Lyophilized Extract from the Larvae of the Blowfly Lucilia sericata as a New Strategy for the Management of Chronic Wounds
Abstract
1. Introduction
2. Materials and Methods
2.1. Development of a Lyophilized Maggot Extract
2.2. Application of Lyophilized Maggot Extract to Patients with Chronic Wounds
- Chronic leg ulcers;
- Refractory to treatment for at least three months;
- No underlying consumptive diseases (e.g., malignancies);
- A three-week pretreatment phase with stage-appropriate wound care demonstrated no significant improvement.
- Comprehensive clinical and wound-specific history;
- Standardized photo documentation;
- Wound exudate collection;
- Microbiological sampling;
- Application of L. sericata extract;
- Dressing with sterile wound coverings.
2.3. Patient Demographics and Wound Characteristics
2.4. Exudate Collection from Ulcers
2.5. Bacterial Colonization Analysis
2.6. Effect on Bacterial Biofilms
2.7. Effect on Bacterial Growth
3. Results
3.1. Isolated Bacterial Species and Effects of Maggot Extracts on Bacterial Colonization In Vivo
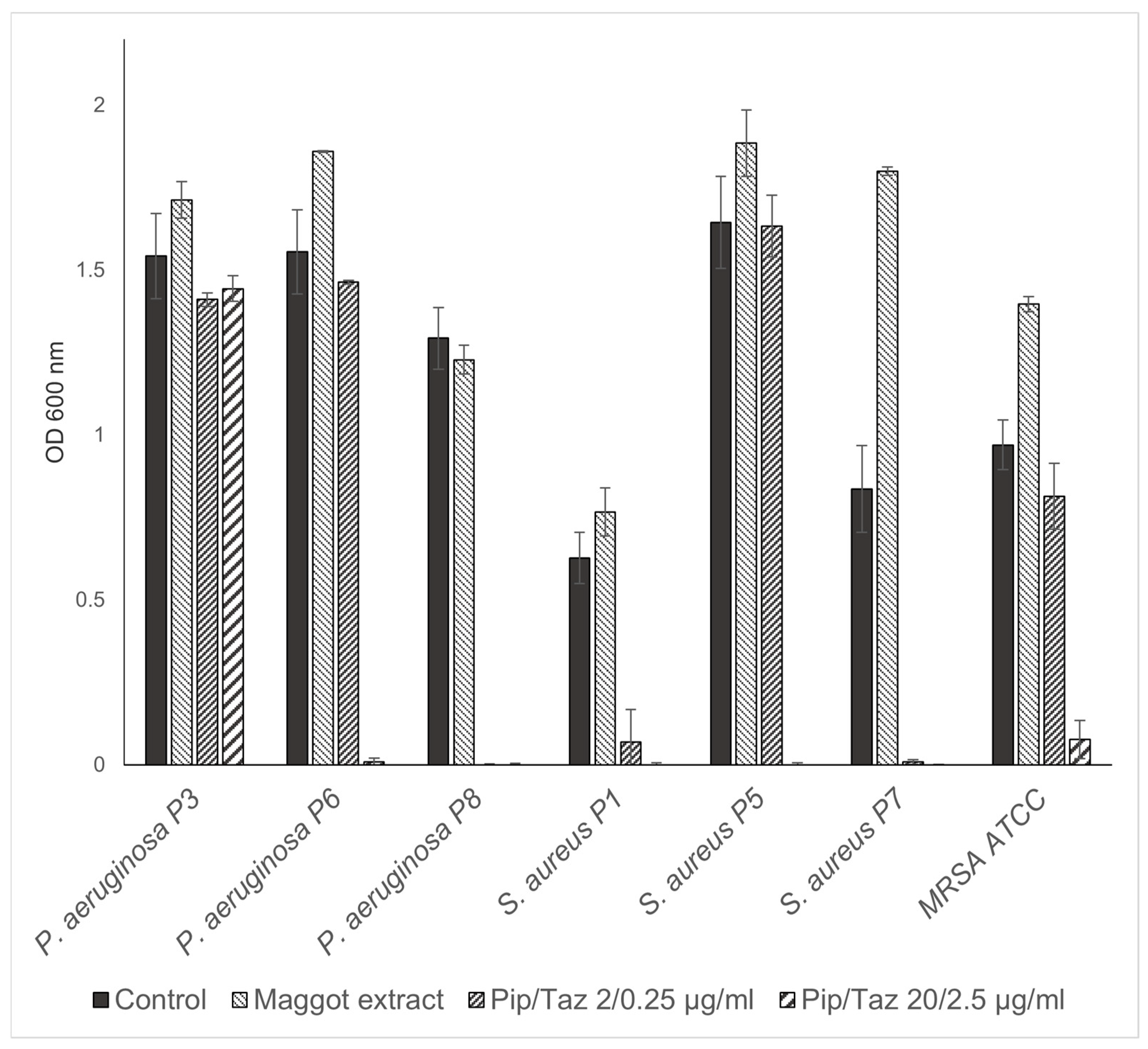
3.2. Effects of Maggot Extracts on Bacterial Growth In Vitro
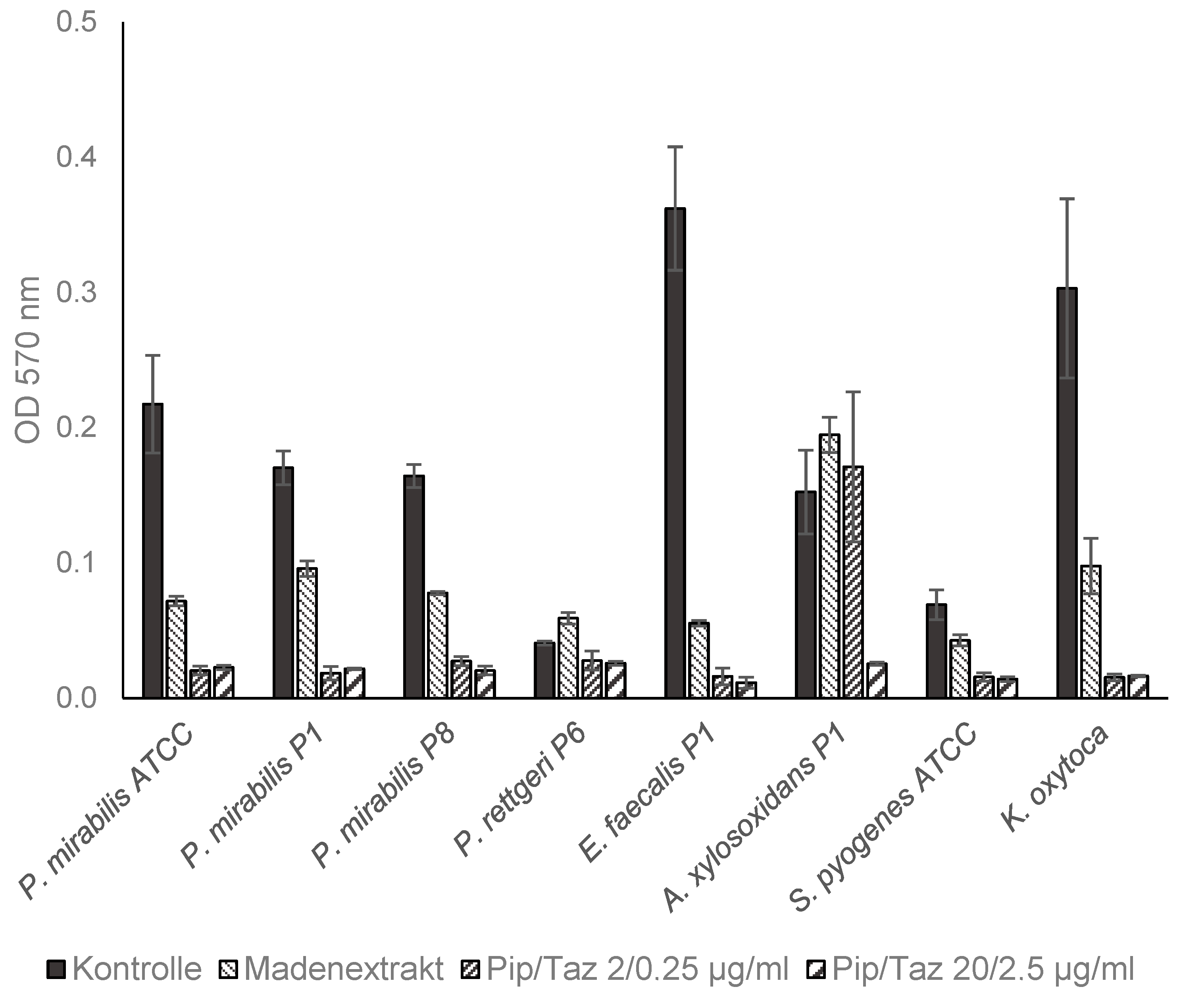
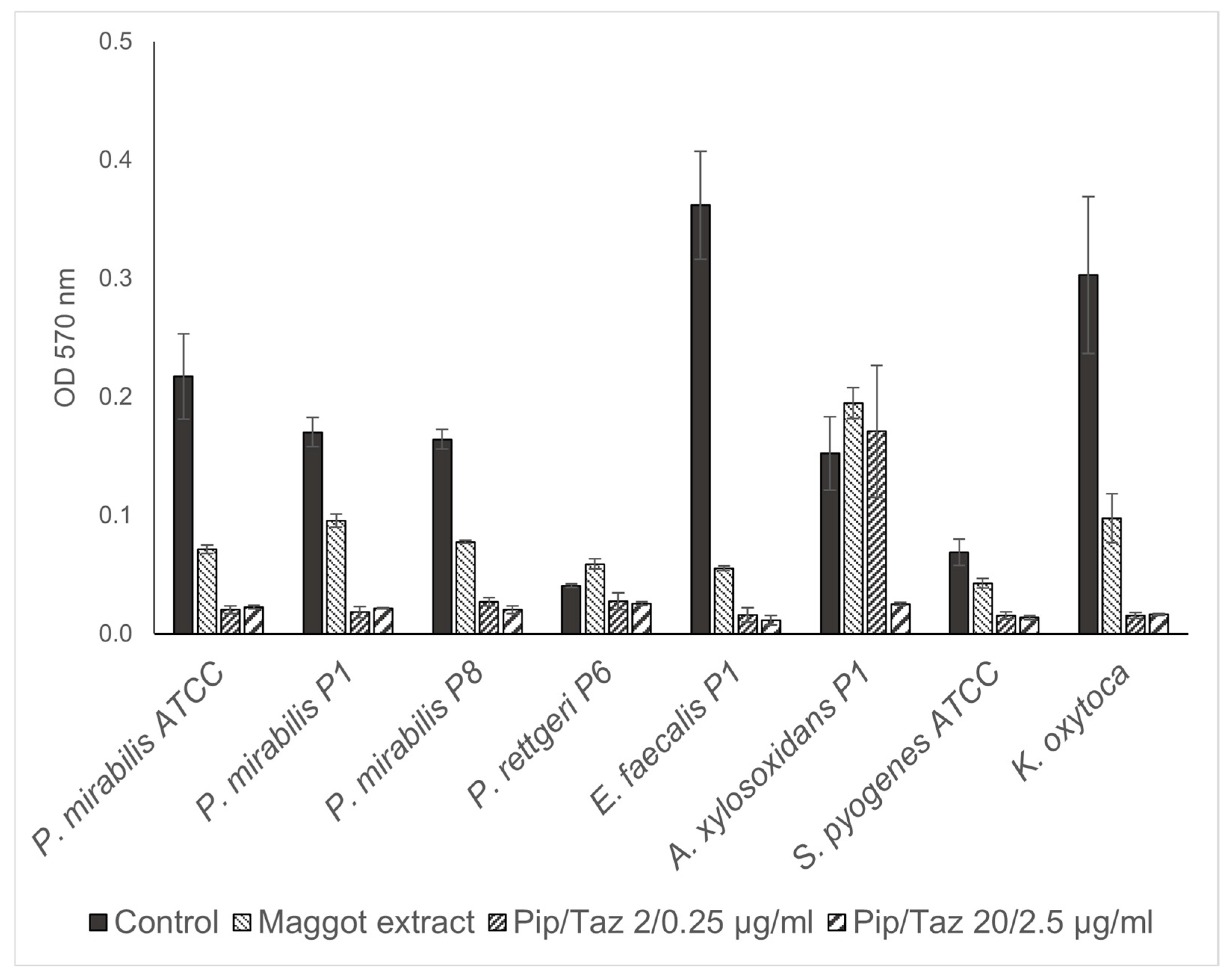
3.3. Effects of Lyophilized Maggot Extracts on Bacterial Biofilm Formation In Vitro
3.4. Clinical Course of Lower Leg Ulcerations During Treatment with Lyophilized Maggot Extract
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| DOAJ | Directory of open-access journals |
| 3MRGNs | Gram-negative rods that are resistant to three of the four antibiotic classes |
| AMP | Antimicrobial peptides |
| ATCC | American-Type Culture Collection |
| Bcl-2 | B-cell lymphoma 2 protein |
| CLSI | Clinical and Laboratory Standards Institute |
| EOT | End of treatment |
| ESs | Excretions/secretions |
| GMP | Good manufacturing practice |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| MSSA | Methicillin-sensitive Staphylococcus aureus |
