Applying Wearable Sensors and Machine Learning to the Diagnostic Challenge of Distinguishing Parkinson’s Disease from Other Forms of Parkinsonism
Abstract
1. Introduction
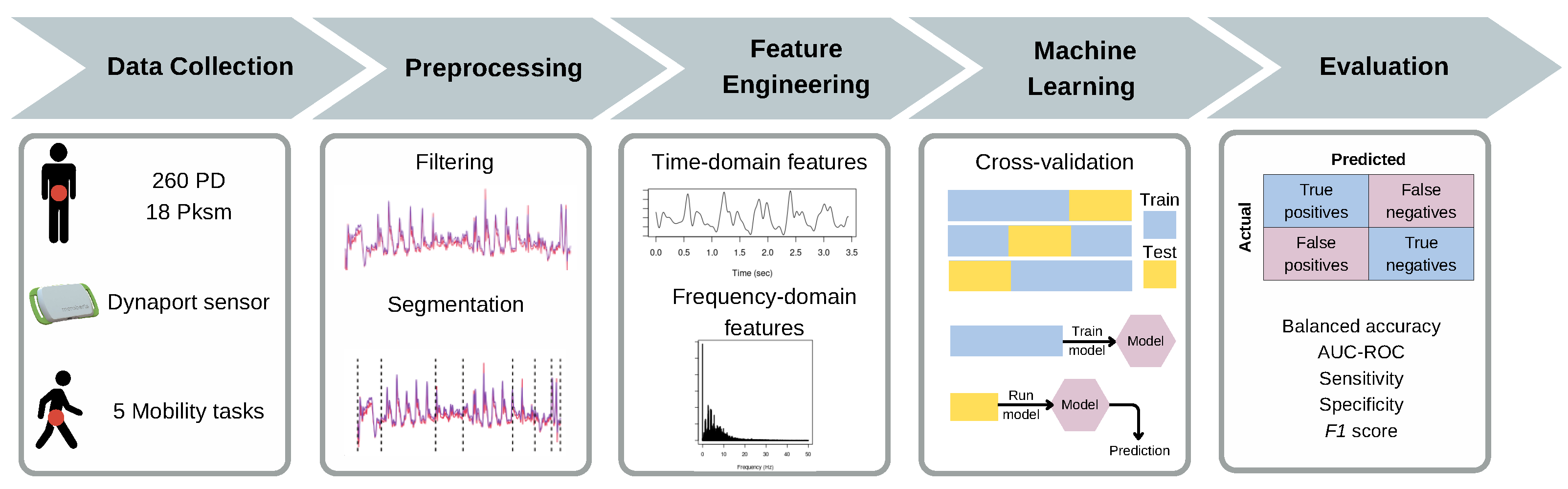
2. Materials and Methods
2.1. Participants
2.2. Experimental Procedure
2.3. Feature Engineering
2.4. Mutual Information-Based Feature Selection for EasyEnsemble (MIEE)
2.5. Machine Learning Model
2.6. Group Feature Importance
2.7. Statistical Analysis
3. Results
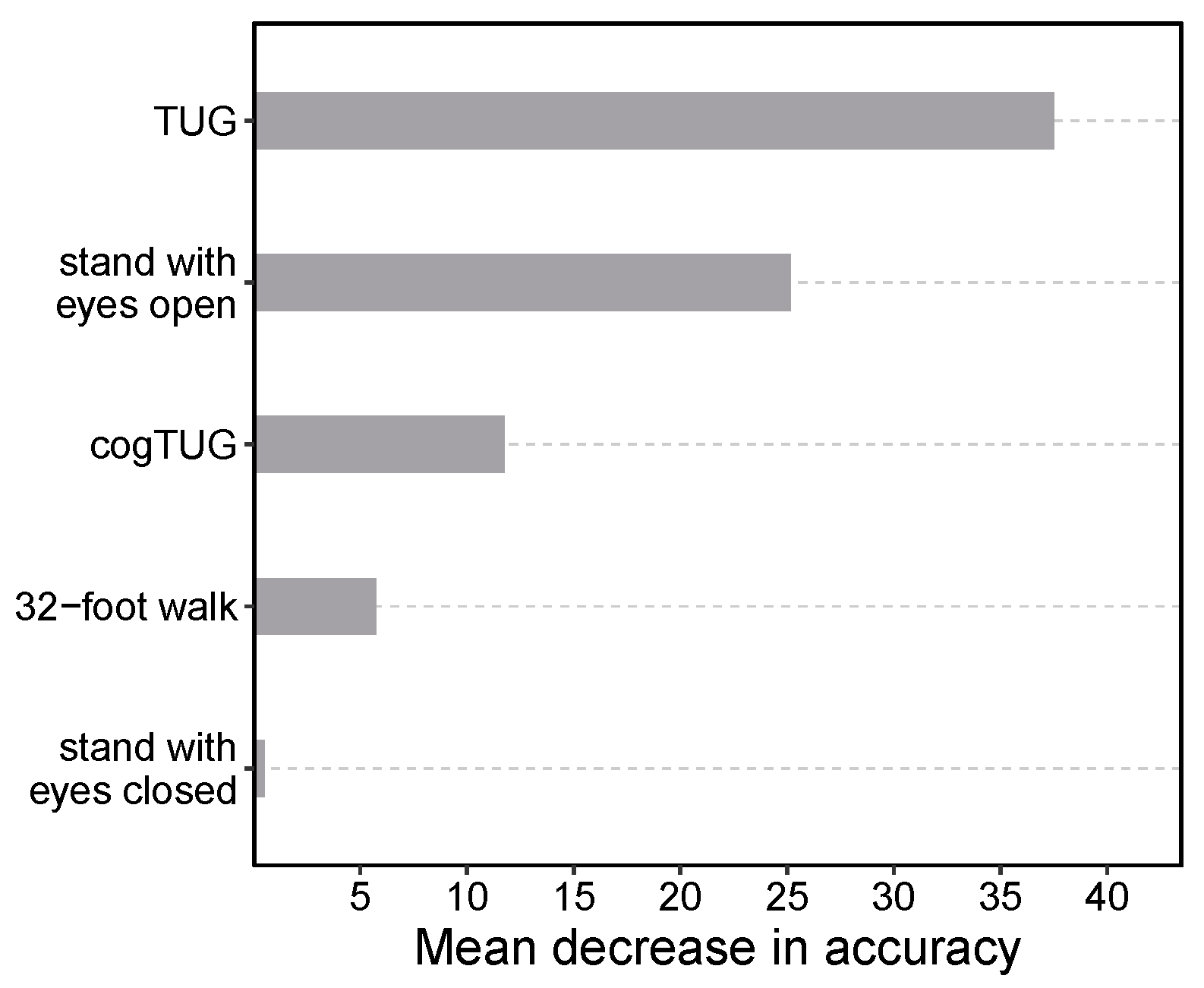
3.1. Feature Importance Analysis
3.2. Model Performance
3.3. Misclassification Analysis
3.4. Model Predictions for Challenging Cases
3.5. The Performance of Alternative Models
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tolosa, E.; Garrido, A.; Scholz, S.W.; Poewe, W. Challenges in the diagnosis of Parkinson’s disease. Lancet Neurol. 2021, 20, 385. [Google Scholar] [CrossRef] [PubMed]
- Tarakad, A.; Jankovic, J. Essential Tremor and Parkinson’s Disease: Exploring the Relationship. Tremor Other Hyperkinetic Movements 2019, 8, 589. [Google Scholar] [CrossRef] [PubMed]
- Levin, J.; Kurz, A.; Arzberger, T.; Giese, A.; Höglinger, G.U. The differential diagnosis and treatment of atypical parkinsonism. Dtsch. Ärzteblatt Int. 2016, 113, 61. [Google Scholar] [CrossRef] [PubMed]
- Hobson, D.E. Clinical manifestations of Parkinson’s disease and parkinsonism. Can. J. Neurol. Sci. 2003, 30, S2–S9. [Google Scholar] [CrossRef]
- Mahoney, J.; Verghese, J.; Holtzer, R.; Allali, G. The evolution of mild parkinsonian signs in aging. J. Neurol. 2014, 261, 1922–1928. [Google Scholar] [CrossRef][Green Version]
- Caslake, R.; Moore, J.N.; Gordon, J.C.; Harris, C.E.; Counsell, C. Changes in diagnosis with follow-up in an incident cohort of patients with parkinsonism. J. Neurol. Neurosurg. Psychiatry 2008, 79, 1202–1207. [Google Scholar] [CrossRef][Green Version]
- Adler, C.H.; Beach, T.G.; Hentz, J.G.; Shill, H.A.; Caviness, J.N.; Driver-Dunckley, E.; Sabbagh, M.N.; Sue, L.I.; Jacobson, S.A.; Belden, C.M.; et al. Low clinical diagnostic accuracy of early vs advanced Parkinson disease: Clinicopathologic study. Neurology 2014, 83, 406–412. [Google Scholar] [CrossRef]
- Rizzo, G.; Copetti, M.; Arcuti, S.; Martino, D.; Fontana, A.; Logroscino, G. Accuracy of clinical diagnosis of Parkinson disease: A systematic review and meta-analysis. Neurology 2016, 86, 566–576. [Google Scholar] [CrossRef]
- Tolosa, E.; Wenning, G.; Poewe, W. The diagnosis of Parkinson’s disease. Lancet Neurol. 2006, 5, 75–86. [Google Scholar] [CrossRef]
- Keshtkarjahromi, M.; Abraham, D.S.; Gruber-Baldini, A.L.; Schrader, K.; Reich, S.G.; Savitt, J.M.; Coelln, R.V.; Shulman, L.M. Confirming Parkinson disease diagnosis: Patterns of diagnostic changes by movement disorder specialists. Park. Dis. 2022, 2022, 5535826. [Google Scholar] [CrossRef]
- Stathaki, M.; Koukouraki, S.; Simos, P.; Boura, I.; Papadaki, E.; Bourogianni, O.; Tsaroucha, A.; Kapsoritakis, N.; Mitsias, P.; Spanaki, C. Is There Any Clinical Value of Adding 123I-Metaiodobenzylguanidine Myocardial Scintigraphy to 123I-Ioflupane (DaTscan) in the Differential Diagnosis of Parkinsonism? Clin. Nucl. Med. 2020, 45, 588–593. [Google Scholar] [CrossRef] [PubMed]
- Mirelman, A.; Bonato, P.; Camicioli, R.; Ellis, T.D.; Giladi, N.; Hamilton, J.L.; Hass, C.J.; Hausdorff, J.M.; Pelosin, E.; Almeida, Q.J. Gait impairments in Parkinson’s disease. Lancet Neurol. 2019, 18, 697–708. [Google Scholar] [CrossRef] [PubMed]
- Heldman, D.A.; Espay, A.J.; LeWitt, P.A.; Giuffrida, J.P. Clinician versus machine: Reliability and responsiveness of motor endpoints in Parkinson’s disease. Park. Relat. Disord. 2014, 20, 590–595. [Google Scholar] [CrossRef] [PubMed]
