Periodic Changes in the Gut Microbiome in Women with the Mixed Type of Irritable Bowel Syndrome
Abstract
1. Introduction
2. Material and Methods
2.1. Participants and Study Design
2.2. Laboratory Tests
2.3. Breathing Tests
2.4. Dysbiosis Test
2.5. Nutritional Recommendations
2.6. Ethical Issues
2.7. Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lacy, B.E.; Patel, N.K. Rome Criteria and a Diagnostic Approach to Irritable Bowel Syndrome. J. Clin. Med. 2017, 6, 99. [Google Scholar] [CrossRef] [PubMed]
- Palsson, O.S.; Baggish, J.S.; Turner, M.J.; Whithead, W.E. Patients Show Frequent Fluctuations between Loose/Watery and Hard/Lumpy Stools: Implications for treatment. Am. J. Gastroenterol. 2012, 107, 286–295. [Google Scholar] [CrossRef] [PubMed]
- Chira, A.; Filip, M.; Dumitrescu, D.L. Patterns of alternation in irritable bowel syndrome. Clujul Med. 2016, 89, 220–223. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Liu, H.; Han, L.; Kang, Z.; Liang, L.; Jianag, S.; Meng, N.; Chen, P.; Xu, Q.; Wu, Q.; et al. Factors Related to Irritable Bowel Syndrome and Differences Among Subtypes: A Cross-Sectional Study in the UK Biobank. Front. Pharmacol. 2022, 13, 905564. [Google Scholar] [CrossRef]
- Mawe, G.M.; Coates, M.D.; Moses, P.L. Review article: Intestinal serotonin signalling in irritable bowel syndrome. Aliment. Pharmacol. Ther. 2006, 23, 1067–1076. [Google Scholar] [CrossRef]
- Sikander, A.; Rana, S.V.; Prasad, K.K. Role of serotonin in gastrointestinal motility and irritable bowel syndrome. Clin. Chim. Acta 2009, 403, 47–55. [Google Scholar] [CrossRef]
- Mezey, E.; Eisenhofer, G.; Hansson, S.; Harta, G.; Hoffman, B.J.; Gallatz, K.; Palkovits, M.; Hunyady, B. Non-neuronal dopamine in the gastrointestinal system. Clin. Exp. Pharmacol. Physiol. 1999, 26, 14–22. [Google Scholar] [PubMed]
- Chojnacki, C.; Poplawski, T.; Blasiak, J.; Fila, M.; Konrad, P.; Chojnacki, J. Alterd Dopamine Signalling in Chronic Epigastric Pain Syndrome. J. Physiol. Pharmacol. 2020, 6, 817–823. [Google Scholar] [CrossRef]
- You, F.Y.; Huang, S.G.; Zhang, H.Y.; Ye, H.; Chi, H.G.; Zou, Y.; Lv, R.X.; Zheng, X.B. Comparison of 5-hydroxytryptamine signalling pathway characteristics in diarrhea-predominant irritable bowel syndrome and ulcerative colitis. World J. Gastroenterol. 2016, 22, 3451–3459. [Google Scholar] [CrossRef]
- Thijssen, A.Y.; Mujagic, Z.; Jonkers, D.M.A.E.; Ludidi, S.; Keszthelyi, D.; Hesselink, M.A.; Clemens, C.H.M.; Conchillo, J.M.; Kruimel, J.W.; Masclee, A.A.M. Alterations in serotonin metabolism in irritable bowel syndrome. Aliment. Pharmacol. Ther. 2016, 43, 272–282. [Google Scholar] [CrossRef]
