The Immune Environment in Colorectal Adenoma: A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Information Sources
2.3. Search Strategy
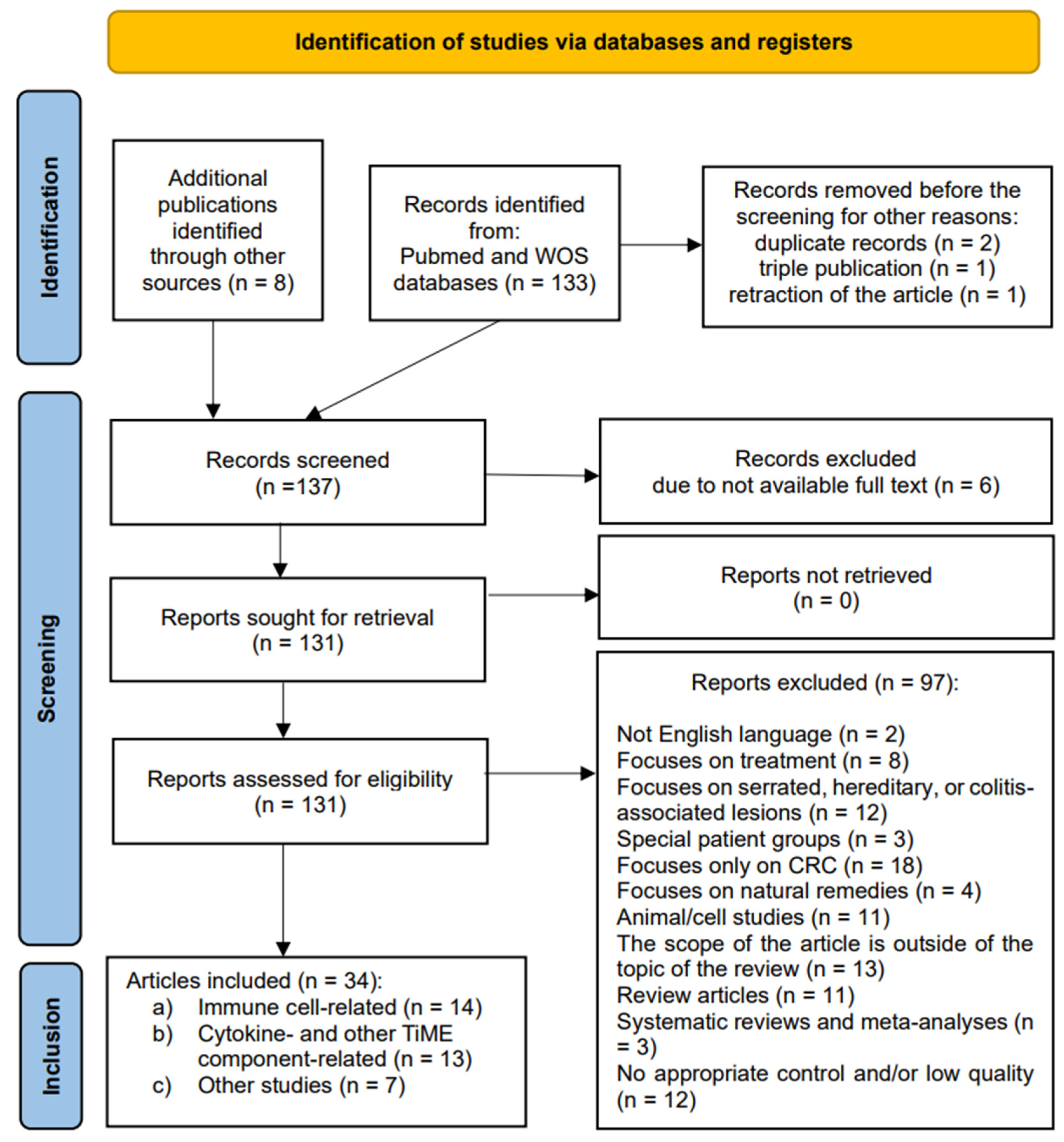
2.4. Selection Process
2.5. Data Collection Process
2.6. Protocol Registration
2.7. Study Quality Assessment and Risk of Bias
3. Results
Search Results and Study Characteristics
4. Discussion
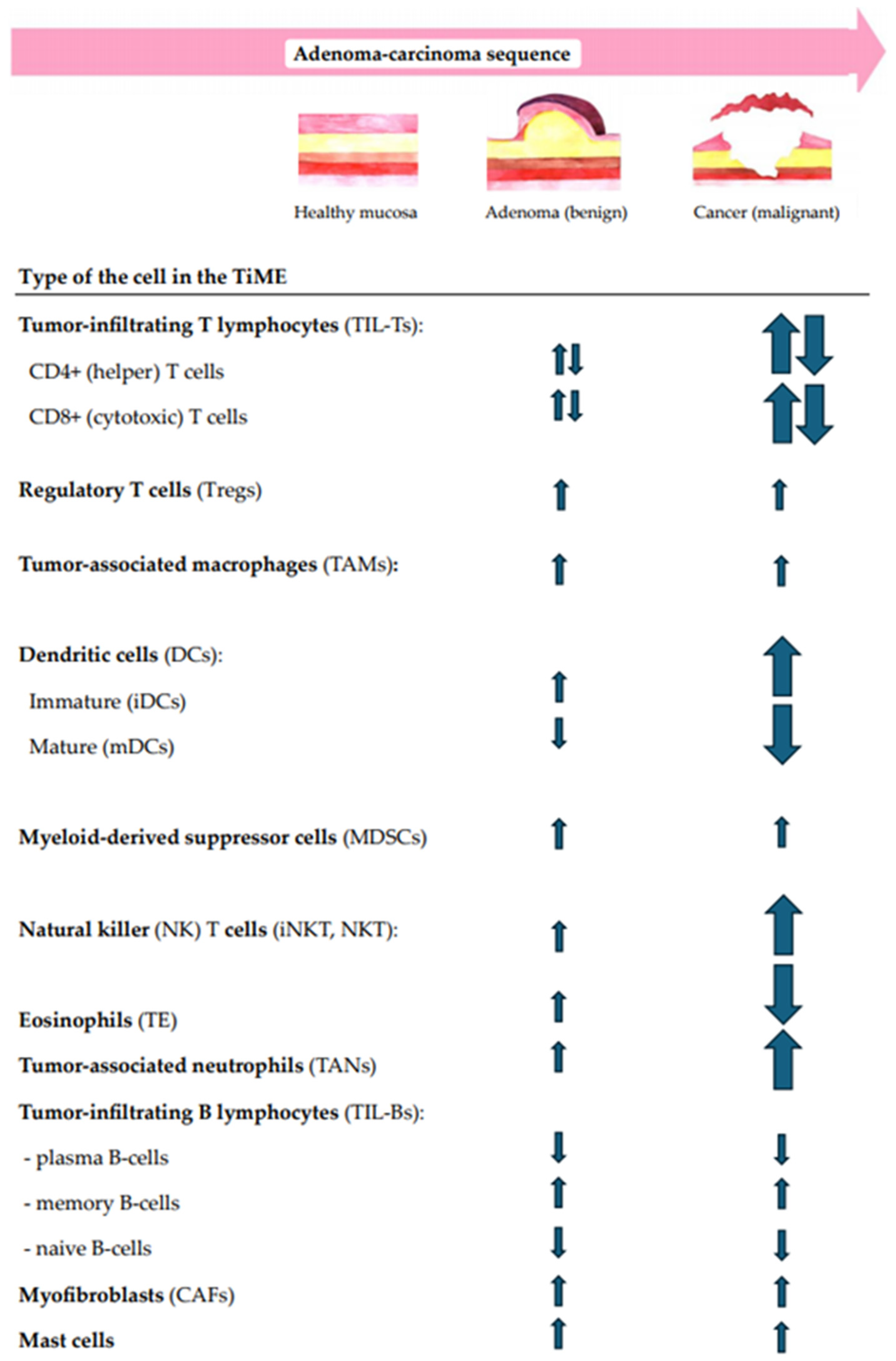
4.1. Immune Cell-Related Changes in Patients with Sporadic Colorectal Adenoma (Along the A–C Pathway)
4.2. Cytokine-Related Immune Responses in Patients with Sporadic CR Adenoma
4.3. Other Immunological Factors in the Progression of Colorectal Adenoma
4.4. Association with Polyp Morphology, Size, Dysplasia Grade, and Location in the Gut
4.5. Limitations of the Review
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Fearon, E.R.; Vogelstein, B. A genetic model for colorectal tumorigenesis. Cell 1990, 61, 759–767. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Yang, M. Molecular Network of Colorectal Cancer and Current Therapeutic Options. Front. Oncol. 2022, 12, 852927. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Goel, A.; Chung, D.C. Pathways of Colorectal Carcinogenesis. Gastroenterology 2020, 158, 291–302. [Google Scholar] [CrossRef]
- Gharib, E.; Robichaud, G.A. From Crypts to Cancer: A Holistic Perspective on Colorectal Carcinogenesis and Therapeutic Strategies. Int. J. Mol. Sci. 2024, 25, 9463. [Google Scholar] [CrossRef]
- Huang, Y.-X.; Wu, J.-H.; Zhao, Y.-Q.; Sui, W.-N.; Tian, T.; Han, W.-X.; Ni, J. An atlas on risk factors for gastrointestinal cancers: A systematic review of Mendelian randomization studies. Prev. Med. 2024, 189, 108147. [Google Scholar] [CrossRef]
- Nguyen, L.; Shanmugan, S. A Review Article: The Relationship Between Obesity and Colorectal Cancer. Curr. Diabetes Rep. 2024, 25, 8. [Google Scholar] [CrossRef]
- Molla, M.D.; Symonds, E.L.; Winter, J.M.; Debie, A.; Wassie, M.M. Metabolic risk factors of colorectal cancer: Umbrella review. Crit. Rev. Oncol. Hematol. 2024, 204, 104502. [Google Scholar] [CrossRef]
- Zeng, W.; Liu, H.; Mao, Y.; Jiang, S.; Yi, H.; Zhang, Z.; Wang, M.; Zong, Z. Myeloid-derived suppressor cells: Key immunosuppressive regulators and therapeutic targets in colorectal cancer (Review). Int. J. Oncol. 2024, 65, 85. [Google Scholar] [CrossRef]
- Jiang, C.; Zhou, Q.; Yi, K.; Yuan, Y.; Xie, X. Colorectal cancer initiation: Understanding early-stage disease for intervention. Cancer Lett. 2024, 589, 216831. [Google Scholar] [CrossRef]
- Galon, J.; Costes, A.; Sanchez-Cabo, F.; Kirilovsky, A.; Mlecnik, B.; Lagorce-Pagès, C.; Tosolini, M.; Camus, M.; Berger, A.; Wind, P.; et al. The type, density, and location of immune cells within human colorectal tumors predict clinical outcomes. Science 2006, 313, 1960–1964. [Google Scholar] [CrossRef] [PubMed]
- Ferkel, S.A.; Holman, E.A.; Sojwal, R.S.; Rubin, S.J.; Rogalla, S. Tumor-Infiltrating Immune Cells in Colorectal Cancer. Neoplasia 2024, 59, 101091. [Google Scholar] [CrossRef]
- Pieren, D.K.J.; Boer, M.C.; de Wit, J. The adaptive immune system in early life: The shift makes it count. Front. Immunol. 2022, 13, 1031924. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Wang, Y.; Tiruthani, K. Tumor immune microenvironment and nano-immunotherapeutics in colorectal cancer. Nanomedicine 2019, 21, 102034. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; He, X.; Wang, Y.; Hu, Z.; Huang, H.; Zhao, S.; Wei, P.; Li, D. Warburg effect in colorectal cancer: The emerging roles in tumor microenvironment and therapeutic implications. J. Hematol. Oncol. 2022, 15, 160. [Google Scholar] [CrossRef]
- Wozniakova, M.; Skarda, J.; Raska, M. The Role of Tumor Microenvironment and Immune Response in Colorectal Cancer Development and Prognosis. Pathol. Oncol. Res. 2022, 28, 1610502. [Google Scholar] [CrossRef]
- Picard, E.; Verschoor, C.P.; Ma, G.W.; Pawelec, G. Relationships Between Immune Landscapes, Genetic Subtypes and Responses to Immunotherapy in Colorectal Cancer. Front. Immunol. 2020, 11, 369. [Google Scholar] [CrossRef]
- Burgos-Molina, A.M.; Santana, T.T.; Redondo, M.; Romero, M.J.B. The Crucial Role of Inflammation and the Immune System in Colorectal Cancer Carcinogenesis: A Comprehensive Perspective. Int. J. Mol. Sci. 2024, 25, 6188. [Google Scholar] [CrossRef]
- Rasmusson, A.; Zilenaite, D.; Nestarenkaite, A.; Augulis, R.; Laurinaviciene, A.; Ostapenko, V.; Poskus, T.; Laurinavicius, A. Immunogradient Indicators for Antitumor Response Assessment by Automated Tumor-Stroma Interface Zone Detection. Am. J. Pathol. 2020, 190, 1309–1322. [Google Scholar] [CrossRef]
- Guo, L.; Wang, C.; Qiu, X.; Pu, X.; Chang, P. Colorectal Cancer Immune Infiltrates: Significance in Patient Prognosis and Immunotherapeutic Efficacy. Front. Immunol. 2020, 11, 1052. [Google Scholar] [CrossRef]
- Chalabi, M.; Fanchi, L.F.; Dijkstra, K.K.; Van Den Berg, J.G.; Aalbers, A.G.; Sikorska, K.; Lopez-Yurda, M.; Grootscholten, C.; Beets, G.L.; Snaebjornsson, P.; et al. Neoadjuvant immunotherapy leads to pathological responses in MMR-proficient and MMR-deficient early-stage colon cancers. Nat. Med. 2020, 26, 566–576. [Google Scholar] [CrossRef] [PubMed]
- Le, D.T.; Uram, J.N.; Wang, H.; Bartlett, B.; Kemberling, H.; Eyring, A.; Skora, A.; Azad, N.S.; Laheru, D.A.; Donehower, R.C.; et al. PD-1 blockade in tumors with mismatch repair deficiency. N. Engl. J. Med. 2015, 33, LBA100. [Google Scholar] [CrossRef]
- Llosa, N.J.; Cruise, M.; Tam, A.; Wicks, E.C.; Hechenbleikner, E.M.; Taube, J.M.; Blosser, R.L.; Fan, H.; Wang, H.; Luber, B.S.; et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov. 2015, 5, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, K.; Stadler, Z.K.; Cercek, A.; Mendelsohn, R.B.; Shia, J.; Segal, N.H.; Diaz, L.A., Jr. Immunotherapy in colorectal cancer: Rationale, challenges and potential. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 361–375. [Google Scholar] [CrossRef]
- Spranger, S.; Gajewski, T.F. Impact of oncogenic pathways on evasion of antitumour immune responses. Nat. Rev. Cancer 2018, 18, 139–147. [Google Scholar] [CrossRef]
- Bollrath, J.; Phesse, T.J.; von Burstin, V.A.; Putoczki, T.; Bennecke, M.; Bateman, T.; Nebelsiek, T.; Lundgren-May, T.; Canli, Ö.; Schwitalla, S.; et al. gp130-mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell 2009, 15, 91–102. [Google Scholar] [CrossRef]
