Absorption of Toxicants from the Ocular Surface: Potential Applications in Toxicology
Abstract
1. Introduction
The Principle of 3Rs (Reduction, Refinement, and Replacement)
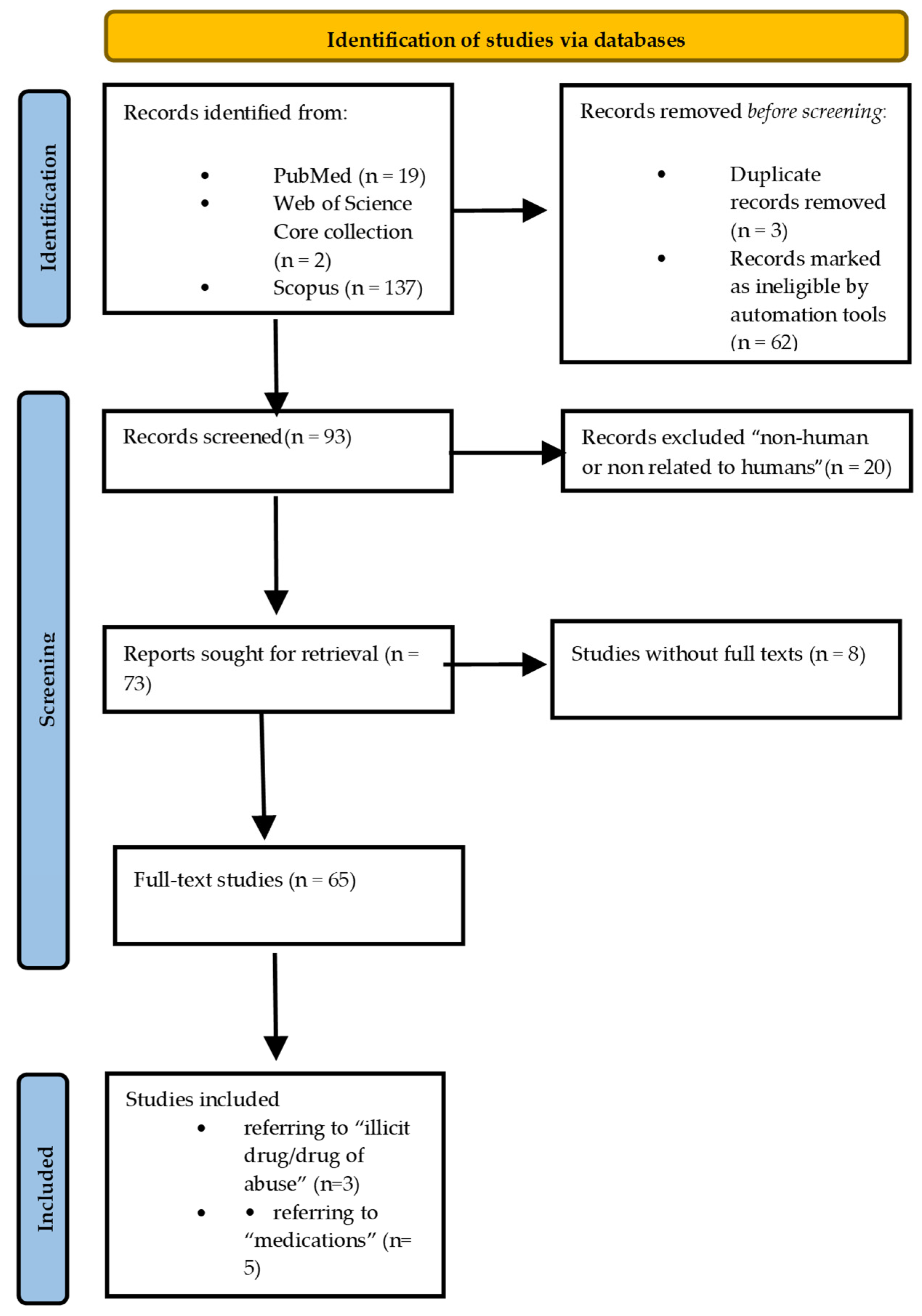
2. Literature Review
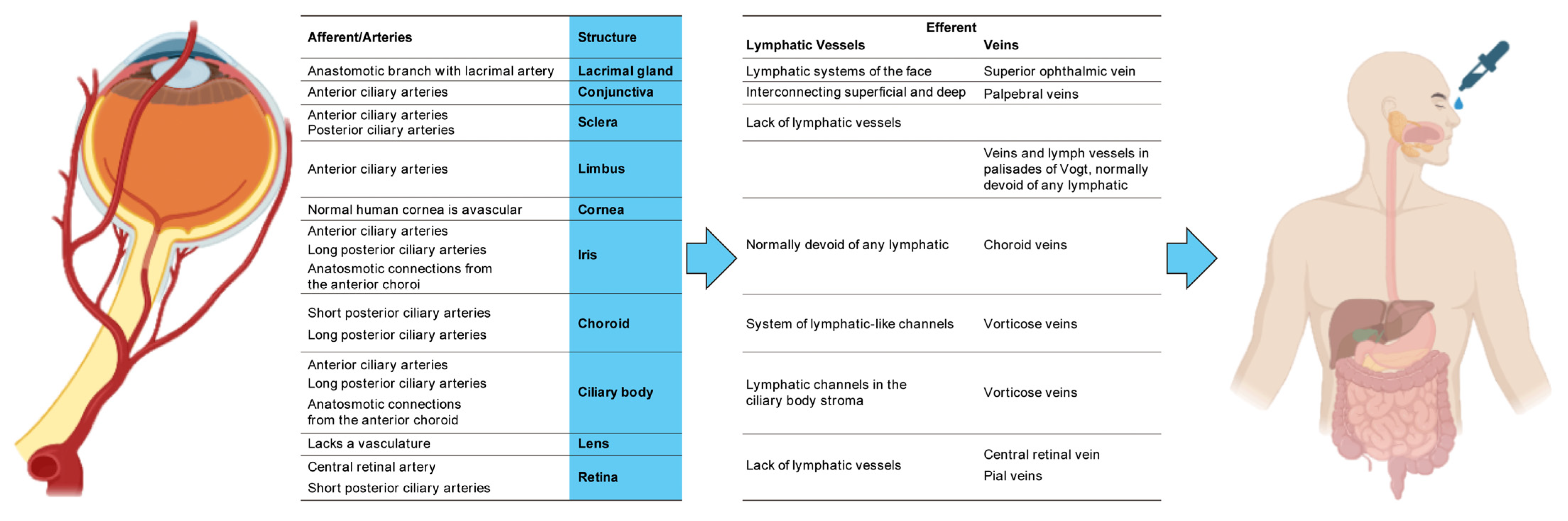
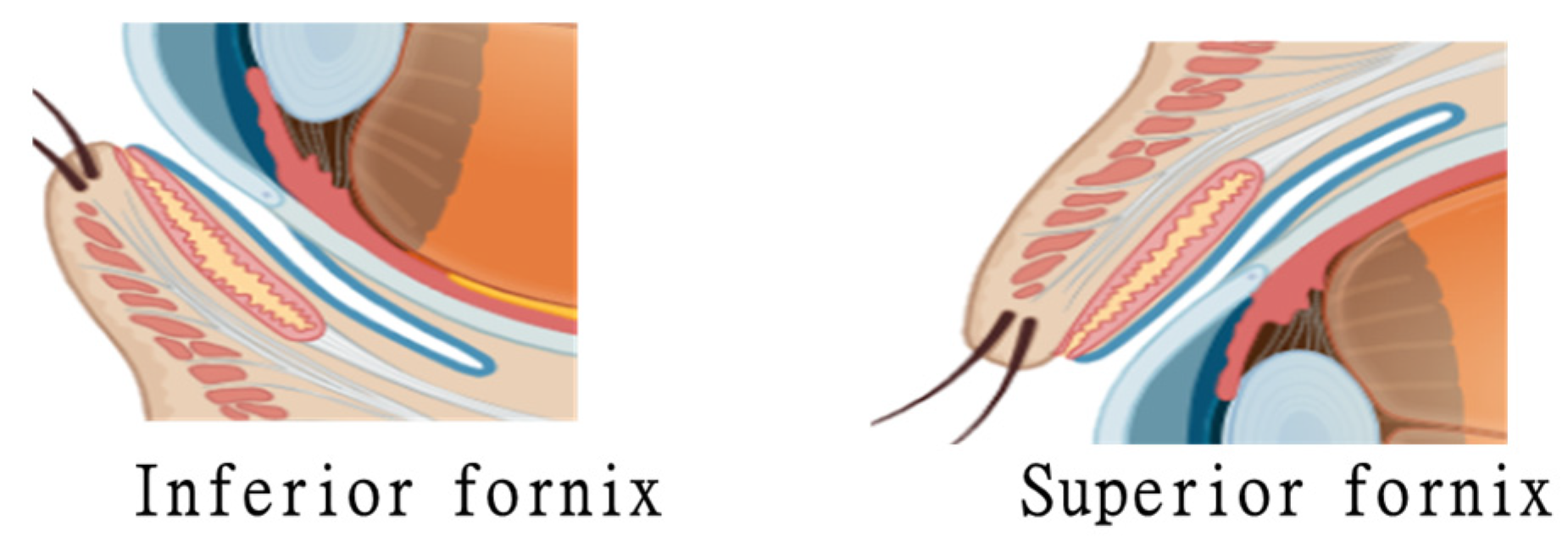
3. Matrix
Methods of Fluid Collection
4. Toxicant
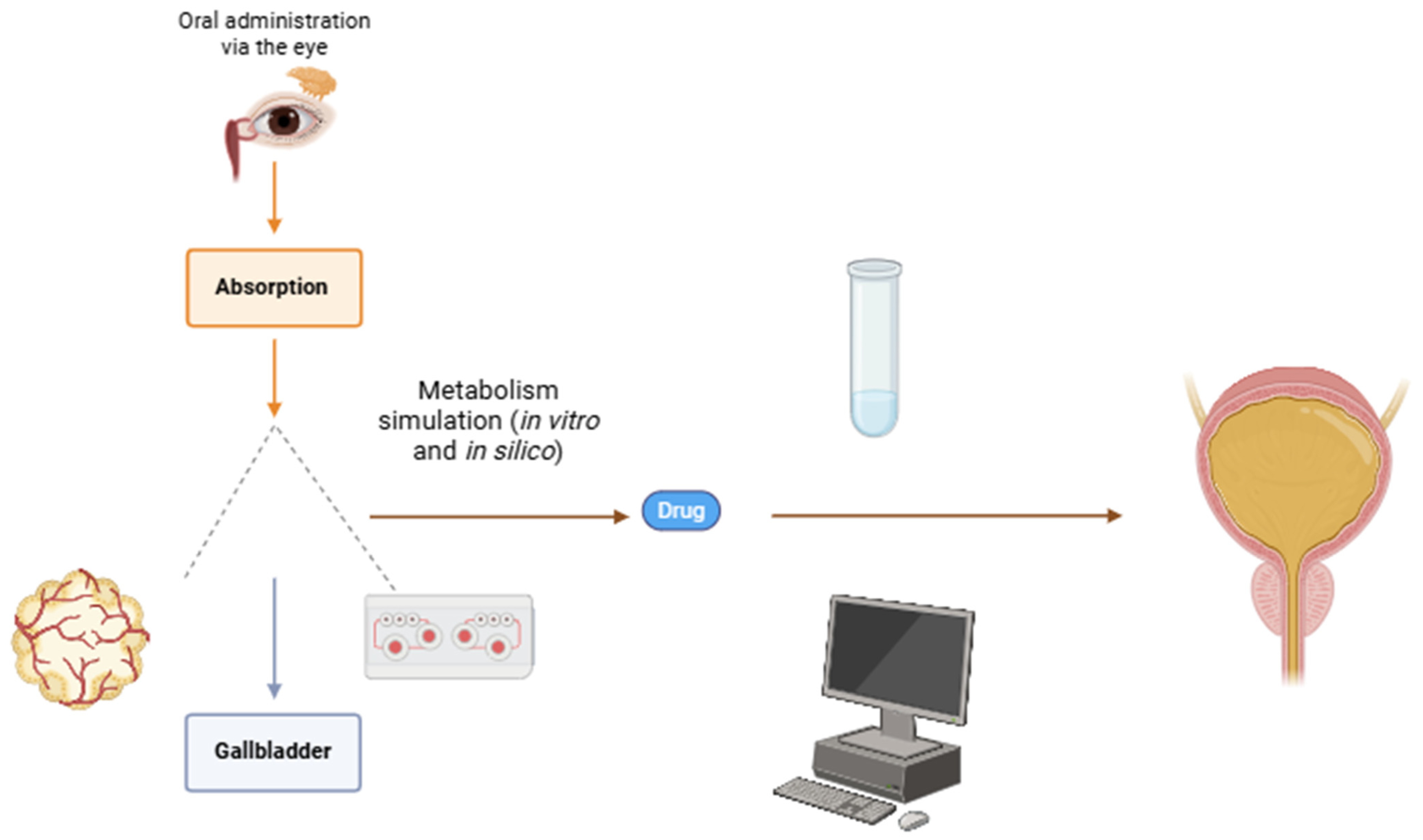
5. Interface with the Organism
6. Medications
| Study | Type of Study | Intervention | Outcome | |
|---|---|---|---|---|
| Abd-Elhakim, Y.M. [71] | Animal | Tartrazine and chlorophyll in rats | Serum levels of immunoglobulins, levels of expression of genes containing interleukins, enzyme-linked immunoassay | |
| Alqaissy, W.Q.M. [72] | Animal | Treatment of infections of the urinary tract in rats induced by pathogenic E. coli | ||
| Gameli, P.S. [73] | In silico metabolite prediction | Metabolism of thieno-triazolo diazepine in human hepatocytes | Web-based in silico prediction | High-resolution mass spectrometry |
| Heo, D. [74] | Metabolism of vardenafil analogs | Toxicity, safety, efficacy, side effects, drug interaction, and metabolism study | Mass spectrometry and liquid chromatography | |
Route for Illicit Drugs
7. In Vitro Models
8. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Machiele, R.; Lopez, M.J.; Czyz, C.N. Anatomy, Head and Neck: Eye Lacrimal Gland. Available online: https://www.ncbi.nlm.nih.gov/books/NBK532914 (accessed on 28 August 2024).