- Schlachetzki, J.C.; Barth, J.; Marxreiter, F.; Gossler, J.; Kohl, Z.; Reinfelder, S.; Gassner, H.; Aminian, K.; Eskofier, B.M.; Winkler, J.; et al. Wearable sensors objectively measure gait parameters in Parkinson’s disease. PLoS ONE 2017, 12, e183989. [Google Scholar] [CrossRef]
- von Coelln, R.; Dawe, R.J.; Leurgans, S.E.; Curran, T.A.; Truty, T.; Yu, L.; Barnes, L.L.; Shulman, J.M.; Shulman, L.M.; Bennett, D.A.; et al. Quantitative mobility metrics from a wearable sensor predict incident parkinsonism in older adults. Park. Relat. Disord. 2019, 65, 190–196. [Google Scholar] [CrossRef]
- Raccagni, C.; Gaßner, H.; Eschlboeck, S.; Boesch, S.; Krismer, F.; Seppi, K.; Poewe, W.; Eskofier, B.M.; Winkler, J.; Wenning, G.; et al. Sensor-based gait analysis in atypical parkinsonian disorders. Brain Behav. 2018, 8, e00977. [Google Scholar] [CrossRef]
- Gaßner, H.; Raccagni, C.; Eskofier, B.M.; Klucken, J.; Wenning, G.K. The Diagnostic Scope of Sensor-Based Gait Analysis in Atypical Parkinsonism: Further Observations. Front. Neurol. 2019, 10, 5. [Google Scholar] [CrossRef]
- Borm, C.D.; Krismer, F.; Wenning, G.K.; Seppi, K.; Poewe, W.; Pellecchia, M.T.; Barone, P.; Johnsen, E.L.; Østergaard, K.; Gurevich, T.; et al. Axial motor clues to identify atypical parkinsonism: A multicentre European cohort study. Park. Relat. Disord. 2018, 56, 33–40. [Google Scholar] [CrossRef]
- Amboni, M.; Ricciardi, C.; Picillo, M.; Santis, C.D.; Ricciardelli, G.; Abate, F.; Tepedino, M.F.; D’Addio, G.; Cesarelli, G.; Volpe, G.; et al. Gait analysis may distinguish progressive supranuclear palsy and Parkinson disease since the earliest stages. Sci. Rep. 2021, 11, 9297. [Google Scholar] [CrossRef]
- Dale, M.L.; Silva-Batista, C.; de Almeida, F.O.; Horak, F.B. Balance and gait in progressive supranuclear palsy: A narrative review of objective metrics and exercise interventions. Front. Neurol. 2023, 14, 1212185. [Google Scholar] [CrossRef]
- Bzdok, D.; Altman, N.; Krzywinski, M. Statistics versus machine learning. Nat. Methods 2018, 15, 233–234. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Sun, H.; Wang, T.; Tang, M.; Bohnen, N.; Muller, M.; Herman, T.; Giladi, N.; Kalinin, A.; Spino, C.; et al. Model-based and Model-free Machine Learning Techniques for Diagnostic Prediction and Classification of Clinical Outcomes in Parkinson’s Disease. Sci. Rep. 2018, 8, 7129. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Desrosiers, C.; Frasnelli, J. Machine learning for the diagnosis of Parkinson’s disease: A review of literature. Front. Aging Neurosci. 2021, 13, 633752. [Google Scholar] [CrossRef] [PubMed]
- Varghese, J.; Brenner, A.; Fujarski, M.; van Alen, C.M.; Plagwitz, L.; Warnecke, T. Machine Learning in the Parkinson’s disease smartwatch (PADS) dataset. Npj Park. Dis. 2024, 10, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Kim, J.; Lee, M.J.; Ahn, J.H.; Lee, D.Y.; Youn, J.; Chung, M.J.; Kim, Z.; Cho, J.W. Differential diagnosis between Parkinson’s disease and atypical parkinsonism based on gait and postural instability: Artificial intelligence using an enhanced weight voting ensemble model. Park. Relat. Disord. 2022, 98, 32–37. [Google Scholar] [CrossRef]
- Fernandes, C.; Ferreira, F.; Lopes, R.L.; Bicho, E.; Erlhagen, W.; Sousa, N.; Gago, M.F. Discrimination of idiopathic Parkinson’s disease and vascular parkinsonism based on gait time series and the levodopa effect. J. Biomech. 2021, 125, 110214. [Google Scholar] [CrossRef]
- Duque, J.D.; Egea, A.J.; Reeb, T.; Rojas, H.A.; Gonzalez-Vargas, A.M. Angular velocity analysis boosted by machine learning for helping in the differential diagnosis of Parkinson’s disease and essential tremor. IEEE Access 2020, 8, 88866–88875. [Google Scholar] [CrossRef]