- Grivennikov, S.; Karin, E.; Terzic, J.; Mucida, D.; Yu, G.-Y.; Vallabhapurapu, S.; Scheller, J.; Rose-John, S.; Cheroutre, H.; Eckmann, L.; et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell 2009, 15, 103–113. [Google Scholar] [CrossRef]
- Canli, Ö.; Nicolas, A.M.; Gupta, J.; Finkelmeier, F.; Goncharova, O.; Pesic, M.; Neumann, T.; Horst, D.; Löwer, M.; Sahin, U.; et al. Myeloid cell-derived reactive oxygen species induce epithelial mutagenesis. Cancer Cell 2017, 32, 869–883.e5. [Google Scholar] [CrossRef]
- Kartikasari, A.E.R.; Huertas, C.S.; Mitchell, A.; Plebanski, M. Tumor-induced inflammatory cytokines and the emerging diagnostic devices for cancer detection and prognosis. Front. Oncol. 2021, 11, 692142. [Google Scholar] [CrossRef]
- Henry, C.J.; Sedjo, R.L.; Rozhok, A.; Salstrom, J.; Ahnen, D.; Levin, T.R.; D’AgostinoJr, R.; Haffner, S.; DeGregori, J.; Byers, T. Lack of significant association between serum inflammatory cytokine profiles and the presence of colorectal adenoma. BMC Cancer 2015, 15, 123. [Google Scholar] [CrossRef]
- Tse, B.C.Y.; Welham, Z.; Engel, A.F.; Molloy, M.P. Genomic, microbial, and immunological microenvironment of colorectal polyps. Cancers 2021, 13, 3382. [Google Scholar] [CrossRef]
- Rubinkiewicz, M.; Migaczewski, M.; Hankus, J.; Dembiński, M.; Pędziwiatr, M.; Okoń, K.; Pisarska, M.; Budzyński, A. The number of regulatory Foxp3+ T-cells in different stages of malignant transformation of large intestinal polyps. Adv. Med. Sci. 2016, 61, 306–310. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.; Willis, J.; Reumers, J.; Taggart, M.; Lucas, F.S.; Thirumurthi, S.; Kanth, P.; Delker, D.; Hagedorn, C.; Lynch, P.; et al. Colorectal premalignancy is associated with consensus molecular subtypes 1 and 2. Ann. Oncol. 2018, 29, 2061–2067. [Google Scholar] [CrossRef] [PubMed]
- Elsayed, I.; Li, L.; Sheahan, K.; Moran, B.; Bakheit, S.; Wang, X. Adenoma to carcinoma: A portrait of molecular and immunological profiles of colorectal sporadic tumors. Int. Immunopharmacol. 2021, 100, 108168. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-Y.; Miyai, K.; Han, H.S.; Hwang, D.-Y.; Seong, M.K.; Chung, H.; Jung, B.H.; Devaraj, B.; McGuire, K.L.; Carethers, J.M. Microsatellite instability, EMAST, and morphology associations with T cell infiltration in colorectal neoplasia. Dig. Dis. Sci. 2011, 57, 72–78. [Google Scholar] [CrossRef]
- Bodduluri, S.R.; Mathis, S.; Maturu, P.; Krishnan, E.; Satpathy, S.R.; Chilton, P.M.; Haribabu, B. Mast cell-dependent CD8(þ) T-cell recruitment mediates immune surveillance of intestinal tumors in Apc(Min/þ) Mice. Cancer Immunol. Res. 2018, 6, 332–347. [Google Scholar] [CrossRef]
- Mehdawi, L.; Osman, J.; Topi, G.; Sjölander, A. High tumor mast cell density is associated with longer survival of colon cancer patients. Acta Oncol. 2016, 55, 1434–1442. [Google Scholar] [CrossRef]
- Dhar, P.; Wu, J.D. NKG2D and its ligands in cancer. Curr. Opin. Immunol. 2018, 51, 55–61. [Google Scholar] [CrossRef]
- Blatner, N.R.; Bonertz, A.; Beckhove, P.; Cheon, E.C.; Krantz, S.B.; Strouch, M.; Weitz, J.; Koch, M.; Halverson, A.L.; Bentrem, D.J.; et al. In colorectal cancer mast cells contribute to systemic regulatory T-cell dysfunction. Proc. Natl. Acad. Sci. USA 2010, 107, 6430–6435. [Google Scholar] [CrossRef]
- Yu, Y.; Blokhuis, B.; Derks, Y.; Kumari, S.; Garssen, J.; Redegeld, F. Human mast cells promote colon cancer growth via bidirectional crosstalk: Studies in 2D and 3D coculture models. OncoImmunology 2018, 7, e1504729. [Google Scholar] [CrossRef]
- Hou, Y.; Huttenlocher, A. Advancing chemokine research: The molecular function of CXCL8. J. Clin. Investig. 2024, 134, e180984. [Google Scholar] [CrossRef] [PubMed]
- Uçmak, F.; Tuncel, E.T. Relationship Between Lesions in Adenomatous Polyp-Dysplasia-Colorectal Cancer Sequence and Neutrophil-to-Lymphocyte Ratio. Med. Sci. Monit. 2016, 22, 4536–4541. [Google Scholar] [CrossRef] [PubMed]
- Akeus, P.; Langenes, V.; von Mentzer, A.; Yrlid, U.; Sjöling, Å.; Saksena, P.; Raghavan, S.; Quiding-Järbrink, M. Altered chemokine production and accumulation of regulatory T cells in intestinal adenomas of APCMin/+ mice. Cancer Immunol. Immunother. 2014, 63, 807–819. [Google Scholar] [CrossRef]
- Biasi, F.; Guina, T.; Maina, M.; Nano, M.; Falcone, A.; Aroasio, E.; Saracco, G.M.; Papotti, M.; Leonarduzzi, G.; Poli, G. Progressive increase of matrix metalloprotease-9 and interleukin-8 serum levels during carcinogenic process in human colorectal tract. PLoS ONE 2012, 7, e41839. [Google Scholar] [CrossRef]
- Sasaki, Y.; Takeda, H.; Sato, T.; Orii, T.; Nishise, S.; Nagino, K.; Iwano, D.; Yaoita, T.; Yoshizawa, K.; Saito, H.; et al. Serum Interleukin-6, insulin, and HOMA-IR in male individuals with colorectal adenoma. Clin. Cancer Res. 2012, 18, 392–399. [Google Scholar] [CrossRef]
- Montazeri, Z.; Theodoratou, E.; Nyiraneza, C.; Timofeeva, M.; Chen, W.; Svinti, V.; Sivakumaran, S.; Gresham, G.; Cubitt, L.; Carvajal-Carmona, L.; et al. Systematic meta-analyses and field synopsis of genetic association studies in colorectal adenomas. Int. J. Epidemiol. 2015, 45, 186–205. [Google Scholar] [CrossRef][Green Version]
- Higgins, J.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.; Welch, V.; Flemyng, E.; Cochrane Handbook for Systematic Reviews of Interventions Version 6.5 (Updated August 2024). Cochrane. Available online: www.training.cochrane.org/handbook (accessed on 6 December 2024).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Moher, D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, 71. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Tugwell, P.; Ga, S.W.; Zello, G.; Petersen, J. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. 2014. Available online: https://api.semanticscholar.org/CorpusID:79550924 (accessed on 6 December 2024).
- Banner, B.F.; Sonmez-Alpan, E.; Yousem, S.A. An immunophenotypic study of the inflammatory cell populations in colon adenomas and carcinomas. Mod. Pathol. 1993, 6, 295–301. [Google Scholar]
- Yuan, A.; Steigen, S.E.; Goll, R.; Vonen, B.; Husbekk, A.; Cui, G.; Florholmen, J. Dendritic cell infiltration pattern along the colorectal adenoma-carcinoma sequence. APMIS 2008, 116, 445–456. [Google Scholar] [CrossRef]
