Multi-Omics Analysis of the Anoikis Gene CASP8 in Prostate Cancer and Biochemical Recurrence (BCR)
Abstract
1. Introduction
2. Methods
2.1. Data Download
2.2. Single-Cell and Spatial Transcriptomic Analysis of Anoikis Gene Set in Prostate Cancer
2.3. eQTL and Prostate Cancer Data Acquisition
2.4. SMR Analysis of eQTLs for Prostate Cancer
2.5. Single-Cell RNA Sequencing of CASP8 in Prostate Normal and Tumor Tissues
2.6. Spatial Transcriptome Analysis of CASP8 in Prostate Cancer
2.7. Constructing and Evaluating Biochemical Recurrence (BCR) Prognostic Models for Prostate Cancer
2.8. Protein Expression Levels of CASP8 and Core Genes in Human Protein Atlas (HPA)
2.9. Gene Set Enrichment Analysis (GSEA)
2.10. Tumor Microenvironment and Immune Checkpoint Analysis
2.11. Drug Sensitivity Analysis and Immunotherapy
2.12. Analysis of the Cancer–Immune Cycle
2.13. Statistical Analyses
3. Results
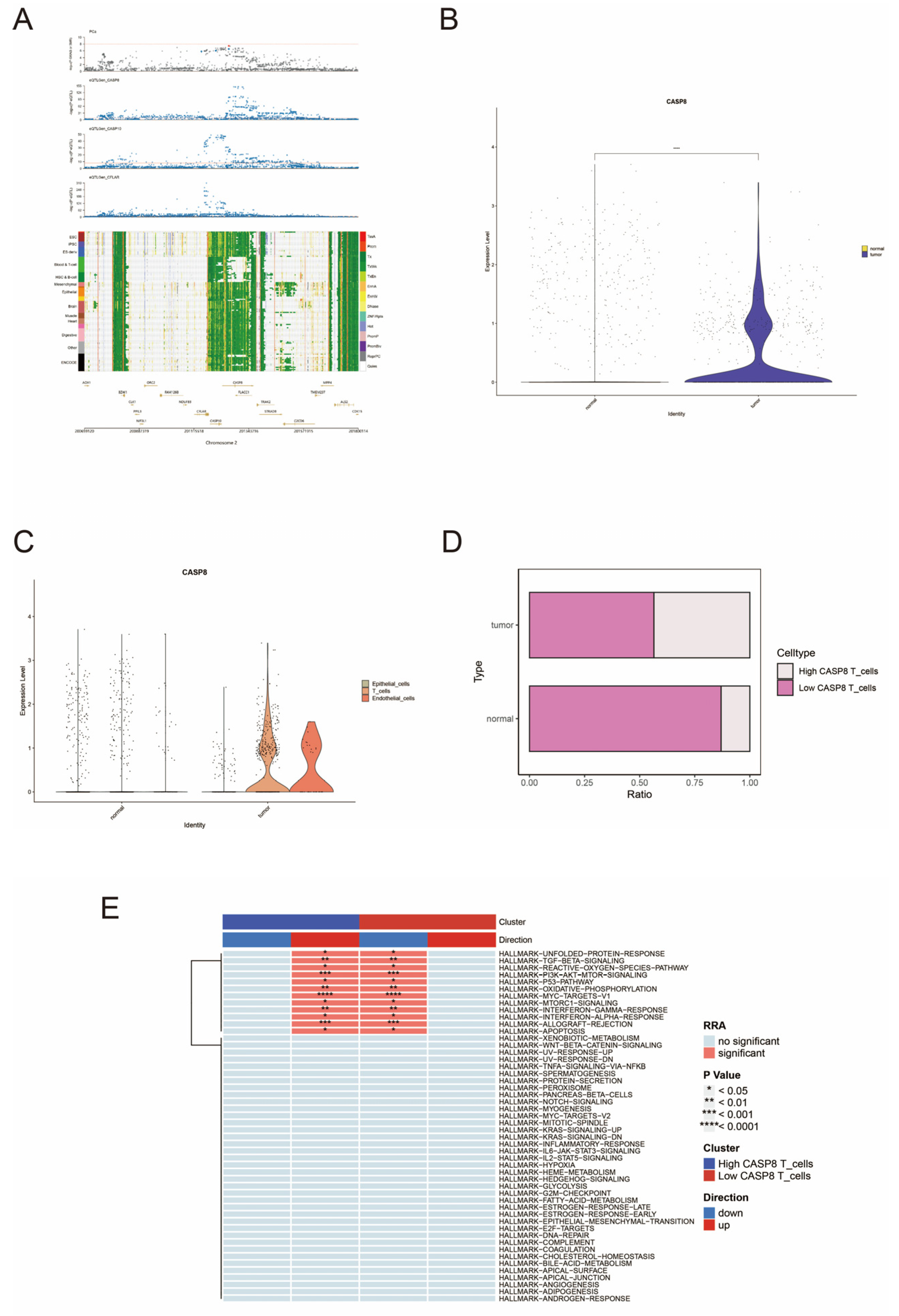
3.1. Single-Cell and Spatial Transcriptome Analysis of the Anoikis Gene Set in Prostate Cancer
3.2. SMR Analysis of Causal Relationship Between eQTL and Prostate Cancer
3.3. Single-Cell Expression Patterns of CASP8 in Prostate Normal and Tumor Tissues
3.4. Spatial Localization and Functional Implications of CASP8 in Prostate Cancer
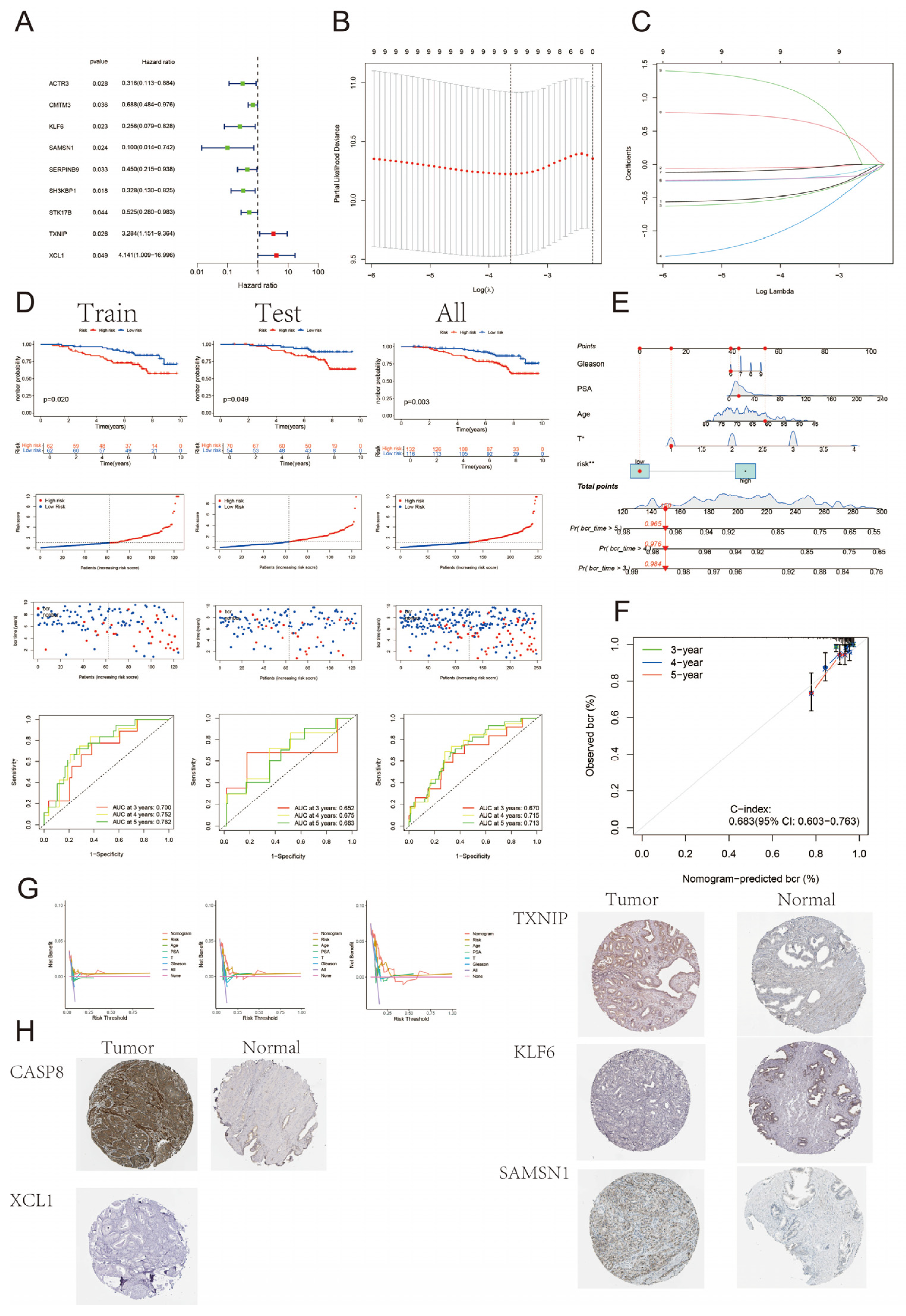
3.5. Construction and Validation of BCR Prognostic Model for Prostate Cancer
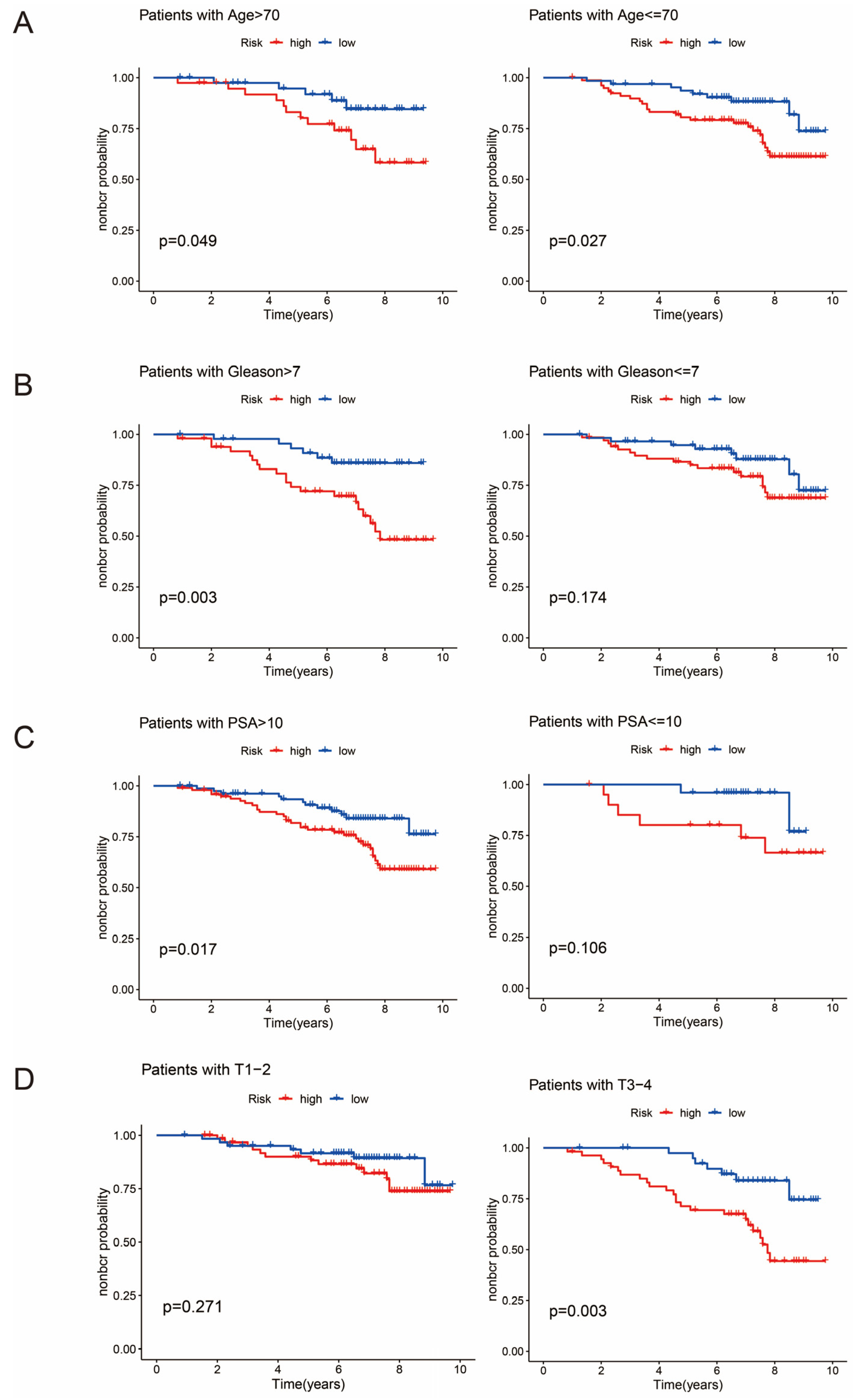
3.6. Clinical Subgroup Analysis
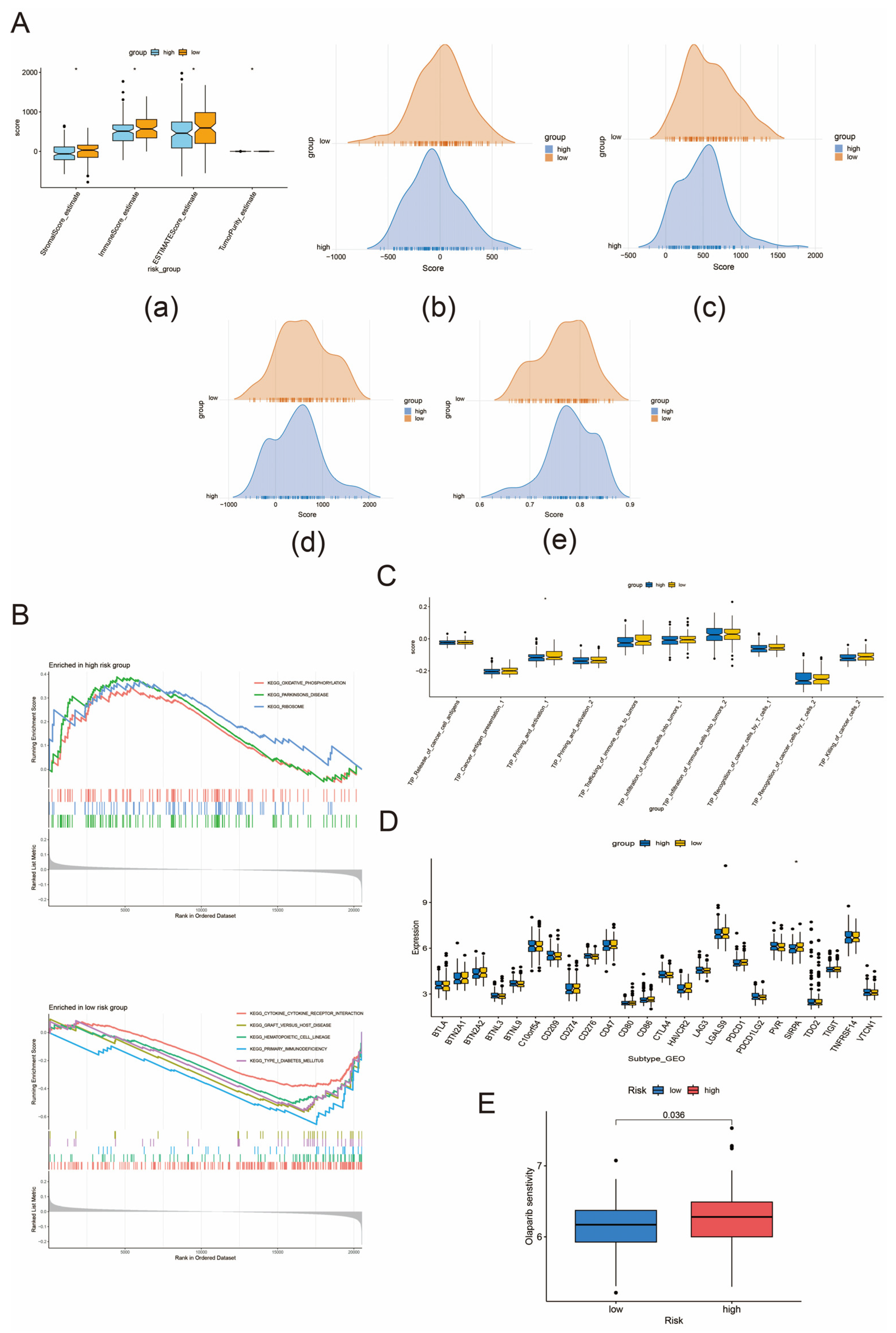
3.7. Tumor Microenvironment Regulation, Immune Checkpoint Analysis, and Immunotherapy
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Heinlein, C.; Chang, C. Androgen receptor in prostate cancer. Endocr. Rev. 2004, 25, 276–308. [Google Scholar] [CrossRef]
- Rawla, P. Epidemiology of Prostate Cancer. World J. Oncol. 2019, 10, 63–89. [Google Scholar] [CrossRef] [PubMed]
- Litwin, M.; Tan, H. The Diagnosis and Treatment of Prostate Cancer A Review. JAMA 2017, 317, 2532–2542. [Google Scholar] [CrossRef] [PubMed]
- Tapper, W.; Carneiro, G.; Mikropoulos, C.; Thomas, S.A.; Evans, P.M.; Boussios, S. The Application of Radiomics and AI to Molecular Imaging for Prostate Cancer. J. Pers. Med. 2024, 14, 287. [Google Scholar] [CrossRef] [PubMed]
