Functional Role of Fatty Acid Synthase for Signal Transduction in Core-Binding Factor Acute Myeloid Leukemia with an Activating c-Kit Mutation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.1.1. Bacterial Strain
2.1.2. Kits
2.1.3. Antibodies
2.1.4. Vectors
2.1.5. Inhibitors
2.2. Methods
2.2.1. Culturing of Cells
2.2.2. Proliferation
2.2.3. Cell Viability Assay
2.2.4. Transformation and Plasmid Preparation
2.2.5. Lentiviral Knockdown of FASN
2.2.6. Immunoblotting
2.2.7. Lipidomic Chromatography
2.3. Mass Spectrometry-Based Differential Quantitative Proteome Analysis
2.3.1. Protein Extraction and Tryptic Digestion
2.3.2. LC-MS/MS Acquisition and Data Processing
2.3.3. Kinome Analysis
2.4. Statistical Analysis
2.4.1. Statistical Analysis of Proteomic Data
2.4.2. Software
3. Results
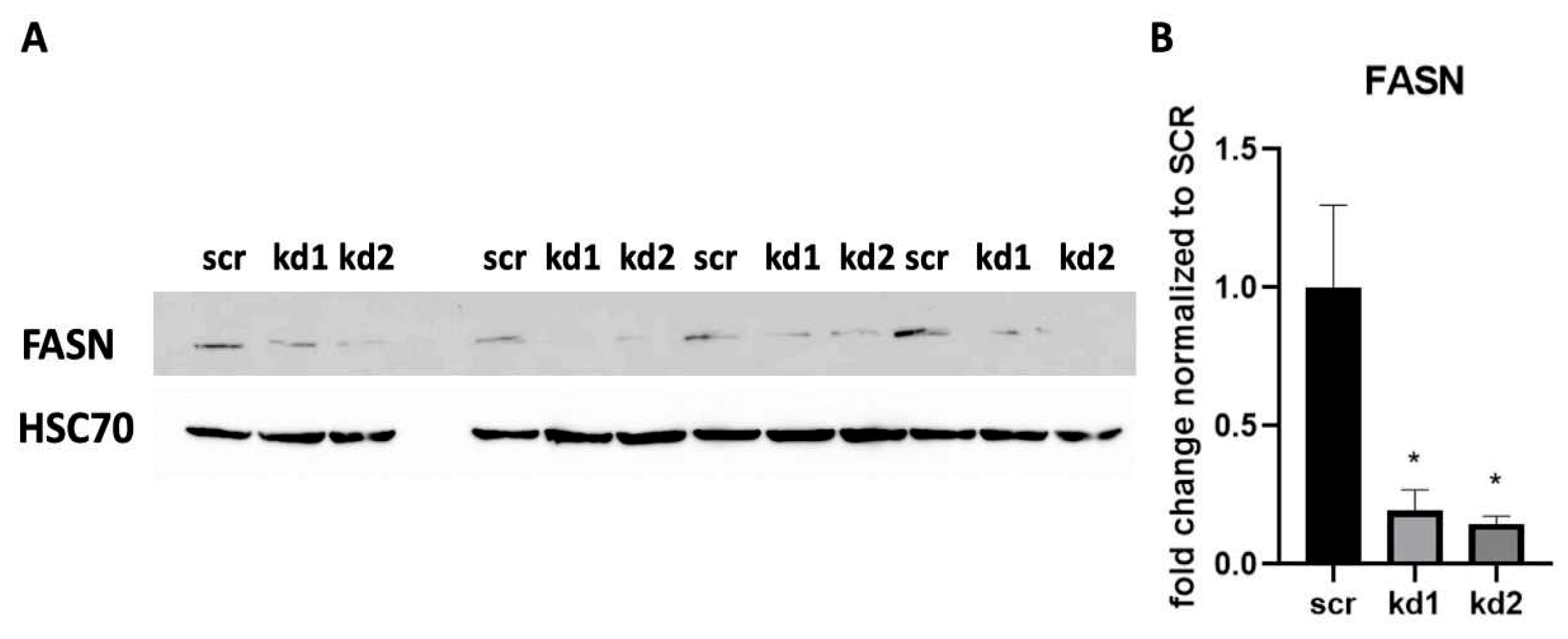
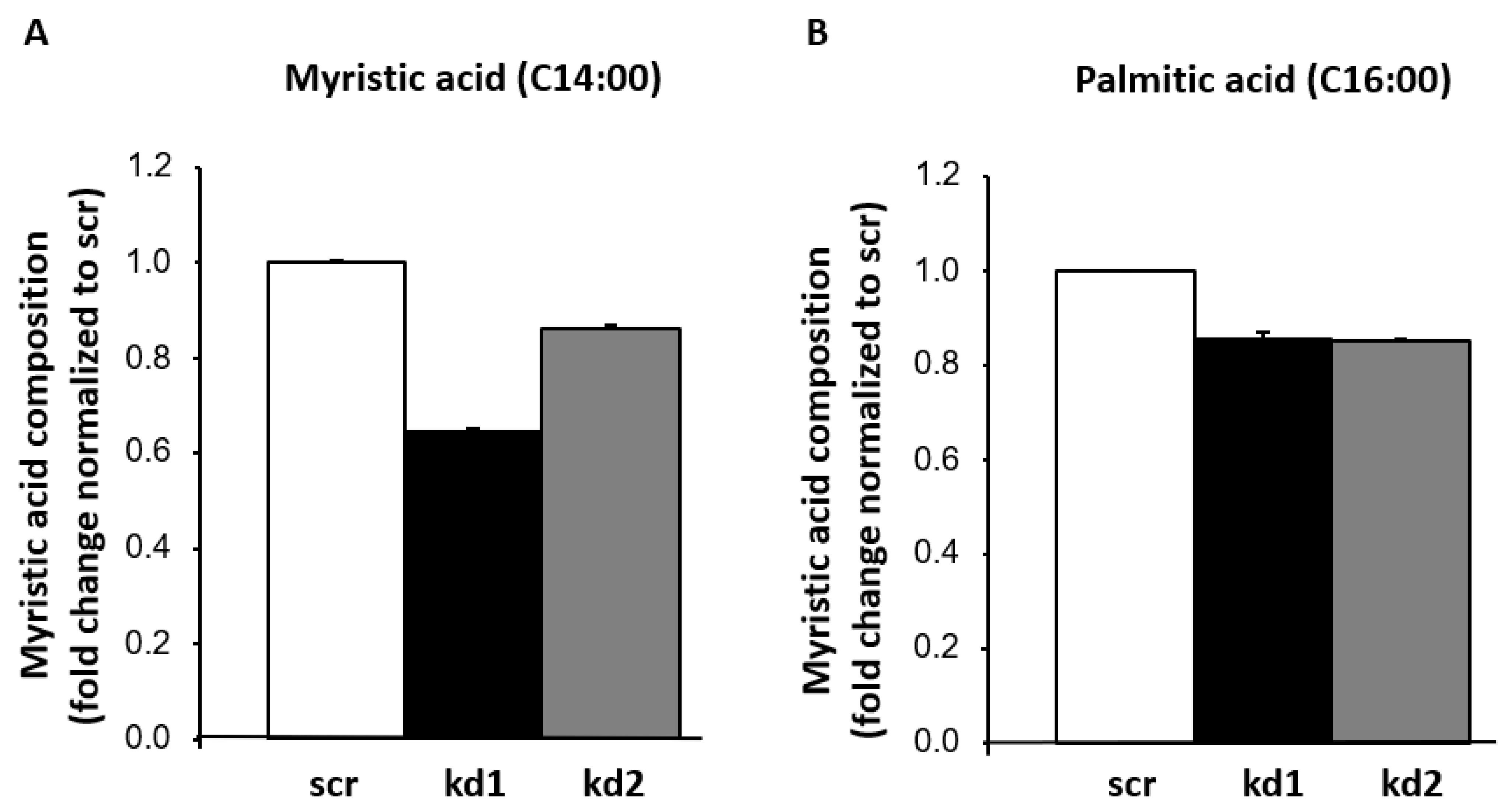
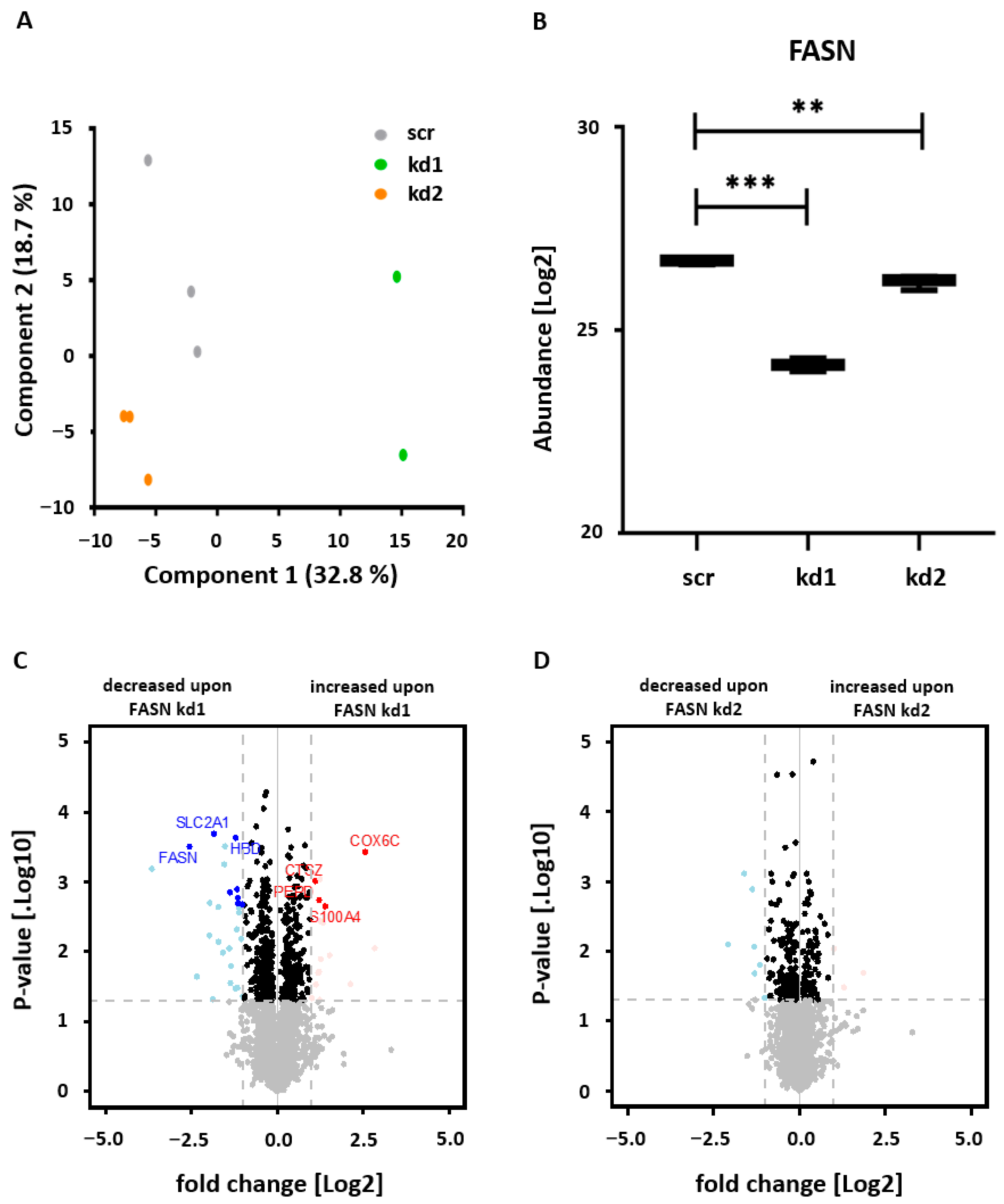
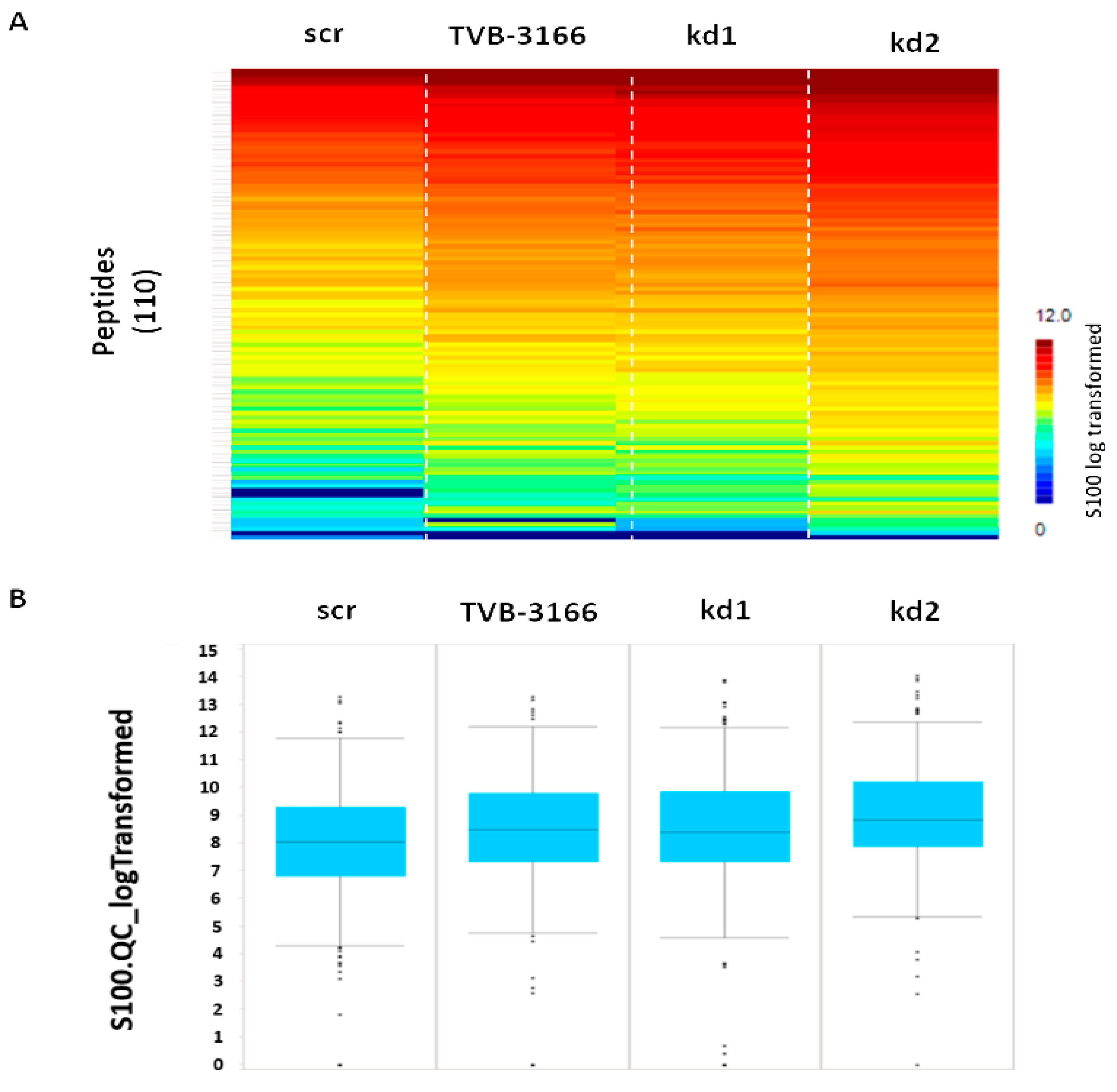
3.1. Establishment of Stable FASN Knockdown in Kasumi1 by Proteomic Analysis
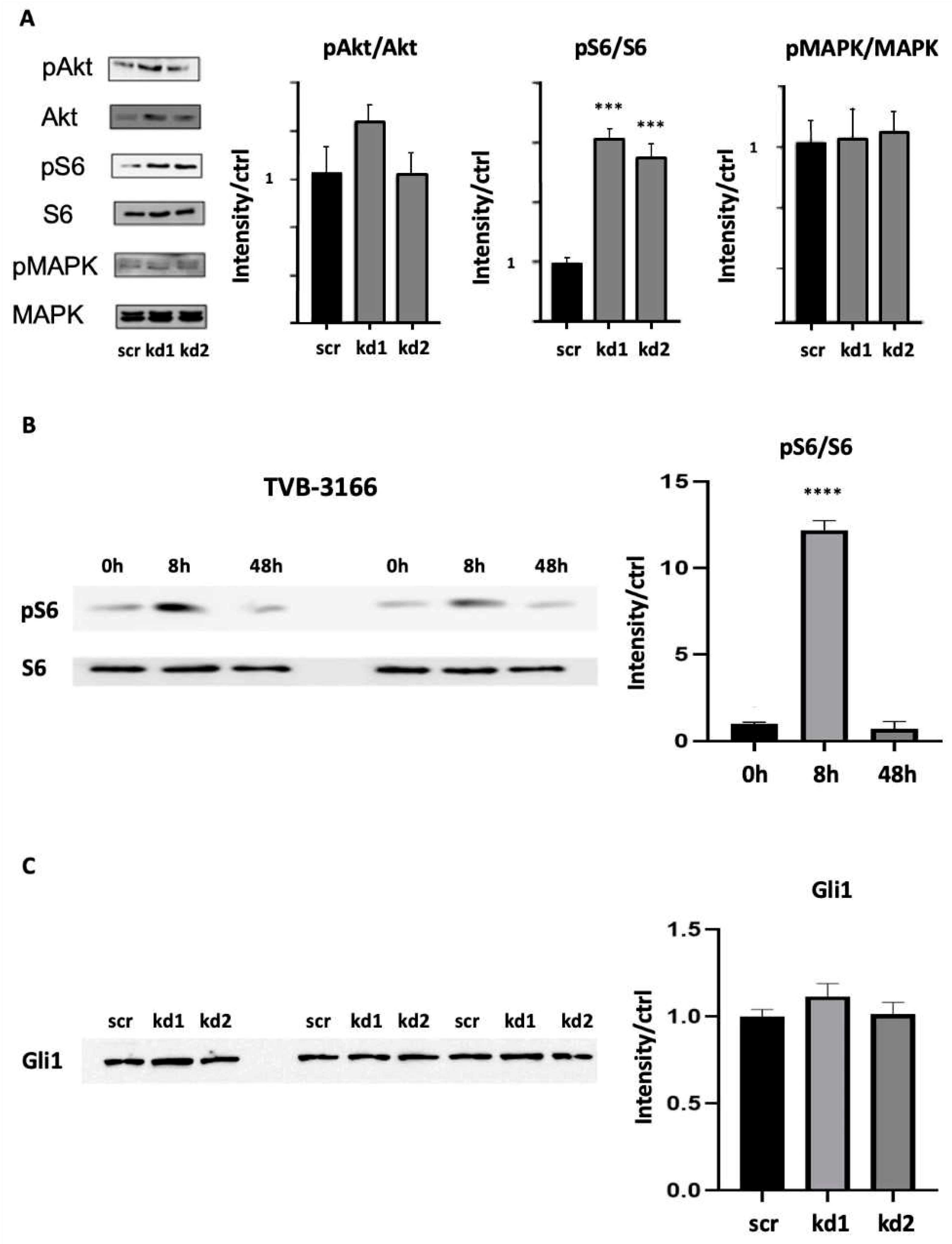
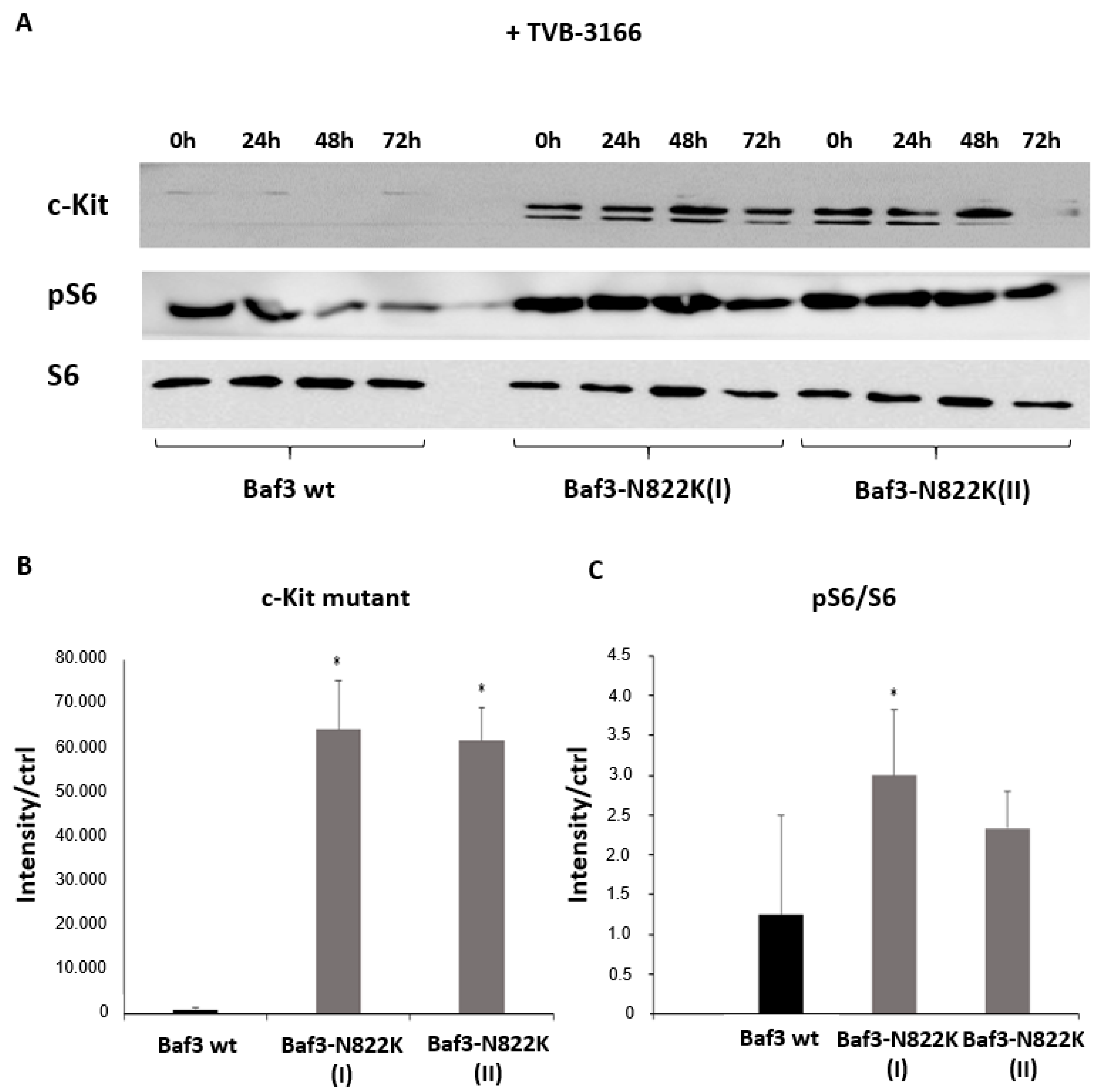
3.2. Upregulation of p-c-Kit, pLyn, pMAPK, and pS6 in Kasumi1, and in Baf3 c-Kit N822K Cells After Pharmacological and Genetic FASN Inhibition
3.3. No Changes in the Proliferation Activity of Kasumi1 Cells After the Inhibition of FASN
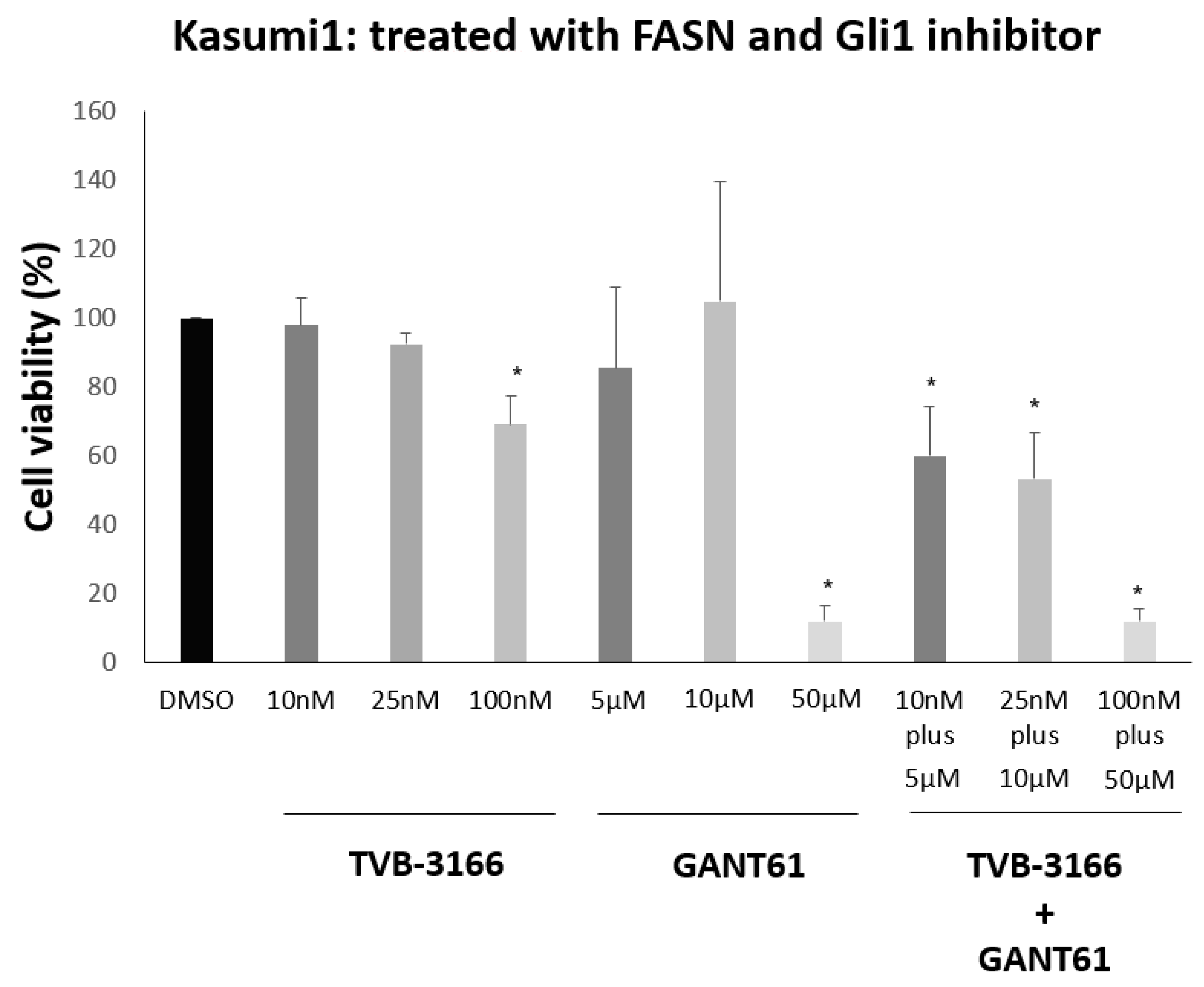
3.4. Reduction in Kasumi1 Cell Viability by Combination of FASN Inhibitor TVB-3166 with Gli1 Inhibitor GANT61
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AML | Acute myeloid leukemia |
| BAD | Bcl-2-associated agonist of cell death protein |
| FLT3 | FMS-like tyrosine kinase 3 |
| FLT3-WT | Wild-type FLT3 |
| FLT3-ITD | Internal tandem duplication |
| JM domain | Juxtamembrane domain |
| FLT3-TKD | Tyrosine kinase domain |
| PM | Plasma membrane |
| ERK | RAS/extracellular signal-regulated kinase |
