022, 27, 381–391. [Google Scholar] [CrossRef]
- Yoo, S.H.; Mohawk, J.A.; Siepka, S.M.; Shan, Y.; Huh, S.K.; Hong, H.K.; Kornblum, I.; Kumar, V.; Koike, N.; Xu, M.; et al. Competing E3 ubiquitin ligases govern circadian periodicity by degradation of CRY in nucleus and cytoplasm. Cell 2013, 152, 1091–1105. [Google Scholar] [CrossRef]
- Reischl, S.; Vanselow, K.; Westermark, P.O.; Thierfelder, N.; Maier, B.; Herzel, H.; Kramer, A. Beta-TrCP1-mediated degradation of PERIOD2 is essential for circadian dynamics. J. Biol. Rhythms 2007, 22, 375–386. [Google Scholar] [CrossRef]
- Xing, W.; Busino, L.; Hinds, T.R.; Marionni, S.T.; Saifee, N.H.; Bush, M.F.; Pagano, M.; Zheng, N. SCF(FBXL3) ubiquitin ligase targets cryptochromes at their cofactor pocket. Nature 2013, 496, 64–68. [Google Scholar] [CrossRef]
- Maronde, E.; Schilling, A.F.; Seitz, S.; Schinke, T.; Schmutz, I.; van der Horst, G.; Amling, M.; Albrecht, U. The clock genes Period 2 and Cryptochrome 2 differentially balance bone formation. PLoS ONE 2010, 5, e11527. [Google Scholar] [CrossRef] [PubMed]
- Srikanta, S.B.; Cermakian, N. To Ub or not to Ub: Regulation of circadian clocks by ubiquitination and deubiquitination. J. Neurochem. 2021, 157, 11–30. [Google Scholar] [CrossRef] [PubMed]
- Jensen, E.D.; Gopalakrishnan, R.; Westendorf, J.J. Regulation of gene expression in osteoblasts. Biofactors 2010, 36, 25–32. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, W.J.; Ryoo, H.M. Post-Translational Regulations of Transcriptional Activity of RUNX2. Mol. Cells 2020, 43, 160–167. [Google Scholar] [CrossRef]
- Zhao, M.; Qiao, M.; Oyajobi, B.O.; Mundy, G.R.; Chen, D. E3 ubiquitin ligase Smurf1 mediates core-binding factor alpha1/Runx2 degradation and plays a specific role in osteoblast differentiation. J. Biol. Chem. 2003, 278, 27939–27944. [Google Scholar] [CrossRef]
- Shimazu, J.; Wei, J.; Karsenty, G. Smurf1 Inhibits Osteoblast Differentiation, Bone Formation, and Glucose Homeostasis through Serine 148. Cell Rep. 2016, 15, 27–35. [Google Scholar] [CrossRef]
