Deciphering Pain and Pruritus in Keloids from the Perspective of Neurological Dysfunction: Where Are We Now?
Abstract
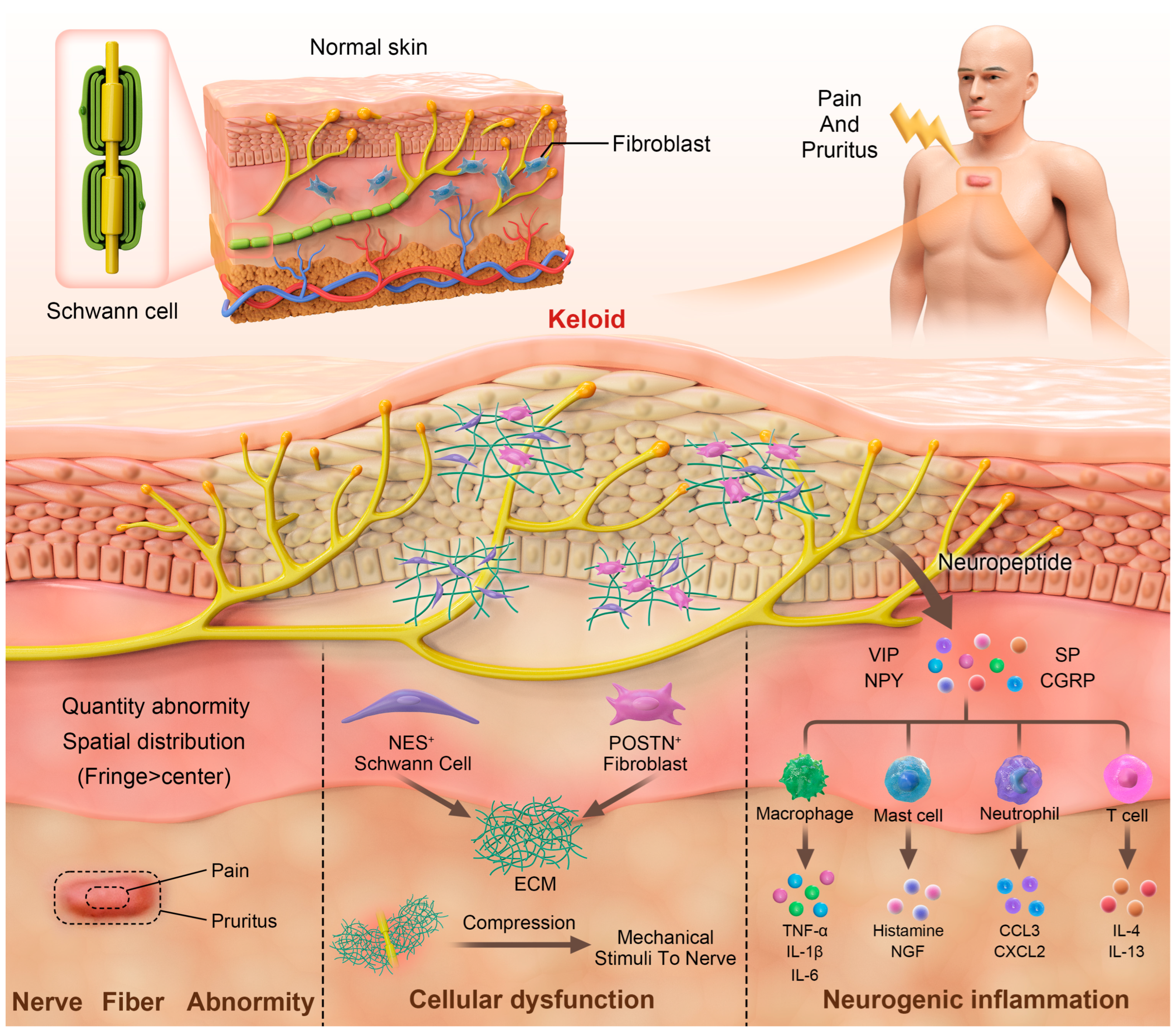
1. Introduction
2. Neuroanatomical Alterations and Dysfunction of Sensory Nerves
3. Neurogenic Inflammation
4. Dysfunction of Schwann Cells and Fibroblasts
4.1. Dysfunction of Schwann Cells During Keloid Formation
4.2. Overactivation of Fibroblasts and Excessive Collagen Deposition
5. Summary and Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Andrews, J.P.; Marttala, J.; Macarak, E.; Rosenbloom, J.; Uitto, J. Keloids: The paradigm of skin fibrosis—Pathomechanisms and treatment. Matrix Biol. 2016, 51, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Kim, Y.H. Comprehensive Insights into Keloid Pathogenesis and Advanced Therapeutic Strategies. Int. J. Mol. Sci. 2024, 25, 8776. [Google Scholar] [CrossRef]
- Feng, C.; Shan, M.; Xia, Y.; Zheng, Z.; He, K.; Wei, Y.; Song, K.; Meng, T.; Liu, H.; Hao, Y.; et al. Single-cell RNA sequencing reveals distinct immunology profiles in human keloid. Front. Immunol. 2022, 13, 940645. [Google Scholar] [CrossRef]
- Liu, X.; Chen, W.; Zeng, Q.; Ma, B.; Li, Z.; Meng, T.; Chen, J.; Yu, N.; Zhou, Z.; Long, X.; et al. Single-Cell RNA-Sequencing Reveals Lineage-Specific Regulatory Changes of Fibroblasts and Vascular Endothelial Cells in Keloids. J. Investig. Dermatol. 2022, 142, 124–135.e11. [Google Scholar] [CrossRef]
- Direder, M.; Weiss, T.; Copic, D.; Vorstandlechner, V.; Laggner, M.; Pfisterer, K.; Mildner, C.S.; Klas, K.; Bormann, D.; Haslik, W.; et al. Schwann cells contribute to keloid formation. Matrix Biol. 2022, 108, 55–76. [Google Scholar] [CrossRef] [PubMed]
- Bijlard, E.; Kouwenberg, C.A.; Timman, R.; Hovius, S.E.; Busschbach, J.J.; Mureau, M.A. Burden of Keloid Disease: A Cross-sectional Health-related Quality of Life Assessment. Acta Derm. Venereol. 2017, 97, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Kassi, K.; Kouame, K.; Kouassi, A.; Allou, A.; Kouassi, I.; Kourouma, S.; Ecra, E.; Sangare, A. Quality of life in black African patients with keloid scars. Dermatol. Rep. 2020, 12, 8312. [Google Scholar] [CrossRef]
- Smith, C.J.; Smith, J.C.; Finn, M.C. The possible role of mast cells (allergy) in the production of keloid and hypertrophic scarring. J. Burn. Care Rehabil. 1987, 8, 126–131. [Google Scholar] [CrossRef]
- Bagabir, R.; Byers, R.J.; Chaudhry, I.H.; Muller, W.; Paus, R.; Bayat, A. Site-specific immunophenotyping of keloid disease demonstrates immune upregulation and the presence of lymphoid aggregates. Br. J. Dermatol. 2012, 167, 1053–1066. [Google Scholar] [CrossRef]