- De Vos, M.; Prince, J.; Buchanan, T.; FitzGerald, J.J.; Antoniades, C.A. Discriminating progressive supranuclear palsy from Parkinson’s disease using wearable technology and machine learning. Gait Posture 2020, 77, 257–263. [Google Scholar] [CrossRef]
- Rovini, E.; Maremmani, C.; Cavallo, F. How wearable sensors can support Parkinson’s disease diagnosis and treatment: A systematic review. Front. Neurosci. 2017, 11, 555. [Google Scholar] [CrossRef]
- Channa, A.; Popescu, N.; Ciobanu, V. Wearable solutions for patients with Parkinson’s disease and neurocognitive disorder: A systematic review. Sensors 2020, 20, 2713. [Google Scholar] [CrossRef]
- Moon, S.; Song, H.J.; Sharma, V.D.; Lyons, K.E.; Pahwa, R.; Akinwuntan, A.E.; Devos, H. Classification of Parkinson’s disease and essential tremor based on balance and gait characteristics from wearable motion sensors via machine learning techniques: A data-driven approach. J. NeuroEngineering Rehabil. 2020, 17, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Surangsrirat, D.; Thanawattano, C.; Pongthornseri, R.; Dumnin, S.; Anan, C.; Bhidayasiri, R. Support vector machine classification of Parkinson’s disease and essential tremor subjects based on temporal fluctuation. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Orlando, FL, USA, 16–20 August 2016; pp. 6389–6392. [Google Scholar]
- Wu, X.; Yu, B.; Liu, P.; Zhu, H.; Li, J.; Wang, H.; Luo, W.; Yun, P. A Wearable Multi-sensor System for Classification of Multiple System Atrophy and Parkinson’s Disease. In Proceedings of the 2022 10th International Conference on Bioinformatics and Computational Biology (ICBCB), Hangzhou, China, 13–15 May 2022; pp. 129–134. [Google Scholar] [CrossRef]
- Russo, M.; Ricciardi, C.; Amboni, M.; Picillo, M.; Ricciardelli, G.; Abate, F.; Tepedino, M.F.; Calabrese, M.C.; Cesarelli, M.; Romano, M. Performing a short sway to distinguish Parkinsonisms. In Proceedings of the 2022 IEEE International Conference on Metrology for Extended Reality, Artificial Intelligence and Neural Engineering (MetroXRAINE), Rome, Italy, 26–28 October 2022; pp. 340–345. [Google Scholar] [CrossRef]
- Lin, S.; Gao, C.; Li, H.; Huang, P.; Ling, Y.; Chen, Z.; Ren, K.; Chen, S. Wearable sensor-based gait analysis to discriminate early Parkinson’s disease from essential tremor. J. Neurol. 2023, 270, 2283–2301. [Google Scholar] [CrossRef] [PubMed]
- Macphee, G.J.A. Diagnosis and differential diagnosis of Parkinson’s disease. In Parkinson’s Disease in the Older Patient; Playfer, J.R., Hindle, J., Eds.; Arnold: London, UK, 2001; pp. 43–77. [Google Scholar]
- Muangpaisan, W.; Mathews, A.; Hori, H.; Seidel, D. A systematic review of the worldwide prevalence and incidence of Parkinson’s disease. J. Med. Assoc. Thail. 2011, 94, 749–755. [Google Scholar]
- de Rijk, M.C.; Tzourio, C.; Breteler, M.M.; Dartigues, J.F.; Amaducci, L.; Lopez-Pousa, S.; Manubens-Bertran, J.M.; Alpérovitch, A.; Rocca, W.A. Prevalence of parkinsonism and Parkinson’s disease in Europe: The EUROPARKINSON Collaborative Study. J. Neurol. Neurosurg. Psychiatry 1997, 62, 10–15. [Google Scholar] [CrossRef]
- Winter, Y.; Bezdolnyy, Y.; Katunina, E.; Avakjan, G.; Reese, J.P.; Klotsche, J.; Oertel, W.H.; Dodel, R.; Gusev, E. Incidence of Parkinson’s disease and atypical parkinsonism: Russian population-based study. Mov. Disord. 2010, 25, 349–356. [Google Scholar] [CrossRef]
- Fleury, V.; Brindel, P.; Nicastro, N.; Burkhard, P.R. Descriptive epidemiology of parkinsonism in the Canton of Geneva, Switzerland. Park. Relat. Disord. 2018, 54, 30–39. [Google Scholar] [CrossRef]
- Linder, J.; Stenlund, H.; Forsgren, L. Incidence of Parkinson’s disease and parkinsonism in northern Sweden: A population-based study. Mov. Disord. 2010, 25, 341–348. [Google Scholar] [CrossRef]