- Itzstein, C.; Coxon, F.P.; Rogers, M.J. The regulation of osteoclast function and bone resorption by small GTPases. Small GTPases 2011, 2, 117–130. [Google Scholar] [CrossRef]
- Gohda, J.; Akiyama, T.; Koga, T.; Takayanagi, H.; Tanaka, S.; Inoue, J. RANK-mediated amplification of TRAF6 signaling leads to NFATc1 induction during osteoclastogenesis. EMBO J. 2005, 24, 790–799. [Google Scholar] [CrossRef] [PubMed]
- Harhaj, E.W.; Dixit, V.M. Regulation of NF-kappaB by deubiquitinases. Immunol. Rev. 2012, 246, 107–124. [Google Scholar] [CrossRef]
- Zhang, H.R.; Wang, Y.H.; Xiao, Z.P.; Yang, G.; Xu, Y.R.; Huang, Z.T.; Wang, W.Z.; He, F. E3 ubiquitin ligases: Key regulators of osteogenesis and potential therapeutic targets for bone disorders. Front. Cell Dev. Biol. 2024, 12, 1447093. [Google Scholar] [CrossRef]
- Soares, A.G.; Aoki, M.S.; Miyabara, E.H.; Deluca, C.V.; Ono, H.Y.; Gomes, M.D.; Moriscot, A.S. Ubiquitin-ligase and deubiquitinating gene expression in stretched rat skeletal muscle. Muscle Nerve 2007, 36, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Ming, J. The role of circadian rhythm in osteoporosis; a review. Front. Cell Dev. Biol. 2022, 10, 960456. [Google Scholar] [CrossRef] [PubMed]
- Severe, N.; Dieudonne, F.X.; Marie, P.J. E3 ubiquitin ligase-mediated regulation of bone formation and tumorigenesis. Cell Death Dis. 2013, 4, e463. [Google Scholar] [CrossRef]
- Lee, Y.; Chun, S.K.; Kim, K. Sumoylation controls CLOCK-BMAL1-mediated clock resetting via CBP recruitment in nuclear transcriptional foci. Biochim. Biophys. Acta 2015, 1853, 2697–2708. [Google Scholar] [CrossRef] [PubMed]
- Lyst, M.J.; Stancheva, I. A role for SUMO modification in transcriptional repression and activation. Biochem. Soc. Trans. 2007, 35, 1389–1392. [Google Scholar] [CrossRef]
