Aspergillus flavus with Mycovirus as an Etiologic Factor for Acute Leukemias in Susceptible Individuals: Evidence and Discussion
Abstract
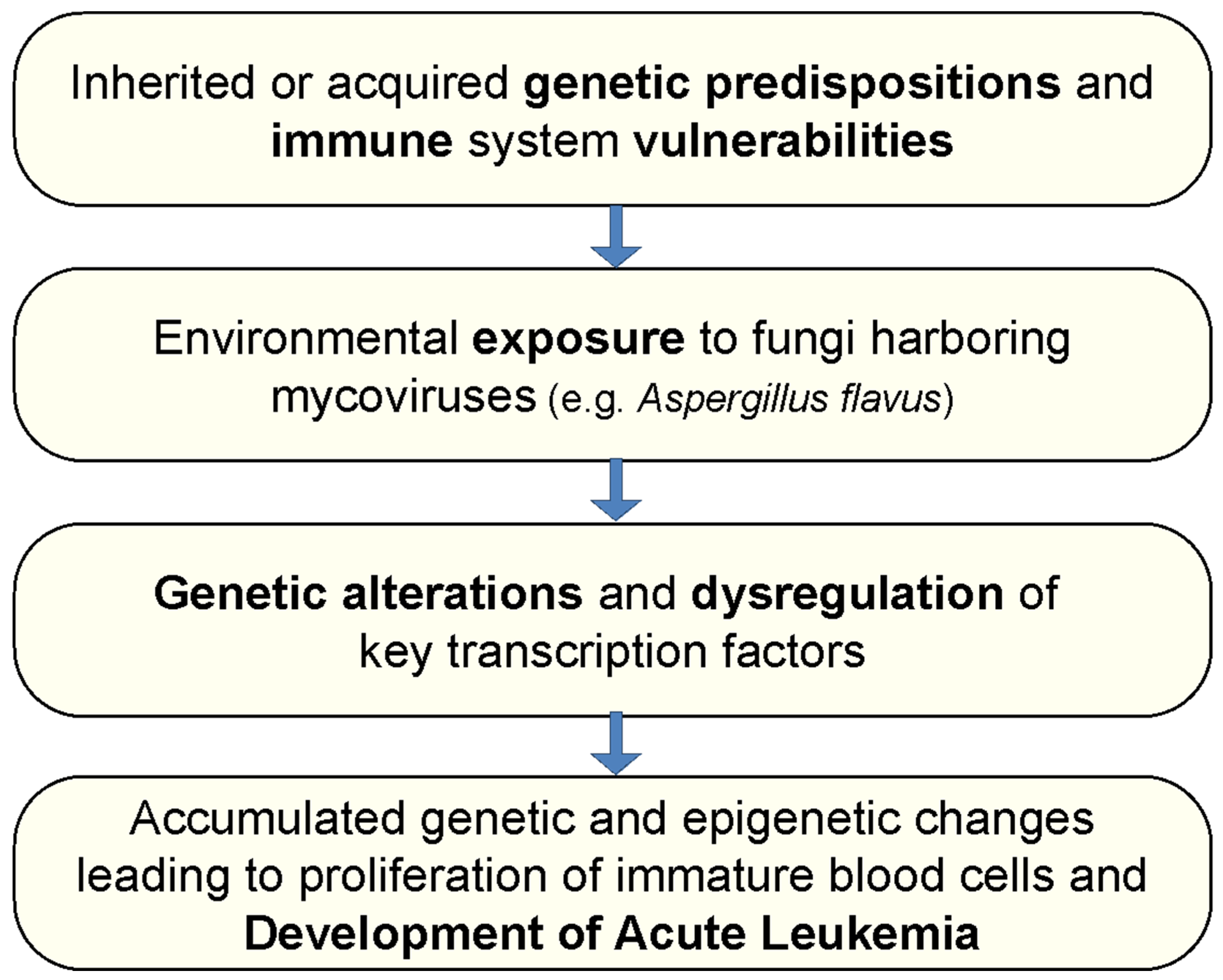
1. Introduction
2. Pathophysiology
2.1. Genetics
2.2. Leukemia in Twins
2.3. Carcinogenesis Attributed to Microbiome Flora
2.4. Mycovirus-Containing Aspergillus flavus and Acute Leukemia
2.5. Fungi in the Environment
2.6. Incidence of Cancer and Leukemia in Asthmatics
2.7. History of Allergy and Mortality from Cancer
2.8. Agricultural Workers and Foresters and Leukemia
3. Conclusions and Hypothesis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef]
- Huang, J.; Chan, S.C.; Ngai, C.H.; Lok, V.; Zhang, L.; Lucero-Prisno, D.E.; Xu, W.; Zheng, Z.-J.; Elcarte, E.; Withers, M.; et al. Disease Burden, Risk Factors, and Trends of Leukaemia: A Global Analysis. Front. Oncol. 2022, 12, 904292. [Google Scholar] [CrossRef] [PubMed]
- Daltveit, D.S.; Morgan, E.; Colombet, M.; Steliarova-Foucher, E.; Bendahhou, K.; Marcos-Gragera, R.; Rongshou, Z.; Smith, A.; Wei, H.; Soerjomataram, I. Global Patterns of Leukemia by Subtype, Age, and Sex in 185 Countries in 2022. Leukemia 2024, 39, 412–419. [Google Scholar] [CrossRef]
- Ruchlemer, R.; Polliack, A. Geography, Ethnicity and “Roots” in Chronic Lymphocytic Leukemia. Leuk. Lymphoma 2013, 54, 1142–1150. [Google Scholar] [CrossRef]
- Mendizabal, A.M.; Younes, N.; Levine, P.H. Geographic and Income Variations in Age at Diagnosis and Incidence of Chronic Myeloid Leukemia. Int. J. Hematol. 2016, 103, 70–78. [Google Scholar] [CrossRef]
- De Braekeleer, M.; De Braekeleer, E.; Douet-Guilbert, N. Geographic/Ethnic Variability of Chromosomal and Molecular Abnormalities in Leukemia. Expert Rev. Anticancer Ther. 2015, 15, 1093–1102. [Google Scholar] [CrossRef] [PubMed]
- Schüz, J.; Erdmann, F. Environmental Exposure and Risk of Childhood Leukemia: An Overview. Arch. Med. Res. 2016, 47, 607–614. [Google Scholar] [CrossRef]
- Stiller, C.A.; Parkin, D.M. Geographic and ethnic variations in the incidence of childhood cancer. Br. Med. Bull. 1996, 52, 682–703. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Bispo, J.A.B.; Pinheiro, P.S.; Kobetz, E.K. Epidemiology and Etiology of Leukemia and Lymphoma. Cold Spring Harb. Perspect. Med. 2020, 10, a034819. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.A. Leukemia in children. Pediatr. Rev. 2019, 40, 319–331. [Google Scholar] [CrossRef]
- Namayandeh, S.M.; Khazaei, Z.; Lari Najafi, M.; Goodarzi, E.; Moslem, A. GLOBAL Leukemia in Children 0–14 Statistics 2018, Incidence and Mortality and Human Development Index (HDI): GLOBOCAN Sources and Methods. Asian Pac. J. Cancer Prev. APJCP 2020, 21, 1487–1494. [Google Scholar] [CrossRef] [PubMed]
- Kojika, S.; Griffin, J.D. Notch Receptors and Hematopoiesis. Exp. Hematol. 2001, 29, 1041–1052. [Google Scholar] [CrossRef]
- Sun, H.; Wei, S.; Yang, L. Dysfunction of Immune System in the Development of Large Granular Lymphocyte Leukemia. Hematology 2019, 24, 139–147. [Google Scholar] [CrossRef]
- Kinlen, L. Epidemiological evidence for an infective basis in childhood leukaemia. J. R. Soc. Health 1996, 116, 393–399. [Google Scholar] [CrossRef]
- Greaves, M.F. Speculations on the cause of childhood acute lymphoblastic leukemia. Leukemia 1988, 2, 120–125. [Google Scholar]
- Greaves, M. Aetiology of acute leukaemia. Lancet 1997, 349, 344–349. [Google Scholar] [CrossRef] [PubMed]
- Buffle, P.A.; Kwan, M.L.; Reynolds, P.; Urayama, K.Y. Environmental and genetic risk factors for childhood leukemia: Appraising the evidence. Cancer Investig. 2005, 23, 60–75. [Google Scholar] [CrossRef]