| OD | Optical density |
| STAT3 | Signal transducer and activator of transcription 3 |
| TSB | Tryptic soy broth |
| VEGF | Vascular endothelial growth factor |
| Pip/Taz | Piperacillin/tazobactam |
References
- Korber, A.; Klode, J.; Al-Benna, S.; Wax, C.; Schadendorf, D.; Steinstraesser, L.; Dissemond, J. Etiology of chronic leg ulcers in 31,619 patients in Germany analyzed by an expert survey. J. Dtsch. Dermatol. Ges. 2011, 9, 116–121. [Google Scholar] [CrossRef]
- Pannier-Fischer, F.; Rabe, E. Epidemiology of chronic venous diseases. Hautarzt 2003, 54, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Müller-Bühl, U.; Leutgeb, R.; Bungartz, J.; Szecsenyi, J.; Laux, G. Expenditure of chronic venous leg ulcer management in German primary care: Results from a population-based study. Int. Wound J. 2013, 10, 52–56. [Google Scholar] [CrossRef] [PubMed]
- Nord, D. Cost-effectiveness in wound care. Zentralbl Chir. 2006, 131 (Suppl. 1), S185–S188. [Google Scholar] [CrossRef]
- Heinlin, J.; Schreml, S.; Babilas, P.; Landthaler, M.; Karrer, S. Cutaneous wound healing. Therapeutic interventions. Hautarzt 2010, 61, 611–626, quiz 627. [Google Scholar] [CrossRef]
- Choudhary, V.; Choudhary, M.; Bollag, W.B. Exploring Skin Wound Healing Models and the Impact of Natural Lipids on the Healing Process. Int. J. Mol. Sci. 2024, 25, 3790. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, G.; Valle, M.F.; Malas, M.; Qazi, U.; Maruthur, N.M.; Doggett, D.; Fawole, O.A.; Bass, E.B.; Zenilman, J. Chronic venous leg ulcer treatment: Future research needs. Wound Repair. Regen. 2014, 22, 34–42. [Google Scholar] [CrossRef]
- Shamloul, G.; Khachemoune, A. Reappraisal and updated review of maggot debridement therapy in chronic lower extremity ulcers. Int. J. Dermatol. 2023, 62, 962–968. [Google Scholar] [CrossRef] [PubMed]