- Attard, G.; Murphy, L.; Clarke, N.W.; Sachdeva, A.; Jones, C.; Hoyle, A.; Cross, W.; Jones, R.J.; Parker, C.C.; Gillessen, S.; et al. Abiraterone acetate plus prednisolone with or without enzalutamide for patients with metastatic prostate cancer starting androgen deprivation therapy: Final results from two randomised phase 3 trials of the STAMPEDE platform protocol. Lancet Oncol. 2023, 24, 443–456. [Google Scholar] [CrossRef]
- Ruan, X.-H.; Stacia Chun, T.T.; Huang, D.; Wong, H.-L.; Ho, B.S.-H.; Tsang, C.-F.; Lai, T.C.-T.; Ng, A.T.-L.; Na, R.; Tsu, J.H.-L. Outcomes of radical prostatectomy in a 20-year localized prostate cancer single institution series in China. Asian J. Androl. 2023, 25, 345–349. [Google Scholar] [CrossRef]
- Reijnen, C.; Brunenberg, E.J.L.; Kerkmeijer, L.G.W. Advancing the treatment of localized prostate cancer with MR-guided radiotherapy. Prostate Cancer Prostatic Dis. 2023, 26, 50–52. [Google Scholar] [CrossRef]
- Moul, J. Prostate specific antigen only progression of prostate cancer. J. Urol. 2000, 163, 1632–1642. [Google Scholar] [CrossRef]
- Roach, M.; Hanks, G.; Thames, H.; Schellhammer, P.; Shipley, W.; Sokol, G.; Sandler, H. Defining biochemical failure following radiotherapy with or without hormonal therapy in men with clinically localized prostate cancer: Recommendations of the RTOG-ASTRO Phoenix Consensus Conference. Int. J. Radiat. Oncol. Biol. Phys. 2006, 65, 965–974. [Google Scholar] [CrossRef]
- Shao, N.; Zhu, Y.; Wan, F.-N.; Ye, D.-W. Identification of seven long noncoding RNAs signature for prediction of biochemical recurrence in prostate cancer. Asian J. Androl. 2019, 21, 618–622. [Google Scholar] [CrossRef]
- Wang, S.; Su, W.; Zhong, C.; Yang, T.; Chen, W.; Chen, G.; Liu, Z.; Wu, K.; Zhong, W.; Li, B.; et al. An Eight-CircRNA Assessment Model for Predicting Biochemical Recurrence in Prostate Cancer. Front. Cell Dev. Biol. 2020, 8, 599494. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Chen, J.; Lin, Z.; Liu, Q.; Zhong, C.; Cai, Z.; Jia, Z.; Zhong, W.; Liang, Y.; Cai, C. A prognostic signature consisting of N6-methyladenosine modified mRNAs demonstrates clinical potential in prediction of biochemical recurrence and guidance on precision therapy in prostate cancer. Transl. Oncol. 2023, 33, 101670. [Google Scholar] [CrossRef] [PubMed]