- Magro, F.; Vieira-Coelho, M.A.; Fraga, S.; Serrao, M.M.; Veloso, F.T.; Robeiro, T.; Soares da Silva, P. Impaired synthesis or cellular storage of norepinephrine, dopamine, and 5-hydroxytryptamine in human inflammatory bowel diseases. Dig. Dis. Sci. 2002, 47, 216–224. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.S.; Schmauss, C.; Ractliffe, E.; Gershon, M.D. Physiological; modulation of intestinal motility by enteric dopaminergic neurons and D2 receptor: Analysis of dopamine receptor expression, location, development, and function in wild-type and knock-out mice. J. Neurosci. 2006, 26, 2798–2807. [Google Scholar] [CrossRef] [PubMed]
- Strandwitz, P. Neurotransmitterrs modulation by the gut microbiota. Brain Res. 2018, 1693, 128–133. [Google Scholar] [CrossRef]
- Chen, M.; Ruan, G.; Chen, L.; Ying, S.; Li, G.; Xu, F.; Xiao, Z.; Tian, Y.; Lv, L.; Ping, Y.; et al. Naurotransmitters and Intestinal Interactions: Focus on the Microbiota-Gut-Brain Axis in Irritable Bowel Syndrome. Front. Endocrinol. 2021, 13, 817100. [Google Scholar] [CrossRef]
- Cao, H.; Liu, X.; Yingyong, A.; Zhou, G.; Liu, Y.; Dong, W.; Wang, S.; Yan, F.; Jiang, K.; Wang, B. Dysbiosis contributes to chronic constipation development via regulation of serotonin transporter in the intestine. Sci. Rep. 2017, 7, 10322. [Google Scholar] [CrossRef]
- Chojnacki, C.; Błońska, A.; Kaczka, A.; Chojnacki, J.; Stępień, A.; Gąsiorowska, A. Evaluation of serotonin and dopamine secretion and metabolism in patients with irritable bowel syndrome. Pol. Arch. Int. Med. 2018, 128, 711–771. [Google Scholar] [CrossRef]
- Chojnacki, J.; Konrad, P.; Medrek-Socha, M.; Kaczka, A.; Błońska, A.; Zajdel, R.; Chojnacki, M.; Gąsiorowska, A. The Variablity of Tryptophan Metabolism in Patients with Mixed Type of Irritable Bowel Syndrome. Int. J. Mol. Sci. 2023, 24, 2550. [Google Scholar]
- Ljótsson, B.; Jones, M.; Talley, N.J.; Kjellstrom, L.; Ahreus, L.; Andreasson, L. Discriminant and convergent validity of the GSRS-IBS symptom severity measure for irritable bowel syndrome: A population study. United Eur. Gastroenterol. J. 2020, 8, 284–292. [Google Scholar] [CrossRef]
- Pimentel, M.; Saad, R.J.; Long, M.D.; Rao, S.S. ACG Clinical Guideline: Small Intestinal Bacterial Overgrowth. Am. J. Gastroenterol. 2020, 115, 165–178. [Google Scholar] [CrossRef]
- Casén, C.; Vebo, H.C.; Sekelja, M.; Hegge, F.T.; Karlson, M.K.; Ciemniejewska, E.; Dzankovic, S.; Frøyland, C.; Nestestog, R.; Engstrand, L.; et al. Deviations in human gut microbiota: A novel diagnostic test for determining dysbiosis in patients with IBS or IBD. Alement. Pharmacol. Ther. 2015, 42, 71–83. [Google Scholar] [CrossRef]