- Figueroa, S.C.; Kennedy, C.J.; Wesseling, C.; Wiemels, J.M.; Morimoto, L.; Mora, A.M. Early immune stimulation and childhood acute lymphoblastic leukemia in Costa Rica: A comparison of statistical approaches. Environ. Res. 2020, 182, 109023. [Google Scholar] [CrossRef] [PubMed]
- Greaves, M. A causal mechanism for childhood acute lymphoblastic leukaemia. Nat. Rev. Cancer 2018, 18, 471–484. [Google Scholar] [CrossRef] [PubMed]
- Hauer, J.; Fischer, U.; Borkhardt, A. Toward prevention of childhood ALL by early-life immune training. Blood J. Am. Soc. Hematol. 2021, 138, 1412–1428. [Google Scholar] [CrossRef] [PubMed]
- Ajrouche, R.; Rudant, J.; Orsi, L.; Petit, A.; Baruchel, A.; Lambilliotte, A.; Gambart, M.; Michel, G.; Bertrand, Y.; Ducassou, S.; et al. Childhood Acute Lymphoblastic Leukaemia and Indicators of Early Immune Stimulation: The Estelle Study (SFCE). Br. J. Cancer 2015, 112, 1017–1026. [Google Scholar] [CrossRef] [PubMed]
- Rudant, J.; Orsi, L.; Menegaux, F.; Petit, A.; Baruchel, A.; Bertrand, Y.; Lambilliotte, A.; Robert, A.; Michel, G.; Margueritte, G.; et al. Childhood Acute Leukemia, Early Common Infections, and Allergy: The ESCALE Study. Am. J. Epidemiol. 2010, 172, 1015–1027. [Google Scholar] [CrossRef]
- Gale, R.P.; Opelz, G. Commentary: Does Immune Suppression Increase Risk of Developing Acute Myeloid Leukemia? Leukemia 2012, 26, 422–423. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, S.M.; Fouad, T.M.; Summa, V.; Hasan, S.K.; Lo-Coco, F. Acute Myeloid Leukemia Developing in Patients with Autoimmune Diseases. Haematologica 2012, 97, 805–817. [Google Scholar] [CrossRef]
- Derhovanessian, E.; Solana, R.; Larbi, A.; Pawelec, G. Immunity, Ageing and Cancer. Immun. Ageing 2008, 5, 11. [Google Scholar] [CrossRef] [PubMed]
- Hakim, F.T.; Flomerfelt, F.A.; Boyiadzis, M.; Gress, R.E. Aging, Immunity and Cancer. Curr. Opin. Immunol. 2004, 16, 151–156. [Google Scholar] [CrossRef]
- Domer, P.H.; Fakharzadeh, S.S.; Chen, C.S.; Jockel, J.; Johansen, L.; Silverman, G.A.; Kersey, J.H.; Korsmeyer, S.J. Acute Mixed-Lineage Leukemia t(4;11)(Q21;Q23) Generates an MLL-AF4 Fusion Product. Proc. Natl. Acad. Sci. USA 1993, 90, 7884–7888. [Google Scholar] [CrossRef] [PubMed]
- Tebbi, C.K. Etiology of Acute Leukemia: A Review. Cancers 2021, 13, 2256. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.L.; Arts, P.; Carmichael, C.L.; Babic, M.; Dobbins, J.; Chong, C.-E.; Schreiber, A.W.; Feng, J.; Phillips, K.; Wang, P.P.S.; et al. RUNX1-Mutated Families Show Phenotype Heterogeneity and a Somatic Mutation Profile Unique to Germline Predisposed AML. Blood Adv. 2020, 4, 1131–1144. [Google Scholar] [CrossRef]
- Greaves, M. Pre-Natal Origins of Childhood Leukemia. Rev. Clin. Exp. Hematol. 2003, 7, 233–245. [Google Scholar]
- Greaves, M.F.; Maia, A.T.; Wiemels, J.L.; Ford, A.M. Leukemia in Twins: Lessons in Natural History. Blood 2003, 102, 2321–2333. [Google Scholar] [CrossRef]
- Bardini, M.; Fazio, G.; Abascal, L.C.; Meyer, C.; Maglia, O.; Sala, S.; Palamini, S.; Rebellato, S.; Marschalek, R.; Rizzari, C.; et al. Prenatal Origin of NUTM1 Gene Rearrangement in Infant B-Cell Precursor Acute Lymphoblastic Leukaemia. Br. J. Haematol. 2024, 205, 1883–1888. [Google Scholar] [CrossRef] [PubMed]
- Papaemmanuil, E.; Rapado, I.; Li, Y.; Potter, N.E.; Wedge, D.C.; Tubio, J.; Alexandrov, L.B.; Van Loo, P.; Cooke, S.L.; Marshall, J.; et al. RAG-Mediated Recombination Is the Predominant Driver of Oncogenic Rearrangement in ETV6-RUNX1 Acute Lymphoblastic Leukemia. Nat. Genet. 2014, 46, 116–125. [Google Scholar] [CrossRef]