- Benito-León, J.; Bermejo-Pareja, F.; Rodríguez, J.; Molina, J.; Gabriel, R.; Morales, J.; for the Neurological Disorders in Central Spain (NEDICES) Study Group. Prevalence of PD and other types of parkinsonism in three elderly populations of central Spain. Mov. Disord. 2003, 18, 267–274. [Google Scholar] [CrossRef]
- El-Tallawy, H.; Farghaly, W.; Shehata, G.; Rageh, T.; Hakeem, N.; Abd Elhamed, M.; Badry, R. Prevalence of Parkinson’s disease and other types of Parkinsonism in Al Kharga district, Egypt. Neuropsychiatr. Dis. Treat. 2013, 9, 1821–1826. [Google Scholar] [CrossRef]
- Kaur, H.; Pannu, H.S.; Malhi, A.K. A Systematic Review on Imbalanced Data Challenges in Machine Learning: Applications and Solutions. ACM Comput. Surv. 2019, 52, 1–36. [Google Scholar] [CrossRef]
- Ramyachitra, D.; Manikandan, P. Imbalanced dataset classification and solutions: A review. Int. J. Comput. Bus. Res. (IJCBR) 2014, 5, 1–29. [Google Scholar]
- Gibb, W.R.; Lees, A.J. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J. Neurol. Neurosurg. Psychiatry 1988, 51, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Shulman, L.M.; Gruber-Baldini, A.L.; Anderson, K.E.; Vaughan, C.G.; Reich, S.G.; Fishman, P.S.; Weiner, W.J. The evolution of disability in Parkinson disease. Mov. Disord. 2008, 23, 790–796. [Google Scholar] [CrossRef]
- Ghourchian, S.; Gruber-Baldini, A.L.; Shakya, S.; Herndon, J.; Reich, S.G.; von Coelln, R.; Savitt, J.M.; Shulman, L.M. Weight loss and weight gain in Parkinson disease. Park. Relat. Disord. 2021, 83, 31–36. [Google Scholar] [CrossRef]
- Shulman, L.M.; Gruber-Baldini, A.L.; Anderson, K.E.; Fishman, P.S.; Reich, S.G.; Weiner, W.J. The clinically important difference on the unified Parkinson’s disease rating scale. Arch. Neurol. 2010, 67, 64–70. [Google Scholar] [CrossRef]
- Wenning, G.K.; Stankovic, I.; Vignatelli, L.; Fanciulli, A.; Calandra-Buonaura, G.; Seppi, K.; Palma, J.A.; Meissner, W.G.; Krismer, F.; Berg, D.; et al. The movement disorder society criteria for the diagnosis of multiple system atrophy. Mov. Disord. 2022, 37, 1131–1148. [Google Scholar] [CrossRef]
- Höglinger, G.U.; Respondek, G.; Stamelou, M.; Kurz, C.; Josephs, K.A.; Lang, A.E.; Mollenhauer, B.; Müller, U.; Nilsson, C.; Whitwell, J.L.; et al. Clinical diagnosis of progressive supranuclear palsy: The movement disorder society criteria. Mov. Disord. 2017, 32, 853–864. [Google Scholar] [CrossRef]
- McKeith, I.G.; Boeve, B.F.; Dickson, D.W.; Halliday, G.; Taylor, J.P.; Weintraub, D.; Aarsland, D.; Galvin, J.; Attems, J.; Ballard, C.G.; et al. Diagnosis and management of dementia with lewy bodies: Fourth consensus report of the DLB consortium. Neurology 2017, 89, 88–100. [Google Scholar] [CrossRef]
- Armstrong, M.J.; Litvan, I.; Lang, A.E.; Bak, T.H.; Bhatia, K.P.; Borroni, B.; Boxer, A.L.; Dickson, D.W.; Grossman, M.; Hallett, M.; et al. Criteria for the diagnosis of corticobasal degeneration. Neurology 2013, 80, 496–503. [Google Scholar] [CrossRef]
- Bhatia, K.P.; Bain, P.; Bajaj, N.; Elble, R.J.; Hallett, M.; Louis, E.D.; Raethjen, J.; Stamelou, M.; Testa, C.M.; Deuschl, G. Consensus statement on the classification of tremors. From the task force on tremor of the International Parkinson and Movement Disorder Society. Mov. Disord. 2018, 33, 75–87. [Google Scholar] [CrossRef]
- Shin, H.W.; Chung, S.J. Drug-induced parkinsonism. J. Clin. Neurol. 2012, 8, 15–21. [Google Scholar] [CrossRef]
- Khalil, R.M.; Shulman, L.M.; Gruber-Baldini, A.L.; Shakya, S.; Fenderson, R.; Van Hoven, M.; Hausdorff, J.M.; von Coelln, R.; Cummings, M.P. Simplification of Mobility Tests and Data Processing to Increase Applicability of Wearable Sensors as Diagnostic Tools for Parkinson’s Disease. Sensors 2024, 24, 4983. [Google Scholar] [CrossRef]