- Roncucci, L.; Mora, E.; Mariani, F.; Bursi, S.; Pezzi, A.; Rossi, G.; Ponz de Leon, M. Myeloperoxidase-positive cell infiltration in colorectal carcinogenesis as indicator of colorectal cancer risk. Cancer Epidemiol Biomark. Prev. 2008, 17, 2291–2297. [Google Scholar] [CrossRef]
- Cui, G.; Yuan, A.; Vonen, B.; Florholmen, J. Progressive cellular response in the lamina propria of the colorectal adenoma–carcinoma sequence. Histopathology 2009, 54, 550–560. [Google Scholar] [CrossRef] [PubMed]
- McLean, M.H.; Murray, G.I.; Stewart, K.N.; Norrie, G.; Mayer, C.; Hold, G.L.; Thomson, J.; Fyfe, N.; Hope, M.; Mowat, N.A.G.; et al. The inflammatory microenvironment in colorectal neoplasia. PLoS ONE 2011, 6, e15366. [Google Scholar] [CrossRef] [PubMed]
- Mariani, F.; Sena, P.; Pedroni, M.; Benatti, P.; Manni, P.; Di Gregorio, C.; Manenti, A.; Palumbo, C.; de Leon, M.P.; Roncucci, L. Th inducing POZ-Kruppel Factor (ThPOK) is a key regulator of the immune response since the early steps of colorectal carcinogenesis. PLoS ONE 2013, 8, e54488. [Google Scholar] [CrossRef] [PubMed]
- Jang, T.J. Progressive Increase of Regulatory T Cells and Decrease of CD8+ T Cells and CD8+ T Cells/Regulatory T Cells Ratio during Colorectal Cancer Development. Korean J. Pathol. 2013, 47, 443–451. [Google Scholar] [CrossRef]
- Hua, W.; Yuan, A.; Zheng, W.; Li, C.; Cui, J.; Pang, Z.; Zhang, L.; Li, Z.; Goll, R.; Cui, G. Accumulation of FoxP3+ T regulatory cells in the tumor microenvironment of human colorectal adenomas. Pathol. Res. Pract. 2016, 212, 106–112. [Google Scholar] [CrossRef]
- Maglietta, A.; Maglietta, R.; Staiano, T.; Bertoni, R.; Ancona, N.; Marra, G.; Resta, L. The Immune Landscapes of Polypoid and Nonpolypoid Precancerous Colorectal Lesions. PLoS ONE 2016, 11, e0159373. [Google Scholar] [CrossRef]
- Zhu, X.-W.; Zhu, H.-Z.; Zhu, Y.-Q.; Feng, M.-H.; Qi, J.; Chen, Z.-F. Foxp3 expression in CD4+CD25+Foxp3+ regulatory T cells promotes development of colorectal cancer by inhibiting tumor immunity. J. Huazhong Univ. Sci. Technol. Med. Sci. 2016, 36, 677–682. [Google Scholar] [CrossRef]
- Cui, G.; Xu, G.; Zhu, L.; Pang, Z.; Zheng, W.; Li, Z.; Yuan, A. Temporal and spatial changes of cells positive for stem-like markers in different compartments and stages of human colorectal adenoma-carcinoma sequence. Oncotarget 2017, 8, 45311–45322. [Google Scholar] [CrossRef]
- Garcia, D.; Spaans, L.; Miranda, S.; Gonçalves, G.; Reis, J.; Costa, J.L.; Durães, C.; Carneiro, F.; Machado, J.C. The Influence of the Genetic and Immunologic Context in the Development of Colorectal Adenoma: A Case Series Report. Acta Medica Port. 2020, 33, 297–304. [Google Scholar] [CrossRef]
- Chen, B.; Scurrah, C.R.; McKinley, E.T.; Simmons, A.J.; Ramirez-Solano, M.A.; Zhu, X.; Markham, N.O.; Heiser, C.N.; Vega, P.N.; Rolong, A.; et al. Differential pre-malignant programs and microenvironment chart distinct paths to malignancy in human colorectal polyps. Cell 2021, 184, 6262–6280.e26. [Google Scholar] [CrossRef]
- Omran, T.A.; Tunsjø, H.S.; Jahanlu, D.; Brackmann, S.A.; Bemanian, V.; Sæther, P.C. Decoding immune-related gene-signatures in colorectal neoplasia. Front. Immunol. 2024, 15, 1407995. [Google Scholar] [CrossRef] [PubMed]
- Adegboyega, P.A.; Ololade, O.; Saada, J.; Mifflin, R.; Di Mari, J.F.; Powell, D.W. Subepithelial myofibroblasts express cyclooxygenase-2 in colorectal tubular adenomas. Clin. Cancer Res. 2004, 10, 5870–5879. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.; Goll, R.; Olsen, T.; Steigen, S.E.; Husebekk, A.; Vonen, B.; Florholmen, J. Reduced expression of microenvironmental Th1 cytokines accompanies adenomas–carcinomas sequence of colorectum. Cancer Immunol. Immunother. 2006, 56, 985–995. [Google Scholar] [CrossRef]
- Cui, G.; Yuan, A.; Goll, R.; Vonen, B.; Florholmen, J. Dynamic changes of interleukin-8 network along the colorectal adenoma–carcinoma sequence. Cancer Immunol. Immunother. 2009, 58, 1897–1905. [Google Scholar] [CrossRef]
- Cui, G.; Yuan, A.; Goll, R.; Florholmen, J. IL-17A in the tumor microenvironment of the human colorectal adenoma–carcinoma sequence. Scand. J. Gastroenterol. 2012, 47, 1304–1312. [Google Scholar] [CrossRef]
- Wang, J.; Xu, K.; Wu, J.; Luo, C.; Li, Y.; Wu, X.; Gao, H.; Feng, G.; Yuan, B.-Z. The changes of Th17 cells and the related cytokines in the progression of human colorectal cancers. BMC Cancer 2012, 12, 418. [Google Scholar] [CrossRef]
- Cui, G.; Qi, H.; Gundersen, M.D.; Yang, H.; Christiansen, I.; Sørbye, S.W.; Goll, R.; Florholmen, J. Dynamics of the IL-33/ST2 network in the progression of human colorectal adenoma to sporadic colorectal cancer. Cancer Immunol. Immunother. 2014, 64, 181–190. [Google Scholar] [CrossRef]
- Xie, Z.; Qu, Y.; Leng, Y.; Sun, W.; Ma, S.; Wei, J.; Hu, J.; Zhang, X. Human colon carcinogenesis is associated with increased interleukin-17-driven inflammatory responses. Drug Des. Dev. Ther. 2015, 9, 1679–1689. [Google Scholar] [CrossRef]
- Cui, G.; Yuan, A.; Zhu, L.; Florholmen, J.; Goll, R. Increased expression of interleukin-21 along colorectal adenoma-carcinoma sequence and its predicating significance in patients with sporadic colorectal cancer. Clin. Immunol. 2017, 183, 266–272. [Google Scholar] [CrossRef]
- Cui, G.; Yuan, A.; Li, Z.; Goll, R.; Florholmen, J. ST2 and regulatory T cells in the colorectal adenoma/carcinoma microenvironment: Implications for diseases progression and prognosis. Sci. Rep. 2020, 10, 5892. [Google Scholar] [CrossRef]
- Cui, G.; Li, Z.; Florholmen, J.; Goll, R. Dynamic stromal cellular reaction throughout human colorectal adenoma-carcinoma sequence: A role of TH17/IL-17A. Biomed. Pharmacother. 2021, 140, 111761. [Google Scholar] [CrossRef] [PubMed]
- Youssef, H.M.K.; Radi, D.A.; El-Azeem, M.A.A. Expression of TSP50, SERCA2 and IL-8 in Colorectal Adenoma and Carcinoma: Correlation to Clinicopathological Factors. Pathol. Oncol. Res. 2021, 27, 1609990. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.; Li, G.; Pang, Z.; Florholmen, J.; Goll, R. The presentation and regulation of the IL-8 network in the epithelial cancer stem-like cell niche in patients with colorectal cancer. Biomed. Pharmacother. 2022, 152, 113252. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, X.-L.; Liu, J.-J.; Qian, Z.-Y.; Pan, Z.-Y.; Song, N.-P.; Chen, H.-Y.; Zhang, W.; Zhang, X. ICOS/ICOSLG and PD-1 Co-Expression is Associated with the Progression of Colorectal Precancerous- Carcinoma Immune Microenvironment. J. Inflamm. Res. 2023, 16, 977–992. [Google Scholar] [CrossRef]