- Sanap, A.R. A Review on Ophthalmic Drug Delivery System. Res. J. Sci. Technol. 2024, 16, 79–86. [Google Scholar] [CrossRef]
- Uddin, N.; Rutar, M. Ocular Lymphatic and Glymphatic Systems: Implications for Retinal Health and Disease. Int. J. Mol. Sci. 2022, 23, 10139. [Google Scholar] [CrossRef] [PubMed]
- Anatomica, T. Terminologica anatomica. In Federative Committe on Anatomical Terminology; Thieme: Stuggart, Germany, 1998. [Google Scholar]
- Greathouse, D.G.; Halle, J.S.; Dalley, A.F. Terminologia Anatomica: Revised anatomical terminology. J. Orthop. Sports Phys. Ther. 2004, 34, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Del Amo, E.M.; Vellonen, K.S.; Urtti, A.; Terasaki, T.; Hammid, A.; Honkakoski, P.; Auriola, S. Mass spectrometry in ocular drug research. Mass. Spectrom. Rev. 2023. [Google Scholar] [CrossRef]
- Stefanac, T.; Grgas, D.; Landeka Dragicevic, T. Xenobiotics-Division and Methods of Detection: A Review. J. Xenobiot. 2021, 11, 130–141. [Google Scholar] [CrossRef]
- van Haeringen, N.J. Secretion of drugs in tears. Curr. Eye Res. 1985, 4, 485–488. [Google Scholar] [CrossRef]
- Russell, W.M.S.; Burch, R.L.; Hume, C.W. The Principles of Humane Experimental Technique; Methuen: London, UK, 1959; Volume 238. [Google Scholar]
- Louzao, M.C.; Costas, C. Environmental Toxicology: Non-Bacterial Toxins; 4.1. 1 The Three Rs; Walter de Gruyter: Berlin, Germany, 2024; Volume 103. [Google Scholar]
- Christophe, J. La Directive 2010/63/UE–un tournant pour la primatologie? Rev. Primatol. 2011, 3. [Google Scholar] [CrossRef]
- Hubrecht, R.C.; Carter, E. The 3Rs and Humane Experimental Technique: Implementing Change. Animals 2019, 9, 754. [Google Scholar] [CrossRef]
- Loscher, M.; Seiz, C.; Hurst, J.; Schnichels, S. Topical Drug Delivery to the Posterior Segment of the Eye. Pharmaceutics 2022, 14, 134. [Google Scholar] [CrossRef]
- Herman, T.F.; Santos, C. First-Pass Effect. Available online: https://www.ncbi.nlm.nih.gov/pubmed/31869143 (accessed on 16 August 2024).
- Xie, L.; Diao, Z.; Xia, J.; Zhang, J.; Xu, Y.; Wu, Y.; Liu, Z.; Jiang, C.; Peng, Y.; Song, Z.; et al. Comprehensive Evaluation of Metabolism and the Contribution of the Hepatic First-Pass Effect in the Bioavailability of Glabridin in Rats. J. Agric. Food Chem. 2023, 71, 1944–1956. [Google Scholar] [CrossRef]
- Shumway, C.L.; Motlagh, M.; Wade, M. Anatomy, Head and Neck, Eye Conjunctiva. Available online: https://www.ncbi.nlm.nih.gov/books/NBK519502/ (accessed on 28 August 2024).
- Tong, L.; Lan, W.; Petznick, A. Definition of the ocular surface. In Ocular Surface Anatomy and Physiology, Disorders and Therapeutic Care; CRC Press: Boca Raton, FL, USA, 2012. [Google Scholar]
- Croker, C. Ocular Surface-Tear Film Interface. 2024. Available online: https://synapse.org.za/cpd-activities/croker-ocular-surface-2.pdf (accessed on 3 March 2025).