- Maeda, D.; Kubo, T.; Kiya, K.; Kawai, K.; Matsuzaki, S.; Kobayashi, D.; Fujiwara, T.; Katayama, T.; Hosokawa, K. Periostin is induced by IL-4/IL-13 in dermal fibroblasts and promotes RhoA/ROCK pathway-mediated TGF-beta1 secretion in abnormal scar formation. J. Plast. Surg. Hand Surg. 2019, 53, 288–294. [Google Scholar] [CrossRef]
- Akaishi, S.; Ogawa, R.; Hyakusoku, H. Keloid and hypertrophic scar: Neurogenic inflammation hypotheses. Med. Hypotheses 2008, 71, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, R. Keloid and Hypertrophic Scars Are the Result of Chronic Inflammation in the Reticular Dermis. Int. J. Mol. Sci. 2017, 18, 606. [Google Scholar] [CrossRef]
- Huu, N.D.; Huu, S.N.; Thi, X.L.; Van, T.N.; Minh, P.P.T.; Minh, T.T.; Van, T.H.; Cam, V.T.; Huyen, M.L.; Hau, K.T.; et al. Successful Treatment of Intralesional Triamcilonon Acetonide Injection in Keloid Patients. Open Access Maced. J. Med. Sci. 2019, 7, 275–278. [Google Scholar]
- Choi, J.E.; Di Nardo, A. Skin neurogenic inflammation. Semin. Immunopathol. 2018, 40, 249–259. [Google Scholar] [CrossRef]
- Chen, Z.F. A neuropeptide code for itch. Nat. Rev. Neurosci. 2021, 22, 758–776. [Google Scholar] [CrossRef] [PubMed]
- Steinhoff, M.; Oaklander, A.L.; Szabo, I.L.; Stander, S.; Schmelz, M. Neuropathic itch. Pain 2019, 160 (Suppl. S1), S11–S16. [Google Scholar] [CrossRef] [PubMed]
- Sutaria, N.; Adawi, W.; Goldberg, R.; Roh, Y.S.; Choi, J.; Kwatra, S.G. Itch: Pathogenesis and treatment. J. Am. Acad. Dermatol. 2022, 86, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.P.; Derichs, L.; Meyer Zu Horste, G.; Agelopoulos, K.; Stander, S. Generalized chronic itch induced by small-fibre neuropathy: Clinical profile and proposed diagnostic criteria. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 1795–1802. [Google Scholar] [CrossRef]
- Terkelsen, A.J.; Karlsson, P.; Lauria, G.; Freeman, R.; Finnerup, N.B.; Jensen, T.S. The diagnostic challenge of small fibre neuropathy: Clinical presentations, evaluations, and causes. Lancet Neurol. 2017, 16, 934–944. [Google Scholar] [CrossRef]
- Hochman, B.; Nahas, F.X.; Sobral, C.S.; Arias, V.; Locali, R.F.; Juliano, Y.; Ferreira, L.M. Nerve fibres: A possible role in keloid pathogenesis. Br. J. Dermatol. 2008, 158, 651–652. [Google Scholar] [CrossRef]
- Tey, H.L.; Maddison, B.; Wang, H.; Ishiju, Y.; McMichael, A.; Marks, M.; Willford, P.; Maruzivab, D.; Ferdinando, D.; Dick, J.; et al. Cutaneous innervation and itch in keloids. Acta Derm. Venereol. 2012, 92, 529–531. [Google Scholar] [CrossRef]
- Saffari, T.M.; Bijlard, E.; Van Bodegraven, E.A.M.; Mureau, M.A.M.; Hovius, S.E.R.; Huygen, F. Sensory perception and nerve fibre innervation in patients with keloid scars: An investigative study. Eur. J. Dermatol. 2018, 28, 828–829. [Google Scholar] [CrossRef] [PubMed]