- Moezzi, J.; Gopalswamy, N.; Haas, R.J.; Markert, R.J.; Suryaprasad, S.; Bhutani, M.S. Stromal Eosinophilia in Colonic Epithelial Neoplasms. Am. J. Gastroenterol. 2000, 95, 520–523. [Google Scholar] [CrossRef]
- Kiziltaş, S.; Sezgin Ramadan, S.; Topuzoğlu, A.; Küllü, S. Does the severity of tissue eosinophilia of colonic neoplasms reflect their malignancy potential? Turk. J. Gastroenterol. Off. J. Turk. Soc. Gastroenterol. 2008, 19, 239–244. [Google Scholar]
- Freitas, J.A.; Gullo, I.; Garcia, D.; Miranda, S.; Spaans, L.; Pinho, L.; Reis, J.; Sousa, F.; Baptista, M.; Resende, C.; et al. The Adaptive Immune Landscape of the Colorectal Adenoma–Carcinoma Sequence. Int. J. Mol. Sci. 2021, 22, 9791. [Google Scholar] [CrossRef]
- Mohamed, A.S.E.D.; El-Rebey, H.S.; AboElnasr, L.S.a.; Abdou, A.G. The role and relationship between programmed death ligand 1 and cytotoxic T lymphocyte-associated antigen-4 immunohistochemical expression in colorectal carcinoma patients; an impact on outcome. Ecancermedicalscience 2021, 15, 1323. [Google Scholar] [CrossRef]
- Wallace, K.; El Nahas, G.J.; Bookhout, C.; Thaxton, J.E.; Lewin, D.N.; Nikolaishvili-Feinberg, N.; Cohen, S.M.; Brazeal, J.G.; Hill, E.G.; Wu, J.D.; et al. Immune Responses Vary in Preinvasive Colorectal Lesions by Tumor Location and Histology. Cancer Prev. Res. 2021, 14, 885–892. [Google Scholar] [CrossRef]
- Wallace, K.; Nahhas, G.J.; Bookhout, C.; Lewin, D.N.; Paulos, C.M.; Nikolaishvili-Feinberg, N.; Cohen, S.M.; Guglietta, S.; Bakhtiari, A.; Camp, E.R.; et al. Preinvasive Colorectal Lesions of African Americans Display an Immunosuppressive Signature Compared to Caucasian Americans. Front. Oncol. 2021, 11, 659036. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, X.; Jin, Z.; Chen, H.; Zhang, C.; Wang, W.; Jing, J.; Pan, W. Clinical Impact of X-Ray Repair Cross-Complementary 1 (XRCC1) and the Immune Environment in Colorectal Adenoma–Carcinoma Pathway Progression. J. Inflamm. Res. 2021, 14, 5403–5417. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Xie, K.; Liu, T. Cancer Immunotherapies: From Efficacy to Resistance Mechanisms—Not Only Checkpoint Matters. Front. Immunol. 2021, 12, 690112. [Google Scholar] [CrossRef] [PubMed]
- Cui, G.; Shi, Y.; Cui, J.; Tang, F.; Florholmen, J. Immune microenvironmental shift along human colorectal adenoma–carcinoma sequence: Is it relevant to tumor development, biomarkers and biotherapeutic targets? Scand. J. Gastroenterol. 2012, 47, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, M.; Greten, F.R. The inflammatory pathogenesis of colorectal cancer. Nat. Rev. Immunol. 2021, 21, 653–667. [Google Scholar] [CrossRef]
- Cui, G. Immune battle at the premalignant stage of colorectal cancer: Focus on immune cell compositions, functions and cytokine products. Am. J. Cancer Res. 2020, 10, 1308–1320. [Google Scholar]
- Preza, G.C.; Yang, O.O.; Elliott, J.; Anton, P.A.; Ochoa, M.T. T lymphocyte density and distribution in human colorectal mucosa, and inefficiency of current cell isolation protocols. PLoS ONE 2015, 10, e0122723. [Google Scholar] [CrossRef]
- Kryczek, I.; Lin, Y.; Nagarsheth, N.; Peng, D.; Zhao, L.; Zhao, E.; Vatan, L.; Szeliga, W.; Dou, Y.; Owens, S.; et al. IL-22+CD4+ T cells promote colorectal cancer stemness via STAT3 transcription factor activation and induction of the methyltransferase DOT1L. Immunity 2014, 40, 772–784. [Google Scholar] [CrossRef]
- Li, Q.; Geng, S.; Luo, H.; Wang, W.; Mo, Y.-Q.; Luo, Q.; Wang, L.; Song, G.-B.; Sheng, J.-P.; Xu, B. Signaling pathways involved in colorectal cancer: Pathogenesis and targeted therapy. Signal Transduct. Target. Ther. 2024, 9, 266. [Google Scholar] [CrossRef]
- Cui, G. TH9, TH17, and TH22 Cell Subsets and Their Main Cytokine Products in the Pathogenesis of Colorectal Cancer. Front. Oncol. 2019, 9, 1002. [Google Scholar] [CrossRef]
- Liu, F.; Hu, X.; Zimmerman, M.; Waller, J.L.; Wu, P.; Hayes-Jordan, A.; Lev, D.; Liu, K. TNFalpha cooperates with IFN-gamma to repress Bcl-xL expression to sensitize metastatic colon carcinoma cells to TRAIL-mediated apoptosis. PLoS ONE 2011, 6, e16241. [Google Scholar]
- Oshi, M.; Sarkar, J.; Wu, R.; Tokumaru, Y.; Yan, L.; Nakagawa, K.; Ishibe, A.; Matsuyama, R.; Endo, I.; Takabe, K. Intratumoral density of regulatory T cells is a predictor of host immune response and chemotherapy response in colorectal cancer. Am. J. Cancer Res. 2022, 12, 490–503. [Google Scholar] [PubMed]
- Strizova, Z.; Benesova, I.; Bartolini, R.; Novysedlak, R.; Cecrdlova, E.; Foley, L.K.; Striz, I. M1/M2 macrophages and their overlaps—Myth or reality? Clin. Sci. 2023, 137, 1067–1093. [Google Scholar] [CrossRef] [PubMed]
- Konstantinov, A.S.; Kovaleva, O.; Samoilova, D.; Shelekhova, K. Role of macrophages in progression of colorectal cancer: A contrast with the traditional paradigm. Int. J. Clin. Exp. Pathol. 2022, 15, 403–411. [Google Scholar]
- Soncin, I.; Sheng, J.; Chen, Q.; Foo, S.; Duan, K.; Lum, J.; Poidinger, M.; Zolezzi, F.; Karjalainen, K.; Ruedl, C. The tumour microenvironment creates a niche for the self-renewal of tumour-promoting macrophages in colon adenoma. Nat. Commun. 2018, 9, 582. [Google Scholar] [CrossRef]
- Wang, H.; Tian, T.; Zhang, J. Tumor-Associated Macrophages (TAMs) in Colorectal Cancer (CRC): From Mechanism to Therapy and Prognosis. Int. J. Mol. Sci. 2021, 22, 8470. [Google Scholar] [CrossRef]
- Forssell, J.; Öberg, A.; Henriksson, M.L.; Stenling, R.; Jung, A.; Palmqvist, R. High macrophage infiltration along the tumor front correlates with improved survival in colon cancer. Clin. Cancer Res. 2007, 13, 1472–1479. [Google Scholar] [CrossRef]
- Zhou, Q.; Peng, R.-Q.; Wu, X.-J.; Xia, Q.; Hou, J.-H.; Ding, Y.; Zhou, Q.-M.; Zhang, X.; Pang, Z.-Z.; Wan, D.-S.; et al. The density of macrophages in the invasive front is inversely correlated to liver metastasis in colon cancer. J. Transl. Med. 2010, 8, 13. [Google Scholar] [CrossRef]
- Norton, S.E.; Ward-Hartstonge, K.A.; Taylor, E.S.; A Kemp, R. Immune cell interplay in colorectal cancer prognosis. World J. Gastrointest. Oncol. 2015, 7, 221–232. [Google Scholar] [CrossRef]