- Yin, W.; Chen, G.; Li, Y.; Li, R.; Jia, Z.; Zhong, C.; Wang, S.; Mao, X.; Cai, Z.; Deng, J.; et al. Identification of a 9-gene signature to enhance biochemical recurrence prediction in primary prostate cancer: A benchmarking study using ten machine learning methods and twelve patient cohorts. Cancer Lett. 2024, 588, 216739. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Zhang, Z.; Wang, P.; Zhao, F.; Miao, L.; Wang, Y.; Li, Y.; Li, Y.; Gao, Z. Advancements in precision nanomedicine design targeting the anoikis-platelet interface of circulating tumor cells. Acta Pharm. Sin. B 2024, 14, 3457–3475. [Google Scholar] [CrossRef]
- Mei, J.; Jiang, X.-Y.; Tian, H.-X.; Rong, D.-C.; Song, J.-N.; Wang, L.; Chen, Y.-S.; Wong, R.C.B.; Guo, C.-X.; Wang, L.-S.; et al. Anoikis in cell fate, physiopathology, and therapeutic interventions. MedComm 2024, 5, e718. [Google Scholar] [CrossRef]
- Cox, A.; Dunning, A.; Garcia-Closas, M.; Balasubramanian, S.; Reed, M.; Pooley, K.; Scollen, S.; Baynes, C.; Ponder, B.; Chanock, S.; et al. A common coding variant in CASP8 is associated with breast cancer risk. Nat. Genet. 2007, 39, 352–358. [Google Scholar] [CrossRef]
- MacPherson, G.; Healey, C.; Teare, M.; Balasubramanian, S.; Reed, M.; Pharoah, P.; Ponder, B.; Meath, M.; Bhattacharyya, N.; Cox, A. Association of a common variant of the CASP8 gene with reduced risk of breast cancer. JNCI-J. Natl. Cancer Inst. 2004, 96, 1866–1869. [Google Scholar] [CrossRef]
- Ulybina, Y.; Kuligina, E.; Mitiushkina, N.; Rozanov, M.; Ivantsov, A.; Ponomariova, D.; Togo, A.; Levchenko, E.; Shutkin, V.; Brenister, S.; et al. Coding polymorphisms in Casp5, CASP8 and DR4 genes may play a role in predisposition to lung cancer. Cancer Lett. 2009, 278, 183–191. [Google Scholar] [CrossRef]
- Hart, K.; Landvik, N.; Lind, H.; Skaug, V.; Haugen, A.; Zienolddiny, S. A combination of functional polymorphisms in the CASP8, MMP1, IL10 and SEPS1 genes affects risk of non-small cell lung cancer. Lung Cancer 2011, 71, 123–129. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, Z.; Tian, Y.; Shao, J.; Zhang, Z. A Six-Nucleotide Insertion-Deletion Polymorphism in the CASP8 Promoter Associated with Risk and Progression of Bladder Cancer. Clin. Cancer Res. 2009, 15, 2567–2572. [Google Scholar] [CrossRef]