- Eckert, R.L.; Broome, A.M.; Ruse, M.; Robinson, N.; Ryan, D.; Lee, K. S100 proteins in the epidermis. J. Investig. Dermatol. 2004, 123, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.C.; Hsueh, H.W.; Kan, H.W.; Liao, C.H.; Jiang, H.H.; Chiang, H.; Lin, W.M.; Yeh, T.Y.; Lin, Y.H.; Cheng, Y.Y.; et al. Skin nerve pathology: Biomarkers of premanifest and manifest amyloid neuropathy. Ann. Neurol. 2019, 85, 560–573. [Google Scholar] [CrossRef]
- Nadal-Nicolas, F.M.; Galindo-Romero, C.; Lucas-Ruiz, F.; Marsh-Amstrong, N.; Li, W.; Vidal-Sanz, M.; Agudo-Barriuso, M. Pan-retinal ganglion cell markers in mice, rats, and rhesus macaques. Zool. Res. 2023, 44, 226–248. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Han, M.; Lou, F.; Sun, Y.; Yin, Q.; Sun, L.; Wang, Z.; Li, X.; Zhou, H.; Xu, Z.; et al. Tenascin C(+) papillary fibroblasts facilitate neuro-immune interaction in a mouse model of psoriasis. Nat. Commun. 2023, 14, 2004. [Google Scholar] [CrossRef]
- Lee, S.S.; Yosipovitch, G.; Chan, Y.H.; Goh, C.L. Pruritus, pain, and small nerve fiber function in keloids: A controlled study. J. Am. Acad. Dermatol. 2004, 51, 1002–1006. [Google Scholar] [CrossRef]
- Limandjaja, G.C.; Niessen, F.B.; Scheper, R.J.; Gibbs, S. The Keloid Disorder: Heterogeneity, Histopathology, Mechanisms and Models. Front. Cell Dev. Biol. 2020, 8, 360. [Google Scholar] [CrossRef]
- Ogawa, R.; Akita, S.; Akaishi, S.; Aramaki-Hattori, N.; Dohi, T.; Hayashi, T.; Kishi, K.; Kono, T.; Matsumura, H.; Muneuchi, G.; et al. Diagnosis and Treatment of Keloids and Hypertrophic Scars-Japan Scar Workshop Consensus Document 2018. Burn. Trauma 2019, 7, 39. [Google Scholar] [CrossRef]
- Lee, Y.I.; Yang, Y.; Ham, S.; Shim, J.E.; Lee, S.G.; Lee, S.H.; Kim, T.G.; Lee, W.J.; Kim, D.Y.; Lee, J.H. Heterogeneity in Keloid Scars: Influence of Mechanical Stretching on Keloids Arising from Different Anatomical Sites. J. Investig. Dermatol. 2025, 145, 710–713.e7. [Google Scholar] [CrossRef]
- Yagmur, C.; Akaishi, S.; Ogawa, R.; Guneren, E. Mechanical receptor-related mechanisms in scar management: A review and hypothesis. Plast. Reconstr. Surg. 2010, 126, 426–434. [Google Scholar] [CrossRef] [PubMed]
- Scott, G.D.; Fryer, A.D.; Jacoby, D.B. Quantifying nerve architecture in murine and human airways using three-dimensional computational mapping. Am. J. Respir. Cell Mol. Biol. 2013, 48, 10–16. [Google Scholar] [CrossRef]
- Bikis, C.; Degrugillier, L.; Thalmann, P.; Schulz, G.; Muller, B.; Hieber, S.E.; Kalbermatten, D.F.; Madduri, S. Three-dimensional imaging and analysis of entire peripheral nerves after repair and reconstruction. J. Neurosci. Methods 2018, 295, 37–44. [Google Scholar] [CrossRef]