| C563 | Cysteine at position 563 |
| CBF-AML | Core-binding factor AML |
| MAPK | Mitogen-activated protein kinase |
| SFKs | Src family kinases |
| FASN | Fatty acid synthase |
| RPS6 | Ribosomal Protein S6 |
| CD117 | Cluster of differentiation 117 |
| CBF | Core-binding factor |
References
- Koenig, K.L.; Sahasrabudhe, K.D.; Sigmund, A.M.; Bhatnagar, B. AML with Myelodysplasia-Related Changes: Development, Challenges, and Treatment Advances. Genes 2020, 11, 845. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, M.; Wang, E.S. Novel therapies for AML: A round-up for clinicians. Expert Rev. Clin. Pharmacol. 2020, 13, 1389–1400. [Google Scholar] [CrossRef] [PubMed]
- Döhner, H.; Wei, A.H.; Appelbaum, F.R.; Craddock, C.; DiNardo, C.D.; Dombret, H.; Ebert, B.L.; Fenaux, P.; Godley, L.A.; Hasserjian, R.P.; et al. Diagnosis management of AML in adults: 2022 recommendations from an international expert panel on behalf of the, ELN. Blood 2022, 140, 1345–1377. [Google Scholar] [CrossRef] [PubMed]
- Weinberg, O.K.; Porwit, A.; Orazi, A.; Hasserjian, R.P.; Foucar, K.; Duncavage, E.J.; Arber, D.A. The International Consensus Classification of acute myeloid leukemia. Virchows Arch. 2023, 482, 27–37. [Google Scholar] [CrossRef]
- Papaemmanuil, E.; Gerstung, M.; Bullinger, L.; Gaidzik, V.I.; Paschka, P.; Roberts, N.D.; Potter, N.E.; Heuser, M.; Thol, F.; Bolli, N.; et al. Genomic Classification and Prognosis in Acute Myeloid Leukemia. N. Engl. J. Med. 2016, 374, 2209–2221. [Google Scholar] [CrossRef]
- The Cancer Genome Atlas Research Network. Genomic and epigenomic landscapes of adult de novo acute myeloid leukemia. N. Engl. J. Med. 2013, 368, 2059–2074. [Google Scholar] [CrossRef]
- Papaemmanuil, E.; Gerstung, M.; Malcovati, L.; Tauro, S.; Gundem, G.; Van Loo, P.; Yoon, C.J.; Ellis, P.; Wedge, D.C.; Pellagatti, A.; et al. Clinical and biological implications of driver mutations in myelodysplastic syndromes. Blood 2013, 122, 3616–3627, quiz 99. [Google Scholar] [CrossRef]