- Zhang, Y.; Ji, S.; Miao, G.; Du, S.; Wang, H.; Yang, X.; Li, A.; Lu, Y.; Wang, X.; Zhao, X. The current role of dendritic cells in the progression and treatment of colorectal cancer. Cancer Biol. Med. 2024, 21, 20240188. [Google Scholar] [CrossRef]
- Legitimo, A.; Consolini, R.; Failli, A.; Orsini, G.; Spisni, R. Dendritic cell defects in the colorectal cancer. Hum. Vaccines Immunother. 2014, 10, 3224–3235. [Google Scholar] [CrossRef]
- Sieminska, I.; Baran, J. Myeloid-Derived Suppressor Cells in Colorectal Cancer. Front. Immunol. 2020, 11, 1526. [Google Scholar] [CrossRef]
- Ma, P.; Beatty, P.L.; McKolanis, J.; Brand, R.; Schoen, R.E.; Finn, O.J. Circulating myeloid derived suppressor cells (MDSC) that accumulate in premalignancy share phenotypic and functional characteristics with MDSC in cancer. Front. Immunol. 2019, 10, 1401. [Google Scholar] [CrossRef] [PubMed]
- Ghazvinian, Z.; Abdolahi, S.; Tokhanbigli, S.; Tarzemani, S.; Piccin, A.; Zali, M.R.; Verdi, J.; Baghaei, K. Contribution of natural killer cells in innate immunity against colorectal cancer. Front. Oncol. 2023, 12, 1077053. [Google Scholar] [CrossRef] [PubMed]
- Reid, F.S.W.; Egoroff, N.; Pockney, P.G.; Smith, S.R. A systematic scoping review on natural killer cell function in colorectal cancer. Cancer Immunol. Immunother. 2020, 70, 597–606. [Google Scholar] [CrossRef]
- Della Chiesa, M.; Setti, C.; Giordano, C.; Obino, V.; Greppi, M.; Pesce, S.; Marcenaro, E.; Rutigliani, M.; Provinciali, N.; Paleari, L.; et al. NK Cell-Based Immunotherapy in Colorectal Cancer. Vaccines 2022, 10, 1033. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Sedimbi, S.; Löfbom, L.; Singh, A.K.; A Porcelli, S.; Cardell, S.L. Unique invariant natural killer T cells promote intestinal polyps by suppressing TH1 immunity and promoting regulatory T cells. Mucosal Immunol. 2017, 11, 131–143. [Google Scholar] [CrossRef]
- Lopez-Perez, D.; Prados-Lopez, B.; Galvez, J.; Leon, J.; Carazo, A. Eosinophils in Colorectal Cancer: Emerging Insights into Anti-Tumoral Mechanisms and Clinical Implications. Int. J. Mol. Sci. 2024, 25, 6098. [Google Scholar] [CrossRef]
- Saraiva, A.L.; Carneiro, F. New Insights into the Role of Tissue Eosinophils in the Progression of Colorectal Cancer: A Literature Review. Acta Medica Port. 2018, 31, 329–337. [Google Scholar] [CrossRef]
- Prizment, A.E.; Vierkant, R.A.; Smyrk, T.C.; Tillmans, L.S.; Lee, J.J.; Sriramarao, P.; Nelson, H.H.; Lynch, C.F.; Thibodeau, S.N.; Church, T.R.; et al. Tumor eosinophil infiltration and improved survival of colorectal cancer patients: Iowa Women’s Health Study. Mod. Pathol. 2016, 29, 516–527. [Google Scholar] [CrossRef]
- Cho, H.; Lim, S.-J.; Won, K.Y.; Bae, G.E.; Kim, G.Y.; Min, J.W.; Noh, B.-J. Eosinophils in Colorectal Neoplasms Associated with Expression of CCL11 and CCL24. J. Pathol. Transl. Med. 2016, 50, 45–51. [Google Scholar] [CrossRef]
- Reichman, H.; Itan, M.; Rozenberg, P.; Yarmolovski, T.; Brazowski, E.; Varol, C.; Gluck, N.; Shapira, S.; Arber, N.; Qimron, U.; et al. Activated Eosinophils Exert Antitumorigenic Activities in Colorectal Cancer. Cancer Immunol. Res. 2019, 7, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Peng, K.; Song, Y.; Yang, W.; Shu, W.; Yu, T.; Yu, L.; Lin, M.; Wei, Q.; Chen, C.; et al. CD177+ neutrophils suppress epithelial cell tumourigenesis in colitis-associated cancer and predict good prognosis in colorectal cancer. Carcinogenesis 2018, 39, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, R.; Kawada, K.; Itatani, Y.; Ogawa, R.; Kiyasu, Y.; Sakai, Y. The Role of Tumor-Associated Neutrophils in Colorectal Cancer. Int. J. Mol. Sci. 2019, 20, 529. [Google Scholar] [CrossRef] [PubMed]
- Tanoglu, A.; Karagoz, E. Predictive role of the neutrophil-to-lymphocyte ratio in patients with advanced hepatocellular carcinoma receiving sorafenib. Asian Pac. J. Cancer Prev. 2014, 15, 1063. [Google Scholar] [CrossRef]
- Shibutani, M.; Maeda, K.; Nagahara, H.; Fukuoka, T.; Nakao, S.; Matsutani, S.; Hirakawa, K.; Ohira, M. The peripheral monocyte count is associated with the density of tumor-associated macrophages in the tumor microenvironment of colorectal cancer: A retrospective study. BMC Cancer 2017, 17, 404. [Google Scholar] [CrossRef]
- Xue, D.; Hu, S.; Zheng, R.; Luo, H.; Ren, X. Tumor-infiltrating B cells: Their dual mechanistic roles in the tumor microenvironment. Biomed. Pharmacother. 2024, 179, 117436. [Google Scholar] [CrossRef]
- Hu, A.; Nussbaum, Y.I.; Mitchem, J.; Yoo, J. Colorectal Cancer-Associated Myofibroblasts Exhibit Enhanced Angiogenin Expression and Signaling via the PLXNB2 Receptor. J. Surg. Res. 2024, 296, 273–280. [Google Scholar] [CrossRef]
- Cui, J.-Y.; Ma, J.; Gao, X.-X.; Sheng, Z.-M.; Pan, Z.-X.; Shi, L.-H.; Zhang, B.-G. Unraveling the role of cancer-associated fibroblasts in colorectal cancer. World J. Gastrointest. Oncol. 2024, 16, 4565–4578. [Google Scholar] [CrossRef]
- Liu, X.; Li, X.; Wei, H.; Liu, Y.; Li, N. Mast cells in colorectal cancer tumour progression, angiogenesis, and lymphangiogenesis. Front. Immunol. 2023, 14, 1209056. [Google Scholar] [CrossRef]
- Kucukzeybek, B.B.; Dere, Y.; Sari, A.A.; Ocal, I.; Avcu, E.; Dere, O.; Calli, A.O.; Dinckal, C.; Tunakan, M.; Kucukzeybek, Y. The prognostic significance of CD117-positive mast cells and microvessel density in colorectal cancer. Medicine 2024, 103, e38997. [Google Scholar] [CrossRef]
- Borowczak, J.; Szczerbowski, K.; Maniewski, M.; Kowalewski, A.; Janiczek-Polewska, M.; Szylberg, A.; Marszałek, A.; Szylberg, Ł. The Role of Inflammatory Cytokines in the Pathogenesis of Colorectal Carcinoma—Recent Findings and Review. Biomedicines 2022, 10, 1670. [Google Scholar] [CrossRef] [PubMed]
- Braumüller, H.; Mauerer, B.; Andris, J.; Berlin, C.; Wieder, T.; Kesselring, R. The Cytokine Network in Colorectal Cancer: Implications for New Treatment Strategies. Cells 2022, 12, 138. [Google Scholar] [CrossRef] [PubMed]
- Maryam, S.; Krukiewicz, K.; Haq, I.U.; Khan, A.A.; Yahya, G.; Cavalu, S. Interleukins (Cytokines) as Biomarkers in Colorectal Cancer: Progression, Detection, and Monitoring. J. Clin. Med. 2023, 12, 3127. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, H.; Nishikata, R.; Senju, S.; Nishimura, Y. Myeloid-Derived Suppressor Cells Attenuate TH1 Development through IL-6 Production to Promote Tumor Progression. Cancer Immunol. Res. 2013, 1, 64–76. [Google Scholar] [CrossRef]