- Yamazaki, T.; Li, W.; Yang, L.; Li, P.; Cao, H.; Motegi, S.I.; Udey, M.C.; Bernhard, E.; Nakamura, T.; Mukouyama, Y.S. Whole-Mount Adult Ear Skin Imaging Reveals Defective Neuro-Vascular Branching Morphogenesis in Obese and Type 2 Diabetic Mouse Models. Sci. Rep. 2018, 8, 430. [Google Scholar] [CrossRef]
- Goswami, R.; Anastakis, D.J.; Katz, J.; Davis, K.D. A longitudinal study of pain, personality, and brain plasticity following peripheral nerve injury. Pain 2016, 157, 729–739. [Google Scholar] [CrossRef]
- Mucke, M.; Cuhls, H.; Radbruch, L.; Baron, R.; Maier, C.; Tolle, T.; Treede, R.D.; Rolke, R. Quantitative sensory testing (QST). English version. Schmerz 2021, 35 (Suppl. S3), 153–160. [Google Scholar] [CrossRef] [PubMed]
- Pfab, F.; Valet, M.; Sprenger, T.; Huss-Marp, J.; Athanasiadis, G.I.; Baurecht, H.J.; Konstantinow, A.; Zimmer, C.; Behrendt, H.; Ring, J.; et al. Temperature modulated histamine-itch in lesional and nonlesional skin in atopic eczema—A combined psychophysical and neuroimaging study. Allergy 2010, 65, 84–94. [Google Scholar] [CrossRef] [PubMed]
- Chun, K.S.; Kang, Y.J.; Lee, J.Y.; Nguyen, M.; Lee, B.; Lee, R.; Jo, H.H.; Allen, E.; Chen, H.; Kim, J.; et al. A skin-conformable wireless sensor to objectively quantify symptoms of pruritus. Sci. Adv. 2021, 7, eabf9405. [Google Scholar] [CrossRef]
- Gupta, K.; Harvima, I.T. Mast cell-neural interactions contribute to pain and itch. Immunol. Rev. 2018, 282, 168–187. [Google Scholar] [CrossRef]
- Ogawa, R. Mechanobiology of scarring. Wound Repair. Regen. 2011, 19 (Suppl. S1), s2–s9. [Google Scholar] [CrossRef]
- Wallengren, J.; Chen, D.; Sundler, F. Neuropeptide-containing C-fibres and wound healing in rat skin. Neither capsaicin nor peripheral neurotomy affect the rate of healing. Br. J. Dermatol. 1999, 140, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Kwak, I.S.; Choi, Y.H.; Jang, Y.C.; Lee, Y.K. Immunohistochemical analysis of neuropeptides (protein gene product 9.5, substance P and calcitonin gene-related peptide) in hypertrophic burn scar with pain and itching. Burns 2014, 40, 1661–1667. [Google Scholar] [CrossRef]
- Suarez, E.; Syed, F.; Alonso-Rasgado, T.; Bayat, A. Identification of biomarkers involved in differential profiling of hypertrophic and keloid scars versus normal skin. Arch. Dermatol. Res. 2015, 307, 115–133. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Jin, M.; Luo, Y.; Jin, Z.; Pi, L. Integrated bioinformatics analysis of core regulatory elements involved in keloid formation. BMC Med. Genom. 2021, 14, 239. [Google Scholar] [CrossRef]
- Crowe, R.; Parkhouse, N.; Mcgrouther, D.; Burnstock, G. Neuropeptide-containing nerves in painful hypertrophic human scar tissue. Br. J. Dermatol. 1994, 130, 444–452. [Google Scholar] [CrossRef]