- Paschka, P.; Schlenk, R.F.; Weber, D.; Benner, A.; Bullinger, L.; Heuser, M.; I Gaidzik, V.; Thol, F.; Agrawal, M.; Teleanu, V.; et al. Adding dasatinib to intensive treatment in core-binding factor acute myeloid leukemia-results of the AMLSG 11-08 trial. Leukemia 2018, 32, 1621–1630. [Google Scholar] [CrossRef]
- Masson, K.; Ronnstrand, L. Oncogenic signaling from the hematopoietic growth factor receptors c-Kit and Flt3. Cell. Signal. 2009, 21, 1717–1726. [Google Scholar] [CrossRef]
- Linnekin, D. Early signaling pathways activated by c-Kit in hematopoietic cells. Int. J. Biochem. Cell Biol. 1999, 31, 1053–1074. [Google Scholar] [CrossRef]
- Wandzioch, E.; Edling, C.E.; Palmer, R.H.; Carlsson, L.; Hallberg, B. Activation of the MAP kinase pathway by c-Kit is PI-3 kinase dependent in hematopoietic progenitor/stem cell lines. Blood 2004, 104, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Pathania, S.; Rawal, R.K. An update on chemical classes targeting ERK1/2 for the management of cancer. Future Med. Chem. 2020, 12, 593–611. [Google Scholar] [CrossRef] [PubMed]
- Carrière, A.; Cargnello, M.; Julien, L.-A.; Gao, H.; Bonneil, E.; Thibault, P.; Roux, P.P. Oncogenic MAPK signaling stimulates mTORC1 activity by promoting RSK-mediated raptor phosphorylation. Curr. Biol. 2008, 18, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Lai, K.O.; Liang, Z.; Fei, E.; Huang, H.; Ip, N.Y. Cyclin-dependent Kinase 5 (Cdk5)-dependent Phosphorylation of p70 Ribosomal S6 Kinase 1 (S6K) Is Required for Dendritic Spine Morphogenesis. J. Biol. Chem. 2015, 290, 14637–14646. [Google Scholar] [CrossRef]
- Mukhopadhyay, N.K.; Price, D.J.; Kyriakis, J.M.; Pelech, S.; Sanghera, J.; Avruch, J. An array of insulin-activated, proline-directed serine/threonine protein kinases phosphorylate the p70 S6 kinase. J. Biol. Chem. 1992, 267, 3325–3335. [Google Scholar] [CrossRef]