- Pflugfelder, S.C.; Stern, M.E. Biological functions of tear film. Exp. Eye Res. 2020, 197, 108115. [Google Scholar] [CrossRef] [PubMed]
- King-Smith, P.E.; Begley, C.G.; Braun, R.J. High Resolution Images of Human Meibum Spread on Saline. Investig. Ophthalmol. Vis. Sci. 2024, 65, 41. [Google Scholar] [CrossRef] [PubMed]
- Cher, I. Fluids of the ocular surface: Concepts, functions and physics. Clin. Exp. Ophthalmol. 2012, 40, 634–643. [Google Scholar] [CrossRef] [PubMed]
- Abbey, A.M.; Yoo, S.H. Corneal Anatomy and Optics. In Corneal Topography; CRC Press: Boca Raton, FL, USA, 2024; pp. 11–19. [Google Scholar]
- Abtahi, M.A.; Beheshtnejad, A.H.; Latifi, G.; Akbari-Kamrani, M.; Ghafarian, S.; Masoomi, A.; Sonbolastan, S.A.; Jahanbani-Ardakani, H.; Atighechian, M.; Banan, L.; et al. Corneal Epithelial Thickness Mapping: A Major Review. J. Ophthalmol. 2024, 2024, 6674747. [Google Scholar] [CrossRef]
- Maharana, P.K.; Sharma, N. Applied Anatomy and Physiology of the Cornea. In Mastering Corneal Surgery; CRC Press: Boca Raton, FL, USA, 2024; pp. 3–9. [Google Scholar]
- Shen, S.; Zhang, Y. Restoration of corneal epithelial barrier function: A possible target for corneal neovascularization. Ocul. Surf. 2024, 34, 38–49. [Google Scholar] [CrossRef]
- Jutley, G.; Carpenter, D.; Hau, S.; Booth, D.; Jasim, H.A.; Tay, E.; Daniel, C.; Saw, V. Upper and lower conjunctival fornix depth in healthy white caucasian eyes: A method of objective assessment. Eye 2016, 30, 1351–1358. [Google Scholar] [CrossRef]
- Abdu, N.; Weldemariam, D.G.; Goitom Tesfagaber, A.; Tewelde, T.; Tesfamariam, E.H. A cross-sectional study on the proper administration of eye medications and its determinants among outpatients attending Brhan Aini Ophthalmic National Referral Hospital in Asmara, Eritrea. BMJ Open 2024, 14, e084168. [Google Scholar] [CrossRef]
- Akhter, M.H.; Ahmad, I.; Alshahrani, M.Y.; Al-Harbi, A.I.; Khalilullah, H.; Afzal, O.; Altamimi, A.S.A.; Najib Ullah, S.N.M.; Ojha, A.; Karim, S. Drug Delivery Challenges and Current Progress in Nanocarrier-Based Ocular Therapeutic System. Gels 2022, 8, 82. [Google Scholar] [CrossRef]
- Yartsev, V.D.; Atkova, E.L.; Ekaterinchev, M.A. Topographic and anatomical features of the nasolacrimal duct obstruction due to radioiodine treatment. Int. Ophthalmol. 2023, 43, 3385–3390. [Google Scholar] [CrossRef]
- Park, J.H.; Huh, J.A.; Piao, J.F.; Lee, H.; Baek, S.H. Measuring nasolacrimal duct volume using computed tomography images in nasolacrimal duct obstruction patients in Korean. Int. J. Ophthalmol. 2019, 12, 100–105. [Google Scholar] [CrossRef]
- Dorota, K.; Łucja, N.; Ewa, F.; Anna, N.; Piotr, M. Tear Film—Physiology and Disturbances in Various Diseases and Disorders. In Ocular Surface Diseases; Dorota, K., Ed.; IntechOpen: Rijeka, Croatia, 2020; p. 1. [Google Scholar]
- Amini, P.; Okeme, J.O. Tear Fluid as a Matrix for Biomonitoring Environmental and Chemical Exposures. Curr. Environ. Health Rep. 2024, 11, 340–355. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhao, S.Z.; Koh, S.K.; Chen, L.; Vaz, C.; Tanavde, V.; Li, X.R.; Beuerman, R.W. In-depth analysis of the human tear proteome. J. Proteom. 2012, 75, 3877–3885. [Google Scholar] [CrossRef] [PubMed]
- Bachhuber, F.; Huss, A.; Senel, M.; Tumani, H. Diagnostic biomarkers in tear fluid: From sampling to preanalytical processing. Sci. Rep. 2021, 11, 10064. [Google Scholar] [CrossRef] [PubMed]
- Barmada, A.; Shippy, S.A. Tear analysis as the next routine body fluid test. Eye 2020, 34, 1731–1733. [Google Scholar] [CrossRef]
- Yao, Y.N.; Di, D.; Yuan, Z.C.; Wu, L.; Hu, B. Schirmer Paper Noninvasive Microsampling for Direct Mass Spectrometry Analysis of Human Tears. Anal. Chem. 2020, 92, 6207–6212. [Google Scholar] [CrossRef]
- Berra, M.; Galperin, G.; Dawidowski, L.; Tau, J.; Marquez, I.; Berra, A. Impact of wildfire smoke in Buenos Aires, Argentina, on ocular surface. Arq. Bras. Oftalmol. 2015, 78, 110–114. [Google Scholar] [CrossRef]
- Galperin, G.; Berra, M.; Marquez, M.I.; Mandaradoni, M.; Tau, J.; Berra, A. Impact of environmental pollution on the ocular surface of Sjogren’s syndrome patients. Arq. Bras. Oftalmol. 2018, 81, 481–489. [Google Scholar] [CrossRef]
- Rummenie, V.T.; Matsumoto, Y.; Dogru, M.; Wang, Y.; Hu, Y.; Ward, S.K.; Igarashi, A.; Wakamatsu, T.; Ibrahim, O.; Goto, E.; et al. Tear cytokine and ocular surface alterations following brief passive cigarette smoke exposure. Cytokine 2008, 43, 200–208. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, J.H.; Nam, W.H.; Yi, K.; Choi, D.G.; Hyon, J.Y.; Wee, W.R.; Shin, Y.J. Oral alcohol administration disturbs tear film and ocular surface. Ophthalmology 2012, 119, 965–971. [Google Scholar] [CrossRef]
- Gutierrez, M.L.A.; Colman Lerner, J.E.; Giuliani, D.S.; Porta, A.A.; Andrinolo, D. Comparative study of tear lipid composition in two human populations with different exposure to particulate matter in La Plata, Argentina. Environ. Sci. Pollut. Res. Int. 2019, 26, 6948–6956. [Google Scholar] [CrossRef]
- Chen, Y.J.; Chen, Y.Y.; Lai, C.H. Clinical association between trace elements of tear and dry eye metrics. Sci. Rep. 2022, 12, 18052. [Google Scholar] [CrossRef] [PubMed]