- Ford, A.M.; Colman, S.; Greaves, M. Covert Pre-Leukaemic Clones in Healthy Co-Twins of Patients with Childhood Acute Lymphoblastic Leukaemia. Leukemia 2023, 37, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Kirkham, C.M.; Scott, J.N.F.; Wang, X.; Smith, A.L.; Kupinski, A.P.; Ford, A.M.; Westhead, D.R.; Stockley, P.G.; Tuma, R.; Boyes, J. Cut-and-Run: A Distinct Mechanism by Which V(D)J Recombination Causes Genome Instability. Mol. Cell 2019, 74, 584–597.e9. [Google Scholar] [CrossRef] [PubMed]
- Paulsson, K.; Lilljebjörn, H.; Biloglav, A.; Olsson, L.; Rissler, M.; Castor, A.; Barbany, G.; Fogelstrand, L.; Nordgren, A.; Sjögren, H.; et al. The Genomic Landscape of High Hyperdiploid Childhood Acute Lymphoblastic Leukemia. Nat. Genet. 2015, 47, 672–676. [Google Scholar] [CrossRef]
- Kaczmarska, A.; Derebas, J.; Pinkosz, M.; Niedźwiecki, M.; Lejman, M. The Landscape of Secondary Genetic Rearrangements in Pediatric Patients with B-Cell Acute Lymphoblastic Leukemia with t(12;21). Cells 2023, 12, 357. [Google Scholar] [CrossRef]
- Aydin, C.; Cetin, Z.; Manguoglu, A.E.; Tayfun, F.; Clark, O.A.; Kupesiz, A.; Akkaya, B.; Karauzum, S.B. Evaluation of ETV6/RUNX1 Fusion and Additional Abnormalities Involving ETV6 and/or RUNX1 Genes Using FISH Technique in Patients with Childhood Acute Lymphoblastic Leukemia. Indian J. Hematol. Blood Transfus. 2016, 32, 154–161. [Google Scholar] [CrossRef]
- Parker, H.; An, Q.; Barber, K.; Case, M.; Davies, T.; Konn, Z.; Stewart, A.; Wright, S.; Griffiths, M.; Ross, F.M.; et al. The Complex Genomic Profile of ETV6-RUNX1 Positive Acute Lymphoblastic Leukemia Highlights a Recurrent Deletion of TBL1XR1. Genes Chromosomes Cancer 2008, 47, 1118–1125. [Google Scholar] [CrossRef] [PubMed]
- Sundaresh, A.; Williams, O. Mechanism of ETV6-RUNX1 leukemia. In RUNX Proteins in Development and Cancer; Springer: Singapore, 2017; pp. 201–216. [Google Scholar]
- Schäfer, D.; Olsen, M.; Lähnemann, D.; Stanulla, M.; Slany, R.; Schmiegelow, K.; Borkhardt, A.; Fischer, U. Five percent of healthy newborns have an ETV6-RUNX1 fusion as revealed by DNA-based GIPFEL screening. Blood J. Am. Soc. Hematol. 2018, 131, 821–826. [Google Scholar] [CrossRef]
- Dobbins, S.E.; Sherborne, A.L.; Ma, Y.P.; Bardini, M.; Biondi, A.; Cazzaniga, G.; Lloyd, A.; Chubb, D.; Greaves, M.F.; Houlston, R.S. The Silent Mutational Landscape of Infant MLL-AF4 pro-B Acute Lymphoblastic Leukemia. Genes Chromosomes Cancer 2013, 52, 954–960. [Google Scholar] [CrossRef] [PubMed]
- Andersson, A.K.; Ma, J.; Wang, J.; Chen, X.; Gedman, A.L.; Dang, J.; Nakitandwe, J.; Holmfeldt, L.; Parker, M.; Easton, J.; et al. The Landscape of Somatic Mutations in Infant MLL-Rearranged Acute Lymphoblastic Leukemias. Nat. Genet. 2015, 47, 330–337. [Google Scholar] [CrossRef]
- Mullighan, C.G.; Miller, C.B.; Radtke, I.; Phillips, L.A.; Dalton, J.; Ma, J.; White, D.; Hughes, T.P.; Le Beau, M.M.; Pui, C.-H.; et al. BCR-ABL1 Lymphoblastic Leukaemia Is Characterized by the Deletion of Ikaros. Nature 2008, 453, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Mullighan, C.G.; Su, X.; Zhang, J.; Radtke, I.; Phillips, L.A.A.; Miller, C.B.; Ma, J.; Liu, W.; Cheng, C.; Schulman, B.A.; et al. Deletion of IKZF1 and Prognosis in Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2009, 360, 470–480. [Google Scholar] [CrossRef] [PubMed]