- Fu, G.; Tang, J.; Wang, M.; Qin, C.; Yan, F.; Ding, Q.; Yin, C.; Wang, X.; Zhang, Z. CASP8 promoter polymorphism, mRNA expression and risk of prostate cancer among Chinese men. J. Biomed. Res. 2011, 25, 128–134. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, S.; Garcia-Marques, F.; Zhang, C.; Lee, J.; Nolley, R.; Shen, M.; Hsu, E.; Aslan, M.; Koul, K.; Pitteri, S.; et al. Discovery of CASP8 as a potential biomarker for high-risk prostate cancer through a high-multiplex immunoassay. Sci. Rep. 2021, 11, 7612. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Gou, X.; Lai, G.; Li, K.; Zhu, X.; Liu, N.; Kuang, Y.; Ren, K.; Xie, Y.; Xu, Y.; et al. Single-nucleus sequencing unveils heterogeneity in renal cell carcinomas microenvironment: Insights into pathogenic origins and treatment-responsive cellular subgroups. Cancer Lett. 2024, 604, 217259. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Zhang, F.; Hu, H.; Bakshi, A.; Robinson, M.R.; Powell, J.E.; Montgomery, G.W.; Goddard, M.E.; Wray, N.R.; Visscher, P.M.; et al. Integration of summary data from GWAS and eQTL studies predicts complex trait gene targets. Nat. Genet. 2016, 48, 481–487. [Google Scholar] [CrossRef]
- Zhang, F.; Li, X.; Tian, W. Unsupervised Inference of Developmental Directions for Single Cells Using VECTOR. Cell Rep. 2020, 32, 108069. [Google Scholar] [CrossRef]
- Ergün, S.; Aslan, S.; Demir, D.; Kayaoğlu, S.; Saydam, M.; Keleş, Y.; Kolcuoğlu, D.; Taşkurt Hekim, N.; Güneş, S. Beyond Death: Unmasking the Intricacies of Apoptosis Escape. Mol. Diagn. Ther. 2024, 28, 403–423. [Google Scholar] [CrossRef]
- Fuentes-Prior, P.; Salvesen, G.S. The protein structures that shape caspase activity, specificity, activation and inhibition. Biochem. J. 2004, 384, 201–232. [Google Scholar] [CrossRef]
- Lens, S.M.A.; Kataoka, T.; Fortner, K.A.; Tinel, A.; Ferrero, I.; MacDonald, R.H.; Hahne, M.; Beermann, F.; Attinger, A.; Orbea, H.-A.; et al. The caspase 8 inhibitor c-FLIP(L) modulates T-cell receptor-induced proliferation but not activation-induced cell death of lymphocytes. Mol. Cell. Biol. 2002, 22, 5419–5433. [Google Scholar] [CrossRef]
- Mellman, I.; Chen, D.S.; Powles, T.; Turley, S.J. The cancer-immunity cycle: Indication, genotype, and immunotype. Immunity 2023, 56, 2188–2205. [Google Scholar] [CrossRef]
- Boussios, S.; Rassy, E.; Shah, S.; Ioannidou, E.; Sheriff, M.; Pavlidis, N. Aberrations of DNA repair pathways in prostate cancer: A cornerstone of precision oncology. Expert Opin. Ther. Targets 2021, 25, 329–333. [Google Scholar] [CrossRef]