- Pittayanon, R.; Lau, J.T.; Yuan, Y.; Leontiadis, G.I.; Tse, F.; Surette, M.; Moayyedi, P. Gut microbiota in Patients with Irritable Bowel Syndrome. A Systemic Review. Gastroenterology 2019, 157, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, I.B.; Das, A.; O’Herlihy, E.; Coughlan, S.; Cisek, K.; Moore, M.; Bradley, F.; Carty, T.; Pradhan, M.; Dwibedi, C.; et al. Differences in Fecal Microbiomes and Metabolomes of People With vs Without Irritable Bowel Syndrome and Bile Acid Malabsorption. Gastroenterology 2020, 158, 1016–1028.e8. [Google Scholar] [CrossRef] [PubMed]
- Duan, R.; Zhu, S.; Wang, B.; Duan, L. Alterations of Gut Microbiota in Patients With Irritable Bowel Syndrome Based on 16S rRNA-Target Sequencing: A Systemic Review. Clin. Transl. Gastroenterol. 2019, 10, e00012. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Alammar, N.; Singh, R.; Nanavati, J.; Chaudhary, R.; Mullin, G.E. Gut Microbial Dysbiosis in the Irritable Bowel Syndrome: A Systemic Review and Meta-Analysis of Case-Control Studies. J. Acad. Nutr. Diet. 2020, 120, 565–586. [Google Scholar] [CrossRef]
- Zhuang, X.; Tian, Z.; Li, L.; Chen, M.; Xiong, L. Fecal Microbiota Alterations Associated with Diarrhea-Predominant Irritable Bowel Syndrome. Front. Microbiol. 2018, 9, 1600. [Google Scholar] [CrossRef]
- Caroll, I.M.; Ringel-Kulka, T.; Siddle, J.P.; Ringel, Y. Alterations in Composition and Diversity of the Intestinal Microbiota in Patients with Diarrhea-Predominant Irritable Bowel Syndrome. Neurogastroenterol. Motil. 2012, 24, 521-e248. [Google Scholar] [CrossRef]
- Shukla, R.; Ghoshal, U.; Dhole, T.N.; Ghoshal, U.C. Fecal Microbiota in Patients with Irritable Bowel Syndrome Compared with Healthy Controls Using Real-Time Polymerase Chain Reaction. An Evidence of Dysbiosis. Dig. Dis. Sci. 2015, 60, 2953–2962. [Google Scholar] [CrossRef]
- Liu, H.N.; Wu, H.; Chen, Y.J.; Shen, X.Z.; Liu, T.T. Altered Molecular Signature of Intestinal Microbiota in Irritable Bowel Syndrome Patients Compared wih Healthy Controls: A systematic Review and Meta-Analysis. Dig. Liver Dis. 2017, 49, 331–337. [Google Scholar] [CrossRef]
- Pozuelo, M.; Panda, S.; Santiago, A.; Mendez, S.; Accarino, A.; Santos, J.; Guarner, F.; Azpiroz, F.; Manichanh, C. Reduction of Butyrate and Methane- Producing Microorganisms in Patients with Irritable Bowel Syndrome. Sci. Rep. 2015, 5, 12693. [Google Scholar] [CrossRef]
- Rajilic-Stojanovic, M.; Biagi, E.; Heilig, H.G.H.J.; Kajander, K.; Kekkonen, R.A.; Tims, S.; De Vos, W.M. Global and Deep Molecular Analysis of Microbiota Signatures in Fecal Samples from Patients with Irritable Bowel Syndrome. Gastroenterology 2011, 141, 1729–1801. [Google Scholar] [CrossRef]
- Parkes, G.C.; Rayment, N.B.; Hudspith, B.N.; Petrowska, L.; Lomer, M.C.; Brostoff, J.; Whelan, K.; Sanderson, J.D. Distinct Microbial Populations Exist in the Mucosa-Associated Microbiota of Sub-Groups of Irritable Bowel Syndrome: Distinct Mucosal Microbiota in IBS. Neurogastroenterol. Motil. 2012, 24, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Chojnacki, J.; Popławski, T.; Kaczka, A.; Romanowska, N.; Chojnacki, C.; Gąsiorowska, A. Assessment of Urinary Dopamine and Serotonin Metabolites in Relation to Dysbiosis Indicators in Patients with Functional Constipation. Nutrients 2024, 16, 2981. [Google Scholar] [CrossRef] [PubMed]