- Leevers, S.J.; Marshall, C.J. Activation of extracellular signal-regulated kinase, ERK2, by p21ras oncoprotein. EMBO J. 1992, 11, 569–574. [Google Scholar] [CrossRef]
- Lobell, R.B.; A Omer, C.; Abrams, M.T.; Bhimnathwala, H.G.; Brucker, M.J.; A Buser, C.; Davide, J.P.; Desolms, S.J.; Dinsmore, C.J.; Ellis-Hutchings, M.S.; et al. Evaluation of farnesyl:protein transferase and geranylgeranyl:protein transferase inhibitor combinations in preclinical models. Cancer Res. 2001, 61, 8758–8768. [Google Scholar]
- Choi, W.-I.; Jeon, B.-N.; Park, H.; Yoo, J.-Y.; Kim, Y.-S.; Koh, D.-I.; Kim, M.-H.; Kim, Y.-R.; Lee, C.-E.; Kim, K.-S.; et al. SwissPalm: Protein Palmitoylation database. F1000Research 2015, 4, 261. [Google Scholar] [CrossRef]
- Choi, W.-I.; Jeon, B.-N.; Park, H.; Yoo, J.-Y.; Kim, Y.-S.; Koh, D.-I.; Kim, M.-H.; Kim, Y.-R.; Lee, C.-E.; Kim, K.-S.; et al. Proto-oncogene FBI-1 (Pokemon) and SREBP-1 synergistically activate transcription of fatty-acid synthase gene (FASN). J. Biol. Chem. 2008, 283, 29341–29354. [Google Scholar] [CrossRef]
- Van de Sande, T.; Roskams, T.; Lerut, E.; Joniau, S.; Van Poppel, H.; Verhoeven, G.; Swinnen, J.V. High-level expression of fatty acid synthase in human prostate cancer tissues is linked to activation and nuclear localization of Akt/PKB. J. Pathol. 2005, 206, 214–219. [Google Scholar] [CrossRef]
- Ventura, R.; Mordec, K.; Waszczuk, J.; Wang, Z.; Lai, J.; Fridlib, M.; Buckley, D.; Kemble, G.; Heuer, T.S. Inhibition of de novo Palmitate Synthesis by Fatty Acid Synthase Induces Apoptosis in Tumor Cells by Remodeling Cell Membranes, Inhibiting Signaling Pathways, and Reprogramming Gene Expression. EBioMedicine 2015, 2, 808–824. [Google Scholar] [CrossRef] [PubMed]
- Cammenga, J.; Horn, S.; Bergholz, U.; Sommer, G.; Besmer, P.; Fiedler, W.; Stocking, C. Extracellular KIT receptor mutants, commonly found in core binding factor AML, are constitutively active and respond to imatinib mesylate. Blood 2005, 106, 3958–3961. [Google Scholar] [CrossRef] [PubMed]