- Galluzzi, L.; López-Soto, A.; Kumar, S.; Kroemer, G. Caspases Connect Cell-Death Signaling to Organismal Homeostasis. Immunity 2016, 44, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Vince, J.E. The role of caspase-8 in inflammatory signalling and pyroptotic cell death. Semin. Immunol. 2023, 70, 101832. [Google Scholar] [CrossRef] [PubMed]
- Mandal, R.; Barrón, J.C.; Kostova, I.; Becker, S.; Strebhardt, K. Caspase-8: The double-edged sword. Biochim. Biophys. Acta Rev. Cancer 2020, 1873, 188357. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Zhang, J.; Wang, L.; Liu, H.; Wang, J.; Liu, J.; Liu, Z.; Zhu, Y.; Xu, Y.; Yang, W.; et al. Non-apoptotic function of caspase-8 confers prostate cancer enzalutamide resistance via NF-κB activation. Cell Death Dis. 2021, 12, 833. [Google Scholar] [CrossRef]
- Helfer, B.; Boswell, B.C.; Finlay, D.; Cipres, A.; Vuori, K.; Bong Kang, T.; Wallach, D.; Dorfleutner, A.; Lahti, J.M.; Flynn, D.C.; et al. Caspase-8 promotes cell motility and calpain activity under nonapoptotic conditions. Cancer Res. 2006, 66, 4273–4278. [Google Scholar] [CrossRef]
- Koschny, R.; Brost, S.; Hinz, U.; Sykora, J.; Batke, E.M.; Singer, S.; Breuhahn, K.; Stremmel, W.; Walczak, H.; Schemmer, P.; et al. Cytosolic and nuclear caspase-8 have opposite impact on survival after liver resection for hepatocellular carcinoma. BMC Cancer 2013, 13, 532. [Google Scholar] [CrossRef]
- Manzo-Merino, J.; Massimi, P.; Lizano, M.; Banks, L. The human papillomavirus (HPV) E6 oncoproteins promotes nuclear localization of active caspase 8. Virology 2014, 450–451, 146–152. [Google Scholar] [CrossRef]
- Müller, I.; Strozyk, E.; Schindler, S.; Beissert, S.; Oo, H.Z.; Sauter, T.; Lucarelli, P.; Raeth, S.; Hausser, A.; Al Nakouzi, N.; et al. Cancer Cells Employ Nuclear Caspase-8 to Overcome the p53-Dependent G2/M Checkpoint through Cleavage of USP28. Mol. Cell 2020, 77, 970–984. [Google Scholar] [CrossRef]
- Deng, J.; Pan, T.; Liu, Z.; McCarthy, C.; Vicencio, J.M.; Cao, L.; Alfano, G.; Suwaidan, A.A.; Yin, M.; Beatson, R.; et al. The role of TXNIP in cancer: A fine balance between redox, metabolic, and immunological tumor control. Br. J. Cancer 2023, 129, 1877–1892. [Google Scholar] [CrossRef]
- Zhou, Q.; Nguyen, T.T.T.; Mun, J.-Y.; Siegelin, M.D.; Greene, L.A. DPEP Inhibits Cancer Cell Glucose Uptake, Glycolysis and Survival by Upregulating Tumor Suppressor TXNIP. Cells 2024, 13, 1025. [Google Scholar] [CrossRef]