- Buchman, A.S.; Leurgans, S.E.; Weiss, A.; VanderHorst, V.; Mirelman, A.; Dawe, R.; Barnes, L.L.; Wilson, R.S.; Hausdorff, J.M.; Bennett, D.A. Associations between quantitative mobility measures derived from components of conventional mobility testing and parkinsonian gait in older adults. PLoS ONE 2014, 9, e86262. [Google Scholar] [CrossRef]
- Dawe, R.J.; Leurgans, S.E.; Yang, J.; Bennett, J.M.; Hausdorff, J.M.; Lim, A.S.; Gaiteri, C.; Bennett, D.A.; Buchman, A.S. Association between quantitative gait and balance measures and total daily physical activity in community-dwelling older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 636–642. [Google Scholar] [CrossRef]
- Butterworth, S. On the theory of filter amplifiers. Exp. Wirel. Wirel. Eng. 1930, 7, 536–541. [Google Scholar]
- Zijlstra, W.; Hof, A.L. Assessment of spatio-temporal gait parameters from trunk accelerations during human walking. Gait Posture 2003, 18, 1–10. [Google Scholar] [CrossRef]
- Palmerini, L.; Mellone, S.; Avanzolini, G.; Valzania, F.; Chiari, L. Quantification of motor impairment in Parkinson’s disease using an instrumented timed up and go test. IEEE Trans. Neural Syst. Rehabil. Eng. 2013, 21, 664–673. [Google Scholar] [CrossRef]
- Antunes, T.P.C.; van Kesteren, K.P. Prediction of trip severity based on tri-axial accelerometry in healthy older adults. J. Hum. Growth Dev. 2015, 25, 75–81. [Google Scholar] [CrossRef][Green Version]
- Toebes, M.J.; Hoozemans, M.J.; Furrer, R.; Dekker, J.; van Dieën, J.H. Associations between measures of gait stability, leg strength and fear of falling. Gait Posture 2015, 41, 76–80. [Google Scholar] [CrossRef]
- Koop, M.M.; Ozinga, S.J.; Rosenfeldt, A.B.; Alberts, J.L. Quantifying turning behavior and gait in Parkinson’s disease using mobile technology. IBRO Rep. 2018, 5, 10–16. [Google Scholar] [CrossRef]
- Burden, R.L.; Faires, J. Numerical Analysis, 9th ed.; Brooks/Cole: Pacific Grove, CA, USA, 2015. [Google Scholar]
- Lomb, N.R. Least-squares frequency analysis of unequally spaced data. Astrophys. Space Sci. 1976, 39, 447–462. [Google Scholar] [CrossRef]
- Scargle, J.D. Studies in astronomical time series analysis. II. Statistical aspects of spectral analysis of unevenly spaced data. Astrophys. J. 1982, 263, 835–853. [Google Scholar] [CrossRef]
- Liu, T.Y. EasyEnsemble and Feature Selection for Imbalance Data Sets. In Proceedings of the 2009 International Joint Conference on Bioinformatics, Systems Biology and Intelligent Computing, Shanghai, China, 3–5 August 2009; pp. 517–520. [Google Scholar] [CrossRef]
- Liu, X.Y.; Wu, J.; Zhou, Z.H. Exploratory Under-Sampling for Class-Imbalance Learning. In Proceedings of the Sixth International Conference on Data Mining, ICDM ’06, Bethesda, MD, USA, 18–22 December 2006; pp. 965–969. [Google Scholar] [CrossRef]
- Cover, T.; Thomas, J. Elements of Information Theory; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 1991. [Google Scholar]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Hastie, T.; Tibshirani, R.; Friedman, J. The Elements of Statistical Learning: Data Mining, Inference, and Prediction, 2nd ed.; Springer Series in Statistics; Springer: Berlin, Germany, 2009. [Google Scholar]
- Weiss, G.M. Mining with rarity: A unifying framework. ACM SIGKDD Explor. Newsl. 2004, 6, 7–19. [Google Scholar] [CrossRef]
- Gregorutti, B.; Michel, B.; Saint-Pierre, P. Grouped variable importance with random forests and application to multiple functional data analysis. Comput. Stat. Data Anal. 2015, 90, 15–35. [Google Scholar] [CrossRef]
- Canty, A.; Ripley, B.D. boot: Bootstrap R (S-Plus) Functions, R package version 1.3-30. 2024. Available online: https://cran.r-project.org/web/packages/boot/index.html (accessed on 21 February 2025).