- Peltonen, S.; Kari, O.; Jarva, H.; Mussalo-Rauhamaa, H.; Haahtela, T.; Meri, S. Complement activation in tear fluid during occupational mold challenge. Ocul. Immunol. Inflamm. 2008, 16, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, M.; Bonatti, R.; Marquezini, M.V.; Garcia, M.L.; Santos, U.P.; Braga, A.L.; Alves, M.R.; Saldiva, P.H.; Monteiro, M.L. Lacrimal Cytokines Assessment in Subjects Exposed to Different Levels of Ambient Air Pollution in a Large Metropolitan Area. PLoS ONE 2015, 10, e0143131. [Google Scholar] [CrossRef] [PubMed]
- Jing, D.; Jiang, X.; Zhou, P.; Ren, X.; Su, J.; Hao, R.; Zhang, M.; Wan, Y.; Li, X. Evidence of air pollution-related ocular signs and altered inflammatory cytokine profile of the ocular surface in Beijing. Sci. Rep. 2022, 12, 18359. [Google Scholar] [CrossRef]
- Paananen, R.O.; Rantamaki, A.H.; Parshintsev, J.; Holopainen, J.M. The Effect of Ambient Ozone on Unsaturated Tear Film Wax Esters. Investig. Ophthalmol. Vis. Sci. 2015, 56, 8054–8062. [Google Scholar] [CrossRef][Green Version]
- Girshevitz, O.; Cohen-Sinai, N.; Zahavi, A.; Vardizer, Y.; Fixler, D.; Goldenberg-Cohen, N. Trace Elements in Tears: Comparison of Rural and Urban Populations Using Particle Induced X-ray Emission. J. Pers. Med. 2022, 12, 1633. [Google Scholar] [CrossRef]
- Avula, A.; Galor, A.; Blackwelder, P.; Carballosa-Gautam, M.; Hackam, A.S.; Jeng, B.; Kumar, N. Application of Scanning Electron Microscopy With Energy-Dispersive X-Ray Spectroscopy for Analyzing Ocular Surface Particles on Schirmer Strips. Cornea 2017, 36, 752–756. [Google Scholar] [CrossRef]
- Kaplan, C.; Galor, A.; Blackwelder, P.; Hackam, A.S.; Jeng, B.H.; Menendez, D.; Kim, S.J.; Kumar, N. Human Ocular Surface Particulate Composition in the Clinical Versus Home Environment. Cornea 2019, 38, 1266–1272. [Google Scholar] [CrossRef]
- Henderson, M.A.; Gillon, S.; Al-Haddad, M. Organization and composition of body fluids. Anaesth. Intensive Care Med. 2024, 25, 432–438. [Google Scholar] [CrossRef]
- Costin, G.-E.; Raabe, H. In Vitro Toxicology Models for Acute Eye and Skin Irritation Assessment; The Royal Society of Chemistry: London, UK, 2013. [Google Scholar]
- Lin, C.C.; Chiu, C.C.; Lee, P.Y.; Chen, K.J.; He, C.X.; Hsu, S.K.; Cheng, K.C. The Adverse Effects of Air Pollution on the Eye: A Review. Int. J. Environ. Res. Public Health 2022, 19, 1186. [Google Scholar] [CrossRef]
- Vitar, R.M.L.; Arana, A.G.H.; Marchini, T.O.; Evelson, P.A.; Ferreira, S.M. The Ocular Surface as a Target of Air Pollution. In Environmental Stressors and OxInflammatory Tissues Responses; CRC Press: Boca Raton, FL, USA, 2024; pp. 80–88. [Google Scholar]
- Alves, M.; Asbell, P.; Dogru, M.; Giannaccare, G.; Grau, A.; Gregory, D.; Kim, D.H.; Marini, M.C.; Ngo, W.; Nowinska, A.; et al. TFOS Lifestyle Report: Impact of environmental conditions on the ocular surface. Ocul. Surf. 2023, 29, 1–52. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Song, M.S.; Lee, Y.; Paik, H.J.; Song, J.S.; Choi, Y.H.; Kim, D.H. Adverse effects of meteorological factors and air pollutants on dry eye disease: A hospital-based retrospective cohort study. Sci. Rep. 2024, 14, 17776. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.W.; Fu, J.; Liu, X.F.; Cui, Z.H.; Chen, W.W.; Guo, L.; Li, X.L.; Ren, Y.; Shao, F.; Chen, L.N.; et al. Impacts of air pollution and meteorological conditions on dry eye disease among residents in a northeastern Chinese metropolis: A six-year crossover study in a cold region. Light. Sci. Appl. 2023, 12, 186. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Jia, H.; Han, J.; Zhang, Z.; Yin, X.; Mu, N.; Zhu, Y.; Li, M. Correlation Between Air Quality Index and Tear Film Lipid Layer Thickness: Comparison Between Patients with Sjogren’s Syndrome and with Meibomian Gland Dysfunction. Curr. Eye Res. 2023, 48, 447–455. [Google Scholar] [CrossRef]
- Hao, R.; Zhang, M.; Zhao, L.; Liu, Y.; Sun, M.; Dong, J.; Xu, Y.; Wu, F.; Wei, J.; Xin, X.; et al. Impact of Air Pollution on the Ocular Surface and Tear Cytokine Levels: A Multicenter Prospective Cohort Study. Front. Med. 2022, 9, 909330. [Google Scholar] [CrossRef]
- Ma, Q.; Liu, Y.; Shi, J.; Qu, G.; Jiang, G. Effect-directed analysis in environment research: Current status and future challenges. Sci. Sin. Chim. 2018, 48, 1195–1206. [Google Scholar] [CrossRef]
- Lai, H.; Liu, X.; Qu, M. Nanoplastics and Human Health: Hazard Identification and Biointerface. Nanomaterials 2022, 12, 1298. [Google Scholar] [CrossRef]
- Dehghanian, Z.; Asgari Lajayer, B.; Biglari Quchan Atigh, Z.; Nayeri, S.; Ahmadabadi, M.; Taghipour, L.; Senapathi, V.; Astatkie, T.; Price, G.W. Micro (nano) plastics uptake, toxicity and detoxification in plants: Challenges and prospects. Ecotoxicol. Environ. Saf. 2023, 268, 115676. [Google Scholar] [CrossRef]
- Moore, S.; Paalanen, L.; Melymuk, L.; Katsonouri, A.; Kolossa-Gehring, M.; Tolonen, H. The Association between ADHD and Environmental Chemicals-A Scoping Review. Int. J. Environ. Res. Public Health 2022, 19, 2849. [Google Scholar] [CrossRef]
- Botelho, R.M.; Silva, A.L.M.; Borbely, A.U. The Autism Spectrum Disorder and Its Possible Origins in Pregnancy. Int. J. Environ. Res. Public Health 2024, 21, 244. [Google Scholar] [CrossRef]