- Nenoff, P.; Herrmann, A.; Gerlach, C.; Herrmann, J.; Simon, J.C. Biosurgical débridement using Lucilia sericata-maggots—An update. Wien. Med. Wochenschr. 2010, 160, 578–585. [Google Scholar] [CrossRef]
- Tombulturk, F.K.; Kanigur-Sultuybek, G. A molecular approach to maggot debridement therapy with Lucilia sericata and its excretions/secretions in wound healing. Wound Repair. Regen. 2021, 29, 1051–1061. [Google Scholar] [CrossRef]
- Cytryńska, M.; Rahnamaeian, M.; Zdybicka-Barabas, A.; Dobslaff, K.; Züchner, T.; Sacheau, G.; Innis, C.A.; Vilcinskas, A. Proline-Rich Antimicrobial Peptides in Medicinal Maggots of Lucilia sericata Interact with Bacterial DnaK but Do Not Inhibit Protein Synthesis. Front. Pharmacol. 2020, 11, 532. [Google Scholar] [CrossRef]
- Babiarczyk, B.; Tobiczyk, J. Patient Perceptions and Experiences with Maggot Debridement Therapy for Managing Chronic Wounds. J. Wound Ostomy Continence Nurs. 2024, 51, 180–184. [Google Scholar] [CrossRef]
- Mumford, Z.; Nigam, Y. Maggots in Medicine: A Narrative Review Discussing the Barriers to Maggot Debridement Therapy and Its Utilisation in the Treatment of Chronic Wounds. J. Clin. Med. 2024, 13, 6746. [Google Scholar] [CrossRef] [PubMed]
- Sherman, R.A.; Stadler, F. Expanding access to maggot containment dressings through redesign and innovation. Int. Wound J. 2025, 22, e70100. [Google Scholar] [CrossRef] [PubMed]
- Steenvoorde, P.; van Doorn, L.P. Maggot debridement therapy: Serious bleeding can occur: Report of a case. J. Wound Ostomy Continence Nurs. 2008, 35, 412–414. [Google Scholar] [CrossRef]
- Wu, M.L.; Yang, Z.M.; Dong, H.C.; Zhang, H.; Zheng, X.; Yuan, B.; Yang, Y.; Liu, J.; Li, P.N. Maggot extract accelerates skin wound healing of diabetic rats via enhancing STAT3 signaling. PLoS ONE 2024, 19, e0309903. [Google Scholar] [CrossRef] [PubMed]
- Becerikli, M.; Wallner, C.; Dadras, M.; Wagner, J.M.; Dittfeld, S.; Jettkant, B.; Gestmann, F.; Mehlhorn, H.; Mehlhorn-Diehl, T.; Lehnhardt, M.; et al. Maggot Extract Interrupts Bacterial Biofilm Formation and Maturation in Combination with Antibiotics by Reducing the Expression of Virulence Genes. Life 2022, 12, 237. [Google Scholar] [CrossRef] [PubMed]