- Perez-Riverol, Y.; Bai, J.; Bandla, C.; García-Seisdedos, D.; Hewapathirana, S.; Kamatchinathan, S.; Kundu, D.J.; Prakash, A.; Frericks-Zipper, A.; Eisenacher, M.; et al. The PRIDE database resources in 2022: A hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 2022, 50, D543–D552. [Google Scholar] [CrossRef]
- Bußmann, L.; Hoffer, K.; von Bargen, C.M.; Droste, C.; Lange, T.; Kemmling, J.; Schröder-Schwarz, J.; Vu, A.T.; Akingunsade, L.; Nollau, P.; et al. Analyzing tyrosine kinase activity in head and neck cancer by functional kinomics: Identification of hyperactivated Src family kinases as prognostic markers and potential targets. Int. J. Cancer 2021, 149, 1166–1180. [Google Scholar] [CrossRef] [PubMed]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 24 February 2025).
- Ayatollahi, H.; Shajiei, A.; Sadeghian, M.H.; Sheikhi, M.; Yazdandoust, E.; Ghazanfarpour, M.; Shams, S.F.; Shakeri, S. Prognostic Importance of C-KIT Mutations in Core Binding Factor Acute Myeloid Leukemia: A Systematic Review. Hematol. Oncol. Stem Cell Ther. 2017, 10, 1–7. [Google Scholar] [CrossRef]
- Paschka, P.; Du, J.; Schlenk, R.F.; Gaidzik, V.I.; Bullinger, L.; Corbacioglu, A.; Späth, D.; Kayser, S.; Schlegelberger, B.; Krauter, J.; et al. Secondary genetic lesions in acute myeloid leukemia with inv(16) or t(16;16): A study of the German-Austrian AML Study Group (AMLSG). Blood 2013, 121, 170–177. [Google Scholar] [CrossRef]
- Ikeda, K.; Nakayama, Y.; Togashi, Y.; Obata, Y.; Kuga, T.; Kasahara, K.; Fukumoto, Y.; Yamaguchi, N. Nuclear localization of Lyn tyrosine kinase mediated by inhibition of its kinase activity. Exp. Cell Res. 2008, 314, 3392–3404. [Google Scholar] [CrossRef]