- Ma, X.N.; Li, M.Y.; Qi, G.Q.; Wei, L.N.; Zhang, D.K. SUMOylation at the crossroads of gut health: Insights into physiology and pathology. Cell Commun. Signal 2024, 22, 404. [Google Scholar] [CrossRef]
- Lee, J.; Lee, Y.; Lee, M.J.; Park, E.; Kang, S.H.; Chung, C.H.; Lee, K.H.; Kim, K. Dual modification of BMAL1 by SUMO2/3 and ubiquitin promotes circadian activation of the CLOCK/BMAL1 complex. Mol. Cell Biol. 2008, 28, 6056–6065. [Google Scholar] [CrossRef]
- Cardone, L.; Hirayama, J.; Giordano, F.; Tamaru, T.; Palvimo, J.J.; Sassone-Corsi, P. Circadian clock control by SUMOylation of BMAL1. Science 2005, 309, 1390–1394. [Google Scholar] [CrossRef]
- Kim, J.H.; Jang, J.W.; Lee, Y.S.; Lee, J.W.; Chi, X.Z.; Li, Y.H.; Kim, M.K.; Kim, D.M.; Choi, B.S.; Kim, J.; et al. RUNX family members are covalently modified and regulated by PIAS1-mediated sumoylation. Oncogenesis 2014, 3, e101. [Google Scholar] [CrossRef]
- Hosoya, A.; Yukita, A.; Ninomiya, T.; Hiraga, T.; Yoshiba, K.; Yoshiba, N.; Kasahara, E.; Nakamura, H. Localization of SUMOylation factors and Osterix in odontoblast lineage cells during dentin formation and regeneration. Histochem. Cell Biol. 2013, 140, 201–211. [Google Scholar] [CrossRef]
- Nayak, A.; Glockner-Pagel, J.; Vaeth, M.; Schumann, J.E.; Buttmann, M.; Bopp, T.; Schmitt, E.; Serfling, E.; Berberich-Siebelt, F. Sumoylation of the transcription factor NFATc1 leads to its subnuclear relocalization and interleukin-2 repression by histone deacetylase. J. Biol. Chem. 2009, 284, 10935–10946. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, N. Regulation of NFATc1 in Osteoclast Differentiation. J. Bone Metab. 2014, 21, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wang, P.; Xu, S.; Li, Z.; Duan, D.D.; Ye, J.; Li, J.; Ding, Y.; Zhang, W.; Lu, J.; et al. The cross-talk between PARylation and SUMOylation in C/EBPbeta at K134 site participates in pathological cardiac hypertrophy. Int. J. Biol. Sci. 2022, 18, 783–799. [Google Scholar] [CrossRef]
