Exploring Neutrophil Heterogeneity and Plasticity in Health and Disease
Abstract
1. Introduction
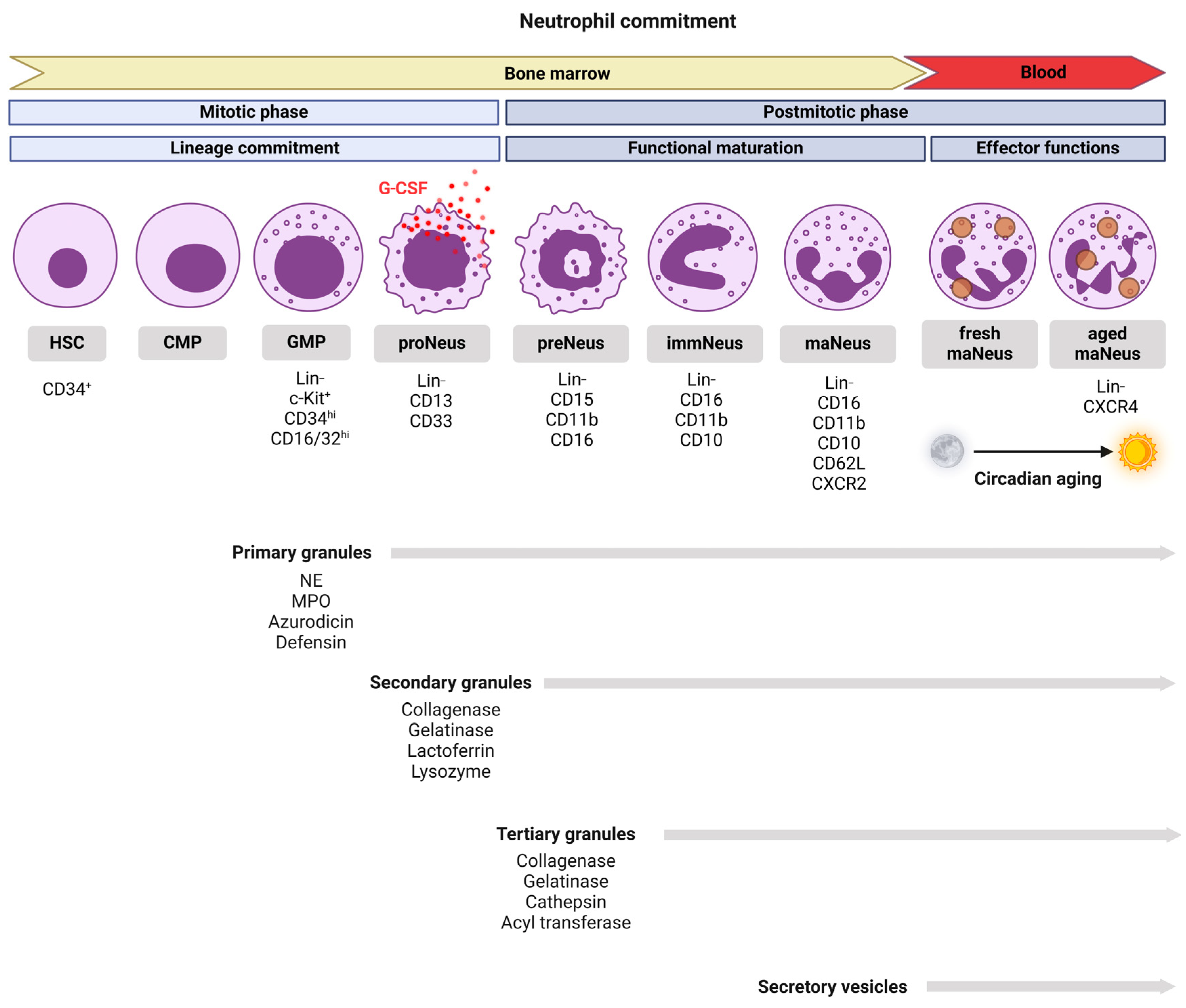
2. Neutrophil Development and Lifespan
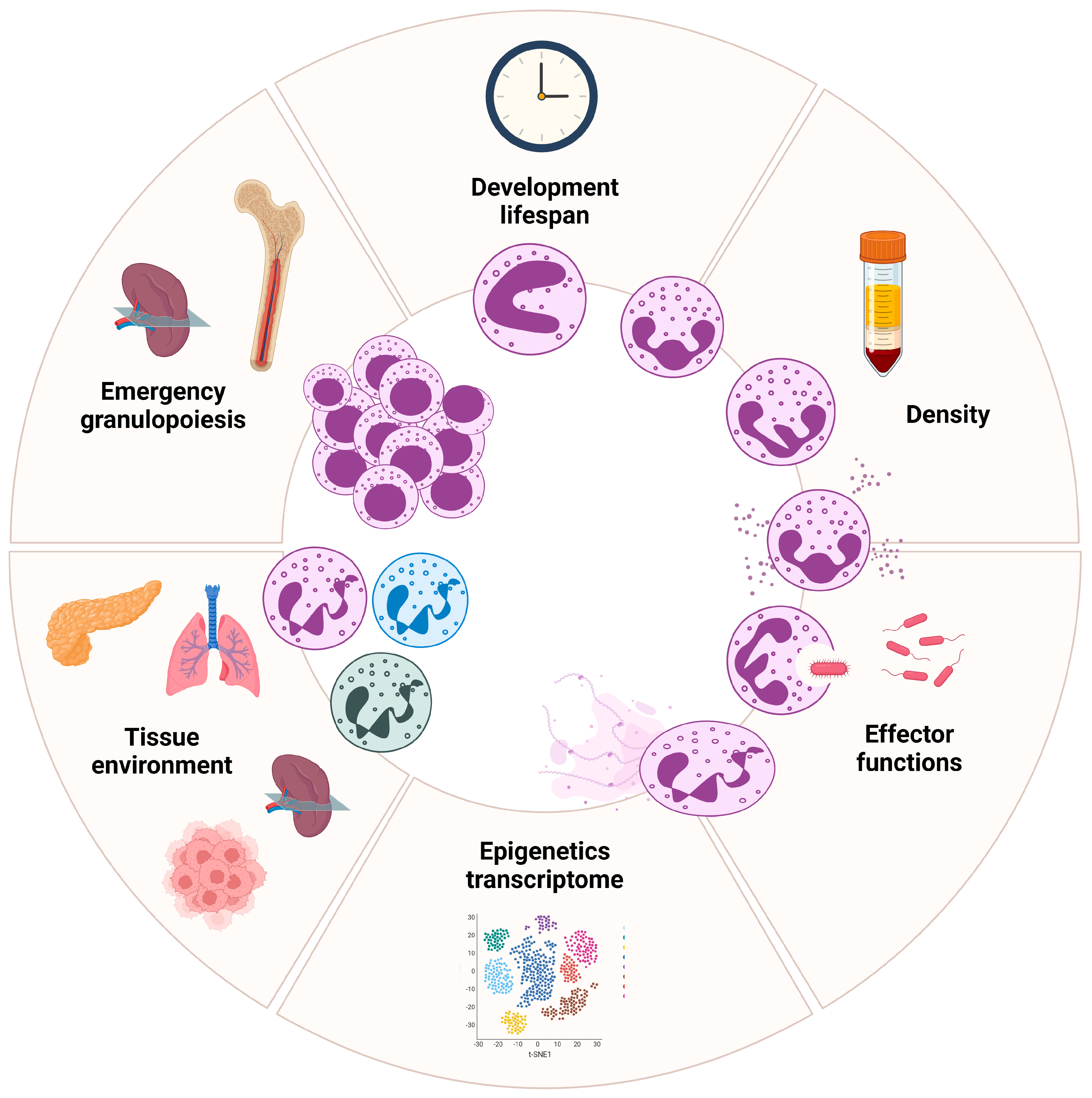
3. Density-Based Heterogeneity in Neutrophils
3.1. High-Density Neutrophils
3.2. Low-Density Neutrophils
3.3. Mechanisms Underlying Neutrophil Density Heterogeneity
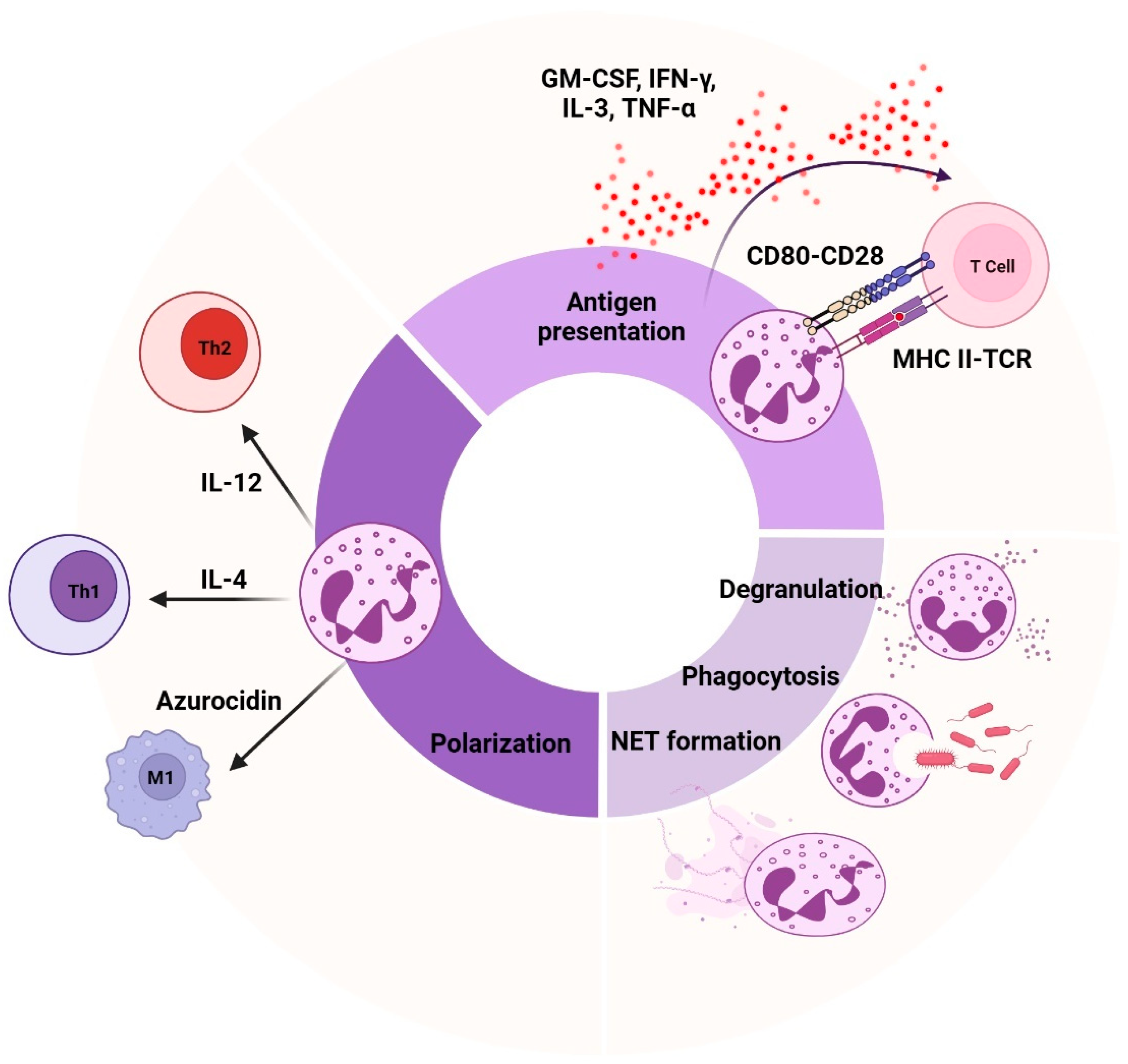
4. Functional Heterogeneity in Neutrophils
5. Transcriptional and Phenotypic Heterogeneity in Neutrophils
5.1. Neutrophil Epigenetic and Transcriptional Regulation, from Bone Marrow to Circulation
5.2. Neutrophil Reprogramming in Tissue Microenvironment
5.3. Emergency Granulopoiesis by Extrinsic Cues
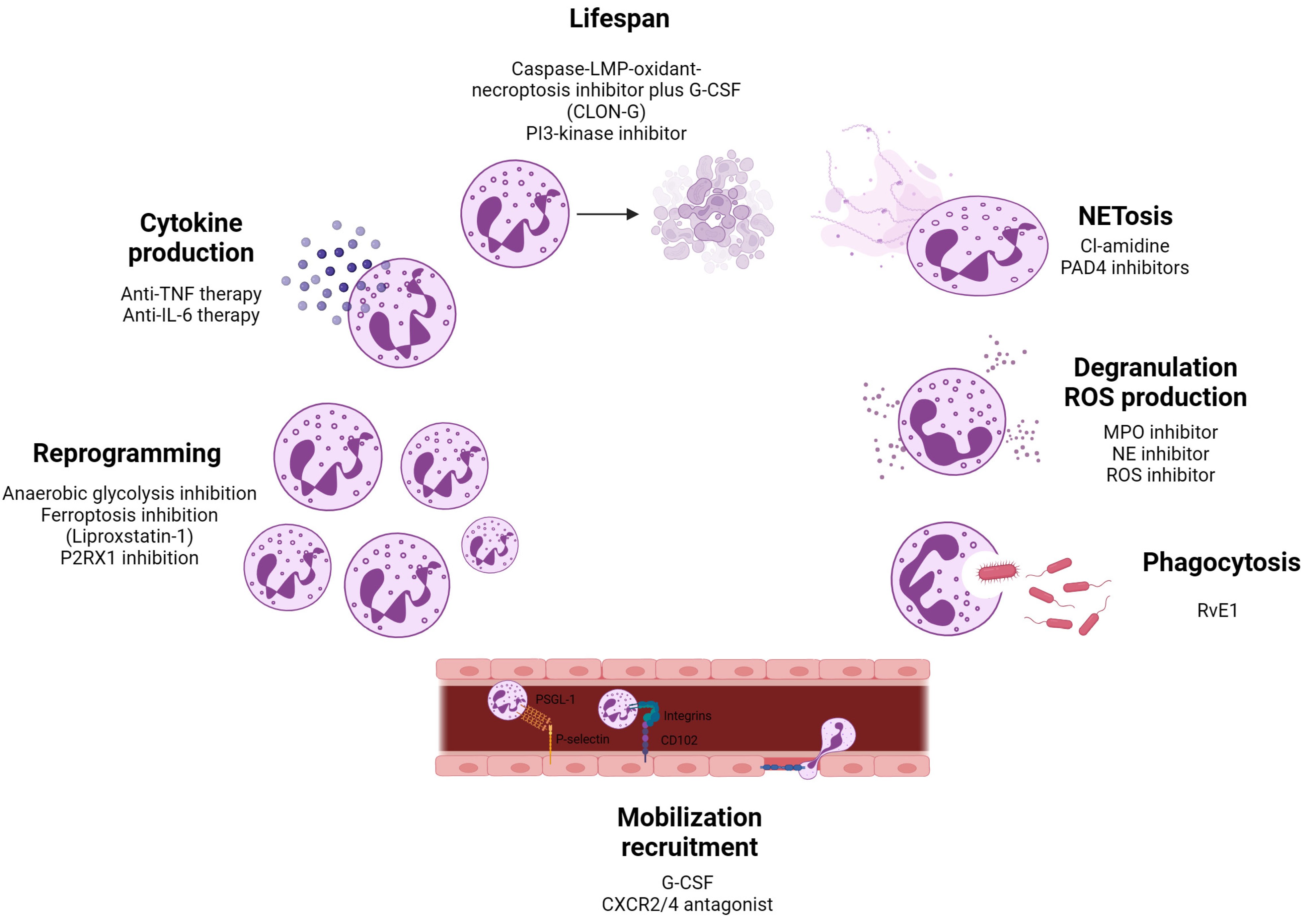
6. Neutrophils as Therapeutic Targets in Disease Contexts
6.1. Sepsis
6.2. Alzheimer’s Disease
6.3. Vasculitis
6.4. Systemic Lupus Erythematosus
6.5. Rheumatoid Arthritis
6.6. Multiple Sclerosis
6.7. Type 1 Diabetes
6.8. Cancer
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Deniset, F.J.; Kubes, P. Neutrophil heterogeneity: Bona fide subsets or polarization states? J. Leukoc. Biol. 2018, 103, 829–838. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, K.; Joshi, B.M. Neutrophil sub-types in maintaining immune homeostasis during steady state, infections and sterile inflammation. Inflamm. Res. 2023, 72, 1175–1192. [Google Scholar] [CrossRef] [PubMed]
- Aroca-Crevillén, A.; Vicanolo, T.; Ovadia, S.; Hidalgo, A. Neutrophils in physiology and pathology. Annu. Rev. Pathol. Mech. Dis. 2024, 19, 227–259. [Google Scholar] [CrossRef] [PubMed]
- Sreejit, G.; Johnson, J.; Jaggers, M.R.; Dahdah, A.; Murphy, J.A.; Hanssen, J.M.N.; Nagareddy, R.P. Neutrophils in cardiovascular disease: Warmongers, peacemakers, or both? Cardiovasc. Res. 2022, 118, 2596–2609. [Google Scholar] [CrossRef]
- Lehman, K.H.; Segal, H.B. The role of neutrophils in host defense and disease. J. Allergy Clin. Immunol. 2020, 145, 1535–1544. [Google Scholar] [CrossRef]
- Akashi, K.; Traver, D.; Miyamoto, T.; Weissman, L.I. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature 2000, 404, 193–197. [Google Scholar] [CrossRef]
- Hock, H.; Hamblen, J.M.; Rooke, M.H.; Traver, D.; Bronson, T.R.; Cameron, S.; Orkin, H.S. Intrinsic requirement for zinc finger transcription factor Gfi-1 in neutrophil differentiation. Immunity 2003, 18, 109–120. [Google Scholar] [CrossRef]
- Sierra, L.L.D.M.; Sakakibara, S.; Gasperini, P.; Salvucci, O.; Jiang, K.; Mccormick, J.P.; Segarra, M.; Stone, J.; Maric, D.; Zhu, J.; et al. The transcription factor Gfi1 regulates G-CSF signaling and neutrophil development through the Ras activator RasGRP1. Blood 2010, 115, 3970–3979. [Google Scholar] [CrossRef]
- Schnoor, M.; Vadillo, E.; Guerrero-Fonseca, M.I. The extravasation cascade revisited from a neutrophil perspective. Curr. Opin. Physiol. 2021, 19, 119–128. [Google Scholar] [CrossRef]
- Nourshargh, S.; Alon, R. Leukocyte migration into inflamed tissues. Immunity 2014, 41, 694–707. [Google Scholar] [CrossRef]
- Maas, L.S.; Soehnlein, O.; Viola, R.J. Organ-specific mechanisms of transendothelial neutrophil migration in the lung, liver, kidney, and aorta. Front. Immunol. 2018, 9, 2739. [Google Scholar] [CrossRef] [PubMed]
- Nourshargh, S.; Renshaw, A.S.; Imhof, A.B. Reverse migration of neutrophils: Where, when, how, and why? Trends Immunol. 2016, 37, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Ovadia, S.; Özcan, A.; Hidalgo, A. The circadian neutrophil, inside-out. J. Leukoc. Biol. 2023, 113, 555–566. [Google Scholar] [CrossRef]
- Ella, K.; Csépányi-Kömi, R.; Káldi, K. Circadian regulation of human peripheral neutrophils. Brain Behav. Immun. 2016, 57, 209–221. [Google Scholar] [CrossRef]
- Casanova-Acebes, M.; Pitaval, C.; Weiss, A.L.; Nombela-Arrieta, C.; Chèvre, R.; A-González, N.; Kunisaki, Y.; Zhang, D.; Rooijen, V.N.; Silberstein, E.L.; et al. Rhythmic modulation of the hematopoietic niche through neutrophil clearance. Cell 2013, 153, 1025–1035. [Google Scholar] [CrossRef]
- Pember, S.; Barnes, K.; Brandt, S.; Kinkade, J.J. Density heterogeneity of neutrophilic polymorphonuclear leukocytes: Gradient fractionation and relationship to chemotactic stimulation. Blood 1983, 61, 1105–1115. [Google Scholar] [CrossRef]
- Hacbarth, E.; Kajdacsy-Balla, A. Low density neutrophils in patients with systemic lupus erythematosus, rheumatoid arthritis, and acute rheumatic fever. Arthritis Rheum. 1986, 29, 1334–1342. [Google Scholar] [CrossRef]