- Davison, A.C.; Hinkley, D.V. Bootstrap Methods and Their Applications; Cambridge University Press: Cambridge, UK, 1997. [Google Scholar]
- Goetz, C.G. The unified Parkinson’s disease rating scale (UPDRS): Status and recommendations. Mov. Disord. 2003, 18, 738–750. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The montreal cognitive assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.D.; Paradis, C.F.; Houck, P.R.; Mazumdar, S.; Stack, J.A.; Rifai, A.; Mulsant, B.; Reynolds, C.F. Rating chronic medical illness burden in geropsychiatric practice and research: Application of the cumulative illness rating scale. Psychiatry Res. 1992, 41, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Hoehn, M.M.; Yahr, M.D. Parkinsonism: Onset, progression, and mortality. Neurology 1967, 17, 427–442. [Google Scholar] [CrossRef] [PubMed]
- Goetz, C.G.; Poewe, W.; Rascol, O.; Sampaio, C.; Stebbins, G.T.; Counsell, C.; Giladi, N.; Holloway, R.G.; Moore, C.G.; Wenning, G.K.; et al. Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: Status and recommendations. Mov. Disord. 2004, 19, 1020–1028. [Google Scholar] [CrossRef]
- Kamalov, F.; Thabtah, F.; Leung, H.H. Feature Selection in Imbalanced Data. Ann. Data Sci. 2023, 10, 1527–1541. [Google Scholar] [CrossRef]
- Akaike, H. Information Theory and an Extension of the Maximum Likelihood Principle. In Selected Papers of Hirotugu Akaike; Springer: New York, NY, USA, 1998; pp. 199–213. [Google Scholar] [CrossRef]
- Junsomboon, N.; Phienthrakul, T. Combining Over-Sampling and Under-Sampling Techniques for Imbalance Dataset. In Proceedings of the 9th International Conference on Machine Learning and Computing, Singapore, 24–26 January 2025; Association for Computing Machinery: New York, NY, USA, 2017; pp. 243–247. [Google Scholar] [CrossRef]
- Shanab, A.A.; Khoshgoftaar, T.M.; Wald, R.; Van Hulse, J. Comparison of approaches to alleviate problems with high-dimensional and class-imbalanced data. In Proceedings of the 2011 IEEE International Conference on Information Reuse & Integration, Las Vegas, NV, USA, 3–5 August 2011; pp. 234–239. [Google Scholar] [CrossRef]
- Laurikkala, J. Improving Identification of Difficult Small Classes by Balancing Class Distribution. In Proceedings of the Artificial Intelligence in Medicine, Cascais, Portugal, 1–4 July 2001; Quaglini, S., Barahona, P., Andreassen, S., Eds.; Springer: Berlin, Heidelberg, 2001; pp. 63–66. [Google Scholar]
- Chawla, N.V.; Bowyer, K.W.; Hall, L.O.; Kegelmeyer, W.P. SMOTE: Synthetic Minority Over-sampling Technique. J. Artif. Intell. Res. 2002, 16, 321–357. [Google Scholar] [CrossRef]
- Sharma, M.; Mishra, R.K.; Hall, A.J.; Casado, J.; Cole, R.; Nunes, A.S.; Barchard, G.; Vaziri, A.; Pantelyat, A.; Wills, A.M. Remote at-home wearable-based gait assessments in progressive supranuclear palsy compared to Parkinson’s disease. BMC Neurol. 2023, 23, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Hatanaka, N.; Sato, K.; Hishikawa, N.; Takemoto, M.; Ohta, Y.; Yamashita, T.; Abe, K. Comparative gait analysis in progressive supranuclear palsy and Parkinson’s disease. Eur. Neurol. 2016, 75, 282–289. [Google Scholar] [CrossRef]
- Sidoroff, V.; Raccagni, C.; Kaindlstorfer, C.; Eschlboeck, S.; Fanciulli, A.; Granata, R.; Eskofier, B.; Seppi, K.; Poewe, W.; Willeit, J.; et al. Characterization of gait variability in multiple system atrophy and Parkinson’s disease. J. Neurol. 2021, 268, 1770–1779. [Google Scholar] [CrossRef]
- Khalil, R.M.; Shulman, L.M.; Gruber-Baldini, A.L.; Shakya, S.; Hausdorff, J.M.; von Coelln, R.; Cummings, M.P. Machine Learning and Statistical Analyses of Sensor Data Reveal Variability Between Repeated Trials in Parkinson’s Disease Mobility Assessments. Sensors 2024, 24, 8096. [Google Scholar] [CrossRef]
- Bäckström, D.; Granåsen, G.; Domellöf, M.E.; Linder, J.; Mo, S.J.; Riklund, K.; Zetterberg, H.; Blennow, K.; Forsgren, L. Early predictors of mortality in parkinsonism and Parkinson disease. Neurology 2018, 91, e2045–e2056. [Google Scholar] [CrossRef]
- Fielding, S.; Macleod, A.; Counsell, C. Medium-term prognosis of an incident cohort of parkinsonian patients compared to controls. Park. Relat. Disord. 2016, 32, 36–41. [Google Scholar] [CrossRef]
- Cooley, J.W.; Tukey, J.W. An algorithm for the machine calculation of complex fourier series. Math. Comput. 1965, 19, 297–301. [Google Scholar] [CrossRef]
- Phinyomark, A.; Phukpattaranont, P.; Limsakul, C. Feature reduction and selection for EMG signal classification. Expert Syst. Appl. 2012, 39(8), 7420–7431. [Google Scholar] [CrossRef]