- Scott, J.R.; Muangman, P.R.; Tamura, R.N.; Zhu, K.Q.; Liang, Z.; Anthony, J.; Engrav, L.H.; Gibran, N.S. Substance P levels and neutral endopeptidase activity in acute burn wounds and hypertrophic scar. Plast. Reconstr. Surg. 2005, 115, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Aubert, A.; Goeres, J.; Liu, A.; Kao, M.; Richardson, K.C.; Jung, K.; Hinz, B.; Crawford, R.I.; Granville, D.J. Potential implications of granzyme B in keloids and hypertrophic scars through extracellular matrix remodeling and latent TGF-beta activation. Front. Immunol. 2024, 15, 1484462. [Google Scholar]
- Suvas, S. Role of Substance P Neuropeptide in Inflammation, Wound Healing, and Tissue Homeostasis. J. Immunol. 2017, 199, 1543–1552. [Google Scholar] [CrossRef]
- Schlereth, T.; Schukraft, J.; Kramer-Best, H.H.; Geber, C.; Ackermann, T.; Birklein, F. Interaction of calcitonin gene related peptide (CGRP) and substance P (SP) in human skin. Neuropeptides 2016, 59, 57–62. [Google Scholar] [CrossRef]
- Baliu-Pique, M.; Jusek, G.; Holzmann, B. Neuroimmunological communication via CGRP promotes the development of a regulatory phenotype in TLR4-stimulated macrophages. Eur. J. Immunol. 2014, 44, 3708–3716. [Google Scholar] [CrossRef]
- Ding, W.; Stohl, L.L.; Xu, L.; Zhou, X.K.; Manni, M.; Wagner, J.A.; Granstein, R.D. Calcitonin Gene-Related Peptide-Exposed Endothelial Cells Bias Antigen Presentation to CD4+ T Cells toward a Th17 Response. J. Immunol. 2016, 196, 2181–2194. [Google Scholar] [CrossRef] [PubMed]
- Welsh, S.E.; Xiao, C.; Kaden, A.R.; Brzezynski, J.L.; Mohrman, M.A.; Wang, J.; Smieszek, S.P.; Przychodzen, B.; Stander, S.; Polymeropoulos, C.; et al. Neurokinin-1 receptor antagonist tradipitant has mixed effects on itch in atopic dermatitis: Results from EPIONE, a randomized clinical trial. J. Eur. Acad. Dermatol. Venereol. 2021, 35, e338–e340. [Google Scholar] [CrossRef]
- Direder, M.; Wielscher, M.; Weiss, T.; Laggner, M.; Copic, D.; Klas, K.; Bormann, D.; Vorstandlechner, V.; Tschachler, E.; Ankersmit, H.J.; et al. The transcriptional profile of keloidal Schwann cells. Exp. Mol. Med. 2022, 54, 1886–1900. [Google Scholar] [CrossRef] [PubMed]
- Parfejevs, V.; Debbache, J.; Shakhova, O.; Schaefer, S.M.; Glausch, M.; Wegner, M.; Suter, U.; Riekstina, U.; Werner, S.; Sommer, L. Injury-activated glial cells promote wound healing of the adult skin in mice. Nat. Commun. 2018, 9, 236. [Google Scholar] [CrossRef] [PubMed]
- Taveggia, C.; Feltri, M.L. Beyond Wrapping: Canonical and Noncanonical Functions of Schwann Cells. Annu. Rev. Neurosci. 2022, 45, 561–580. [Google Scholar] [CrossRef]