- Zaheer, J.; Kim, H.; Ko, I.O.; Jo, E.K.; Choi, E.J.; Lee, H.J.; Shim, I.; Woo, H.J.; Choi, J.; Kim, G.H.; et al. Pre/post-natal exposure to microplastic as a potential risk factor for autism spectrum disorder. Environ. Int. 2022, 161, 107121. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Lin, Y.; Shen, H. Exposure to Polystyrene Microplastics Promotes the Progression of Cognitive Impairment in Alzheimer’s Disease: Association with Induction of Microglial Pyroptosis. Mol. Neurobiol. 2024, 61, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.H.; Kim, D.Y.; Choi, J.H. Roles of Vascular Smooth Muscle Cells in Atherosclerotic Calcification. J. Lipid Atheroscler. 2023, 12, 106–118. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Yoon, H.K.; Kim, D.Y.; Jeong, T.S.; Park, Y.S. An Emerging Role of Micro- and Nanoplastics in Vascular Diseases. Life 2024, 14, 255. [Google Scholar] [CrossRef]
- Millen, A.E.; Dighe, S.; Kordas, K.; Aminigo, B.Z.; Zafron, M.L.; Mu, L. Air Pollution and Chronic Eye Disease in Adults: A Scoping Review. Ophthalmic Epidemiol. 2024, 31, 1–10. [Google Scholar] [CrossRef]
- Hu, C.; Yang, S.; Zhang, T.; Ge, Y.; Chen, Z.; Zhang, J.; Pu, Y.; Liang, G. Organoids and organoids-on-a-chip as the new testing strategies for environmental toxicology-applications & advantages. Environ. Int. 2024, 184, 108415. [Google Scholar] [CrossRef]
- Varela-Fernandez, R.; Diaz-Tome, V.; Luaces-Rodriguez, A.; Conde-Penedo, A.; Garcia-Otero, X.; Luzardo-Alvarez, A.; Fernandez-Ferreiro, A.; Otero-Espinar, F.J. Drug Delivery to the Posterior Segment of the Eye: Biopharmaceutic and Pharmacokinetic Considerations. Pharmaceutics 2020, 12, 269. [Google Scholar] [CrossRef]
- Abd-Elhakim, Y.M.; Hashem, M.M.; El-Metwally, A.E.; Anwar, A.; Abo-El-Sooud, K.; Moustafa, G.G.; Ali, H.A. Comparative haemato-immunotoxic impacts of long-term exposure to tartrazine and chlorophyll in rats. Int. Immunopharmacol. 2018, 63, 145–154. [Google Scholar] [CrossRef]
- Alqaissy, W.Q.M.; Najim, T.M.; Al-Shammari, B.F.M.; Hasan, M.S.; Jead, M. Clinical Treatment of UTI in Rats Induced by Pathogenic E. coli. Indian J. Forensic Med. Toxicol. 2020, 14, 1034–1038. [Google Scholar] [CrossRef]
- Gameli, P.S.; Kutzler, J.; Berardinelli, D.; Carlier, J.; Auwarter, V.; Busardo, F.P. Exploring the Metabolism of Flubrotizolam, a Potent Thieno-Triazolo Diazepine, Using Human Hepatocytes and High-Resolution Mass Spectrometry. Metabolites 2024, 14, 506. [Google Scholar] [CrossRef]
- Heo, D.; Kang, J.S.; Choe, S.; Lee, S.; Kim, K.M.; Pyo, J. Metabolic study of vardenafil analogues: Pseudovardenafil and hydroxyvardenafil. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2020, 1139, 121940. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, S.P.; Rao, S.K. Ocular surface staining: Current concepts and techniques. Indian. J. Ophthalmol. 2023, 71, 1080–1089. [Google Scholar] [CrossRef] [PubMed]
- Mofidfar, M.; Abdi, B.; Ahadian, S.; Mostafavi, E.; Desai, T.A.; Abbasi, F.; Sun, Y.; Manche, E.E.; Ta, C.N.; Flowers, C.W. Drug delivery to the anterior segment of the eye: A review of current and future treatment strategies. Int. J. Pharm. 2021, 607, 120924. [Google Scholar] [CrossRef] [PubMed]
- Santana, C.P.; Matter, B.A.; Patil, M.A.; Silva-Cunha, A.; Kompella, U.B. Corneal Permeability and Uptake of Twenty-Five Drugs: Species Comparison and Quantitative Structure-Permeability Relationships. Pharmaceutics 2023, 15, 1646. [Google Scholar] [CrossRef]
- Agrahari, V.; Mandal, A.; Agrahari, V.; Trinh, H.M.; Joseph, M.; Ray, A.; Hadji, H.; Mitra, R.; Pal, D.; Mitra, A.K. A comprehensive insight on ocular pharmacokinetics. Drug Deliv. Transl. Res. 2016, 6, 735–754. [Google Scholar] [CrossRef]
- Hardie, K.R.; Fenn, S.J. JMM profile: Rifampicin: A broad-spectrum antibiotic. J. Med. Microbiol. 2022, 71, 001566. [Google Scholar] [CrossRef]
- Ponte, C.; Pi, C.; Palmaro, A.; Jouanjus, E.; Lapeyre-Mestre, M.; French Addictovigilance, N. Early signal of diverted use of tropicamide eye drops in France. Br. J. Clin. Pharmacol. 2017, 83, 1791–1800. [Google Scholar] [CrossRef]
- Bellman, V.; Ukolova, A.; Erovichenkova, E.; Lam, S.; Srivastava, H.K.; Bruce, J.; Burgess, D.M. Abuse of tropicamide eye drops: Review of clinical data. Braz. J. Psychiatry 2022, 44, 522–531. [Google Scholar] [CrossRef]
- American Optometric Association. ‘Why Would Someone Try to Get Tropicamide Illegally?’ Doctor’s Question Leads to Surprising Answer. Available online: https://www.aoa.org/news/practice-management/perfect-your-practice/identity-theft?sso=y (accessed on 19 September 2024).
- Bozkurt, M.; Karabulut, V.; Evren, C.; Seker, M.; Kan, H. Intravenous Abuse of Tropicamide in Opioid Use Disorder: Presentation of 2 Cases. Subst. Abus. 2015, 36, 170–173. [Google Scholar] [CrossRef]
- Sefi-Yurdakul, N.; Sancakli, O. Tropicamide and anaphylaxis: A case report. Saudi J. Ophthalmol. 2021, 35, 71–72. [Google Scholar] [CrossRef]
- Yelne, S.; Pendam, M. Allergic Reaction to Tropicamide Eye Drops: A Case Report. Cureus 2024, 16, e57945. [Google Scholar] [CrossRef] [PubMed]
- Bersani, F.S.; Imperatori, C.; Prilutskaya, M.; Kuliev, R.; Corazza, O. Injecting eye-drops: A mini-review on the non-clinical use of tropicamide. Hum. Psychopharmacol. 2015, 30, 262–264. [Google Scholar] [CrossRef] [PubMed]
- Eid, A.; Ahmad, M.A.; Ghareeb, D.; Ali, M.R. The impact of several urine adulterants on samples that test positive for Tetrahydrocannabinol using screening tests. Zagazig J. Forensic Med. 2023, 21, 212–242. [Google Scholar] [CrossRef]
- Spiller, H.A.; Siewert, D.J. Drug-facilitated sexual assault using tetrahydrozoline. J. Forensic Sci. 2012, 57, 835–838. [Google Scholar] [CrossRef]