- Altin, C.; Er, O. Comparison of different time and frequency domain feature extraction methods on elbow gesture’s EMG. Eur. J. Interdiscip. Stud. 2016, 5, 35. [Google Scholar] [CrossRef]
- Oung, Q.; Hariharan, M.; Lee, H.; Basah, S.; Sarillee, M.; Lee, C. Wearable multimodal sensors for evaluation of patients with Parkinson disease. In Proceedings of the 2015 IEEE International Conference on Control System, Computing and Engineering (ICCSCE), Penang, Malaysia, 27–29 November 2015; pp. 269–274. [Google Scholar] [CrossRef]
- Sinderby, C.; Lindstrom, L.; Grassino, A.E. Automatic assessment of electromyogram quality. J. Appl. Physiol. 1995, 79, 1803–1815. [Google Scholar] [CrossRef] [PubMed]
- Pepa, L.; Ciabattoni, L.; Verdini, F.; Capecci, M.; Ceravolo, M. Smartphone based fuzzy logic freezing of gait detection in Parkinson’s disease. In Proceedings of the 2014 IEEE/ASME 10th International Conference on Mechatronic and Embedded Systems and Applications (MESA), Ancona, Italy, 10–12 September 2014; pp. 1–6. [Google Scholar] [CrossRef]
- Mehta, A.; Vaddadi, S.K.; Sharma, V.; Kala, P. A Phase-Wise Analysis of Machine Learning Based Human Activity Recognition using Inertial Sensors. In Proceedings of the 2020 IEEE 17th India Council International Conference (INDICON), New Delhi, India, 10–13 December 2020; pp. 1–7. [Google Scholar] [CrossRef]
- Bao, L.; Intille, S.S. Activity recognition from user-annotated acceleration data. In Proceedings of the International Conference on Pervasive Computing, Vienna, Austria, 21–23 April 2004; pp. 1–17. [Google Scholar] [CrossRef]
- Arora, S.; Venkataraman, V.; Donohue, S.; Biglan, K.M.; Dorsey, E.R.; Little, M.A. High Accuracy Discrimination of Parkinson’s Disease Participants from Healthy Controls using Smartphones. In Proceedings of the 2014 IEEE International Conference on Acoustics, Speech and Signal Processing (ICASSP), Florence, Italy, 4–9 May 2014; pp. 3641–3644. [Google Scholar] [CrossRef]
- Aich, S.; Youn, J.; Chakraborty, S.; Pradhan, P.M.; Park, J.H.; Park, S.; Park, J. A supervised machine learning approach to detect the on/off state in Parkinson’s disease using wearable based gait signals. Diagnostics 2020, 10, 421. [Google Scholar] [CrossRef] [PubMed]
- Phinyomark, A.; Phukpattaranont, P.; Limsakul, C. Fractal analysis features for weak and single-channel upper-limb EMG signals. Expert Syst. Appl. 2012, 39(12), 11156–11163. [Google Scholar] [CrossRef]
- Hasni, H.; Yahya, N.; Asirvadam, V.S.; Jatoi, M.A. Analysis of electromyogram (EMG) for detection of neuromuscular disorders. In Proceedings of the 2018 International Conference on Intelligent and Advanced System (ICIAS), Kuala Lumpur, Malaysia, 13–14 August 2018; pp. 1–6. [Google Scholar] [CrossRef]
- Sukumar, N.; Taran, S.; Bajaj, V. Physical actions classification of surface EMG signals using VMD. In Proceedings of the 2018 International Conference on Communication and Signal Processing (ICCSP), Chennai, India, 3–5 April 2018; pp. 0705–0709. [Google Scholar] [CrossRef]
- Kaiser, J. On a simple algorithm to calculate the ’energy’ of a signal. In Proceedings of the International Conference on Acoustics, Speech and Signal Processing (ICASSP), Albuquerque, NM, USA, 3–6 April 1990; vol. 1, pp. 381–384. [Google Scholar] [CrossRef]
- Penzel, T.; Kantelhardt, J.; Grote, L.; Peter, J.; Bunde, A. Comparison of detrended fluctuation analysis and spectral analysis for heart rate variability in sleep and sleep apnea. IEEE Trans. Biomed. Eng. 2003, 50, 1143–1151. [Google Scholar] [CrossRef]
- Higuchi, T. Approach to an irregular time series on the basis of the fractal theory. Phys. D Nonlinear Phenom. 1988, 31, 277–283. [Google Scholar] [CrossRef]
- Katz, M.J. Fractals and the analysis of waveforms. Comput. Biol. Med. 1988, 18, 145–156. [Google Scholar] [CrossRef]
- Gneiting, T.; Ševčíková, H.; Percival, D.B. Estimators of fractal dimension: Assessing the roughness of time series and spatial data. Stat. Sci. 2012, 27, 247–277. [Google Scholar] [CrossRef]
- Quiroz, J.C.; Banerjee, A.; Dascalu, S.M.; Lau, S.L. Feature selection for activity recognition from smartphone accelerometer data. Intell. Autom. Soft Comput. 2017, 24, 785–793. [Google Scholar] [CrossRef]
- Ayman, A.; Attalah, O.; Shaban, H. An efficient human activity recognition framework based on wearable IMU wrist sensors. In Proceedings of the 2019 IEEE International Conference on Imaging Systems and Techniques (IST), Abu Dhabi, United Arab Emirates, 9–10 December 2019; pp. 1–5. [Google Scholar] [CrossRef]