- Ren, Q.; Liu, Z.; Wu, L.; Yin, G.; Xie, X.; Kong, W.; Zhou, J.; Liu, S. C/EBPbeta: The structure, regulation, and its roles in inflammation-related diseases. Biomed. Pharmacother. 2023, 169, 115938. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Duan, P.; Yao, L.; Hou, H. Shiftwork-Mediated Disruptions of Circadian Rhythms and Sleep Homeostasis Cause Serious Health Problems. Int. J. Genomics 2018, 2018, 8576890. [Google Scholar] [CrossRef]
- Okamoto-Uchida, Y.; Izawa, J.; Nishimura, A.; Hattori, A.; Suzuki, N.; Hirayama, J. Post-translational Modifications are Required for Circadian Clock Regulation in Vertebrates. Curr. Genomics 2019, 20, 332–339. [Google Scholar] [CrossRef]
- Garrigue-Antar, L.; Hartigan, N.; Kadler, K.E. Post-translational modification of bone morphogenetic protein-1 is required for secretion and stability of the protein. J. Biol. Chem. 2002, 277, 43327–43334. [Google Scholar] [CrossRef]
- Unnanuntana, A.; Rebolledo, B.J.; Khair, M.M.; DiCarlo, E.F.; Lane, J.M. Diseases affecting bone quality: Beyond osteoporosis. Clin. Orthop. Relat. Res. 2011, 469, 2194–2206. [Google Scholar] [CrossRef]
- Lamande, S.R.; Bateman, J.F. Genetic Disorders of the Extracellular Matrix. Anat. Rec. 2020, 303, 1527–1542. [Google Scholar] [CrossRef]
- Alcorta-Sevillano, N.; Infante, A.; Macias, I.; Rodriguez, C.I. Murine Animal Models in Osteogenesis Imperfecta: The Quest for Improving the Quality of Life. Int. J. Mol. Sci. 2022, 24, 184. [Google Scholar] [CrossRef]
- Dudek, M.; Swift, J.; Meng, Q.J. The circadian clock and extracellular matrix homeostasis in aging and age-related diseases. Am. J. Physiol. Cell Physiol. 2023, 325, C52–C59. [Google Scholar] [CrossRef]
- Matsushita, T.; Wilcox, W.R.; Chan, Y.Y.; Kawanami, A.; Bukulmez, H.; Balmes, G.; Krejci, P.; Mekikian, P.B.; Otani, K.; Yamaura, I.; et al. FGFR3 promotes synchondrosis closure and fusion of ossification centers through the MAPK pathway. Hum. Mol. Genet. 2009, 18, 227–240. [Google Scholar] [CrossRef] [PubMed]