- de Rooij, J.D.E.; Beuling, E.; van den Heuvel-Eibrink, M.M.; Obulkasim, A.; Baruchel, A.; Trka, J.; Reinhardt, D.; Sonneveld, E.; Gibson, B.E.S.; Pieters, R.; et al. Recurrent Deletions of IKZF1 in Pediatric Acute Myeloid Leukemia. Haematologica 2015, 100, 1151–1159. [Google Scholar] [CrossRef]
- Kuiper, R.P.; Waanders, E.; van der Velden, V.H.J.; van Reijmersdal, S.V.; Venkatachalam, R.; Scheijen, B.; Sonneveld, E.; van Dongen, J.J.M.; Veerman, A.J.P.; van Leeuwen, F.N.; et al. IKZF1 Deletions Predict Relapse in Uniformly Treated Pediatric Precursor B-ALL. Leukemia 2010, 24, 1258–1264. [Google Scholar] [CrossRef]
- Dupuis, A.; Gaub, M.P.; Legrain, M.; Drenou, B.; Mauvieux, L.; Lutz, P.; Herbrecht, R.; Chan, S.; Kastner, P. Biclonal and Biallelic Deletions Occur in 20% of B-ALL Cases with IKZF1 Mutations. Leukemia 2013, 27, 503–507. [Google Scholar] [CrossRef][Green Version]
- Dörge, P.; Meissner, B.; Zimmermann, M.; Möricke, A.; Schrauder, A.; Bouquin, J.-P.; Schewe, D.; Harbott, J.; Teigler-Schlegel, A.; Ratei, R.; et al. IKZF1 Deletion Is an Independent Predictor of Outcome in Pediatric Acute Lymphoblastic Leukemia Treated According to the ALL-BFM 2000 Protocol. Haematologica 2013, 98, 428–432. [Google Scholar] [CrossRef]
- Gupta, S.K.; Bakhshi, S.; Kumar, L.; Seth, R.; Kumar, R. IKZF1 (IKAROS) deletions in B-ALL and its clinical correlation: A prospective study from a tertiary care centre in Northern India. Leuk. Res. 2016, 41, 7–11. [Google Scholar] [CrossRef]
- Kuehn, H.S.; Boisson, B.; Cunningham-Rundles, C.; Reichenbach, J.; Stray-Pedersen, A.; Gelfand, E.W.; Maffucci, P.; Pierce, K.R.; Abbott, J.K.; Voelkerding, K.V.; et al. Loss of B Cells in Patients with Heterozygous Mutations in IKAROS. N. Engl. J. Med. 2016, 374, 1032–1043. [Google Scholar] [CrossRef] [PubMed]
- Churchman, M.L.; Qian, M.; Te Kronnie, G.; Zhang, R.; Yang, W.; Zhang, H.; Lana, T.; Tedrick, P.; Baskin, R.; Verbist, K.; et al. Germline Genetic IKZF1 Variation and Predisposition to Childhood Acute Lymphoblastic Leukemia. Cancer Cell 2018, 33, 937–948.e8. [Google Scholar] [CrossRef] [PubMed]
- Moriyama, T.; Metzger, M.L.; Wu, G.; Nishii, R.; Qian, M.; Devidas, M.; Yang, W.; Cheng, C.; Cao, X.; Quinn, E.; et al. Germline Genetic Variation in ETV6 and Risk of Childhood Acute Lymphoblastic Leukaemia: A Systematic Genetic Study. Lancet Oncol. 2015, 16, 1659–1666. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Schrader, K.A.; Waanders, E.; Timms, A.E.; Vijai, J.; Miething, C.; Wechsler, J.; Yang, J.; Hayes, J.; Klein, R.J.; et al. A Recurrent Germline PAX5 Mutation Confers Susceptibility to Pre-B Cell Acute Lymphoblastic Leukemia. Nat. Genet. 2013, 45, 1226–1231. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, B.D.; Boyse, E.A. Possible Explanation of the High Concoddance for Acute Leukaemia in Monozygotic Twins. Lancet 1971, 1, 699–701. [Google Scholar] [CrossRef]
- de Smith, A.J.; Wiemels, J.L.; Mead, A.J.; Roberts, I.; Roy, A.; Spector, L.G. Backtracking to the future: Unraveling the origins of childhood leukemia. Leukemia 2024, 38, 416–419. [Google Scholar] [CrossRef]
- Hemminki, K.; Jiang, Y. Risks among siblings and twins for childhood acute lymphoid leukaemia: Results from the Swedish Family-Cancer Database. Leukemia 2002, 16, 297–298. [Google Scholar] [CrossRef][Green Version]
- Falletta, J.M.; Starling, K.A.; Fernbach, D.J. Leukemia in Twins. Pediatrics 1973, 52, 846–849. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Dobbins, S.E.; Sherborne, A.L.; Chubb, D.; Galbiati, M.; Cazzaniga, G.; Micalizzi, C.; Tearle, R.; Lloyd, A.L.; Hain, R.; et al. Developmental Timing of Mutations Revealed by Whole-Genome Sequencing of Twins with Acute Lymphoblastic Leukemia. Proc. Natl. Acad. Sci. USA 2013, 110, 7429–7433. [Google Scholar] [CrossRef] [PubMed]