- He, W.; Yan, L.; Hu, D.; Hao, J.; Liou, Y.C.; Luo, G. Neutrophil heterogeneity and plasticity: Unveiling the multifaceted roles in health and disease. MedComm (2020) 2025, 6, e70063. [Google Scholar] [CrossRef]
- Hassani, M.; Hellebrekers, P.; Chen, N.; Aalst, V.C.; Bongers, S.; Hietbrink, F.; Koenderman, L.; Vrisekoop, N. On the origin of low-density neutrophils. J. Leukoc. Biol. 2020, 107, 809–818. [Google Scholar] [CrossRef]
- Sagiv, Y.J.; Michaeli, J.; Assi, S.; Mishalian, I.; Kisos, H.; Levy, L.; Damti, P.; Lumbroso, D.; Polyansky, L.; Sionov, V.R.; et al. Phenotypic diversity and plasticity in circulating neutrophil subpopulations in cancer. Cell Rep. 2015, 10, 562–573. [Google Scholar] [CrossRef]
- Blanco-Camarillo, C.; Alemán, R.O.; Rosales, C. Low-density neutrophils in healthy individuals display a mature primed phenotype. Front. Immunol. 2021, 12, 672520. [Google Scholar] [CrossRef] [PubMed]
- Waight, D.J.; Hu, Q.; Miller, A.; Liu, S.; Abrams, I.S. Tumor-derived G-csf facilitates neoplastic growth through a granulocytic myeloid-derived suppressor cell-dependent mechanism. PLoS ONE 2011, 6, e27690. [Google Scholar] [CrossRef] [PubMed]
- Denny, F.M.; Yalavarthi, S.; Zhao, W.; Thacker, G.S.; Anderson, M.; Sandy, R.A.; Mccune, J.W.; Kaplan, J.M. A distinct subset of proinflammatory neutrophils isolated from patients with systemic lupus erythematosus induces vascular damage and synthesizes type I IFNs. J. Immunol. 2010, 184, 3284–3297. [Google Scholar] [CrossRef] [PubMed]
- Othman, A.; Sekheri, M.; Filep, G.J. Roles of neutrophil granule proteins in orchestrating inflammation and immunity. FEBS J. 2022, 289, 3932–3953. [Google Scholar] [CrossRef]
- Rawat, K.; Syeda, S.; Shrivastava, A. Neutrophil-derived granule cargoes: Paving the way for tumor growth and progression. Cancer Metastasis Rev. 2021, 40, 221–244. [Google Scholar] [CrossRef]
- Borregaard, N.; Cowland, B.J. Granules of the human neutrophilic polymorphonuclear leukocyte. Blood 1997, 89, 3503–3521. [Google Scholar] [CrossRef]
- Kjeldsen, L.; Sengeløv, H.; Lollike, K.; Borregaard, N. Granules and secretory vesicles in human neonatal neutrophils. Pediatr. Res. 1996, 40, 120–129. [Google Scholar] [CrossRef][Green Version]
- Uribe-Querol, E.; Rosales, C. Phagocytosis: Our current understanding of a universal biological process. Front. Immunol. 2020, 11, 1066. [Google Scholar] [CrossRef]
- Rohrbach, S.A.; Slade, J.D.; Thompson, R.P.; Mowen, A.K. Activation of PAD4 in NET formation. Front. Immunol. 2012, 3, 360. [Google Scholar] [CrossRef]
- Eichelberger, R.K.; Goldman, E.W. Manipulating neutrophil degranulation as a bacterial virulence strategy. PLOS Pathog. 2020, 16, e1009054. [Google Scholar] [CrossRef]
- Yousefi, S.; Simon, D.; Stojkov, D.; Karsonova, A.; Karaulov, A.; Simon, H.-U. In vivo evidence for extracellular DNA trap formation. Cell Death Dis. 2020, 11, 300. [Google Scholar] [CrossRef] [PubMed]
- Bert, S.; Nadkarni, S.; Perretti, M. Neutrophil-T cell crosstalk and the control of the host inflammatory response. Immunol. Rev. 2023, 314, 36–49. [Google Scholar] [CrossRef]
- Puga, I.; Cols, M.; Barra, M.C.; He, B.; Cassis, L.; Gentile, M.; Comerma, L.; Chorny, A.; Shan, M.; Xu, W.; et al. B cell–helper neutrophils stimulate the diversification and production of immunoglobulin in the marginal zone of the spleen. Nat. Immunol. 2012, 13, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Gisbergen, V.P.J.M.K.; Sanchez-Hernandez, M.; Geijtenbeek, B.H.T.; Kooyk, V.Y. Neutrophils mediate immune modulation of dendritic cells through glycosylation-dependent interactions between Mac-1 and DC-SIGN. J. Exp. Med. 2005, 201, 1281–1292. [Google Scholar] [CrossRef]
- Kumar, P.K.; Nicholls, J.A.; Wong, Y.H.C. Partners in crime: Neutrophils and monocytes/macrophages in inflammation and disease. Cell Tissue Res. 2018, 371, 551–565. [Google Scholar] [CrossRef]
- Vono, M.; Lin, A.; Norrby-Teglund, A.; Koup, A.R.; Liang, F.; Loré, K. Neutrophils acquire the capacity for antigen presentation to memory CD4+ T cells in vitro and ex vivo. Blood 2017, 129, 1991–2001. [Google Scholar] [CrossRef]
- Matsushima, H.; Geng, S.; Lu, R.; Okamoto, T.; Yao, Y.; Mayuzumi, N.; Kotol, F.P.; Chojnacki, J.B.; Miyazaki, T.; Gallo, L.R.; et al. Neutrophil differentiation into a unique hybrid population exhibiting dual phenotype and functionality of neutrophils and dendritic cells. Blood 2013, 121, 1677–1689. [Google Scholar] [CrossRef]
- Lad, M.; Beniwal, A.S.; Jain, S.; Shukla, P.; Kalistratova, V.; Jung, J.; Shah, S.S.; Yagnik, G.; Saha, A.; Sati, A.; et al. Glioblastoma induces the recruitment and differentiation of dendritic-like "hybrid" neutrophils from skull bone marrow. Cancer Cell 2024, 42, 1549–1569.e1516. [Google Scholar] [CrossRef]