- Batool, M.; Jalal, A.; Kim, K. Sensors technologies for human activity analysis based on SVM optimized by PSO algorithm. In Proceedings of the 2019 International Conference on Applied and Engineering Mathematics (ICAEM), Taxila, Pakistan, 27–29 August 2019; pp. 145–150. [Google Scholar] [CrossRef]
- Venables, W.; Ripley, B. Modern Applied Statistics with S; Springer: New York, NY, USA, 2002. [Google Scholar]
| Feature | PD () | non-PD Parkinsonism () | p |
|---|---|---|---|
| Age (years, ) | 66.8 ± 9.3 | 68.7 ± 8.2 | 0.377 |
| Gender (%male) | 62.3 | 38.9 | 0.093 |
| Height (cm, ) | 172.2 ± 10.4 | 165.3 ± 13.8 | 0.059 |
| UPDRS (total, ) | 35.1 ± 17.1 | 47.9 ± 22.4 | 0.033 |
| UPDRS (motor-part III, ) | 22.1 ± 11.8 | 28.8 ± 14.9 | 0.085 |
| Disease duration (years, ) | 7.8 ± 6.5 | 1.7 ± 2.1 | 4.2 × 10−10 |
| H&Y () | 2.2 ± 0.62 | 2.9 ± 0.77 | 0.005 |
| stage 1 (n) | 12 | 0 | |
| stage 1.5 (n) | 4 | 0 | |
| stage 2 (n) | 168 | 6 | |
| stage 2.5 (n) | 35 | 3 | |
| stage 3 (n) | 24 | 5 | |
| stage 4 (n) | 17 | 4 |
| All Tasks | TUG-Only | |
|---|---|---|
| Balanced accuracy (CI) [%] | 72.9 (60.2, 81.4) | 78.2 (65.7, 85.6) |
| AUC-ROC (CI) | 0.73 (0.63, 0.83) | 0.78 (0.69, 0.87) |
| Sensitivity (CI) | 0.68 (0.62, 0.74) | 0.73 (0.67, 0.78) |
| Specificity (CI) | 0.78 (0.50, 0.93) | 0.83 (0.56, 0.95) |
| F1 score (CI) | 0.80 (0.76, 0.84) | 0.84 (0.80, 0.87) |
| (a) All Tasks | (b) TUG-Only | ||||
|---|---|---|---|---|---|
| PD | Pksm | PD | Pksm | ||
| PD | 177 | 83 | PD | 190 | 70 |
| Pksm | 4 * | 14 | Pksm | 3 * | 15 |
| PD | Non-PD Parkinsonism | |||||
|---|---|---|---|---|---|---|
| Correctly Classified | Incorrectly Classified | Correctly Classified | Incorrectly Classified | |||
| UPDRS_PIII | 19.7 ± 10.9 | 28.7 ± 11.4 | 3.8 × 10−8 | 30.5 ± 14.9 | 17.7 ± 5.7 | 0.016 |
| MoCA | 27.6 ± 2.8 | 26.5 ± 3.5 | 0.02 | 26.3 ± 4.9 | 23.3 ± 2.1 | 0.127 |
| CIRS-G | 4.5 ± 3.2 | 5.7 ± 3.9 | 0.035 | 8.0 ± 4.6 | 7.2 ± 3.6 | 0.73 |
| Age | 66.0 ± 8.9 | 69.1 ± 9.9 | 0.023 | 67.6 ± 8.1 | 74.0 ± 5.2 | 0.15 |
| Disease duration | 7.1 ± 5.9 | 8.7 ± 6.9 | 0.038 | 2.5 ± 2.3 | 2.3 ± 2.5 | 0.47 |
| Sex (% male) | 59.5 | 70.0 | 0.184 | 33.3 | 100.0 | 0.053 |
| H&Y (n) | 1.3 × 10−6 | 0.40 | ||||
| 1 | 10 | 2 | 0 | 0 | ||
| 1.5 | 3 | 1 | 0 | 0 | ||
| 2 | 138 | 30 | 4 | 2 | ||
| 2.5 | 27 | 8 | 2 | 1 | ||
| 3 | 8 | 16 | 5 | 0 | ||
| 4 | 4 | 13 | 4 | 0 | ||
| Unsupervised RF FS with Max Bacc | MI-Based Ranking for Top FS | F1 Ranking Using DT for Feature Scoring | Supervised RF FS with Min AIC | |
|---|---|---|---|---|
| Balanced accuracy (%) | 67.8 | 68.2 | 68.3 | 52.1 |
| AUC-ROC | 0.68 | 0.68 | 0.68 | 0.52 |
| Sensitivity | 0.80 | 0.92 | 0.87 | 0.93 |
| Specificity | 0.56 | 0.44 | 0.50 | 0.11 |
| F1 score | 0.87 | 0.94 | 0.91 | 0.93 |
| Sampling Before FS, Model Trained on Original Data | Sampling Before FS, Model Trained on Sampled Data | FS Before Sampling, Model Trained on Sampled Data | |
|---|---|---|---|
| Balanced accuracy (%) | 56.4 | 49.6 | 59.4 |
| AUC-ROC | 0.56 | 0.50 | 0.59 |
| Sensitivity | 0.96 | 0.99 | 0.97 |
| Specificity | 0.17 | 0.00 | 0.22 |
| F1 score | 0.95 | 0.96 | 0.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalil, R.M.; Shulman, L.M.; Gruber-Baldini, A.L.; Reich, S.G.; Savitt, J.M.; Hausdorff, J.M.; Coelln, R.v.; Cummings, M.P. Applying Wearable Sensors and Machine Learning to the Diagnostic Challenge of Distinguishing Parkinson’s Disease from Other Forms of Parkinsonism. Biomedicines 2025, 13, 572. https://doi.org/10.3390/biomedicines13030572
Khalil RM, Shulman LM, Gruber-Baldini AL, Reich SG, Savitt JM, Hausdorff JM, Coelln Rv, Cummings MP. Applying Wearable Sensors and Machine Learning to the Diagnostic Challenge of Distinguishing Parkinson’s Disease from Other Forms of Parkinsonism. Biomedicines. 2025; 13(3):572. https://doi.org/10.3390/biomedicines13030572
Chicago/Turabian StyleKhalil, Rana M., Lisa M. Shulman, Ann L. Gruber-Baldini, Stephen G. Reich, Joseph M. Savitt, Jeffrey M. Hausdorff, Rainer von Coelln, and Michael P. Cummings. 2025. "Applying Wearable Sensors and Machine Learning to the Diagnostic Challenge of Distinguishing Parkinson’s Disease from Other Forms of Parkinsonism" Biomedicines 13, no. 3: 572. https://doi.org/10.3390/biomedicines13030572
APA StyleKhalil, R. M., Shulman, L. M., Gruber-Baldini, A. L., Reich, S. G., Savitt, J. M., Hausdorff, J. M., Coelln, R. v., & Cummings, M. P. (2025). Applying Wearable Sensors and Machine Learning to the Diagnostic Challenge of Distinguishing Parkinson’s Disease from Other Forms of Parkinsonism. Biomedicines, 13(3), 572. https://doi.org/10.3390/biomedicines13030572