- Xie, M.; Xie, R.; Xie, S.; Wu, Y.; Wang, W.; Li, X.; Xu, Y.; Liu, B.; Zhou, Y.; Wang, T.; et al. Thioredoxin interacting protein (TXNIP) acts as a tumor suppressor in human prostate cancer. Cell Biol. Int. 2020, 44, 2094–2106. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Liu, J.; Wang, Y.; Liu, H.; Fu, J.; Zhao, Y.; Huang, Y. METTL3-mediated m6A modification of pri-miR-148a-3p affects prostate cancer progression by regulating TXNIP. Environ. Toxicol. 2023, 38, 2377–2390. [Google Scholar] [CrossRef] [PubMed]
- Chou, W.-C.; Hsiung, C.-N.; Chen, W.-T.; Tseng, L.-M.; Wang, H.-C.; Chu, H.-W.; Hou, M.-F.; Yu, J.-C.; Shen, C.-Y. A functional variant near XCL1 gene improves breast cancer survival via promoting cancer immunity. Int. J. Cancer 2020, 146, 2182–2193. [Google Scholar] [CrossRef] [PubMed]
- Kamei, M.; Matsuo, K.; Yoshida, Y.; Shimada, K.; Otsuki, M.; Fujimoto, N.; Ishibashi, M.; Quan, Y.-S.; Kamiyama, F.; Hara, Y.; et al. Intratumoral delivery of a highly active form of XCL1 enhances antitumor CTL responses through recruitment of CXCL9-expressing conventional type-1 dendritic cells. Int. J. Cancer 2024, 154, 2176–2188. [Google Scholar] [CrossRef]
- Chen, K.; Wu, Z.; Zhao, H.; Wang, Y.; Ge, Y.; Wang, D.; Li, Z.; An, C.; Liu, Y.; Wang, F.; et al. XCL1/Glypican-3 Fusion Gene Immunization Generates Potent Antitumor Cellular Immunity and Enhances Anti-PD-1 Efficacy. Cancer Immunol. Res. 2020, 8, 81–93. [Google Scholar] [CrossRef]
- Liu, X.; Gomez-Pinillos, A.; Loder, C.; Carrillo-de Santa Pau, E.; Qiao, R.; Unger, P.D.; Kurek, R.; Oddoux, C.; Melamed, J.; Gallagher, R.E.; et al. KLF6 loss of function in human prostate cancer progression is implicated in resistance to androgen deprivation. Am. J. Pathol. 2012, 181, 1007–1016. [Google Scholar] [CrossRef][Green Version]
- Li, C.-R.; Su, J.J.-M.; Wang, W.-Y.; Lee, M.T.-L.; Wang, T.-Y.; Jiang, K.-Y.; Li, C.-F.; Hsu, J.-M.; Chen, C.-K.; Chen, M.; et al. Molecular profiling of prostatic acinar morphogenesis identifies PDCD4 and KLF6 as tissue architecture-specific prognostic markers in prostate cancer. Am. J. Pathol. 2013, 182, 363–374. [Google Scholar] [CrossRef]
- DiFeo, A.; Martignetti, J.A.; Narla, G. The role of KLF6 and its splice variants in cancer therapy. Drug Resist. Updates 2009, 12, 1–7. [Google Scholar] [CrossRef]
- Narla, G.; DiFeo, A.; Yao, S.; Banno, A.; Hod, E.; Reeves, H.L.; Qiao, R.F.; Camacho-Vanegas, O.; Levine, A.; Kirschenbaum, A.; et al. Targeted inhibition of the KLF6 splice variant, KLF6 SV1, suppresses prostate cancer cell growth and spread. Cancer Res. 2005, 65, 5761–5768. [Google Scholar] [CrossRef]