- Bueno, C.; Tejedor, J.R.; Bashford-Rogers, R.; González-Silva, L.; Valdés-Mas, R.; Agraz-Doblás, A.; Díaz de la Guardia, R.; Ribera, J.; Zamora, L.; Bilhou-Nabera, C.; et al. Natural History and Cell of Origin of TC F3-ZN F384 and PTPN11 Mutations in Monozygotic Twins with Concordant BCP-ALL. Blood 2019, 134, 900–905. [Google Scholar] [CrossRef]
- Cazzaniga, G.; van Delft, F.W.; Lo Nigro, L.; Ford, A.M.; Score, J.; Iacobucci, I.; Mirabile, E.; Taj, M.; Colman, S.M.; Biondi, A.; et al. Developmental Origins and Impact of BCR-ABL1 Fusion and IKZF1 Deletions in Monozygotic Twins with Ph+ Acute Lymphoblastic Leukemia. Blood 2011, 118, 5559–5564. [Google Scholar] [CrossRef] [PubMed]
- Alpar, D.; Wren, D.; Ermini, L.; Mansur, M.B.; van Delft, F.W.; Bateman, C.M.; Titley, I.; Kearney, L.; Szczepanski, T.; Gonzalez, D.; et al. Clonal Origins of ETV6-RUNX1+ Acute Lymphoblastic Leukemia: Studies in Monozygotic Twins. Leukemia 2015, 29, 839–846. [Google Scholar] [CrossRef] [PubMed]
- Kadan-Lottick, N.S.; Kawashima, T.; Tomlinson, G.; Friedman, D.L.; Yasui, Y.; Mertens, A.C.; Robison, L.L.; Strong, L.C. The Risk of Cancer in Twins: A Report from the Childhood Cancer Survivor Study. Pediatr. Blood Cancer 2006, 46, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Zella, D.; Gallo, R.C. Viruses and Bacteria Associated with Cancer: An Overview. Viruses 2021, 13, 1039. [Google Scholar] [CrossRef] [PubMed]
- Yusuf, K.; Sampath, V.; Umar, S. Bacterial Infections and Cancer: Exploring This Association And Its Implications for Cancer Patients. Int. J. Mol. Sci. 2023, 24, 3110. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, K.; Ahangari, H.; Chapeland-Leclerc, F.; Ruprich-Robert, G.; Tarhriz, V.; Dilmaghani, A. Role of Fungal Infections in Carcinogenesis and Cancer Development: A Literature Review. Adv. Pharm. Bull. 2022, 12, 747–756. [Google Scholar] [CrossRef] [PubMed]
- Hough, B.; Steenkamp, E.; Wingfield, B.; Read, D. Fungal viruses unveiled: A comprehensive review of mycoviruses. Viruses 2023, 15, 1202. [Google Scholar] [CrossRef]
- Khan, H.A.; Nerva, L.; Bhatti, M.F. The Good, the Bad and the Cryptic: The Multifaceted Roles of Mycoviruses and Their Potential Applications for a Sustainable Agriculture. Virology 2023, 585, 259–269. [Google Scholar] [CrossRef]
- Kotta-Loizou, I.; Coutts, R.H.A. Mycoviruses in Aspergilli: A Comprehensive Review. Front. Microbiol. 2017, 8, 1699. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, F.R. The RNA Interference-Virus Interplay: Tools of Nature for Gene Modulation, Morphogenesis, Evolution and a Possible Mean for Aflatoxin Control. Appl. Microbiol. Biotechnol. 2009, 83, 611–615. [Google Scholar] [CrossRef]
- Schmidt, F.R.; Lemke, P.A.; Esser, K. Viral Influences on Aflatoxin Formation by Aspergillus flavus. Appl. Microbiol. Biotechnol. 1986, 24, 248–252. [Google Scholar] [CrossRef]
- Tebbi, C.K.; Yan, J.; Sahakian, E.; Mediavilla-Varela, M.; Pinilla-Ibarz, J.; Patel, S.; Rottinghaus, G.E.; Liu, R.Y.; Dennison, C. Mycovirus-Containing Aspergillus flavus Alters Transcription Factors in Normal and Acute Lymphoblastic Leukemia Cells. Int. J. Mol. Sci. 2024, 25, 10361. [Google Scholar] [CrossRef]
- Tebbi, C.K.; Sahakian, E.; Yan, J.; Patel, S.; Mediavilla-Varela, M. Exposure to Mycovirus-Containing Aspergillus flavus Alters Transcription Factors in Normal and Leukemia Cell Lines. Proceedings 2024, 100, 19. [Google Scholar] [CrossRef]