- Besharse, J.C. Coupling an activated MAP kinase to circadian clock output. Neuron 2001, 29, 3–4. [Google Scholar] [CrossRef]
- Song, C.; Wang, J.; Kim, B.; Lu, C.; Zhang, Z.; Liu, H.; Kang, H.; Sun, Y.; Guan, H.; Fang, Z.; et al. Insights into the Role of Circadian Rhythms in Bone Metabolism: A Promising Intervention Target? Biomed. Res. Int. 2018, 2018, 9156478. [Google Scholar] [CrossRef] [PubMed]
- Spengler, M.L.; Kuropatwinski, K.K.; Comas, M.; Gasparian, A.V.; Fedtsova, N.; Gleiberman, A.S.; Gitlin, I.I.; Artemicheva, N.M.; Deluca, K.A.; Gudkov, A.V.; et al. Core circadian protein CLOCK is a positive regulator of NF-kappaB-mediated transcription. Proc. Natl. Acad. Sci. USA 2012, 109, E2457–E2465. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Lin, C.; Stavre, Z.; Greenblatt, M.B.; Shim, J.H. Osteoblast-Osteoclast Communication and Bone Homeostasis. Cells 2020, 9, 2073. [Google Scholar] [CrossRef]
- Juliana, N.; Azmi, L.; Effendy, N.M.; Mohd Fahmi Teng, N.I.; Abu, I.F.; Abu Bakar, N.N.; Azmani, S.; Yazit, N.A.A.; Kadiman, S.; Das, S. Effect of Circadian Rhythm Disturbance on the Human Musculoskeletal System and the Importance of Nutritional Strategies. Nutrients 2023, 15, 734. [Google Scholar] [CrossRef]
- Berry, J.L.; Davies, M.; Mee, A.P. Vitamin D metabolism, rickets, and osteomalacia. Semin. Musculoskelet. Radiol. 2002, 6, 173–182. [Google Scholar] [CrossRef]
- Roodman, G.D.; Windle, J.J. Paget disease of bone. J. Clin. Investig. 2005, 115, 200–208. [Google Scholar] [CrossRef]
- Orsini, F.; Crotti, C.; Cincinelli, G.; Di Taranto, R.; Amati, A.; Ferrito, M.; Varenna, M.; Caporali, R. Bone Involvement in Rheumatoid Arthritis and Spondyloartritis: An Updated Review. Biology 2023, 12, 1320. [Google Scholar] [CrossRef]
- Wang, X.L.; Li, L. Circadian Clock Regulates Inflammation and the Development of Neurodegeneration. Front. Cell Infect. Microbiol. 2021, 11, 696554. [Google Scholar] [CrossRef] [PubMed]