- Dos Santos, C.; Demur, C.; Bardet, V.; Prade-Houdellier, N.; Payrastre, B.; Recher, C. A critical role for Lyn in acute myeloid leukemia. Blood 2008, 111, 2269–2279. [Google Scholar] [CrossRef]
- Okamoto, M.; Hayakawa, F.; Miyata, Y.; Watamoto, K.; Emi, N.; Abe, A.; Kiyoi, H.; Towatari, M.; Naoe, T. Lyn is an important component of the signal transduction pathway specific to FLT3/ITD and can be a therapeutic target in the treatment of AML with FLT3/ITD. Leukemia 2007, 21, 403–410. [Google Scholar] [CrossRef]
- Ali, A.; Levantini, E.; Teo, J.T.; Goggi, J.; Clohessy, J.G.; Wu, C.S.; Chen, P.L.; Yang, H.; Krishnan, I.; Kocher, O.; et al. Fatty acid synthase mediates EGFR palmitoylation in EGFR mutated non-small cell lung cancer. EMBO Mol. Med. 2018, 10, e8313. [Google Scholar] [CrossRef]
- Falchook, G.; Infante, J.; Arkenau, H.-T.; Patel, M.R.; Dean, E.; Borazanci, E.; Brenner, A.; Cook, N.; Lopez, J.; Pant, S.; et al. First-in-human study of the safety, pharmacokinetics, and pharmacodynamics of first-in-class fatty acid synthase inhibitor TVB-2640 alone and with a taxane in advanced tumors. EClinicalMedicine 2021, 34, 100797. [Google Scholar] [CrossRef] [PubMed]
- Kelly, W.; Duque, A.E.D.; Michalek, J.; Konkel, B.; Caflisch, L.; Chen, Y.; Pathuri, S.C.; Madhusudanannair-Kunnuparampil, V.; Floyd, J.; Brenner, A. Phase II Investigation of TVB-2640 (Denifanstat) with Bevacizumab in Patients with First Relapse High-Grade Astrocytoma. Clin. Cancer Res. 2023, 29, 2419–2425. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.E.; Jung, B.H.; Park, J.; Kang, S.; Lee, H. Deciphering Fatty Acid Synthase Inhibition-Triggered Metabolic Flexibility in Prostate Cancer Cells through Untargeted Metabolomics. Cells 2020, 9, 2447. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.-G.; Xing, B.; Lv, K.; O’keefe, R.A.; Wu, M.; Wang, R.; Bauer, K.M.; Ghazaryan, A.; Burslem, G.M.; Zhang, J.; et al. RAB27B controls palmitoylation-dependent NRAS trafficking and signaling in myeloid leukemia. J. Clin. Investig. 2023, 133, e165510. [Google Scholar] [CrossRef]