- Abdo, H.; Calvo-Enrique, L.; Lopez, J.M.; Song, J.; Zhang, M.D.; Usoskin, D.; El Manira, A.; Adameyko, I.; Hjerling-Leffler, J.; Ernfors, P. Specialized cutaneous Schwann cells initiate pain sensation. Science 2019, 365, 695–699. [Google Scholar] [CrossRef]
- Hewson, D.W.; Bedforth, N.M.; Hardman, J.G. Peripheral nerve injury arising in anaesthesia practice. Anaesthesia 2018, 73 (Suppl. S1), 51–60. [Google Scholar] [CrossRef]
- Britto, J.A.; Elliot, D. Aggressive keloid scarring of the Caucasian wrist and palm. Br. J. Plast. Surg. 2001, 54, 461–462. [Google Scholar] [CrossRef]
- Russo, B.; Borowczyk, J.; Cacialli, P.; Moguelet, P.; Truchetet, M.E.; Modarressi, A.; Brembilla, N.C.; Bertrand, J.; Boehncke, W.H.; Chizzolini, C. IL-25 participates in keratinocyte-driven dermal matrix turnover and is reduced in systemic sclerosis epidermis. Rheumatology 2022, 61, 4558–4569. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Y.; Jin, H.; Khodeiry, M.M.; Kong, W.; Wang, N.; Lee, J.K.; Lee, R.K. Reactive Fibroblasts in Response to Optic Nerve Crush Injury. Mol. Neurobiol. 2021, 58, 1392–1403. [Google Scholar] [CrossRef]
- Lim, T.K.; Shi, X.Q.; Johnson, J.M.; Rone, M.B.; Antel, J.P.; David, S.; Zhang, J. Peripheral nerve injury induces persistent vascular dysfunction and endoneurial hypoxia, contributing to the genesis of neuropathic pain. J. Neurosci. 2015, 35, 3346–3359. [Google Scholar] [CrossRef]
- Que, J.; Cao, Q.; Sui, T.; Du, S.; Zhang, A.; Kong, D.; Cao, X. Tacrolimus reduces scar formation and promotes sciatic nerve regeneration. Neural Regen. Res. 2012, 7, 2500–2506. [Google Scholar]
- Que, J.; Cao, Q.; Sui, T.; Du, S.; Kong, D.; Cao, X. Effect of FK506 in reducing scar formation by inducing fibroblast apoptosis after sciatic nerve injury in rats. Cell Death Dis. 2013, 4, e526. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Tian, L.; Zhang, Y.; Zhang, X.; Kang, J.; Dong, H.; Huang, N.; Pan, L.; Ning, B. Apigenin inhibits fibrous scar formation after acute spinal cord injury through TGFbeta/SMADs signaling pathway. CNS Neurosci. Ther. 2022, 28, 1883–1894. [Google Scholar] [CrossRef] [PubMed]
- Deng, C.C.; Hu, Y.F.; Zhu, D.H.; Cheng, Q.; Gu, J.J.; Feng, Q.L.; Zhang, L.X.; Xu, Y.P.; Wang, D.; Rong, Z.; et al. Single-cell RNA-seq reveals fibroblast heterogeneity and increased mesenchymal fibroblasts in human fibrotic skin diseases. Nat. Commun. 2021, 12, 3709. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Mishra, S.K.; Olivry, T.; Yosipovitch, G. Periostin, an Emerging Player in Itch Sensation. J. Investig. Dermatol. 2021, 141, 2338–2343. [Google Scholar] [CrossRef]
- Gong, T.; Wang, Y.; Dong, S.; Ma, X.; Du, D.; Zou, C.; Zheng, Q.; Wen, Z. Single-cell RNA-seq reveals the communications between extracellular matrix-related components and Schwann cells contributing to the earlobe keloid formation. Front. Med. 2022, 9, 1000324. [Google Scholar] [CrossRef]