- Tebbi, C.K.; Badiga, A.; Sahakian, E.; Arora, A.I.; Nair, S.; Powers, J.J.; Achille, A.N.; Jaglal, M.V.; Patel, S.; Migone, F. Plasma of Acute Lymphoblastic Leukemia Patients React to the Culture of a Mycovirus Containing Aspergillus flavus. J. Pediatr. Hematol. Oncol. 2020, 42, 350–358. [Google Scholar] [CrossRef]
- Tebbi, C.K.; Badiga, A.; Sahakian, E.; Powers, J.J.; Achille, A.N.; Patel, S.; Migone, F. Exposure to a Mycovirus Containing Aspergillus flavus Reproduces Acute Lymphoblastic Leukemia Cell Surface and Genetic Markers in Cells from Patients in Remission and Not Controls. Cancer Treat. Res. Commun. 2021, 26, 100279. [Google Scholar] [CrossRef] [PubMed]
- Saftien, A.; Puschhof, J.; Elinav, E. Fungi and cancer. Gut 2023, 72, 1410–1425. [Google Scholar] [CrossRef]
- Huët, M.A.L.; Lee, C.Z.; Rahman, S. A Review on Association of Fungi with the Development and Progression of Carcinogenesis in the Human Body. Curr. Res. Microb. Sci. 2022, 3, 100090. [Google Scholar] [CrossRef]
- Tebbi, C.K. Carcinogenesis and Leukemogenesis of Microorganisms: A Review. 21st Century Pathol. 2022, 2, 1–11. [Google Scholar]
- Di Cosola, M.; Cazzolla, A.P.; Charitos, I.A.; Ballini, A.; Inchingolo, F.; Santacroce, L. Candida albicans and Oral Carcinogenesis. A Brief Review. J. Fungi 2021, 7, 476. [Google Scholar] [CrossRef]
- Tebbi, C.K. Mycoviruses in Fungi: Carcinogenesis of Fungal Agents May Not Always Be Mycotoxin Related. J. Fungi 2023, 9, 368. [Google Scholar] [CrossRef] [PubMed]
- Kiruthiga, C.; Devi, K.P. Mechanisms Involved in Carcinogenesis. In Nutraceuticals and Cancer Signaling: Clinical Aspects and Mode of Action; Jafari, S.M., Nabavi, S.M., Silva, A.S., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 11–36. ISBN 978-3-030-74035-1. [Google Scholar]
- Vallianou, N.; Kounatidis, D.; Christodoulatos, G.S.; Panagopoulos, F.; Karampela, I.; Dalamaga, M. Mycobiome and Cancer: What Is the Evidence? Cancers 2021, 13, 3149. [Google Scholar] [CrossRef]
- Klimesova, K.; Jiraskova Zakostelska, Z.; Tlaskalova-Hogenova, H. Oral Bacterial and Fungal Microbiome Impacts Colorectal Carcinogenesis. Front. Microbiol. 2018, 9, 774. [Google Scholar] [CrossRef]
- Takahashi, Y.; Nagata, N.; Shimbo, T.; Nishijima, T.; Watanabe, K.; Aoki, T.; Sekine, K.; Okubo, H.; Watanabe, K.; Sakurai, T.; et al. Long-Term Trends in Esophageal Candidiasis Prevalence and Associated Risk Factors with or without HIV Infection: Lessons from an Endoscopic Study of 80,219 Patients. PLoS ONE 2015, 10, e0133589. [Google Scholar] [CrossRef] [PubMed]
- Li, M.X.; Cheng, S.J. Carcinogenesis of Esophageal Cancer in Linxian, China. Chin. Med. J. 1984, 97, 311–316. [Google Scholar]
- Ribeiro, U.; Posner, M.C.; Safatle-Ribeiro, A.V.; Reynolds, J.C. Risk Factors for Squamous Cell Carcinoma of the Oesophagus. Br. J. Surg. 1996, 83, 1174–1185. [Google Scholar] [PubMed]
- Yang, C.S. Research on Esophageal Cancer in China: A Review. Cancer Res. 1980, 40, 2633–2644. [Google Scholar]
- Sánchez-Alonzo, K.; Parra-Sepúlveda, C.; Vega, S.; Bernasconi, H.; Campos, V.L.; Smith, C.T.; Sáez, K.; García-Cancino, A. In vitro incorporation of Helicobacter pylori into Candida albicans caused by acidic pH stress. Pathogens 2020, 9, 489. [Google Scholar] [CrossRef] [PubMed]
- Coker, O.O. Non-bacteria microbiome (virus, fungi, and archaea) in gastrointestinal cancer. J. Gastroenterol. Hepatol. 2022, 37, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Ding, T.; Liu, C.; Li, Z. The mycobiome in human cancer: Analytical challenges, molecular mechanisms, and therapeutic implications. Mol. Cancer 2025, 24, 18. [Google Scholar] [CrossRef]