- Claudio, J.O.; Zhu, Y.X.; Benn, S.J.; Shukla, A.H.; McGlade, C.J.; Falcioni, N.; Stewart, A.K. HACS1 encodes a novel SH3-SAM adaptor protein differentially expressed in normal and malignant hematopoietic cells. Oncogene 2001, 20, 5373–5377. [Google Scholar] [CrossRef]
- Zhu, Y.X.; Benn, S.; Li, Z.H.; Wei, E.; Masih-Khan, E.; Trieu, Y.; Bali, M.; McGlade, C.J.; Claudio, J.O.; Stewart, A.K. The SH3-SAM adaptor HACS1 is up-regulated in B cell activation signaling cascades. J. Exp. Med. 2004, 200, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Yanagisawa, K.; Tokumaru, S.; Taguchi, A.; Nimura, Y.; Osada, H.; Nagino, M.; Takahashi, T. Detailed characterization of a homozygously deleted region corresponding to a candidate tumor suppressor locus at 21q11-21 in human lung cancer. Genes Chromosomes Cancer 2008, 47, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Noll, J.E.; Hewett, D.R.; Williams, S.A.; Vandyke, K.; Kok, C.; To, L.B.; Zannettino, A.C.W. SAMSN1 is a tumor suppressor gene in multiple myeloma. Neoplasia 2014, 16, 572–585. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Liu, C.-H.; Lei, M.; Zeng, Q.; Li, L.; Tang, H.; Zhang, N. Metabolic regulation of the immune system in health and diseases: Mechanisms and interventions. Signal Transduct. Target. Ther. 2024, 9, 268. [Google Scholar] [CrossRef]
- Zhao, X.; Ren, T.; Li, S.; Wang, X.; Hou, R.; Guan, Z.; Liu, D.; Zheng, J.; Shi, M. A new perspective on the therapeutic potential of tumor metastasis: Targeting the metabolic interactions between TAMs and tumor cells. Int. J. Biol. Sci. 2024, 20, 5109–5126. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Jiang, Z.; He, J.; Zhang, B.; Wang, L.; Long, C.; Zhao, B.; Yang, Y.; Du, L.; Luo, W.; Hu, J.; et al. A Potential “Anti-Warburg Effect” in Circulating Tumor Cell-mediated Metastatic Progression? Aging Dis. 2024, 16, 269. [Google Scholar] [CrossRef]
- Hong, X.; Roh, W.; Sullivan, R.J.; Wong, K.H.K.; Wittner, B.S.; Guo, H.; Dubash, T.D.; Sade-Feldman, M.; Wesley, B.; Horwitz, E.; et al. The Lipogenic Regulator SREBP2 Induces Transferrin in Circulating Melanoma Cells and Suppresses Ferroptosis. Cancer Discov. 2021, 11, 678–695. [Google Scholar] [CrossRef]