- Pillay, J.; Tak, T.; Kamp, M.V.; Koenderman, L. Immune suppression by neutrophils and granulocytic myeloid-derived suppressor cells: Similarities and differences. Cell. Mol. Life Sci. 2013, 70, 3813–3827. [Google Scholar] [CrossRef]
- Moses, K.; Brandau, S. Human neutrophils: Their role in cancer and relation to myeloid-derived suppressor cells. Semin. Immunol. 2016, 28, 187–196. [Google Scholar] [CrossRef]
- Müller, I.; Munder, M.; Kropf, P.; Hänsch, M.G. Polymorphonuclear neutrophils and T lymphocytes: Strange bedfellows or brothers in arms? Trends Immunol. 2009, 30, 522–530. [Google Scholar] [CrossRef] [PubMed]
- Soehnlein, O.; Zernecke, A.; Eriksson, E.E.; Rothfuchs, G.A.; Pham, T.C.; Herwald, H.; Bidzhekov, K.; Rottenberg, E.M.; Weber, C.; Lindbom, L. Neutrophil secretion products pave the way for inflammatory monocytes. Blood 2008, 112, 1461–1471. [Google Scholar] [CrossRef] [PubMed]
- Jaillon, S.; Galdiero, R.M.; Prete, D.D.; Cassatella, A.M.; Garlanda, C.; Mantovani, A. Neutrophils in innate and adaptive immunity. Semin. Immunopathol. 2013, 35, 377–394. [Google Scholar] [CrossRef] [PubMed]
- Maskarinec, A.S.; Mckelvy, M.; Boyle, K.; Hotchkiss, H.; Duarte, E.M.; Addison, B.; Amato, N.; Khandelwal, S.; Arepally, M.G.; Lee, M.G. Neutrophil functional heterogeneity is a fixed phenotype and is associated with distinct gene expression profiles. J. Leukoc. Biol. 2022, 112, 1485–1495. [Google Scholar] [CrossRef] [PubMed]
- Silvestre-Roig, C.; Fridlender, G.Z.; Glogauer, M.; Scapini, P. Neutrophil Diversity in Health and Disease. Trends Immunol. 2019, 40, 565–583. [Google Scholar] [CrossRef]
- Scheiber, A.; Trebo, M.; Pittl, A.; Heidegger, I.; Hautz, T.; Oberhuber, R.; Trajanoski, Z.; Augustin, F.; Sopper, S.; Wolf, D.; et al. Profiling low-mRNA content cells in complex human tissues using BD Rhapsody single-cell analysis. STAR Protoc. 2024, 5, 103475. [Google Scholar] [CrossRef]
- Wigerblad, G.; Cao, Q.; Brooks, S.; Naz, F.; Gadkari, M.; Jiang, K.; Gupta, S.; O’Neil, L.; Dell’Orso, S.; Kaplan, J.M.; et al. Single-cell analysis reveals the range of transcriptional states of circulating human neutrophils. J. Immunol. 2022, 209, 772–782. [Google Scholar] [CrossRef]
- Vietinghoff, V.S.; Ley, K. Homeostatic regulation of blood neutrophil counts. J. Immunol. 2008, 181, 5183–5188. [Google Scholar] [CrossRef]
- Devi, S.; Wang, Y.; Chew, K.W.; Lima, R.; A-González, N.; Mattar, N.Z.C.; Chong, Z.S.; Schlitzer, A.; Bakocevic, N.; Chew, S.; et al. Neutrophil mobilization via plerixafor-mediated CXCR4 inhibition arises from lung demargination and blockade of neutrophil homing to the bone marrow. J. Exp. Med. 2013, 210, 2321–2336. [Google Scholar] [CrossRef]
- Evrard, M.; Kwok, W.H.I.; Chong, Z.S.; Teng, W.W.K.; Becht, E.; Chen, J.; Sieow, L.J.; Penny, L.H.; Ching, C.G.; Devi, S.; et al. Developmental analysis of bone marrow neutrophils reveals populations specialized in expansion, trafficking, and effector functions. Immunity 2018, 48, 364–379.e368. [Google Scholar] [CrossRef]
- Kwok, I.; Becht, E.; Xia, Y.; Ng, M.; Teh, C.Y.; Tan, L.; Evrard, M.; Li, L.Y.J.; Tran, T.N.H.; Tan, Y.; et al. Combinatorial single-cell analyses of granulocyte-monocyte progenitor heterogeneity reveals an early uni-potent neutrophil progenitor. Immunity 2020, 53, 303–318.e305. [Google Scholar] [CrossRef] [PubMed]
- Ai, Z. Revealing key regulators of neutrophil function during inflammation by re-analysing single-cell RNA-seq. PLoS ONE 2022, 17, e0276460. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Shi, Q.; Wu, P.; Zhang, X.; Kambara, H.; Su, J.; Yu, H.; Park, S.-Y.; Guo, R.; Ren, Q.; et al. Single-cell transcriptome profiling reveals neutrophil heterogeneity in homeostasis and infection. Nat. Immunol. 2020, 21, 1119–1133. [Google Scholar] [CrossRef]
- Kim, M.; Lu, J.R.; Benayoun, A.B. Single-cell RNA-seq of primary bone marrow neutrophils from female and male adult mice. Sci. Data 2022, 9, 442. [Google Scholar] [CrossRef]
- Grieshaber-Bouyer, R.; Radtke, A.F.; Cunin, P.; Stifano, G.; Levescot, A.; Vijaykumar, B.; Nelson-Maney, N.; Blaustein, B.R.; Monach, A.P.; Nigrovic, A.P.; et al. The neutrotime transcriptional signature defines a single continuum of neutrophils across biological compartments. Nat. Commun. 2021, 12, 2856. [Google Scholar] [CrossRef]
- Calzetti, F.; Finotti, G.; Tamassia, N.; Bianchetto-Aguilera, F.; Castellucci, M.; Canè, S.; Lonardi, S.; Cavallini, C.; Matte, A.; Gasperini, S.; et al. CD66b−CD64dimCD115− cells in the human bone marrow represent neutrophil-committed progenitors. Nat. Immunol. 2022, 23, 679–691. [Google Scholar] [CrossRef]