- Hirano, A.; Fu, Y.H.; Ptacek, L.J. The intricate dance of post-translational modifications in the rhythm of life. Nat. Struct. Mol. Biol. 2016, 23, 1053–1060. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Nakajima, R.; Shirasawa, M.; Fikriyanti, M.; Zhao, L.; Iwanaga, R.; Bradford, A.P.; Kurayoshi, K.; Araki, K.; Ohtani, K. Expanding Roles of the E2F-RB-p53 Pathway in Tumor Suppression. Biology 2023, 12, 1511. [Google Scholar] [CrossRef] [PubMed]
- El-Tanani, M.; Rabbani, S.A.; Ali, A.A.; Alfaouri, I.G.A.; Al Nsairat, H.; Al-Ani, I.H.; Aljabali, A.A.; Rizzo, M.; Patoulias, D.; Khan, M.A.; et al. Circadian rhythms and cancer: Implications for timing in therapy. Discov. Oncol. 2024, 15, 767. [Google Scholar] [CrossRef]
- Chiang, C.K.; Xu, B.; Mehta, N.; Mayne, J.; Sun, W.Y.; Cheng, K.; Ning, Z.; Dong, J.; Zou, H.; Cheng, H.M.; et al. Phosphoproteome Profiling Reveals Circadian Clock Regulation of Posttranslational Modifications in the Murine Hippocampus. Front. Neurol. 2017, 8, 110. [Google Scholar] [CrossRef]
- Wu, X.; Yang, X.; Dai, Y.; Zhao, Z.; Zhu, J.; Guo, H.; Yang, R. Single-cell sequencing to multi-omics: Technologies and applications. Biomark. Res. 2024, 12, 110. [Google Scholar] [CrossRef]
- Fadul, S.M.; Arshad, A.; Mehmood, R. CRISPR-based epigenome editing: Mechanisms and applications. Epigenomics 2023, 15, 1137–1155. [Google Scholar] [CrossRef]
- Aye-Han, N.N.; Ni, Q.; Zhang, J. Fluorescent biosensors for real-time tracking of post-translational modification dynamics. Curr. Opin. Chem. Biol. 2009, 13, 392–397. [Google Scholar] [CrossRef]
- Burt, L.A.; Billington, E.O.; Rose, M.S.; Raymond, D.A.; Hanley, D.A.; Boyd, S.K. Effect of High-Dose Vitamin D Supplementation on Volumetric Bone Density and Bone Strength: A Randomized Clinical Trial. J. Am. Med. Assoc. 2019, 322, 736–745. [Google Scholar] [CrossRef]