- Efstathiou, J.A.; Morgans, A.K.; Bland, C.S.; Shore, N.D. Novel hormone therapy and coordination of care in high-risk biochemically recurrent prostate cancer. Cancer Treat. Rev. 2024, 122, 102630. [Google Scholar] [CrossRef]
- Freedland, S.; Humphreys, E.; Mangold, L.; Eisenberger, M.; Dorey, F.; Walsh, P.; Partin, A. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA 2005, 294, 433–439. [Google Scholar] [CrossRef]
- Zhou, Z.; Chen, M.-J.M.; Luo, Y.; Mojumdar, K.; Peng, X.; Chen, H.; Kumar, S.V.; Akbani, R.; Lu, Y.; Liang, H. Tumor-intrinsic SIRPA promotes sensitivity to checkpoint inhibition immunotherapy in melanoma. Cancer Cell 2022, 40, 1324–1340. [Google Scholar] [CrossRef] [PubMed]
- Marshall, C.H.; Teply, B.A.; Lu, J.; Oliveira, L.; Wang, H.; Mao, S.S.; Kelly, W.K.; Paller, C.J.; Markowski, M.C.; Denmeade, S.R.; et al. Olaparib Without Androgen Deprivation for High-Risk Biochemically Recurrent Prostate Cancer Following Prostatectomy: A Nonrandomized Controlled Trial. JAMA Oncol. 2024, 10, 1400–1408. [Google Scholar] [CrossRef] [PubMed]
- Bilen, M.A.; Khilfeh, I.; Rossi, C.; Morrison, L.; Diaz, L.; Hilts, A.; Lefebvre, P.; Pilon, D.; George, D.J. Treatment patterns for patients with BRCA1/2-positive metastatic castration-resistant prostate cancer. Oncologist 2024, 30, oyae183. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Rachmat, R.; Enyioma, S.; Ghose, A.; Revythis, A.; Boussios, S. BRCA Mutations in Prostate Cancer: Assessment, Implications and Treatment Considerations. Int. J. Mol. Sci. 2021, 22, 12628. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, S.; Yin, H. Multi-Omics Analysis of the Anoikis Gene CASP8 in Prostate Cancer and Biochemical Recurrence (BCR). Biomedicines 2025, 13, 661. https://doi.org/10.3390/biomedicines13030661
Huang S, Yin H. Multi-Omics Analysis of the Anoikis Gene CASP8 in Prostate Cancer and Biochemical Recurrence (BCR). Biomedicines. 2025; 13(3):661. https://doi.org/10.3390/biomedicines13030661
Chicago/Turabian StyleHuang, Shan, and Hang Yin. 2025. "Multi-Omics Analysis of the Anoikis Gene CASP8 in Prostate Cancer and Biochemical Recurrence (BCR)" Biomedicines 13, no. 3: 661. https://doi.org/10.3390/biomedicines13030661
APA StyleHuang, S., & Yin, H. (2025). Multi-Omics Analysis of the Anoikis Gene CASP8 in Prostate Cancer and Biochemical Recurrence (BCR). Biomedicines, 13(3), 661. https://doi.org/10.3390/biomedicines13030661