- Scheiermann, C.; Kunisaki, Y.; Lucas, D.; Chow, A.; Jang, J.-E.; Zhang, D.; Hashimoto, D.; Merad, M.; Frenette, S.P. Adrenergic nerves govern circadian leukocyte recruitment to tissues. Immunity 2012, 37, 290–301. [Google Scholar] [CrossRef]
- Kim, D.N.; Luster, D.A. The role of tissue resident cells in neutrophil recruitment. Trends Immunol. 2015, 36, 547–555. [Google Scholar] [CrossRef]
- Bae, H.G.; Kim, S.Y.; Park, Y.J.; Lee, M.; Lee, K.S.; Kim, C.J.; Kim, G.J.; Shin, J.Y.; Lee, H.; Kim, S.-Y.; et al. Unique characteristics of lung-resident neutrophils are maintained by PGE2/PKA/Tgm2-mediated signaling. Blood 2022, 140, 889–899. [Google Scholar] [CrossRef]
- Ballesteros, I.; Rubio-Ponce, A.; Genua, M.; Lusito, E.; Kwok, I.; Fernández-Calvo, G.; Khoyratty, E.T.; Grinsven, V.E.; González-Hernández, S.; Nicolás-Ávila, Á.J.; et al. Co-option of neutrophil fates by tissue environments. Cell 2020, 183, 1282–1297.e1218. [Google Scholar] [CrossRef]
- Massena, S.; Christoffersson, G.; Vågesjö, E.; Seignez, C.; Gustafsson, K.; Binet, F.; Hidalgo, H.C.; Giraud, A.; Lomei, J.; Weström, S.; et al. Identification and characterization of VEGF-A–responsive neutrophils expressing CD49d, VEGFR1, and CXCR4 in mice and humans. Blood 2015, 126, 2016–2026. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Kubo, H.; Kobayashi, S.; Ishizawa, K.; He, M.; Suzuki, T.; Fujino, N.; Kunishima, H.; Hatta, M.; Nishimaki, K.; et al. The increase in surface CXCR4 expression on lung extravascular neutrophils and its effects on neutrophils during endotoxin-induced lung injury. Cell. Mol. Immunol. 2011, 8, 305–314. [Google Scholar] [CrossRef] [PubMed]
- Fridlender, G.Z.; Sun, J.; Kim, S.; Kapoor, V.; Cheng, G.; Ling, L.; Worthen, S.G.; Albelda, M.S. Polarization of tumor-associated neutrophil phenotype by TGF-β: “N1” versus “N2” TAN. Cancer Cell 2009, 16, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Xue, R.; Zhang, Q.; Cao, Q.; Kong, R.; Xiang, X.; Liu, H.; Feng, M.; Wang, F.; Cheng, J.; Li, Z.; et al. Liver tumour immune microenvironment subtypes and neutrophil heterogeneity. Nature 2022, 612, 141–147. [Google Scholar] [CrossRef]
- Liu, S.; Wu, W.; Du, Y.; Yin, H.; Chen, Q.; Yu, W.; Wang, W.; Yu, J.; Liu, L.; Lou, W.; et al. The evolution and heterogeneity of neutrophils in cancers: Origins, subsets, functions, orchestrations and clinical applications. Mol. Cancer 2023, 22, 148. [Google Scholar] [CrossRef]
- Antuamwine, B.B.; Bosnjakovic, R.; Hofmann-Vega, F.; Wang, X.; Theodosiou, T.; Iliopoulos, I.; Brandau, S. N1 versus N2 and PMN-MDSC: A critical appraisal of current concepts on tumor-associated neutrophils and new directions for human oncology. Immunol. Rev. 2023, 314, 250–279. [Google Scholar] [CrossRef]
- Salcher, S.; Sturm, G.; Horvath, L.; Untergasser, G.; Kuempers, C.; Fotakis, G.; Panizzolo, E.; Martowicz, A.; Trebo, M.; Pall, G.; et al. High-resolution single-cell atlas reveals diversity and plasticity of tissue-resident neutrophils in non-small cell lung cancer. Cancer Cell 2022, 40, 1503–1520.e1508. [Google Scholar] [CrossRef]
- Ng, F.S.M.; Kwok, I.; Tan, L.; Shi, C.; Cerezo-Wallis, D.; Tan, Y.; Leong, K.; Calvo, F.G.; Yang, K.; Zhang, Y.; et al. Deterministic reprogramming of neutrophils within tumors. Science 2024, 383, eadf6493. [Google Scholar] [CrossRef]
- Wu, Y.; Ma, J.; Yang, X.; Nan, F.; Zhang, T.; Ji, S.; Rao, D.; Feng, H.; Gao, K.; Gu, X.; et al. Neutrophil profiling illuminates anti-tumor antigen-presenting potency. Cell 2024, 187, 1422–1439.e1424. [Google Scholar] [CrossRef]
- Manz, G.M.; Boettcher, S. Emergency granulopoiesis. Nat. Rev. Immunol. 2014, 14, 302–314. [Google Scholar] [CrossRef]
- Zhang, F.; Xia, Y.; Su, J.; Quan, F.; Zhou, H.; Li, Q.; Feng, Q.; Lin, C.; Wang, D.; Jiang, Z. Neutrophil diversity and function in health and disease. Signal Transduct. Target. Ther. 2024, 9, 343. [Google Scholar] [CrossRef]
- Guo, R.; Xie, X.; Ren, Q.; Liew, X.P. New insights on extramedullary granulopoiesis and neutrophil heterogeneity in the spleen and its importance in disease. J. Leukoc. Biol. 2024, qiae220. [Google Scholar] [CrossRef]
- Paudel, S.; Ghimire, L.; Jin, L.; Jeansonne, D.; Jeyaseelan, S. Regulation of emergency granulopoiesis during infection. Front. Immunol. 2022, 13, 961601. [Google Scholar] [CrossRef]
- Tsioumpekou, M.; Krijgsman, D.; Leusen, W.H.J.; Olofsen, A.P. The role of cytokines in neutrophil development, tissue homing, function and plasticity in health and disease. Cells 2023, 12, 1981. [Google Scholar] [CrossRef]