- Gupta, N.M.; Chaudhary, A.; Talwar, P. Candidial Obstruction of the Common Bile Duct. Br. J. Surg. 1985, 72, 13. [Google Scholar] [CrossRef]
- Ramirez-Garcia, A.; Rementeria, A.; Aguirre-Urizar, J.M.; Moragues, M.D.; Antoran, A.; Pellon, A.; Abad-Diaz-de-Cerio, A.; Hernando, F.L. Candida albicans and Cancer: Can This Yeast Induce Cancer Development or Progression? Crit. Rev. Microbiol. 2016, 42, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Godoy-Vitorino, F.; Romaguera, J.; Zhao, C.; Vargas-Robles, D.; Ortiz-Morales, G.; Vázquez-Sánchez, F.; Sanchez-Vázquez, M.; de la Garza-Casillas, M.; Martinez-Ferrer, M.; White, J.R.; et al. Cervicovaginal Fungi and Bacteria Associated with Cervical Intraepithelial Neoplasia and High-Risk Human Papillomavirus Infections in a Hispanic Population. Front. Microbiol. 2018, 9, 2533. [Google Scholar] [CrossRef]
- Banerjee, S.; Tian, T.; Wei, Z.; Shih, N.; Feldman, M.D.; Alwine, J.C.; Coukos, G.; Robertson, E.S. The Ovarian Cancer Oncobiome. Oncotarget 2017, 8, 36225–36245. [Google Scholar] [CrossRef]
- Zhong, M.; Xiong, Y.; Zhao, J.; Gao, Z.; Ma, J.; Wu, Z.; Song, Y.; Hong, X. Candida albicans Disorder Is Associated with Gastric Carcinogenesis. Theranostics 2021, 11, 4945–4956. [Google Scholar] [CrossRef] [PubMed]
- Engku Nasrullah Satiman, E.A.F.; Ahmad, H.; Ramzi, A.B.; Abdul Wahab, R.; Kaderi, M.A.; Wan Harun, W.H.A.; Dashper, S.; McCullough, M.; Arzmi, M.H. The Role of Candida albicans Candidalysin ECE1 Gene in Oral Carcinogenesis. J. Oral Pathol. Med. 2020, 49, 835–841. [Google Scholar] [CrossRef]
- Zhu, F.; Willette-Brown, J.; Song, N.-Y.; Lomada, D.; Song, Y.; Xue, L.; Gray, Z.; Zhao, Z.; Davis, S.R.; Sun, Z.; et al. Autoreactive T Cells and Chronic Fungal Infection Drive Esophageal Carcinogenesis. Cell Host Microbe 2017, 21, 478–493.e7. [Google Scholar] [CrossRef] [PubMed]
- Arzmi, M.H.; Dashper, S.; McCullough, M. Polymicrobial Interactions of Candida albicans and Its Role in Oral Carcinogenesis. J. Oral Pathol. Med. 2019, 48, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Webster, J.; Davey, R.A.; Turner, J.C.R. Vapour as the Source of Water in Buller’s Drop. Mycol. Res. 1989, 93, 297–302. [Google Scholar] [CrossRef]
- Langenberg, W.J. Relation of Weather Variables and Periodicities of tryAirborne Spores of Alternaria Dauci. Phytopathology 1977, 77, 879. [Google Scholar] [CrossRef]
- Talley, S.M.; Coley, P.D.; Kursar, T.A. The effects of weather on fungal abundance and richness among 25 communities in the Intermountain West. BMC Ecol. 2002, 2, 7. [Google Scholar] [CrossRef] [PubMed]
- Troutt, C.; Levetin, E. Correlation of Spring Spore Concentrations and Meteorological Conditions in Tulsa, Oklahoma. Int. J. Biometeorol. 2001, 45, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Vélez-Pereira, A.M.; De Linares, C.; Delgado, R.; Belmonte, J. Temporal Trends of the Airborne Fungal Spores in Catalonia (NE Spain), 1995–2013. Aerobiologia 2016, 32, 23–37. [Google Scholar] [CrossRef]
- Baxi, S.N.; Portnoy, J.M.; Larenas-Linnemann, D.; Phipatanakul, W. Environmental Allergens Workgroup Exposure and Health Effects of Fungi on Humans. J. Allergy Clin. Immunol. Pract. 2016, 4, 396–404. [Google Scholar] [CrossRef]
- Kumar, R.; Verma, D.; Singh, B.L.; Kumar, U. Composting of Sugar-Cane Waste by-Products through Treatment with Microorganisms and Subsequent Vermicomposting. Bioresour. Technol. 2010, 101, 6707–6711. [Google Scholar] [CrossRef]