- Benjamin, D.I.; Li, D.S.; Lowe, W.; Heuer, T.; Kemble, G.; Nomura, D.K. Diacylglycerol Metabolism and Signaling Is a Driving Force Underlying FASN Inhibitor Sensitivity in Cancer Cells. ACS Chem. Biol. 2015, 10, 1616–1623. [Google Scholar] [CrossRef]
- Obata, Y.; Hara, Y.; Shiina, I.; Murata, T.; Tasaki, Y.; Suzuki, K.; Ito, K.; Tsugawa, S.; Yamawaki, K.; Takahashi, T.; et al. N822K- or V560G-mutated KIT activation preferentially occurs in lipid rafts of the Golgi apparatus in leukemia cells. Cell Commun. Signal. 2019, 17, 114. [Google Scholar] [CrossRef]
- Li, M.; Zhang, L.; Chen, C.W. Diverse Roles of Protein Palmitoylation in Cancer Progression, Immunity, Stemness, and Beyond. Cells 2023, 12, 2209. [Google Scholar] [CrossRef]
- Xie, J.; Ponuwei, G.A.; Moore, C.E.; Willars, G.B.; Tee, A.R.; Herbert, T.P. cAMP inhibits mammalian target of rapamycin complex-1 and -2 (mTORC1 and 2) by promoting complex dissociation and inhibiting mTOR kinase activity. Cell. Signal. 2011, 23, 1927–1935. [Google Scholar] [CrossRef]
- Ventura, A.P.; Radhakrishnan, S.; Green, A.; Rajaram, S.K.; Allen, A.N.; O’Briant, K.; Schummer, M.; Karlan, B.; Urban, N.; Tewari, M.; et al. Activation of the MEK-S6 pathway in high-grade ovarian cancers. Appl. Immunohistochem. Mol. Morphol. 2010, 18, 499–508. [Google Scholar] [CrossRef]
- Yang, X.; Liu, L.; Sternberg, D.; Tang, L.; Galinsky, I.; DeAngelo, D.; Stone, R. The FLT3 internal tandem duplication mutation prevents apoptosis in interleukin-3-deprived BaF3 cells due to protein kinase A and ribosomal S6 kinase 1-mediated BAD phosphorylation at serine 112. Cancer Res. 2005, 65, 7338–7347. [Google Scholar] [CrossRef]
- Sun, L.; Yao, Y.; Pan, G.; Zhan, S.; Shi, W.; Lu, T.; Yuan, J.; Tian, K.; Jiang, L.; Song, S.; et al. Small interfering RNA-mediated knockdown of fatty acid synthase attenuates the proliferation and metastasis of human gastric cancer cells via the mTOR/Gli1 signaling pathway. Oncol. Lett. 2018, 16, 594–602. [Google Scholar] [CrossRef]