- Gallucci, S.; Meka, S.; Gamero, M.A. Abnormalities of the type I interferon signaling pathway in lupus autoimmunity. Cytokine 2021, 146, 155633. [Google Scholar] [CrossRef]
- Kwok, J.A.; Allcock, A.; Ferreira, C.R.; Cano-Gamez, E.; Smee, M.; Burnham, L.K.; Zurke, Y.-X.; Novak, A.; Darwent, M.; Baron, T.; et al. Neutrophils and emergency granulopoiesis drive immune suppression and an extreme response endotype during sepsis. Nat. Immunol. 2023, 24, 767–779. [Google Scholar] [CrossRef]
- Cuda, M.C.; Pope, M.R.; Perlman, H. The inflammatory role of phagocyte apoptotic pathways in rheumatic diseases. Nat. Rev. Rheumatol. 2016, 12, 543–558. [Google Scholar] [CrossRef]
- Saez, A.; Herrero-Fernandez, B.; Gomez-Bris, R.; Sánchez-Martinez, H.; Gonzalez-Granado, M.J. Pathophysiology of inflammatory bowel disease: Innate immune system. Int. J. Mol. Sci. 2023, 24, 1526. [Google Scholar] [CrossRef]
- Hua, Y.; Yang, S.; Zhang, Y.; Li, J.; Wang, M.; Yeerkenbieke, P.; Liao, Q.; Liu, Q. Modulating ferroptosis sensitivity: Environmental and cellular targets within the tumor microenvironment. J. Exp. Clin. Cancer Res. 2024, 43, 19. [Google Scholar] [CrossRef]
- Nagaraj, S.; Gabrilovich, I.D. Regulation of suppressive function of myeloid-derived suppressor cells by CD4+ T cells. Semin. Cancer Biol. 2012, 22, 282–288. [Google Scholar] [CrossRef][Green Version]
- Gabrilovich, I.D. Myeloid-Derived Suppressor Cells. Cancer Immunol. Res. 2017, 5, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Jennings, R.M.; Munn, D.; Blazeck, J. Immunosuppressive metabolites in tumoral immune evasion: Redundancies, clinical efforts, and pathways forward. J. ImmunoTherapy Cancer 2021, 9, e003013. [Google Scholar] [CrossRef] [PubMed]
- Jablonska, J.; Lang, S.; Sionov, V.R.; Granot, Z. The regulation of pre-metastatic niche formation by neutrophils. Oncotarget 2017, 8, 112132–112144. [Google Scholar] [CrossRef] [PubMed]
- Singer, M.; Deutschman, S.C.; Seymour, W.C.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, R.G.; Chiche, J.-D.; Coopersmith, M.C.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801. [Google Scholar] [CrossRef]
- Sônego, F.; Castanheira, S.E.V.F.; Ferreira, G.R.; Kanashiro, A.; Leite, G.V.A.C.; Nascimento, C.D.; Colón, F.D.; Borges, F.D.V.; Alves-Filho, C.J.; Cunha, Q.F. Paradoxical roles of the neutrophil in sepsis: Protective and deleterious. Front. Immunol. 2016, 7, 155. [Google Scholar] [CrossRef]
- Sun, R.; Huang, J.; Yang, Y.; Liu, L.; Shao, Y.; Li, L.; Sun, B. Dysfunction of low-density neutrophils in peripheral circulation in patients with sepsis. Sci. Rep. 2022, 12, 685. [Google Scholar] [CrossRef]
- Morisaki, T.; Goya, T.; Ishimitsu, T.; Torisu, M. The increase of low density subpopulations and CD10 (CALLA) negative neutrophils in severely infected patients. Surg. Today 1992, 22, 322–327. [Google Scholar] [CrossRef]
- Takizawa, S.; Murao, A.; Ochani, M.; Aziz, M.; Wang, P. Frontline science: Extracellular CIRP generates a proinflammatory Ly6G+ CD11bhi subset of low-density neutrophils in sepsis. J. Leucoc. Biol. 2021, 109, 1019–1032. [Google Scholar] [CrossRef]
- Gurien, D.S.; Aziz, M.; Jin, H.; Wang, H.; He, M.; Al-Abed, Y.; Nicastro, M.J.; Coppa, F.G.; Wang, P. Extracellular microRNA 130b-3p inhibits eCIRP-induced inflammation. EMBO Rep. 2020, 21, e48075. [Google Scholar] [CrossRef]
- Pietronigro, C.E.; Bianca, D.V.; Zenaro, E.; Constantin, G. NETosis in Alzheimer’s Disease. Front. Immunol. 2017, 8, 211. [Google Scholar] [CrossRef]
- Zhang, X.; He, G.; Hu, Y.; Liu, B.; Xu, Y.; Li, X.; Lv, X.; Li, J. Single cell transcriptome analysis identified a unique neutrophil type associated with Alzheimer’s disease. Immun. Ageing 2024, 21, 42. [Google Scholar] [CrossRef] [PubMed]
- Prete, D.A.; Martínez-Muñoz, L.; Mazzon, C.; Toffali, L.; Sozio, F.; Za, L.; Bosisio, D.; Gazzurelli, L.; Salvi, V.; Tiberio, L.; et al. The atypical receptor CCRL2 is required for CXCR2-dependent neutrophil recruitment and tissue damage. Blood 2017, 130, 1223–1234. [Google Scholar] [CrossRef]
- Treppo, E.; Monti, S.; Delvino, P.; Marvisi, C.; Ricordi, C.; Rocca, L.G.; Moretti, M.; Italiano, N.; Cianni, D.F.; Ferro, F.; et al. Systemic vasculitis: One year in review 2024. Clin. Exp. Rheumatol. 2024, 42, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Abdgawad, M.; Gunnarsson, L.; Bengtsson, A.A.; Geborek, P.; Nilsson, L.; Segelmark, M.; Hellmark, T. Elevated neutrophil membrane expression of proteinase 3 is dependent upon CD177 expression. Clin. Exp. Immunol. 2010, 161, 89–97. [Google Scholar] [CrossRef]