- van der Plas, M.J.; Jukema, G.N.; Wai, S.W.; Dogterom-Ballering, H.C.; Lagendijk, E.L.; van Gulpen, C.; van Dissel, J.T.; Bloemberg, G.V.; Nibbering, P.H. Maggot excretions/secretions are differentially effective against biofilms of Staphylococcus aureus and Pseudomonas aeruginosa. J. Antimicrob. Chemother. 2008, 61, 117–122. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, G.A.; Kolter, R. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: A genetic analysis. Mol. Microbiol. 1998, 28, 449–461. [Google Scholar] [CrossRef] [PubMed]
- Eng, Y.A.; Thian, J.Y.; Ignacio, J. Patients with hard-to-heal wounds: A review and synthesis of their experiences and perceptions of maggot debridement. J. Wound Care 2025, 34, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Jiang, K.; Chen, J.; Wu, L.; Lu, H.; Wang, A.; Wang, J. A systematic review of maggot debridement therapy for chronically infected wounds and ulcers. Int. J. Infect. Dis. 2014, 25, 32–37. [Google Scholar] [CrossRef]
- Hurlow, J.; Wolcott, R.D.; Bowler, P.G. Clinical management of chronic wound infections: The battle against biofilm. Wound Repair. Regen. 2025, 33, e13241. [Google Scholar] [CrossRef]
- Gieroń, M.; Słowik-Rylska, M.; Kucharczyk, M.; Cyran-Stemplewska, S.; Gieroń, B.; Czerwonka, G.; Kozieł, D.; Kręcisz, B. The influence of maggot debridement therapy on the bacterial flora of hard-to-heal wounds. J. Wound Care 2024, 33, 778–787. [Google Scholar] [CrossRef]
- Altincicek, B.; Vilcinskas, A. Septic injury-inducible genes in medicinal maggots of the green blow fly Lucilia sericata. Insect Mol. Biol. 2009, 18, 119–125. [Google Scholar] [CrossRef]
- Andersen, A.S.; Sandvang, D.; Schnorr, K.M.; Kruse, T.; Neve, S.; Joergensen, B.; Karlsmark, T.; Krogfelt, K.A. A novel approach to the antimicrobial activity of maggot debridement therapy. J. Antimicrob. Chemother. 2010, 65, 1646–1654. [Google Scholar] [CrossRef]