- Khalif, I.L.; Quigley, E.M.; Konowith, E.A.; Maximowa, I.D. Alterations in the colonic flora and intestinal permeability and evidence of immune activation in chronic constipation. Dig. Liver Dis. 2005, 37, 838–849. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.E.; Choi, S.C.; Park, K.S.; Park, M.I.; Shin, J.E.; Lee, T.H.; Jung, K.W.; Koo, H.S.; Myung, S.J. Change of fecal flora and effectiveness of short-term VSL-3 probiotic treatment in patients with functional constipation. J. Neurogastroenterol. Motil. 2015, 21, 111–120. [Google Scholar] [CrossRef]
- Mancabelli, L.; Milani, C.; Lugli, G.A.; Turroni, F.; Mangifest, M.; Viappiani, A.; Ticinesi, A.; Nouvenne, A.; Meschi, T.; Van Sinderen, D.; et al. Unvelling the gut microbiota composition and functionality associated with constipation trough metagenomic analyses. Sci. Rep. 2017, 7, 9879. [Google Scholar] [CrossRef]
- Pecyna, P.; Gabyel, M.; Mankowska-Wierzbicka, D.; Nowak-Malczewska, D.M.; Jaskiewicz, K.; Jaworska, M.M.; Tomczak, H.; Rydzanicz, M.; Ploski, R.; Grzymislawski, M.; et al. Gender Influences Gut Microbiota among Patients with Irritable Bowel Syndrome. Int. J. Mol. Sci. 2023, 24, 10424. [Google Scholar] [CrossRef]
- Napolitano, M.; Fasulo, E.; Ungaro, F.; Massimino, L.; Sinagra, E.; Danese, S.; Mandarino, F.V. Gut Dysbiosis in Irritable Bowel Syndrome: A Narrative Review on Correlation with Disease Subtypes and Novel Therapeutic Implications. Microorganisms 2023, 11, 2369. [Google Scholar] [CrossRef]
- Teige, E.S.; Sortvik, U.; Lied, G.A. A Systemic Review: Fecal Bacterial Profile in Patients with Irritable Bowel Syndrome Analyzed with the GA-Map Dysbiosis Test Based on the 16S rRNA Gene of Bacterial Species or Groups. Clin. Exper. Gastroenterol. 2024, 17, 109–120. [Google Scholar] [CrossRef]
- Durgam, N.; Dashputre, A.A.; Moshkovich, O.; Rezaie, A.; Martinez, N.; Pedram, E.; Stansbury, J.; Joseph, G. Content validation of a daily patient-reported outcome measure for assessing symptoms in patients with Small Intestinal Bacterial Overgrowth. Qual. Life Res. 2023, 32, 2573–2585. [Google Scholar] [CrossRef]
- Losurdo, G.; Leandro, G.; Ierardi, E.; Perri, F.; Barone, M.; Leo, A.D. Breath Tests for the Non-invasive Diagosis of Small Intestinal Bacterial Overgrowth. A systematic Review with Meta-analysis. J. Neurogastroenterol. Motil. 2020, 26, 16–28. [Google Scholar] [CrossRef]