- Hu, N.; Westra, J.; Huitema, G.M.; Bijl, M.; Brouwer, E.; Stegeman, A.C.; Heeringa, P.; Limburg, C.P.; Kallenberg, M.G.C. Coexpression of CD177 and membrane proteinase 3 on neutrophils in antineutrophil cytoplasmic autoantibody–associated systemic vasculitis: Anti–proteinase 3–mediated neutrophil activation is independent of the role of CD177-expressing neutrophils. Arthritis Rheum. 2009, 60, 1548–1557. [Google Scholar] [CrossRef]
- Amos, A.L.; Ma, Y.F.; Tesch, H.G.; Liles, T.J.; Breckenridge, G.D.; Nikolic-Paterson, J.D.; Han, Y. ASK1 inhibitor treatment suppresses p38/JNK signalling with reduced kidney inflammation and fibrosis in rat crescentic glomerulonephritis. J. Cell. Mol. Med. 2018, 22, 4522–4533. [Google Scholar] [CrossRef]
- Nishide, M.; Nishimura, K.; Matsushita, H.; Kawada, S.; Shimagami, H.; Metsugi, S.; Kato, Y.; Kawasaki, T.; Tsujimoto, K.; Edahiro, R.; et al. Neutrophil single-cell analysis identifies a uniquely primed subset and provides insights into predicting relapse of autoimmune small vessel vasculitis. Nat. Portf. 2024. [Google Scholar] [CrossRef]
- Arnaud, L.; Chasset, F.; Martin, T. Immunopathogenesis of systemic lupus erythematosus: An update. Autoimmun. Rev. 2024, 23, 103648. [Google Scholar] [CrossRef]
- Ohl, K.; Rauen, T.; Tenbrock, K. Dysregulated neutrophilic cell death in SLE: A spotlight on ferroptosis. Signal Transduct. Target. Ther. 2021, 6, 392. [Google Scholar] [CrossRef]
- Li, P.; Jiang, M.; Li, K.; Li, H.; Zhou, Y.; Xiao, X.; Xu, Y.; Krishfield, S.; Lipsky, P.E.; Tsokos, G.C.; et al. Glutathione peroxidase 4–regulated neutrophil ferroptosis induces systemic autoimmunity. Nat. Immunol. 2021, 22, 1107–1117. [Google Scholar] [CrossRef]
- Garcia-Romo, G.S.; Caielli, S.; Vega, B.; Connolly, J.; Allantaz, F.; Xu, Z.; Punaro, M.; Baisch, J.; Guiducci, C.; Coffman, R.L.; et al. Netting neutrophils are major inducers of type I IFN production in pediatric systemic lupus erythematosus. Sci. Transl. Med. 2011, 3, 73ra20. [Google Scholar] [CrossRef] [PubMed]
- Salemme, R.; Peralta, L.N.; Meka, S.H.; Pushpanathan, N.; Alexander, J.J. The Role of NETosis in Systemic Lupus Erythematosus. J. Cell. Immunol. 2019, 1, 33. [Google Scholar] [CrossRef] [PubMed]
- Grayson, C.P.; Schauer, C.; Herrmann, M.; Kaplan, J.M. Review: Neutrophils as Invigorated Targets in Rheumatic Diseases. Arthritis Rheumatol. 2016, 68, 2071–2082. [Google Scholar] [CrossRef] [PubMed]
- Nikolopoulos, D.; Parodis, I. Janus kinase inhibitors in systemic lupus erythematosus: Implications for tyrosine kinase 2 inhibition. Front. Med. 2023, 10, 1217147. [Google Scholar] [CrossRef]
- Hasni, A.S.; Gupta, S.; Davis, M.; Poncio, E.; Temesgen-Oyelakin, Y.; Carlucci, M.P.; Wang, X.; Naqi, M.; Playford, P.M.; Goel, R.R.; et al. Phase 1 double-blind randomized safety trial of the Janus kinase inhibitor tofacitinib in systemic lupus erythematosus. Nat. Commun. 2021, 12, 3391. [Google Scholar] [CrossRef]
- Blanco, P.L.; Pedersen, L.H.; Wang, X.; Lightfoot, L.Y.; Seto, N.; Carmona-Rivera, C.; Yu, Z.X.; Hoffmann, V.; Yuen, T.S.P.; Kaplan, J.M. Improved Mitochondrial Metabolism and Reduced Inflammation Following Attenuation of Murine Lupus With Coenzyme Q10 Analog Idebenone. Arthritis Rheumatol. 2020, 72, 454–464. [Google Scholar] [CrossRef]
- Trigunaite, A.; Khan, A.; Der, E.; Song, A.; Varikuti, S.; J⊘Rgensen, N.T. Gr-1highCD11b+ Cells Suppress B Cell Differentiation and Lupus-like Disease in Lupus-Prone Male Mice. Arthritis Rheum. 2013, 65, 2392–2402. [Google Scholar] [CrossRef]
- Park, M.J.; Lee, S.H.; Kim, E.K.; Lee, E.J.; Park, S.H.; Kwok, S.K.; Cho, M.L. Myeloid-derived suppressor cells induce the expansion of regulatory B cells and ameliorate autoimmunity in the Sanroque mouse model of systemic lupus erythematosus. Arthritis Rheumatol. 2016, 68, 2717–2727. [Google Scholar] [CrossRef]
- Lourenço, V.E.; Wong, M.; Hahn, H.B.; Palma-Diaz, F.M.; Skaggs, J.B. Laquinimod Delays and Suppresses Nephritis in Lupus-Prone Mice and Affects Both Myeloid and Lymphoid Immune Cells. Arthritis Rheumatol. 2014, 66, 674–685. [Google Scholar] [CrossRef]
- Dong, G.; Yang, Y.; Li, X.; Yao, X.; Zhu, Y.; Zhang, H.; Wang, H.; Ma, Q.; Zhang, J.; Shi, H.; et al. Granulocytic myeloid-derived suppressor cells contribute to IFN-I signaling activation of B cells and disease progression through the lncRNA NEAT1-BAFF axis in systemic lupus erythematosus. Biochim. Et Biophys. Acta (BBA)-Mol. Basis Dis. 2020, 1866, 165554. [Google Scholar] [CrossRef]