- Ceřovský, V.; Slaninová, J.; Fučík, V.; Monincová, L.; Bednárová, L.; Maloň, P.; Stokrová, J. Lucifensin, a novel insect defensin of medicinal maggots: Synthesis and structural study. Chembiochem 2011, 12, 1352–1361. [Google Scholar] [CrossRef] [PubMed]
- Valachova, I.; Prochazka, E.; Bohova, J.; Novak, P.; Takac, P.; Majtan, J. Antibacterial properties of lucifensin in Lucilia sericata maggots after septic injury. Asian Pac. J. Trop. Biomed. 2014, 4, 358–361. [Google Scholar] [CrossRef]
- Valachova, I.; Takac, P.; Majtan, J. Midgut lysozymes of Lucilia sericata—New antimicrobials involved in maggot debridement therapy. Insect Mol. Biol. 2014, 23, 779–787. [Google Scholar] [CrossRef] [PubMed]
- Jockenhofer, F.; Gollnick, H.; Herberger, K.; Isbary, G.; Renner, R.; Stucker, M.; Valesky, E.; Wollina, U.; Weichenthal, M.; Karrer, S.; et al. Aetiology, comorbidities and cofactors of chronic leg ulcers: Retrospective evaluation of 1000 patients from 10 specialised dermatological wound care centers in Germany. Int. Wound J. 2016, 13, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Gjødsbøl, K.; Christensen, J.J.; Karlsmark, T.; Jørgensen, B.; Klein, B.M.; Krogfelt, K.A. Multiple bacterial species reside in chronic wounds: A longitudinal study. Int. Wound J. 2006, 3, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Fazli, M.; Bjarnsholt, T.; Kirketerp-Moller, K.; Jorgensen, A.; Andersen, C.B.; Givskov, M.; Tolker-Nielsen, T. Quantitative analysis of the cellular inflammatory response against biofilm bacteria in chronic wounds. Wound Repair. Regen. 2011, 19, 387–391. [Google Scholar] [CrossRef]
- Jeffery Marano, R.; Jane Wallace, H.; Wijeratne, D.; William Fear, M.; San Wong, H.; O’Handley, R. Secreted biofilm factors adversely affect cellular wound healing responses in vitro. Sci. Rep. 2015, 5, 13296. [Google Scholar] [CrossRef] [PubMed]