- Feng, X.; Zhang, Z.; Sun, P.; Song, G.; Wang, L.; Sun, Z.; Yuan, N.; Wang, Q.; Lun, L. Interleukin-18 Is a Prognostic Marker and Plays a Tumor Suppressive Role in Colon Cancer. Dis. Markers 2020, 2020, 6439614. [Google Scholar] [CrossRef]
- Zhu, Z.; Peng, Q.; Duan, X.; Li, J. Interleukin-12: Structure, Function, and Its Impact in Colorectal Cancer. J. Interf. Cytokine Res. 2024, 44, 158–169. [Google Scholar] [CrossRef]
- Jing, Z.-L.; Liu, G.-L.; Zhou, N.; Xu, D.-Y.; Feng, N.; Lei, Y.; Ma, L.-L.; Tang, M.-S.; Tong, G.-H.; Tang, N.; et al. Interferon-γ in the tumor microenvironment promotes the expression of B7H4 in colorectal cancer cells, thereby inhibiting cytotoxic T cells. Sci. Rep. 2024, 14, 6053. [Google Scholar] [CrossRef]
- Qu, N.; Xu, M.; Mizoguchi, I.; Furusawa, J.-I.; Kaneko, K.; Watanabe, K.; Mizuguchi, J.; Itoh, M.; Kawakami, Y.; Yoshimoto, T. Pivotal roles of T-helper 17-related cytokines, IL-17, IL-22, and IL-23, in inflammatory diseases. Clin. Dev. Immunol. 2013, 2013, 968549. [Google Scholar] [CrossRef]
- Stolfi, C.; Pallone, F.; MacDonald, T.T.; Monteleone, G. Interleukin-21 in cancer immunotherapy: Friend or foe? OncoImmunology 2012, 1, 351–354. [Google Scholar] [CrossRef]
- Neurath, M.F. IL-23 in inflammatory bowel diseases and colon cancer. Cytokine Growth Factor Rev. 2018, 45, 1–8. [Google Scholar] [CrossRef]
- Kienzl, M.; Hasenoehrl, C.; Valadez-Cosmes, P.; Maitz, K.; Sarsembayeva, A.; Sturm, E.; Heinemann, A.; Kargl, J.; Schicho, R. IL-33 reduces tumor growth in models of colorectal cancer with the help of eosinophils. OncoImmunology 2020, 9, 1776059. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Yang, M.; Wang, Q. Interleukin-33 in tumorigenesis, tumor immune evasion, and cancer immunotherapy. J. Mol. Med. 2016, 94, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Fasano, M.; Pirozzi, M.; Miceli, C.C.; Cocule, M.; Caraglia, M.; Boccellino, M.; Vitale, P.; De Falco, V.; Farese, S.; Zotta, A.; et al. TGF-β Modulated Pathways in Colorectal Cancer: New Potential Therapeutic Opportunities. Int. J. Mol. Sci. 2024, 25, 7400. [Google Scholar] [CrossRef] [PubMed]
- Bakrim, S.; El Hachlafi, N.; Khalid, A.; Abdalla, A.N.; El Omari, N.; Aboulaghras, S.; Sakran, A.M.; Goh, K.W.; Ming, L.C.; Razi, P.; et al. Recent advances and molecular mechanisms of TGF-β signaling in colorectal cancer, with focus on bioactive compounds targeting. Biomed. Pharmacother. 2024, 177, 116886. [Google Scholar] [CrossRef]
- Sheng, J.; Sun, H.; Yu, F.-B.; Li, B.; Zhang, Y.; Zhu, Y.-T. The Role of Cyclooxygenase-2 in Colorectal Cancer. Int. J. Med. Sci. 2020, 17, 1095–1101. [Google Scholar] [CrossRef]
- Albasri, A.M.; Elkablawy, M.A.; Hussainy, A.S.; Yousif, H.M.; Alhujaily, A.S. Impact of cyclooxygenase-2 over-expression on the prognosis of colorectal cancer patients. An experience from Western Saudi Arabia. Saudi Med. J. 2018, 39, 773–780. [Google Scholar] [CrossRef]
- Chen, X.; Chen, L.-J.; Peng, X.-F.; Deng, L.; Wang, Y.; Li, J.-J.; Guo, D.-L.; Niu, X.-H. Anti-PD-1/PD-L1 therapy for colorectal cancer: Clinical implications and future considerations. Transl. Oncol. 2023, 40, 101851. [Google Scholar] [CrossRef]
- Rosenbaum, M.W.; Bledsoe, J.R.; Morales-Oyarvide, V.; Huynh, T.G.; Mino-Kenudson, M. PD-L1 expression in colorectal cancer is associated with microsatellite instability, BRAF mutation, medullary morphology and cytotoxic tumor-infiltrating lymphocytes. Mod. Pathol. 2016, 29, 1104–1112. [Google Scholar] [CrossRef]
- Fu, X.; Luo, H.; Zheng, Y.; Wang, S.; Zhong, Z.; Wang, Y.; Yang, Y. CTLA-4 immunotherapy exposes differences in immune response along with different tumor progression in colorectal cancer. Aging 2020, 12, 15656–15669. [Google Scholar] [CrossRef]
- Metzger, T.C.; Long, H.; Potluri, S.; Pertel, T.; Bailey-Bucktrout, S.L.; Lin, J.C.; Fu, T.; Sharma, P.; Allison, J.P.; Feldman, R.M. ICOS Promotes the Function of CD4+ Effector T Cells during Anti-OX40–Mediated Tumor Rejection. Cancer Res. 2016, 76, 3684–3689. [Google Scholar] [CrossRef]
- Santaolalla, R.; Fukata, M.; Abreu, M.T. Innate immunity in the small intestine. Curr. Opin. Gastroenterol. 2011, 27, 125–131. [Google Scholar] [CrossRef]
- Xing, C.; Du, Y.; Duan, T.; Nim, K.; Chu, J.; Wang, H.Y.; Wang, R.-F. Interaction between microbiota and immunity and its implication in colorectal cancer. Front. Immunol. 2022, 13, 963819. [Google Scholar] [CrossRef] [PubMed]

| Author | Selection | Comparability | Outcome/ Exposure | Total Score |
|---|---|---|---|---|
| Immune cell-related studies | ||||
| Banner et al. (1993) [50] | *** | * | ** | 6 |
| Yuan et al. (2008) [51] | *** | * | ** | 6 |
| Roncucci et al. (2008) [52] | **** | * | ** | 7 |
| Cui et al. (2009) [53] | *** | ** | * | 6 |
| Mclean al. (2011) [54] | **** | * | ** | 7 |
| Mariani et al. (2013) [55] | *** | * | ** | 6 |
| Jang et al. (2013) [56] | **** | * | ** | 7 |
| Hua et al. (2016) [57] | *** | * | ** | 6 |
| Maglietta et al. (2016) [58] | *** | * | ** | 6 |
| Zhu et al. (2016) [59] | *** | * | * | 5 |
| Cui et al. (2017) [60] | *** | * | ** | 6 |
| Garcia et al. (2020) [61] | ** | * | ** | 5 |
| Chen et al. (2021) [62] | **** | * | ** | 7 |
| Omran et al. (2024) [63] | *** | ** | ** | 7 |
| Cytokine- and other TiME-component-related studies | ||||
| Adegboyega et al. (2004) [64] | **** | * | ** | 7 |
| Cui et al. (2007) [65] | *** | * | ** | 6 |
| Cui et al. (2009) [66] | *** | * | ** | 6 |
| Cui et al. (2012) [67] | **** | * | ** | 7 |
| Wang et al. (2012) [68] | *** | ** | ** | 7 |
| Cui et al. (2015) [69] | **** | * | ** | 7 |
| Xie et al. (2015) [70] | *** | * | ** | 6 |
| Cui et al. (2017) [71] | **** | * | ** | 7 |
| Cui et al. (2020) [72] | **** | * | ** | 7 |
| Cui et al. (2021) [73] | *** | * | ** | 6 |
| Youssef et al. (2021) [74] | **** | * | ** | 7 |
| Cui et al. (2022) [75] | *** | * | ** | 6 |
| Zhang et al. (2023) [76] | **** | * | ** | 7 |
| Relevant studies without a control group | ||||
| Moezzi et al. (2000) [77] | ** | - | *** | 5 |
| Kiziltaş et al. (2008) [78] | ** | - | *** | 5 |
| Freitas et al. (2021) [79] | ** | - | *** | 5 |
| Shams et al. (2021) [80] | ** | - | *** | 5 |
| Wallace et al. (2021) [81] | ** | - | *** | 5 |
| Wallace et al. (2021) [82] | ** | - | *** | 5 |
| Zhang et al. (2021) [83] | ** | - | *** | 5 |
| Author (Publish Date) | NOS ≥5/9 | Study Group Size (n) | Control Group Size (n) | Type of Cell Marker/Cytokine/Other Component | Detection Method | Clinical Evidence (Association with A, and/or CRC) |