- Smolen, S.J.; Aletaha, D.; Barton, A.; Burmester, R.G.; Emery, P.; Firestein, S.G.; Kavanaugh, A.; Mcinnes, B.I.; Solomon, H.D.; Strand, V.; et al. Rheumatoid arthritis. Nat. Rev. Dis. Primers 2018, 4, 18001. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, S.C.; Giaglis, S.; Walker, A.U.; Buser, A.; Hahn, S.; Hasler, P. Enhanced neutrophil extracellular trap generation in rheumatoid arthritis: Analysis of underlying signal transduction pathways and potential diagnostic utility. Arthritis Res. Ther. 2014, 16, R122. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.; Yoo, D.-G.; Kahlenberg, M.J.; Bridges, L.S.; Oni, O.; Huang, H.; Stecenko, A.; Rada, B. Systemic levels of anti-PAD4 autoantibodies correlate with airway obstruction in cystic fibrosis. J. Cyst. Fibros. 2019, 18, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Wright, L.H.; Makki, A.F.; Moots, J.R.; Edwards, W.S. Low-density granulocytes: Functionally distinct, immature neutrophils in rheumatoid arthritis with altered properties and defective TNF signalling. J. Leukoc. Biol. 2017, 101, 599–611. [Google Scholar] [CrossRef] [PubMed]
- Dal-Secco, D.; Wang, J.; Zeng, Z.; Kolaczkowska, E.; Wong, H.Y.C.; Petri, B.; Ransohoff, M.R.; Charo, F.I.; Jenne, N.C.; Kubes, P. A dynamic spectrum of monocytes arising from the in situ reprogramming of CCR2+ monocytes at a site of sterile injury. J. Exp. Med. 2015, 212, 447–456. [Google Scholar] [CrossRef]
- Strauss-Ayali, D.; Conrad, M.S.; Mosser, M.D. Monocyte subpopulations and their differentiation patterns during infection. J. Leukoc. Biol. 2007, 82, 244–252. [Google Scholar] [CrossRef]
- Bluml, S.; Scheinecker, C.; Smolen, S.J.; Redlich, K. Targeting TNF receptors in rheumatoid arthritis. Int. Immunol. 2012, 24, 275–281. [Google Scholar] [CrossRef]
- Wen, L.; Gong, P.; Liang, C.; Shou, D.; Liu, B.; Chen, Y.; Bao, C.; Chen, L.; Liu, X.; Liang, T.; et al. Interplay between myeloid-derived suppressor cells (MDSCs) and Th17 cells: Foe or friend? Oncotarget 2016, 7, 35490–35496. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, J.; Wang, S. The potential therapeutic role of myeloid-derived suppressor cells in autoimmune arthritis. Semin. Arthritis Rheum. 2016, 45, 490–495. [Google Scholar] [CrossRef]
- Moscarello, A.M.; Lei, H.; Mastronardi, G.F.; Winer, S.; Tsui, H.; Li, Z.; Ackerley, C.; Zhang, L.; Raijmakers, R.; Wood, D.D. Inhibition of peptidyl-arginine deiminases reverses protein-hypercitrullination and disease in mouse models of multiple sclerosis. Dis. Models Mech. 2013, 6, 467–478. [Google Scholar] [CrossRef]
- Rumble, M.J.; Huber, K.A.; Krishnamoorthy, G.; Srinivasan, A.; Giles, A.D.; Zhang, X.; Wang, L.; Segal, M.B. Neutrophil-related factors as biomarkers in EAE and MS. J. Exp. Med. 2015, 212, 23–35. [Google Scholar] [CrossRef]
- Fresegna, D.; Bullitta, S.; Musella, A.; Rizzo, R.F.; Vito, D.F.; Guadalupi, L.; Caioli, S.; Balletta, S.; Sanna, K.; Dolcetti, E.; et al. Re-Examining the role of tnf in ms pathogenesis and therapy. Cells 2020, 9, 2290. [Google Scholar] [CrossRef] [PubMed]
- Quan, M.; Zhang, H.; Han, X.; Ba, Y.; Cui, X.; Bi, Y.; Yi, L.; Li, B. Single-cell rna sequencing reveals transcriptional landscape of neutrophils and highlights the role of TREM-1 in EAE. Neurol. Neuroimmunol. Neuroinflamm. 2024, 11, e200278. [Google Scholar] [CrossRef] [PubMed]
- Fan, D.; He, X.; Bian, Y.; Guo, Q.; Zheng, K.; Zhao, Y.; Lu, C.; Liu, B.; Xu, X.; Zhang, G.; et al. Triptolide modulates TREM-1 signal pathway to inhibit the inflammatory response in Rheumatoid Arthritis. Int. J. Mol. Sci. 2016, 17, 498. [Google Scholar] [CrossRef] [PubMed]
- Weber, B.; Schuster, S.; Zysset, D.; Rihs, S.; Dickgreber, N.; Schürch, C.; Riether, C.; Siegrist, M.; Schneider, C.; Pawelski, H.; et al. TREM-1 deficiency can attenuate disease severity without affecting pathogen clearance. PLoS Pathog. 2014, 10, e1003900. [Google Scholar] [CrossRef]
- Valle, A.; Giamporcaro, M.G.; Scavini, M.; Stabilini, A.; Grogan, P.; Bianconi, E.; Sebastiani, G.; Masini, M.; Maugeri, N.; Porretti, L.; et al. Reduction of circulating neutrophils precedes and accompanies type 1 diabetes. Diabetes 2013, 62, 2072–2077. [Google Scholar] [CrossRef]
- Obeagu, I.E.; Obeagu, U.G. Type 1 diabetes mellitus: Roles of neutrophils in the pathogenesis. Medicine 2023, 102, e36245. [Google Scholar] [CrossRef]
- Vecchio, F.; Buono, L.N.; Stabilini, A.; Nigi, L.; Dufort, J.M.; Geyer, S.; Rancoita, M.P.; Cugnata, F.; Mandelli, A.; Valle, A.; et al. Abnormal neutrophil signature in the blood and pancreas of presymptomatic and symptomatic type 1 diabetes. JCI Insight 2018, 3, e122146. [Google Scholar] [CrossRef]