- McCarty, S.M.; Cochrane, C.A.; Clegg, P.D.; Percival, S.L. The role of endogenous and exogenous enzymes in chronic wounds: A focus on the implications of aberrant levels of both host and bacterial proteases in wound healing. Wound Repair. Regen. 2012, 20, 125–136. [Google Scholar] [CrossRef] [PubMed]




| Patient | Sex | Age (Years) | Wound Type | Wound Duration | Treatment Duration (Weeks) | Treatment |
|---|---|---|---|---|---|---|
| 1 | ♀ | 46 | Venous | 12 months | 8 | Clinic |
| 2 | ♀ | 72 | Venous | 12 months | 7 | Clinic |
| 3 | ♀ | 74 | Venous | 6 months | 2 | Clinic |
| 4 | ♂ | 84 | Venous | 12 months | 2 | Clinic |
| 5 | ♂ | 78 | Arterial and venous | 18 months | 8 | Patient/clinic |
| 6 | ♂ | 65 | Venous | 24 months | 8 | Patient/clinic |
| 7 | ♀ | 61 | Postoperative | 3 months | 8 | Clinic |
| 8 | ♀ | 79 | Venous | 36 months | 8 | Clinic |
| 9 | ♂ | 73 | Arterial | 3 months | 7 | Patient/clinic |
| 10 | ♂ | 72 | Venous | 24 months | 8 | Patient/clinic |
| Week 0 | n = 10 | Week 2 | n = 9 | Week 4 | n = 8 | Week 6 | n = 8 | Week 8 | n = 5 | |
|---|---|---|---|---|---|---|---|---|---|---|
| margin | center | margin | center | margin | center | margin | center | margin | center | |
| P. aeruginosa | 7 | 6 | 6 | 5 | 6 | 4 | 3 | 4 | 3 | 3 |
| S. aureus | 6 | 5 | 2 | 2 | 2 | 2 | 4 | 4 | 1 | 1 |
| P. mirabilis | 2 | 1 | 1 | 1 | 0 | 0 | 2 | 1 | 1 | 1 |
| A. xylosoxidans | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| E. faecalis | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| P. rettgeri | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| C. koseri | 1 | 1 | 1 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| K. pneumoniae | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 |
| Gr. G Streptococci | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 | 1 | 0 |
| Dermal flora | 4 | 3 | 3 | 2 | 0 | 0 | 2 | 2 | 2 | 2 |