- Krzyszczyk, P.; Acevedo, A.; Davidoff, E.J.; Timmins, L.M.; Marrero-Berrios, I.; Patel, M.; White, C.; Lowe, C.; Sherba, J.J.; Hartmanshenn, C.; et al. The growing role of precision and personalized medicine for cancer treatment. Technol. Singap. World Sci. 2018, 6, 79–100. [Google Scholar] [CrossRef]
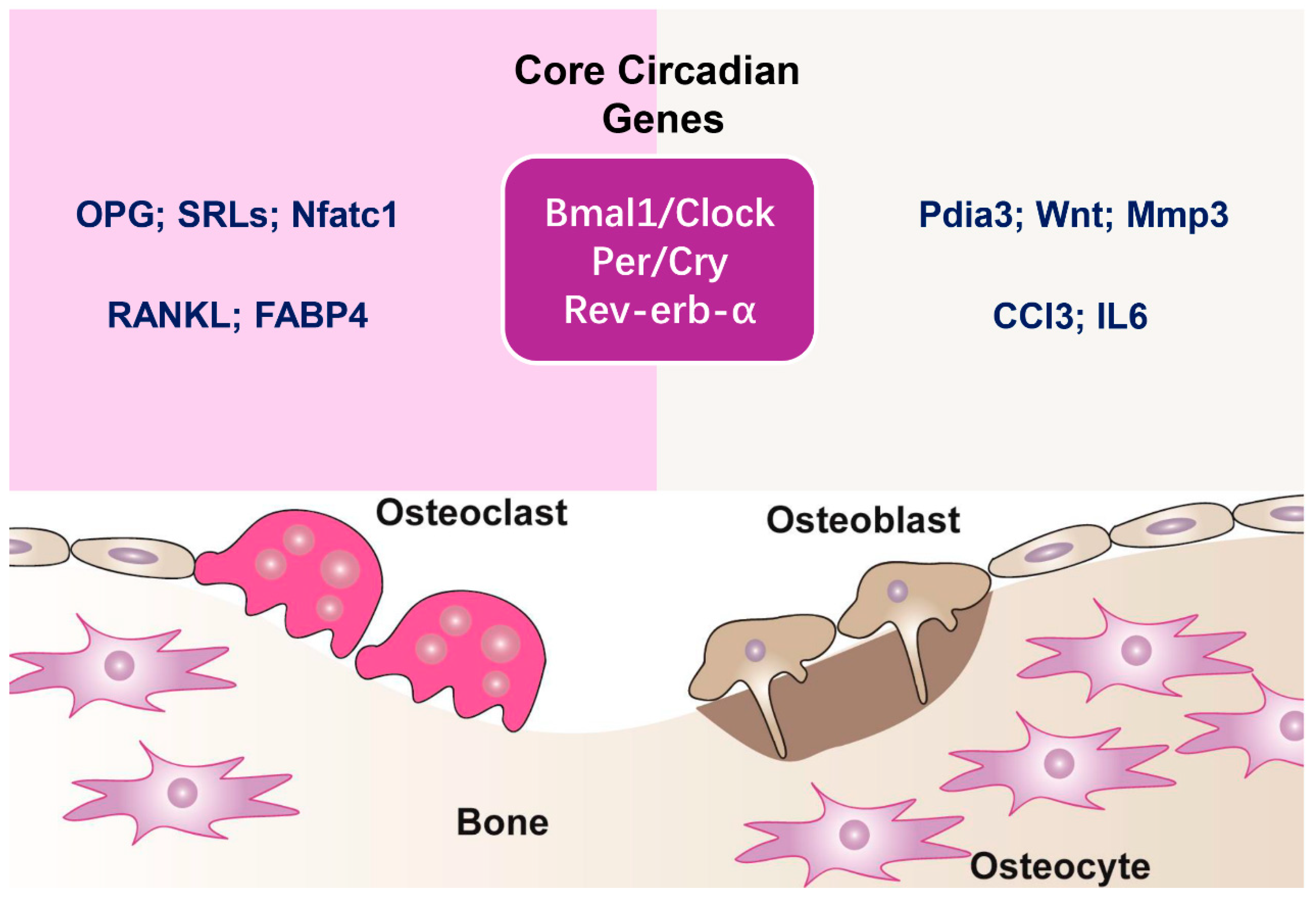
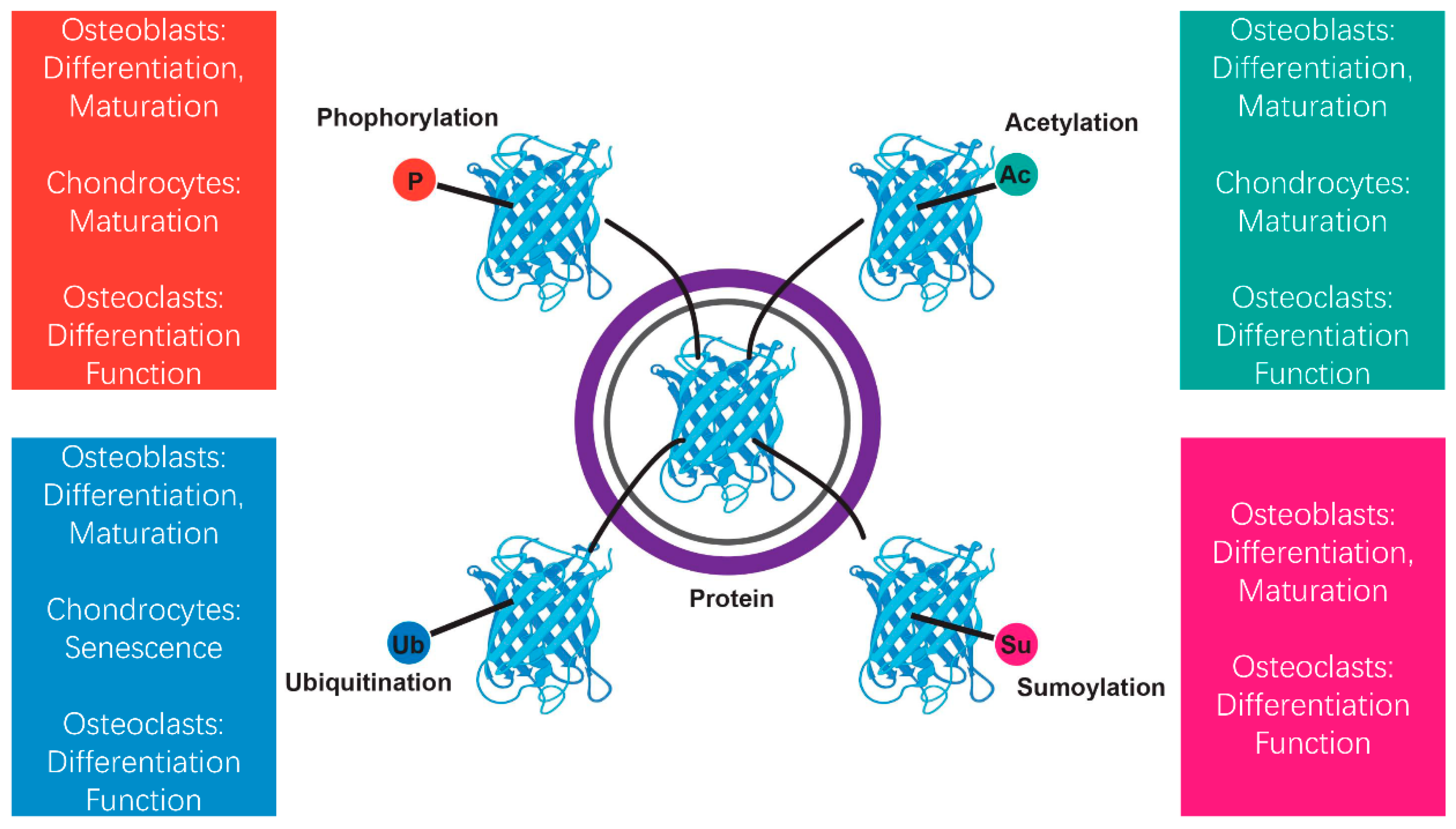
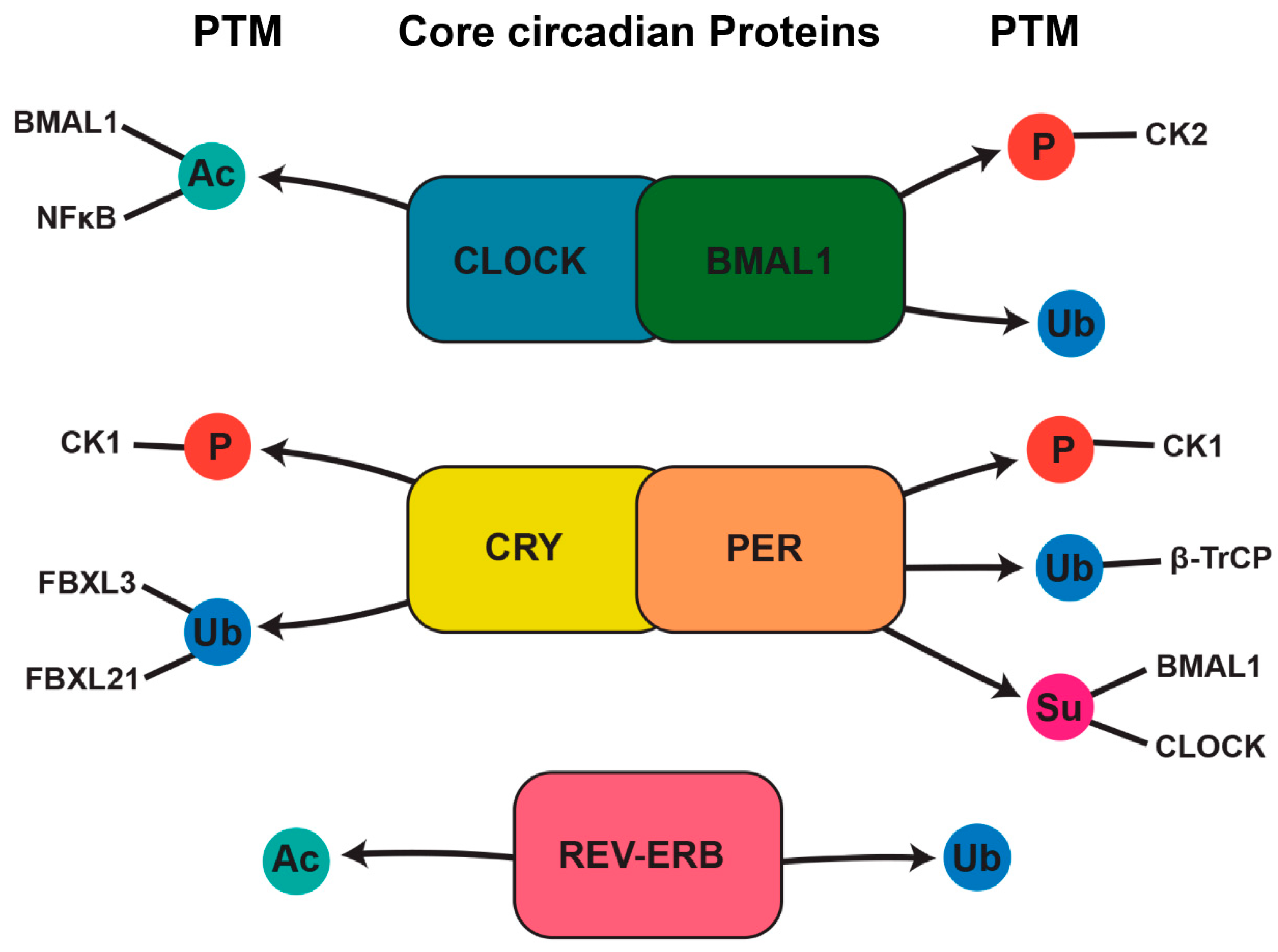

| Molecular Player | Target Bone Cells | Effects on Bone Metabolism | Experimental Evidence |
|---|---|---|---|
| BMAL1/CLOCK | Osteoblasts | Regulates osteoblast differentiation and bone formation | Osteoblast-specific Bmal1 knockout mice exhibit reduced bone mass due to impaired osteoblast differentiation. CLOCK-mutation mice show decreased bone formation, suggesting its role in osteoblast function. |
| Osteoclasts | Influences osteoclast differentiation and bone resorption | Osteoclast-specific Bmal1 knockout mice display high bone mass due to reduced osteoclast differentiation. | |
| PER1/PER2 | Osteoblasts | Regulates osteoblast proliferation and bone formation | Per1/Per2 knockout mice exhibit increased bone mass. |
| CRY1/CRY2 | Osteoblasts | Modulates osteoblast proliferation and bone formation | Cry1/Cry2 double knockout mice show increased bone formation and osteoblast activity. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yuan, V.G. Rhythms in Remodeling: Posttranslational Regulation of Bone by the Circadian Clock. Biomedicines 2025, 13, 705. https://doi.org/10.3390/biomedicines13030705
Yuan VG. Rhythms in Remodeling: Posttranslational Regulation of Bone by the Circadian Clock. Biomedicines. 2025; 13(3):705. https://doi.org/10.3390/biomedicines13030705
Chicago/Turabian StyleYuan, Vincent G. 2025. "Rhythms in Remodeling: Posttranslational Regulation of Bone by the Circadian Clock" Biomedicines 13, no. 3: 705. https://doi.org/10.3390/biomedicines13030705
APA StyleYuan, V. G. (2025). Rhythms in Remodeling: Posttranslational Regulation of Bone by the Circadian Clock. Biomedicines, 13(3), 705. https://doi.org/10.3390/biomedicines13030705