- Wang, Y.; Xiao, Y.; Zhong, L.; Ye, D.; Zhang, J.; Tu, Y.; Bornstein, R.S.; Zhou, Z.; Lam, S.L.K.; Xu, A. Increased neutrophil elastase and proteinase 3 and augmented netosis are closely associated with β-cell autoimmunity in patients with type 1 diabetes. Diabetes 2014, 63, 4239–4248. [Google Scholar] [CrossRef]
- Klocperk, A.; Vcelakova, J.; Vrabcova, P.; Zentsova, I.; Petruzelkova, L.; Sumnik, Z.; Pruhova, S.; Sediva, A.; Parackova, Z. Elevated biomarkers of netosis in the serum of pediatric patients with type 1 diabetes and their first-degree relatives. Front. Immunol. 2021, 12, 699386. [Google Scholar] [CrossRef]
- Qin, J.; Fu, S.; Speake, C.; Greenbaum, C.J.; Odegard, J.M. NETosis-associated serum biomarkers are reduced in type 1 diabetes in association with neutrophil count. Clin. Exp. Immunol. 2016, 184, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Bissenova, S.; Buitinga, M.; Boesch, M.; Korf, H.; Casteels, K.; Teunkens, A.; Mathieu, C.; Gysemans, C. High-throughput analysis of neutrophil extracellular trap levels in subtypes of people with type 1 diabetes. Biology 2023, 12, 882. [Google Scholar] [CrossRef] [PubMed]
- Bissenova, S.; Ellis, D.; Callebaut, A.; Eelen, G.; Derua, R.; Buitinga, M.; Mathieu, C.; Gysemans, C.; Overbergh, L. NET proteome in established type 1 diabetes is enriched in metabolic proteins. Cells 2023, 12, 1319. [Google Scholar] [CrossRef]
- Brown, S.J.; Amend, R.S.; Austin, H.R.; Gatenby, A.R.; Hammarlund, U.E.; Pienta, J.K. Updating the definition of cancer. Mol. Cancer Res. 2023, 21, 1142–1147. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ji, C.; Zhang, H.; Shi, H.; Mao, F.; Qian, H.; Xu, W.; Wang, D.; Pan, J.; Fang, X.; et al. Engineered neutrophil-derived exosome-like vesicles for targeted cancer therapy. Sci. Adv. 2022, 8, eabj8207. [Google Scholar] [CrossRef]
- Zhu, D.; Lu, Y.; Yan, Z.; Deng, Q.; Hu, B.; Wang, Y.; Wang, W.; Wang, Y.; Wang, Y. A beta-Carboline Derivate PAD4 Inhibitor Reshapes Neutrophil Phenotype and Improves the Tumor Immune Microenvironment against Triple-Negative Breast Cancer. J. Med. Chem. 2024, 67, 7973–7994. [Google Scholar] [CrossRef]
- Singhal, S.; Bhojnagarwala, S.P.; O’Brien, S.; Moon, K.E.; Garfall, L.A.; Rao, S.A.; Quatromoni, G.J.; Stephen, L.T.; Litzky, L.; Deshpande, C.; et al. Origin and Role of a subset of tumor-associated neutrophils with antigen-presenting cell features in early-stage human lung cancer. Cancer Cell 2016, 30, 120–135. [Google Scholar] [CrossRef]
- Pylaeva, E.; Korschunow, G.; Spyra, I.; Bordbari, S.; Siakaeva, E.; Ozel, I.; Domnich, M.; Squire, A.; Hasenberg, A.; Thangavelu, K.; et al. During early stages of cancer, neutrophils initiate anti-tumor immune responses in tumor-draining lymph nodes. Cell Rep. 2022, 40, 111171. [Google Scholar] [CrossRef]
- Guo, N.; Ni, K.; Luo, T.; Lan, G.; Arina, A.; Xu, Z.; Mao, J.; Weichselbaum, R.R.; Spiotto, M.; Lin, W. Reprogramming of neutrophils as non-canonical antigen presenting cells by radiotherapy–radiodynamic therapy to facilitate immune-mediated tumor regression. ACS Nano 2021, 15, 17515–17527. [Google Scholar] [CrossRef]
- Huang, S.; Shi, J.; Shen, J.; Fan, X. Metabolic reprogramming of neutrophils in the tumor microenvironment: Emerging therapeutic targets. Cancer Lett. 2025, 612, 217466. [Google Scholar] [CrossRef]
- Benguigui, M.; Cooper, J.T.; Kalkar, P.; Schif-Zuck, S.; Halaban, R.; Bacchiocchi, A.; Kamer, I.; Deo, A.; Manobla, B.; Menachem, R.; et al. Interferon-stimulated neutrophils as a predictor of immunotherapy response. Cancer Cell 2024, 42, 253–265.e212. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, F.; Liu, Z.; Li, M.; Wu, G.; Li, H. P2RX1-Negative neutrophils promote the immunosuppressive microenvironment in Non-Small cell lung cancer by upregulating PD-L1 expression. Hum. Immunol. 2024, 85, 111105. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gysemans, C.; Beya, M.; Pedace, E.; Mathieu, C. Exploring Neutrophil Heterogeneity and Plasticity in Health and Disease. Biomedicines 2025, 13, 597. https://doi.org/10.3390/biomedicines13030597
Gysemans C, Beya M, Pedace E, Mathieu C. Exploring Neutrophil Heterogeneity and Plasticity in Health and Disease. Biomedicines. 2025; 13(3):597. https://doi.org/10.3390/biomedicines13030597
Chicago/Turabian StyleGysemans, Conny, Mateson Beya, Erika Pedace, and Chantal Mathieu. 2025. "Exploring Neutrophil Heterogeneity and Plasticity in Health and Disease" Biomedicines 13, no. 3: 597. https://doi.org/10.3390/biomedicines13030597
APA StyleGysemans, C., Beya, M., Pedace, E., & Mathieu, C. (2025). Exploring Neutrophil Heterogeneity and Plasticity in Health and Disease. Biomedicines, 13(3), 597. https://doi.org/10.3390/biomedicines13030597