|---|---|---|---|---|---|---|
| Human studies focused on tumor-infiltrating immune cell examination in conventional CRA vs. HC/NM (and CRC) | ||||||
| Banner et al. (1993) [50] | 6/9 | HP: 16, A: tubular: 21, tubulovillous: 19, villous: 12, CRC: 17 | NM: 27 | UCHL-1+, L26+, IgG+, IgA+, S-100+, HLA-DR+, KP+, S-100+ | IHC | The diffuse antigen-presenting system and activated immune response are shown in CRA and CRC vs. NM, represented by the expansion and reorganization of the T and B cell compartments and the TAM-cell system. |
| Yuan et al. (2008) [51] | 6/9 | A: 33 CRC: 23 | HC: 19 | mDCs (CD83+, CD208+); iDCs (CD1alpha+); COX-2, PGE2, receptors EP2/EP4 | IHC, qRT-PCR, double IF, Colocalization Analysis | Altered DC infiltration along the ACS. Gradually increased COX-2 expression might contribute to the DC functional defect. |
| Roncucci et al. (2008) [52] | 7/9 | I: 65: A/CRC: 35, IBD: 8; II: 24 aberrant crypt foci: HP: 14, A: 16, CRC: 67 | HC: 22 | MPO+ cells | IHC | MPO+ cells (neutrophils and monocytes) gradually increase along the ACS, a potential marker of CRC risk. MSI seems to influence host immune responses to CRC. |
| Cui et al. (2009) [53] | 6/9 | A: 41, CRC: 25 | HC: 15 | Myofibroblasts, Lymphocytes, TAMs, COX-2 | IHC, double IHC | Progressive cellular (lymphocytes, myofibroblasts, and COX-2) responses in the lamina propria could be involved in the ACS. |
| McLean et al. (2011) [54] | 7/9 | I: A: 65 II: A: LGD: 40, HGD: 40, Invasive CRC: 40 | I: paired adjacent NM: 36; II: NM | CD3+, CD4+, CD8+, CD20+, CD25+, CD56+, CD68+; CXCL1, CXCL2, CXCL3, CCL20, IL8, CL23, CL19, CCL21, CCL5 | IHC, RT-PCR | A phenotypic and genotypic ‘switch’ occurs early in the ACS, with the expression of inflammatory cytokines and chemokines dysregulated in the transition from NM → A, rather than from A → CRC. |
| Mariani et al. (2013) [55] | 6/9 | MA (LGD): 30 (11 patients), CRC 20 (60 samples) | HC: 20 (60 samples) | ThPOK+, CD4+, CD8+, CD56+, GZMB, RUNX3, FOXP3+ | WBA, IF, qRT-PCR, Colocalization analysis | ThPOK may be considered a central regulator of the earliest events in the immune system during CRC development, decreasing the immune response against cancer cells. |
| Jang et al. (2013) [56] | 7/9 | HP: 15, A: LGD: 22, HGD: 27, Intramucosal CRC: 10, Invasive CRC: 32 (T2: 5; T3: 27) | Non-neoplastic mucosa: 17; Adjacent NM: 32 | CD8+, FOXP3+, CD8+/Tregs ratio, COX-2, E-cadherin | IHC | Alteration in the distribution of both CD8+T cells (↓) and Tregs (↑) may generate an immune environment suitable for the development and progression of CRC. |
| Hua et al. (2016) [57] | 6/9 | A: 36 (♂: 26, ♀: 10; avg age: 65 yrs.); CRC: 30 (♂: 18, ♀: 12; avg age: 55.8 yrs.) | HC: 12 (♂: 7, ♀: 5) | FOXP3+, IL-10 | IHC, RT-PCR | Expansion of regulatory T cells is an early event in ACS, presumably playing a role in regulating host immune response to the initiation of CRC. |
| Maglietta et al. (2016) [58] | 6/9 | I: 42: polypoid: 17, nonpolypoid: 25; II: 40: polypoid: 19, nonpolypoid: 21 | I: 42 matched NM; II: None. | CD4+, CD8+, FOXP3+, MHC-I+, CD68+, CD163+ | I: GSEA, II: IHC | The density of CD8+, FOXP3+, CD68+, CD163+, and MHC-I+ increases in the stroma of polypoid precancerous vs. nonpolypoid lesions. Large neoplasms have more immune cells in their stroma than small lesions. CD4+ increases along the conventional ACS in large polypoid vs. nonpolypoid lesions. |
| Zhu et al. (2016) [59] | 5/9 | A: 22, CRC: 48 | NM: 21 | CD4+, CD25+, FOXP3+, IL-10, Stat3 | IHC | CD4+CD25+Foxp3+ Tregs inhibit tumor immunity in combination with IL-10 and Stat3. Expression of all three increases with CRC progression. |
| Cui et al. (2017) [60] | 6/9 | A: 30, CRC: 30 | HC: 12 | CD133+, LGR5+, ALDH1+, Musashi (Msi)+ | IHC, double IHC | Changed temporal and spatial presentation of stem-like markers in different stages of human ACS. |
| Garcia et al. (2020) [61] | 5/9 | A: LGD: 58; HGD: 18 | NM; FAP | CD3+, CD4+, CD8+, CD57+, CD68+, FOXP3. | IHC | Sporadic A contains a higher number of Treg cells, which suggests stronger immune selective pressure, while hereditary lesions have fewer immune infiltrates and seem to benefit from a more tolerant TiME. |
| Chen et al. (2021) [62] | 7/9 | Pre-cancer set: 62 (diverse sex, racial, and age groups): - Conventional A (tubular/tubulovillous), - Serrated polyps (SER) (HP/SSL); Cancer set: 93 | NM: 66 | CD4+, CD8+, CD68+, FOXP3+; Hypermutational status, WNT and serrated pathway activation genes | Multi-assay analysis: scRNA-seq, Whole Exome-seq, MxIF or MxIHC | Most immune cell types were increased in polyps vs. NM. Divergent tumor immune landscapes (in terms of immune cell densities, distribution in epithelial and stromal compartments, and spatial distribution in the glandular crypt and gene expression) in conventional vs. serrated CR lesions were established. |
| Omran et al. (2024) [63] | 7/9 | AA: 25, CRC: 25 | HC: 19; adjacent NM | Expression of 579 immune genes genes (coding cells, cytokines, chemokines, receptors, etc.) | RT-qPCR | Early involvement in carcinogenesis (↑ TAMs, ↓ monocytes, ↑ activated mast cells, ↓ plasma B cells). A distinctive immunological signature in colorectal neoplasia, highlighting CXCL1, CXCL2, IL1B, IL6, CXCL8, PTGS2, and SPP1 as potential CRC biomarkers. |
| Human studies examining cytokine- and other TiME-component-related immune alterations in conventional CRA vs. HC/NM (and CRC) | ||||||
| Adegboyega et al. (2004) [64] | 7/9 | HP: 43, sporadic A: 67, CRC: 39 | NM: 50 | SMA+, COX-2 | IHC | Increased COX-2 expression (specifically to subepithelial intestinal myofibroblasts) is common in sporadic CRA, suggesting that myofibroblasts are important target cells for NSAID-mediated chemoprevention of CRC. |
| Cui et al. (2007) [65] | 6/9 | A: 32, CRC: 20 | HC: 18 | IL-4, IL-10, TNF-α, IFN-γ, IL-12A, IL-18 | Q-PCR, IHC | Distinct changes in the Th1 cytokine profile (slightly increased in CRA and a remarkably decreased in CRC) may reflect a change in the host immune regulatory function along the ACS. |
| Cui et al. (2009) [66] | 6/9 | A: 53, CRC: 44 | HC: 18 | IL-8, receptors IL-8RA and IL-8RB | Q-PCR, IHC, double IHC | The activated IL-8 network in the TiME may function as a significant regulatory factor for the progression of A and AC transition. |