- Kacinko, S.; Lamb, M. Tetrahydrozoline: Death by Eyedrops. Toxicol. Anal. Clin. 2022, 34, S63–S64. [Google Scholar] [CrossRef]
- Menshawey, E.; Menshawey, R. More than meets the eye: A scoping review on the non-medical uses of THZ eye drops. Forensic Sci. Med. Pathol. 2024, 20, 569–578. [Google Scholar] [CrossRef]
- Lusthof, K.J.; Lameijer, W.; Zweipfenning, P.G. Use of clonidine for chemical submission. J. Toxicol. Clin. Toxicol. 2000, 38, 329–332. [Google Scholar] [CrossRef]
- Al-Khalaileh, W.; Abu-Farha, R.; Wazaify, M.; Van Hout, M.C. Ophthalmic drug abuse: An observational study from community pharmacies. Res. Soc. Adm. Pharm. 2019, 15, 943–948. [Google Scholar] [CrossRef]
- Hassan, A.E.; Dibas, M.; Sarraj, A.; Ghozy, S.; El-Qushayri, A.E.; Dmytriw, A.A.; Tekle, W.G. First pass effect vs. multiple passes complete reperfusion: A retrospective study. Neuroradiol. J. 2022, 35, 306–312. [Google Scholar] [CrossRef]
- Bruijn, N.; van Lohuizen, R.; Boron, M.; Fitzek, M.; Gabriele, F.; Giuliani, G.; Melgarejo, L.; Rehulka, P.; Sebastianelli, G.; Triller, P.; et al. Influence of metabolic state and body composition on the action of pharmacological treatment of migraine. J. Headache Pain. 2024, 25, 20. [Google Scholar] [CrossRef]
- Ayon, N.J. High-Throughput Screening of Natural Product and Synthetic Molecule Libraries for Antibacterial Drug Discovery. Metabolites 2023, 13, 625. [Google Scholar] [CrossRef] [PubMed]
- Way, G.P.; Sailem, H.; Shave, S.; Kasprowicz, R.; Carragher, N.O. Evolution and impact of high content imaging. SLAS Discov. 2023, 28, 292–305. [Google Scholar] [CrossRef] [PubMed]
- Ergün, S.; Office, O.E. The Next Generation of Organoids Will Be More Complex and Even Closer to Resembling Real Organs: An Interview with Prof. Dr. Hans Clevers. Organoids 2024, 3, 32–34. [Google Scholar] [CrossRef]
- Yang, S.; Hu, H.; Kung, H.; Zou, R.; Dai, Y.; Hu, Y.; Wang, T.; Lv, T.; Yu, J.; Li, F. Organoids: The current status and biomedical applications. MedComm 2023, 4, e274. [Google Scholar] [CrossRef] [PubMed]
- Hofer, M.; Lutolf, M.P. Engineering organoids. Nat. Rev. Mater. 2021, 6, 402–420. [Google Scholar] [CrossRef]
- Dominguez-Oliva, A.; Hernandez-Avalos, I.; Martinez-Burnes, J.; Olmos-Hernandez, A.; Verduzco-Mendoza, A.; Mota-Rojas, D. The Importance of Animal Models in Biomedical Research: Current Insights and Applications. Animals 2023, 13, 1223. [Google Scholar] [CrossRef]
- Farhang Doost, N.; Srivastava, S.K. A Comprehensive Review of Organ-on-a-Chip Technology and Its Applications. Biosensors 2024, 14, 225. [Google Scholar] [CrossRef]
- Le Dare, B.; Ferron, P.J.; Allard, P.M.; Clement, B.; Morel, I.; Gicquel, T. New insights into quetiapine metabolism using molecular networking. Sci. Rep. 2020, 10, 19921. [Google Scholar] [CrossRef]
- Pognan, F.; Beilmann, M.; Boonen, H.C.M.; Czich, A.; Dear, G.; Hewitt, P.; Mow, T.; Oinonen, T.; Roth, A.; Steger-Hartmann, T.; et al. The evolving role of investigative toxicology in the pharmaceutical industry. Nat. Rev. Drug Discov. 2023, 22, 317–335. [Google Scholar] [CrossRef]
- Hemmerich, J.; Ecker, G.F. In silico toxicology: From structure-activity relationships towards deep learning and adverse outcome pathways. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2020, 10, e1475. [Google Scholar] [CrossRef]
- Rein, J. Organs on a Chip! FDA’s Predictive Toxicology Roadmap; FDLI Update; HeinOnline: Getzville, NY, USA, 2018; Volume 28. [Google Scholar]
- Ingber, D.E. Human organs-on-chips for disease modelling, drug development and personalized medicine. Nat. Rev. Genet. 2022, 23, 467–491. [Google Scholar] [CrossRef] [PubMed]
- Jang, K.J.; Otieno, M.A.; Ronxhi, J.; Lim, H.K.; Ewart, L.; Kodella, K.R.; Petropolis, D.B.; Kulkarni, G.; Rubins, J.E.; Conegliano, D.; et al. Reproducing human and cross-species drug toxicities using a Liver-Chip. Sci. Transl. Med. 2019, 11, eaax5516. [Google Scholar] [CrossRef] [PubMed]
- Hartung, T. The (misleading) role of animal models in drug development. Front. Drug Discov. 2024, 4, 1355044. [Google Scholar] [CrossRef]
- Mukherjee, P.; Roy, S.; Ghosh, D.; Nandi, S.K. Role of animal models in biomedical research: A review. Lab. Anim. Res. 2022, 38, 18. [Google Scholar] [CrossRef]
- Van Norman, G.A. Limitations of Animal Studies for Predicting Toxicity in Clinical Trials: Is it Time to Rethink Our Current Approach? JACC Basic Transl. Sci. 2019, 4, 845–854. [Google Scholar] [CrossRef]
- Clinton, J.W.; Kiparizoska, S.; Aggarwal, S.; Woo, S.; Davis, W.; Lewis, J.H. Drug-Induced Liver Injury: Highlights and Controversies in the Recent Literature. Drug Saf. 2021, 44, 1125–1149. [Google Scholar] [CrossRef]
- de Jongh, D.; Massey, E.K.; Cronin, A.J.; Schermer, M.H.N.; Bunnik, E.M.; Consortium, V. Early-Phase Clinical Trials of Bio-Artificial Organ Technology: A Systematic Review of Ethical Issues. Transpl. Int. 2022, 35, 10751. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Kaneko, M.; Narukawa, M. Approval success rates of drug candidates based on target, action, modality, application, and their combinations. Clin. Transl. Sci. 2021, 14, 1113–1122. [Google Scholar] [CrossRef]
- Sun, D.; Gao, W.; Hu, H.; Zhou, S. Why 90% of clinical drug development fails and how to improve it? Acta Pharm. Sin. B 2022, 12, 3049–3062. [Google Scholar] [CrossRef]