- Birg, A.; Hu, S.; Lin, C. Reevaluating our understanding of lactulose breath tests by incorporating hydrogen sulfide measurements. JGH Open 2019, 3, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Brannelly, N.T.; Hamilton-Shield, J.P.; Killard, A.J. The Measurement of Ammonia in Human Breath and its Potential in Clinical Diagnostics. Crit. Rev. Anal. Chem. 2016, 46, 490–501. [Google Scholar] [CrossRef] [PubMed]
- Furowicz, A.J.; Karakulska, J.; Porużyńska, A. Bacterial urease—A diagnostic marker or factor of enteric bacteria pathogenicity. Med. Wet. 2004, 60, 110–123. [Google Scholar]
- Ahluwalia, B.; Iribarren, C.; Magnusson, M.K.; Sundin, J.; Clevers, E.; Savolainen, O.; Ross, A.B.; Törnblom, H.; Simrén, M.; Öhman, L. A Distinct Faecal Microbiota and Metabolite Profile Linked to Bowel Habits in Patients with Irritable Bowel Syndrome. Cells 2021, 10, 1459. [Google Scholar] [CrossRef]
- Liu, J.; Tan, Y.; Cheng, H.; Zhang, D.; Feng, W.; Peng, C. Functions of Gut Microbiota Metabolites, Current Status and Future Perspectives. Aging Dis. 2022, 13, 1106–1126. [Google Scholar] [CrossRef]
- Conley, T.E.; Slater, R.; Moss, S.; Bulmer, D.C.; Revilla Negro, J.; Ijaz, U.Z.; Pritchard, D.M.; Parkes, M.; Probert, C. Microbiome-driven IBS metabotypes influence response to he low FODMAP diet: Insights from the faecal volatome. EBioMedicine 2024, 107, 105282. [Google Scholar] [CrossRef]
- Hooks, K.B.; O’Malley, M.A. Dysbiosis and Its Discontents. MBio 2017, 8, e01492-17. [Google Scholar] [CrossRef]
- Sun, W.; Ding, D.; Bai, D.; Lin, Y.; Zhang, C.; Zhang, D. Transcriptomics and metabolomics analysis of L-phenyloalanine overproduction in Escherichia coli. Microb. Cell Factories 2023, 22, 65. [Google Scholar] [CrossRef]
- Roager, H.M.; Licht, T.R. Microbial tryptophan catabolites in health and disease. Nat. Commun. 2018, 9, 3294. [Google Scholar] [CrossRef]
- Canakis, A.; Haron, M.; Weber, H.C. Irritable bowel syndrome and gut microbiota. Curr. Opin. Endocrinol. Diabetes Obes. 2020, 27, 28–35. [Google Scholar] [CrossRef]
- Jiang, C.-H.; Wu, H.-Y.; Chen, J.-Y.; Huang, W.; Huang, C.-Y.; Li, Z.-S.; Fang, X. Global Research Trends and Hot Spots in the Overlapping Fields of Functional Bowel Disorders and the Intestinal Microbiota: A Scientometric Analysis. J. Translat. Gastroenterol. 2023, 1, 2–12. [Google Scholar] [CrossRef]
- Xiao, L.; Liu, Q.; Luo, M.; Xiong, L. Gut Microbiota-Derived Metabolites in Irritable Bowel Syndrome. Front. Cell. Infect. Microbiol. 2021, 11, 729346. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, N.; Kanayama, M.; Watanabe, Y.; Yamada, H.; Lili, L.; Torii, A. Effect of Personalize Prebiotic and Supplements on the Symptoms of Irritable Bowel Syndrome: An Open- Label, Single-Arm, Multicenter Clinical Trial. Nutrients 2024, 16, 3333. [Google Scholar] [CrossRef] [PubMed]

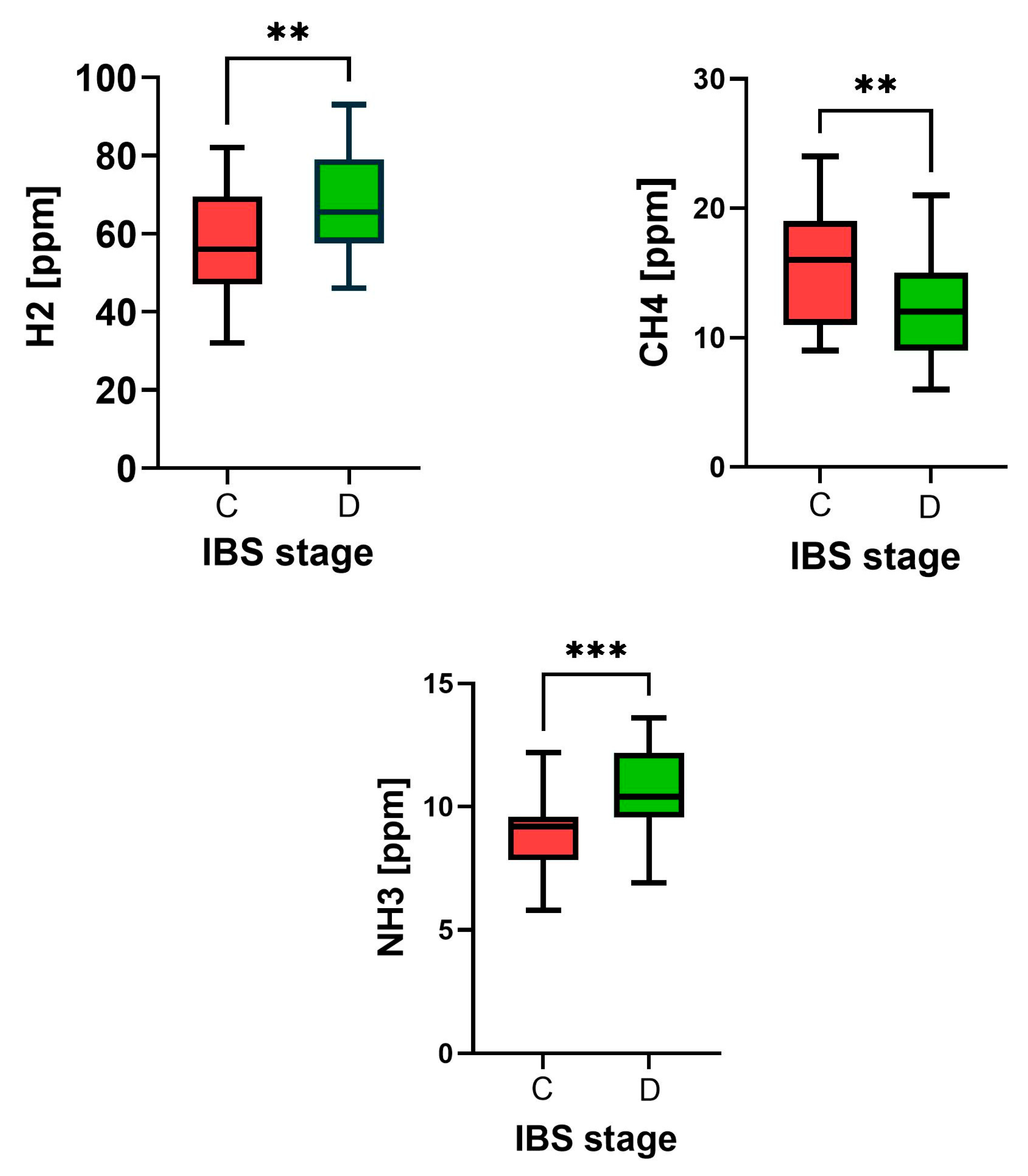