| Patient | Sex | Age (Years) | Wound Size Before Study (cm2) | Wound Size EOT (cm2) | Wound Size EOT (%) | Granulation Tissue Absent/Present |
|---|---|---|---|---|---|---|
| 1 | ♀ | 46 | 288 | 202.5 | −30 | present |
| 2 | ♀ | 72 | 66 | 42.75 | −33 | present |
| 3 | ♀ | 74 | 6.25 | 6.25 | 0 | absent |
| 4 | ♂ | 84 | 143 | 143 | 0 | absent |
| 5 | ♂ | 78 | 12 | 6.75 | −44 | present |
| 6 | ♂ | 65 | 90 | 65 | −28 | present |
| 7 | ♀ | 61 | 8.75 | 0.5 | −94 | present |
| 8 | ♀ | 79 | 7.5 | 0.48 | −93 | present |
| 9 | ♂ | 73 | 8.75 | 41.25 | +471 | absent |
| 10 | ♂ | 72 | 19.25 | 12 | −38 | present |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoff, N.-P.; Gestmann, F.P.; Jansen, T.M.; Janßen, S.; Petersdorf, S.; Homey, B.; Gerber, P.A.; Mehlhorn, H. Lyophilized Extract from the Larvae of the Blowfly Lucilia sericata as a New Strategy for the Management of Chronic Wounds. Biomedicines 2025, 13, 582. https://doi.org/10.3390/biomedicines13030582
Hoff N-P, Gestmann FP, Jansen TM, Janßen S, Petersdorf S, Homey B, Gerber PA, Mehlhorn H. Lyophilized Extract from the Larvae of the Blowfly Lucilia sericata as a New Strategy for the Management of Chronic Wounds. Biomedicines. 2025; 13(3):582. https://doi.org/10.3390/biomedicines13030582
Chicago/Turabian StyleHoff, Norman-Philipp, Falk Peer Gestmann, Theresa Maria Jansen, Sarah Janßen, Sabine Petersdorf, Bernhard Homey, Peter Arne Gerber, and Heinz Mehlhorn. 2025. "Lyophilized Extract from the Larvae of the Blowfly Lucilia sericata as a New Strategy for the Management of Chronic Wounds" Biomedicines 13, no. 3: 582. https://doi.org/10.3390/biomedicines13030582
APA StyleHoff, N.-P., Gestmann, F. P., Jansen, T. M., Janßen, S., Petersdorf, S., Homey, B., Gerber, P. A., & Mehlhorn, H. (2025). Lyophilized Extract from the Larvae of the Blowfly Lucilia sericata as a New Strategy for the Management of Chronic Wounds. Biomedicines, 13(3), 582. https://doi.org/10.3390/biomedicines13030582