- Gallo, K.; Goede, A.; Eckert, O.A.; Gohlke, B.O.; Preissner, R. Withdrawn 2.0-update on withdrawn drugs with pharmacovigilance data. Nucleic Acids Res. 2024, 52, D1503–D1507. [Google Scholar] [CrossRef]
- Horejs, C. Organ chips, organoids and the animal testing conundrum. Nat. Rev. Mater. 2021, 6, 372–373. [Google Scholar] [CrossRef] [PubMed]
- Mahalmani, V.; Prakash, A.; Medhi, B. Do alternatives to animal experimentation replace preclinical research? Indian J. Pharmacol. 2023, 55, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Fernandez, H.; Alhakim-Khalak, F.; Ruiz-Alonso, S.; Diaz, A.; Tamayo, J.; Ramalingam, M.; Larra, E.; Pedraz, J.L. Comprehensive review of the state-of-the-art in corneal 3D bioprinting, including regulatory aspects. Int. J. Pharm. 2024, 662, 124510. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.M.; Saha, A.; Trisha, F.A.; Gonzalez-Andrades, M.; Patra, H.K.; Griffith, M.; Chodosh, J.; Rajaiya, J. An in vitro 3-dimensional Collagen-based Corneal Construct with Innervation Using Human Corneal Cell Lines. Ophthalmol. Sci. 2024, 4, 100544. [Google Scholar] [CrossRef]
- da Fonseca Cardoso, L.M.; da Silva Ferreira, N.C.; de Araripe Lopes Correa, M.; da Silva, S.A.; Alves, L.A. Software for animal randomization: A tool for increasing the reproducibility of science. Lab. Anim. 2024, 58, 164–169. [Google Scholar] [CrossRef]
- Roney, M.; Aluwi, M.F.F.M. The importance of in-silico studies in drug discovery. Intell. Pharm. 2024, 2, 578–579. [Google Scholar] [CrossRef]
- Marques, L.; Costa, B.; Pereira, M.; Silva, A.; Santos, J.; Saldanha, L.; Silva, I.; Magalhaes, P.; Schmidt, S.; Vale, N. Advancing Precision Medicine: A Review of Innovative In Silico Approaches for Drug Development, Clinical Pharmacology and Personalized Healthcare. Pharmaceutics 2024, 16, 332. [Google Scholar] [CrossRef]
- Qureshi, R.; Irfan, M.; Gondal, T.M.; Khan, S.; Wu, J.; Hadi, M.U.; Heymach, J.; Le, X.; Yan, H.; Alam, T. AI in drug discovery and its clinical relevance. Heliyon 2023, 9, e17575. [Google Scholar] [CrossRef]
- Vora, L.K.; Gholap, A.D.; Jetha, K.; Thakur, R.R.S.; Solanki, H.K.; Chavda, V.P. Artificial Intelligence in Pharmaceutical Technology and Drug Delivery Design. Pharmaceutics 2023, 15, 1916. [Google Scholar] [CrossRef]
- Bruni, A.T.; de Carvalho, P.O.M.; Rodrigues, C.H.P.; Leite, V.B.P. methods in forensic science: Quantum chemistry and multivariate analysis applied to infrared spectra of new amphetamine- and cathinone-derived psychoactive substances. Forensic Chem. 2018, 9, 21–34. [Google Scholar] [CrossRef]
- Ellefsen, K.N.; Papsun, D.M.; Mata, D.C.; Ayala, J.L.; Modell, C.; Goodson, L.J.; Truver, M.T. Seized drug reporting of NPS helps to guide regional toxicological practice: A 17 month review between 2022 and 2023. J. Forensic Sci. 2024, 69, 1392–1399. [Google Scholar] [CrossRef] [PubMed]
- Shafi, A.; Berry, A.J.; Sumnall, H.; Wood, D.M.; Tracy, D.K. New psychoactive substances: A review and updates. Ther. Adv. Psychopharmacol. 2020, 10, 2045125320967197. [Google Scholar] [CrossRef]
| Analysis Method | Contaminant | Collection Method | Reference |
|---|---|---|---|
| Agar diffusion assay | Air pollution | Filter paper | Berra et al., 2015 [37]; Galperín et al., 2018 [38] |
| ELISA 1 | Tobacco smoke | Capillary tube | Rummenie et al., 2008 [39] |
| ELISA | Tobacco smoke | Capillary tube | Rummenie et al., 2008 [39] |
| Ethanol assay kit | Alcohol | Capillary tube | Kim et al., 2012 [40] |
| GC-MS 2 | Air pollution | Schirmer strip | Gutierrez et al., 2019 [41] |
| ICP-MS 3 | Trace elements | Capillary tube | Chen et al., 2022 [42] |
| Immunoassay | Mold | Capillary tube | Peltonen et al., 2008 [43] |
| Immunoassay | Air pollution | Capillary tube | Matsuda et al., 2015 [44]; Jing et al., 2022 [45] |
| LC-MS 4 | Ozone | Capillary tube | Paananen et al., 2015 [46] |
| PIXE 5 | Air pollution | Schirmer’s strip | Girshevitz et al., 2022 [47] |
| PSMs 6 | Smoke | Schirmer’s strip | Yao et al., 2020 [36] |
| PSMs | Aerosols | Schirmer’s strip | Yao et al., 2020 [36] |
| PSMs | Drugs of abuse | Schirmer’s strip | Yao et al., 2020 [36] |
| PSMs | Volatile organic compounds | Schirmer’s strip | Yao et al., 2020 [36] |
| SEM/EDS 7 | Particulate matter | Schirmer’s strip | Avula et al., 2017 [48] |
| SEM/EDS | Indoor environment | Schirmer’s strip | Kaplan et al., 2019 [49] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šoša, I.; Perković, M.; Baniček Šoša, I.; Grubešić, P.; Linšak, D.T.; Strenja, I. Absorption of Toxicants from the Ocular Surface: Potential Applications in Toxicology. Biomedicines 2025, 13, 645. https://doi.org/10.3390/biomedicines13030645
Šoša I, Perković M, Baniček Šoša I, Grubešić P, Linšak DT, Strenja I. Absorption of Toxicants from the Ocular Surface: Potential Applications in Toxicology. Biomedicines. 2025; 13(3):645. https://doi.org/10.3390/biomedicines13030645
Chicago/Turabian StyleŠoša, Ivan, Manuela Perković, Ivanka Baniček Šoša, Petra Grubešić, Dijana Tomić Linšak, and Ines Strenja. 2025. "Absorption of Toxicants from the Ocular Surface: Potential Applications in Toxicology" Biomedicines 13, no. 3: 645. https://doi.org/10.3390/biomedicines13030645
APA StyleŠoša, I., Perković, M., Baniček Šoša, I., Grubešić, P., Linšak, D. T., & Strenja, I. (2025). Absorption of Toxicants from the Ocular Surface: Potential Applications in Toxicology. Biomedicines, 13(3), 645. https://doi.org/10.3390/biomedicines13030645