| Feature | Constipation Period | Diarrheal Period | p-Value |
|---|---|---|---|
| Age (years) | 38.1 ± 6.6 | 38.3 ± 7.1 | 0.869 |
| BMI (kg/m2) | 23.2 ± 0.9 | 23.0. ± 1.1 | 0.067 |
| GFR (mL/min) | 99.1 ± 4.9 | 97.9 ± 5.5 | 0.061 |
| ALT (µ/L) | 15.6 ± 3.5 | 14.9 ± 3.8 | 0.059 |
| AST (µ/L) | 13.9 ± 2.1 | 14.3 ± 1.8 | 0.092 |
| CRP (mg/L) | 2.3 ± 1.8 | 3.1 ± 2.3 | 0.188 |
| FC (µg/g) | 26.6 ± 15.7 | 30.9 ± 12.8 | 0.045 * |
| ES (pg/mL) | 118.6 ± 36.2 | 136.8 ± 41.1 | 0.068 |
| FSH (IU/L) | 12.1 ± 10.5 | 14.9 ± 12.9 | 0.089 |
| TSH (µIU/L) | 2.12 ± 0.58 | 3.12 ± 0.83 | 0.079 |
| Bacteria Species | Period-C (No/%) | Period-D (No/%) | p-Value |
|---|---|---|---|
| Bacteroides | 3 (10.0) | 8 (26.6) | 0.1068 |
| Bifidobacterium spp. | 13 (43.3) | 8 (26.6) | 0.0496 * |
| Clostridium spp. | 3 (10.0) | 6 (20.0) | 0.2781 |
| Escherichia spp. | 6 (20.0) | 10 (33.3) | 0.2539 |
| Faecalibacterium prausn. | 7(23.3) | 3 (10.0) | 0.1750 |
| Furmicutes (varia) | 9 (30.0) | 7 (23.3) | 0.5390 |
| Lactobacillus spp. | 12 (40.0) | 4 (13.3) | 0.0178 * |
| Proteobacteria spp. | 4 (13.3) | 8 (26.6) | 0.1974 |
| Ruminococcus (varia) | 4 (13.3) | 6 (20.0) | 0.4861 |
| Streptococcus spp. | 3 (10.0) | 6 (20.0) | 0.2781 |
| DI vs. Urine Metabolites | Rho Spearman/p | |||
|---|---|---|---|---|
| Period-C | p-Value | Period-D | p-Value | |
| H2 | −0.9598 | 0.0089 ** | 0.8425 | 0.0006 *** |
| CH4 | 0.6776 | 0.0360 * | −0.2466 | 0.2570 |
| NH3 | 0.3796 | 0.2675 | 0.1275 | 0.4975 |
| HPA | −0.0313 | 0.0783 | −0.0398 | 0.8467 |
| 3-IS | 0.1504 | 0.1504 | 0.0266 | 0.8946 |
| HA | −0. 1807 | 0.9998 | 0.0647 | 0.7723 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaczka, A.; Błońska, A.; Chojnacki, C.; Gąsiorowska, A.; Błasiak, J.; Popławski, T.; Chojnacki, J. Periodic Changes in the Gut Microbiome in Women with the Mixed Type of Irritable Bowel Syndrome. Biomedicines 2025, 13, 652. https://doi.org/10.3390/biomedicines13030652
Kaczka A, Błońska A, Chojnacki C, Gąsiorowska A, Błasiak J, Popławski T, Chojnacki J. Periodic Changes in the Gut Microbiome in Women with the Mixed Type of Irritable Bowel Syndrome. Biomedicines. 2025; 13(3):652. https://doi.org/10.3390/biomedicines13030652
Chicago/Turabian StyleKaczka, Aleksandra, Aleksandra Błońska, Cezary Chojnacki, Anita Gąsiorowska, Janusz Błasiak, Tomasz Popławski, and Jan Chojnacki. 2025. "Periodic Changes in the Gut Microbiome in Women with the Mixed Type of Irritable Bowel Syndrome" Biomedicines 13, no. 3: 652. https://doi.org/10.3390/biomedicines13030652
APA StyleKaczka, A., Błońska, A., Chojnacki, C., Gąsiorowska, A., Błasiak, J., Popławski, T., & Chojnacki, J. (2025). Periodic Changes in the Gut Microbiome in Women with the Mixed Type of Irritable Bowel Syndrome. Biomedicines, 13(3), 652. https://doi.org/10.3390/biomedicines13030652