- Shim, J.; Oh, S.J.; Yeo, E.; Park, J.H.; Bae, J.H.; Kim, S.H.; Lee, D.; Lee, J.H. Integrated Analysis of Single-Cell and Spatial Transcriptomics in Keloids: Highlights on Fibrovascular Interactions in Keloid Pathogenesis. J. Investig. Dermatol. 2022, 142, 2128–2139.e11. [Google Scholar] [CrossRef]
- Pan, B.; Shi, Z.J.; Yan, J.Y.; Li, J.H.; Feng, S.Q. Long non-coding RNA NONMMUG014387 promotes Schwann cell proliferation after peripheral nerve injury. Neural Regen. Res. 2017, 12, 2084–2091. [Google Scholar]
- Li, G.; Li, X.; Li, Z.; Luo, X.; Jing, L.; Guo, D.; Guan, K.; Yuan, F.; Pan, B. Sox2ot/miR-9/Cthrc1 Promote Proliferation and Migration of Schwann Cells Following Nerve Injury. Neuroscience 2023, 519, 47–59. [Google Scholar] [CrossRef]
- Devigili, G.; Rinaldo, S.; Lombardi, R.; Cazzato, D.; Marchi, M.; Salvi, E.; Eleopra, R.; Lauria, G. Diagnostic criteria for small fibre neuropathy in clinical practice and research. Brain 2019, 142, 3728–3736. [Google Scholar] [CrossRef] [PubMed]
- van Laarhoven, A.I.M.; Marker, J.B.; Elberling, J.; Yosipovitch, G.; Arendt-Nielsen, L.; Andersen, H.H. Itch sensitization? A systematic review of studies using quantitative sensory testing in patients with chronic itch. Pain 2019, 160, 2661–2678. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.P.; Pogatzki-Zahn, E.; Snels, C.; Vu, T.H.; Üçeyler, N.; Loser, K.; Sommer, C.; Evers, A.W.M.; van Laarhoven, A.I.M.; Agelopoulos, K.; et al. There is no functional small-fibre neuropathy in prurigo nodularis despite neuroanatomical alterations. Exp. Dermatol. 2017, 26, 969–971. [Google Scholar] [CrossRef]
- Schneider, G.; Stumpf, A.; Burgmer, M.; Volmering, L.; Broecker, P.; Ständer, S. Relations between a standardized experimental stressor and cutaneous sensory function in patients with chronic pruritus and healthy controls: An experimental case-control study. J. Eur. Acad. Dermatol. 2018, 32, 2230–2236. [Google Scholar] [CrossRef]
- Uyesugi, B.; Lippincott, B.; Dave, S. Treatment of a painful keloid with botulinum toxin type A. Am. J. Phys. Med. Rehabil. 2010, 89, 153–155. [Google Scholar] [CrossRef]
- Zhang, S.; Li, K.; Yu, Z.; Chai, J.; Zhang, Z.; Zhang, Y.; Min, P. Dramatic Effect of Botulinum Toxin Type A on Hypertrophic Scar: A Promising Therapeutic Drug and Its Mechanism Through the SP-NK1R Pathway in Cutaneous Neurogenic Inflammation. Front. Med. 2022, 9, 820817. [Google Scholar] [CrossRef]
- Scala, J.; Vojvodic, A.; Vojvodic, P.; Vlaskovic-Jovicevic, T.; Peric-Hajzler, Z.; Matovic, D.; Dimitrijevic, S.; Vojvodic, J.; Sijan, G.; Stepic, N.; et al. Botulin Toxin Use in Scars/Keloids Treatment. Open Access Maced. J. Med. Sci. 2019, 7, 2979–2981. [Google Scholar] [CrossRef]