| Cui et al. (2012) [67] | 7/9 | A: 50, CRC: 50 | HC: 15 | IL-17A, Th17 | qRT-PCR, s-q IHC | IL-17A and TH17 are highly activated throughout the CR ACS. |
| Wang et al. (2012) [68] | 7/9 | A: 31, CRC: 35 | HC: 24; NM; tumor tissues ex vivo | IL-17A, Th17, anti-CD3, anti-CD28, IL-1β, IL-6, TGF-β, IL-21, IL-23 | Flow cytometry, ELISA | A unique change of Th17 cells (↑), regulated by IL-1β, IL-6, and TGF-β in the progression of CRC. |
| Cui et al. (2015) [69] | 7/9 | A: 50; CRC: 50 | HC: 30 | IL-33, ST2 | qRT-PCR, IHC | Elevated IL-33/ST2 axis expression along CR ACS might be involved in the neoplastic transformation via participation in the regulation of angiogenesis. |
| Xie et al. (2015) [70] | 6/9 | A: 8, CRC: 17, UC: 10 | NM: 16 | IL-17(R)A, ERK, VEGF(R), MMP9, MMP7, MMP2, Bcl-2, cyclin D1, BAX | ELISA, WBA, IHC | IL-17(↑) and its signaling pathways appear as promising new targets in the design and development of drugs for CRC prevention and treatment. |
| Cui et al. (2017) [71] | 7/9 | A: 50, CRC: 50 | HC: 18 | IL-21 | qRT-PCR, Double IF | Increased IL-21 expression within the A/CRC TiME might have a potential predicting significance for survival time in patients with CRC. |
| Cui et al. (2020) [72] | 7/9 | A: 50, CRC: 50 | HC: 30 | IL-33, ST2, FOXP3+ | qRT-PCR, IHC, Double IF | Increased densities of ST2-positive cells relate to Treg accumulation within the A/CRC TiME, suggesting the IL-33/ST2 pathway as a potential contributor to immunosuppressive milieu formation along the ACS. |
| Cui et al. (2021) [73] | 6/9 | A: 50, CRC: 45 | HC: 15 | IL-17A, Ki67, Myofibroblasts, CD146+ | qRT-PCR, IHC, Double IF | The activated TH17/IL-17A network in the TiME is significantly associated with dynamic stromal cellular response throughout the ACS, which might provide a supportive environment for the initiation and progression of CRC. |
| Youssef et al. (2021) [74] | 7/9 | A: 29: LGD: 15, HGD: 14, CRC: 78 | NM: 12 | IL-8, TSP50, SERCA2 | IHC | Increasing expression of IL-8, TSP50, and SERCA2 along ACS is associated with adverse prognostic factors through facilitating several hallmarks of cancer and could be considered independent prognostic factors. |
| Cui et al. (2022) [75] | 6/9 | A: 40, CRC: 37 | HC: 21 | IL-8, IL-1β, capacity of IL-1β to stimulate epithelial IL-8 | q-PCR, IHC, double IF; ELISA | Activation of the IL-8 network in the niche of CSCs from the precancerous A stage to the CRC stage, which IL-1β potentially stimulates. |
| Zhang et al. (2023) [76] | 7/9 | HP: 30, A: LGD: 44, HGD: 29, CRC: 28. | HC: 29 | CD4+, FOXP3+ TILs, and PD-1/PD-L1 immune checkpoints; ICOS, ICOSLG expression | IHC, multiple-IHC | Increased ICOS/ICOSLG expression may be associated with the progressive formation of FOXP3+TILs in the TiME and may further promote the development from precancerous neoplasia to CRC. Elevated co-expression of PD-1+ICOS+ or PD-1+ICOSLG+ contributes to the active TiME along the ACS. |
| Relevant human studies examining various immune infiltration patterns in CRA (and CRC along ACS), though lacking a control group | ||||||
| Moezzi et al. (2000) [77] | 5/9 | HP: 65, A: 313, early CRC in A: 15, CRC: 95 | None | TE%/all immune cells in the stroma | H&E, IHC | There is an inverse relationship between CRC’s invasiveness and stromal eosinophilia’s intensity. |
| Kiziltaş et al. (2008) [78] | 5/9 | HP: 96, SA: 50, A: 257, CRC: 45 | None | TE%/all immune cells in the stroma | H&E, IHC | The intensity of TE is most prominent in A, including serrated adenomas, and is diminished along the ACS. |
| Freitas et al. (2021) [79] | 5/9 | -Sporadic: A: 60: LGD: 30, HGD: 30; CRC: 14; -FAP: 59: LGD: 30, HGD: 22; CRC: 7. | None | CD3+, CD4+, CD8+, FOXP3+, CD57+: TMB; MHC-I expression; PD-L1 expression | H&E, IHC, qPCR | The CR ACS is characterized by a progressive loss of adaptive immune infiltrate and by establishing a progressively immune cold microenvironment. It seems it is unrelated to the loss of tumor cells’ immunogenicity or the onset of an immunosuppressive TiME. |
| Shams et al. (2021) [80] | 5/9 | A: 22, CRC: 103 | Non-neoplastic mucosa: 21 | PD-L1+, CTLA-4+ | 16S | PD-L1+ and CTLA-4+ expression by tumor cells could cooperate in enhancing the progression of CRC, which could lead to poor patient prognosis. Conversely, their expression by TILs could stand against tumor progression. |
| Wallace et al. (2021) [81] | 5/9 | A: tubular: 21, tubulovillous: 37, villous: 36, serrated lesion: 7 | None | CD117+, CD4+/RORC, MICA/B, IL6, IL17A, IFN-γ | IF | Proximal CR lesions were rich in immune infiltrates. The diminishing immune response with increasing villous histology suggests suppressive TiME. |
| Wallace et al. (2021) [82] | 5/9 | Caucasian Americans A (CaAs): 48, African Americans A (AaAs): 47 | None | CD117+, CD4+/RORC, MICA/B, IL6, IL17A, IFN-γ | IF | Decreased immune responses in AaAs vs. CaAs may indicate impaired immune surveillance in early carcinogenesis. Proximal As are more common in AaAs. |
| Zhang et al. (2021) [83] | 5/9 | HP: 30, A: LGD: 44, HGD: 29, CRC: 50 | None | Mutations of 10 genes: XRCC1, TP53, MLH1, MSH, KRAS, GSTP, UMP, THF, DPYD, ABCC2. | IHC, Transcriptome RNA | Increased IEL density and PD-1/PD-L1 expression correlate with cytological dysplasia progression, specifically with the XRCC1 mutation status in CRC, supporting a stepwise ACS and an XRCC1 hypermutated phenotypic mechanism of CR lesions. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silinskaite, U.; Valciukiene, J.; Jakubauskas, M.; Poskus, T. The Immune Environment in Colorectal Adenoma: A Systematic Review. Biomedicines 2025, 13, 699. https://doi.org/10.3390/biomedicines13030699
Silinskaite U, Valciukiene J, Jakubauskas M, Poskus T. The Immune Environment in Colorectal Adenoma: A Systematic Review. Biomedicines. 2025; 13(3):699. https://doi.org/10.3390/biomedicines13030699
Chicago/Turabian StyleSilinskaite, Ugne, Jurate Valciukiene, Matas Jakubauskas, and Tomas Poskus. 2025. "The Immune Environment in Colorectal Adenoma: A Systematic Review" Biomedicines 13, no. 3: 699. https://doi.org/10.3390/biomedicines13030699
APA StyleSilinskaite, U., Valciukiene, J., Jakubauskas, M., & Poskus, T. (2025). The Immune Environment in Colorectal Adenoma: A Systematic Review. Biomedicines, 13(3), 699. https://doi.org/10.3390/biomedicines13030699