- Abraham, A.; Matsui, W. Hedgehog Signaling in Myeloid Malignancies. Cancers 2021, 13, 4888. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhuang, R.; Siebels, B.; Hoffer, K.; Worthmann, A.; Horn, S.; von Bubnoff, N.C.C.; Khandanpour, C.; Gebauer, N.; Gorantla, S.P.; Voss, H.; et al. Functional Role of Fatty Acid Synthase for Signal Transduction in Core-Binding Factor Acute Myeloid Leukemia with an Activating c-Kit Mutation. Biomedicines 2025, 13, 619. https://doi.org/10.3390/biomedicines13030619
Zhuang R, Siebels B, Hoffer K, Worthmann A, Horn S, von Bubnoff NCC, Khandanpour C, Gebauer N, Gorantla SP, Voss H, et al. Functional Role of Fatty Acid Synthase for Signal Transduction in Core-Binding Factor Acute Myeloid Leukemia with an Activating c-Kit Mutation. Biomedicines. 2025; 13(3):619. https://doi.org/10.3390/biomedicines13030619
Chicago/Turabian StyleZhuang, Ruimeng, Bente Siebels, Konstantin Hoffer, Anna Worthmann, Stefan Horn, Nikolas Christian Cornelius von Bubnoff, Cyrus Khandanpour, Niklas Gebauer, Sivahari Prasad Gorantla, Hanna Voss, and et al. 2025. "Functional Role of Fatty Acid Synthase for Signal Transduction in Core-Binding Factor Acute Myeloid Leukemia with an Activating c-Kit Mutation" Biomedicines 13, no. 3: 619. https://doi.org/10.3390/biomedicines13030619
APA StyleZhuang, R., Siebels, B., Hoffer, K., Worthmann, A., Horn, S., von Bubnoff, N. C. C., Khandanpour, C., Gebauer, N., Gorantla, S. P., Voss, H., Schlüter, H., Kriegs, M., Fiedler, W., Bokemeyer, C., Jücker, M., & Kebenko, M. (2025). Functional Role of Fatty Acid Synthase for Signal Transduction in Core-Binding Factor Acute Myeloid Leukemia with an Activating c-Kit Mutation. Biomedicines, 13(3), 619. https://doi.org/10.3390/biomedicines13030619