- Stander, S.; Yosipovitch, G. Substance P and neurokinin 1 receptor are new targets for the treatment of chronic pruritus. Br. J. Dermatol. 2019, 181, 932–938. [Google Scholar] [CrossRef] [PubMed]
- Latorre, R.; Ramirez-Garcia, P.D.; Hegron, A.; Grace, J.L.; Retamal, J.S.; Shenoy, P.; Tran, M.; Aurelio, L.; Flynn, B.; Poole, D.P.; et al. Sustained endosomal release of a neurokinin-1 receptor antagonist from nanostars provides long-lasting relief of chronic pain. Biomaterials 2022, 285, 121536. [Google Scholar] [CrossRef]
- Xie, X.; Pascual, C.; Lieu, C.; Oh, S.; Wang, J.; Zou, B.; Xie, J.; Li, Z.; Xie, J.; Yeomans, D.C.; et al. Analgesic Microneedle Patch for Neuropathic Pain Therapy. ACS Nano 2017, 11, 395–406. [Google Scholar] [CrossRef]
| Author | Year | Disease | Neuropeptide | Techniques |
|---|---|---|---|---|
| Kwak I.S. et al. [42] | 2014 | HS | SP ↑ CGRP ↑ | Immunohistochemical staining |
| Suarez E. et al. [43] | 2015 | HS | SP ↑ CGRP ↑ | Scar tissue qRT-PCR |
| CGRP ↑ | Primary fibroblast qRT-PCR | |||
| NPY ↑ | Primary fibroblast Western blotting | |||
| K | CGRP ↑ | Scar tissue qRT-PCR | ||
| SP ↑ CGRP ↑ NPY ↑ | Primary fibroblast qRT-PCR | |||
| SP ↑ CGRP ↑ | Primary fibroblast Western blotting | |||
| Li C. et al. [44] | 2021 | HS | NPY ↑ | GEO data analysis |
| K | ||||
| Crowe R. et al. [45] | 1994 | HS | NPY ↑ VIP ↑ SP ↑ CGRP ↑ | Immunofluorescence |
| Scott JR et al. [46] | 2005 | PS | SP ↑ | Indirect enzyme-linked immunosorbent assay |
| HS | ||||
| Aubert A et al. [47] | 2025 | HS | SP ↑ | Immunostaining |
| K |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, E.; Xu, R.; Zhang, H.; Xia, W.; Huang, X.; Zan, T. Deciphering Pain and Pruritus in Keloids from the Perspective of Neurological Dysfunction: Where Are We Now? Biomedicines 2025, 13, 663. https://doi.org/10.3390/biomedicines13030663
Yang E, Xu R, Zhang H, Xia W, Huang X, Zan T. Deciphering Pain and Pruritus in Keloids from the Perspective of Neurological Dysfunction: Where Are We Now? Biomedicines. 2025; 13(3):663. https://doi.org/10.3390/biomedicines13030663
Chicago/Turabian StyleYang, En, Ruoqing Xu, Hanrui Zhang, Wenzheng Xia, Xin Huang, and Tao Zan. 2025. "Deciphering Pain and Pruritus in Keloids from the Perspective of Neurological Dysfunction: Where Are We Now?" Biomedicines 13, no. 3: 663. https://doi.org/10.3390/biomedicines13030663
APA StyleYang, E., Xu, R., Zhang, H., Xia, W., Huang, X., & Zan, T. (2025). Deciphering Pain and Pruritus in Keloids from the Perspective of Neurological Dysfunction: Where Are We Now? Biomedicines, 13(3), 663. https://doi.org/10.3390/biomedicines13030663