- Jara, D.; Portnoy, J.; Dhar, M.; Barnes, C. Relation of Indoor and Outdoor Airborne Fungal Spore Levels in the Kansas City Metropolitan Area. Allergy Asthma Proc. 2017, 38, 130–135. [Google Scholar] [CrossRef] [PubMed]
- Rosas, I.; Escamilla, B.; Calderon, C.; Mosiño, P. The Daily Variations of Airborne Fungal Spores in Mexico City. Aerobiologia 1990, 6, 153–158. [Google Scholar] [CrossRef]
- Oliveira, M.; Ribeiro, H.; Abreu, I. Annual Variation of Fungal Spores in Atmosphere of Porto: 2003. Ann. Agric. Environ. Med. 2005, 12, 309–315. [Google Scholar]
- Elbert, W.; Taylor, P.E.; Andreae, M.O.; Pöschl, U. Contribution of Fungi to Primary Biogenic Aerosols in the Atmosphere: Wet and Dry Discharged Spores, Carbohydrates, and Inorganic Ions. Atmos. Chem. Phys. 2007, 7, 4569–4588. [Google Scholar] [CrossRef]
- Bauer, H.; Schüller, E.; Weinke, G.; Berger, A.; Hitzenberger, R.; Marr, I.; Puxbaum, H. Significant Conributions of Fungal Spores to the Organic Carbon and to the Aerosol Mass Balance of the Urban Atmospheric Aerosol. Atmos. Environ. 2008, 42, 5542–5549. [Google Scholar] [CrossRef]
- Womiloju, T.O.; Miller, J.D.; Mayer, P.M.; Brook, J.R. Methods to Determine the Biological Composition of Particulate Matter Collected from Outdoor Air. Atmos. Environ. 2003, 37, 4335–4344. [Google Scholar] [CrossRef]
- Held, A.; Zerrath, A.; McKeon, U.; Fehrenbach, T.; Niessner, R.; Plass-Dülmer, C.; Kaminski, U.; Berresheim, H.; Pöschl, U. Aerosol Size Distributions Measured in Urban, Rural and High-Alpine Air with an Electrical Low Pressure Impactor (ELPI). Atmos. Environ. 2008, 42, 8502–8512. [Google Scholar] [CrossRef]
- Hock, N.; Schneider, J.; Borrmann, S.; Römpp, A.; Moortgat, G.; Franze, T.; Schauer, C.; Pöschl, U.; Plass-Dülmer, C.; Berresheim, H. Rural Continental Aerosol Properties and Processes Observed during the Hohenpeissenberg Aerosol Characterization Experiment (HAZE2002). Atmos. Chem. Phys. 2008, 8, 603–623. [Google Scholar] [CrossRef]
- Crandall, S.G.; Gilbert, G.S. Meteorological Factors Associated with Abundance of Airborne Fungal Spores over Natural Vegetation. Atmos. Environ. 2017, 162, 87–99. [Google Scholar] [CrossRef]
- Picone, C. Diversity and abundance of arbuscular–mycorrhizal fungus spores in tropical forest and pasture 1. Biotropica 2000, 32, 734–750. [Google Scholar]
- Caillaud, D.; Keirsbulck, M.; Leger, C.; Leynaert, B.; Outdoor Mould ANSES Working Group. Outdoor Mold and Respiratory Health: State of Science of Epidemiological Studies. J. Allergy Clin. Immunol. Pract. 2022, 10, 768–784.e3. [Google Scholar] [CrossRef]
- Hyvärinen, A. Characterizing Moisture Damaged Buildings: Environmental and Biological Monitoring. Available online: https://www.julkari.fi/handle/10024/78452 (accessed on 9 December 2024).
- Saad-Hussein, A.; Ibrahim, K.S. Health Impact of Airborne Fungi. In Handbook of Healthcare in the Arab World; Laher, I., Ed.; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–16. ISBN 978-3-319-74365-3. [Google Scholar]
- Tsai, F.C.; Macher, J.M. Concentrations of Airborne Culturable Bacteria in 100 Large US Office Buildings from the BASE studyAbstract. Indoor Air 2005, 15, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, C.G.; Taylor, P.E.; Sá, M.O.; Teixeira, P.R.; Souza, R.A.; Albrecht, R.I.; Godoi, R.H. Identification and quantification of giant bioaerosol particles over the Amazon rainforest. NPJ Clim. Atmos. Sci. 2022, 5, 73. [Google Scholar] [CrossRef]
- Salvaggio, J.E. Inhaled Particles and Respiratory Disease. J. Allergy Clin. Immunol. 1994, 94, 304–309. [Google Scholar] [CrossRef]
- Schuyler, M.; Salvaggio, J.E. Hypersensitivity Pneumonitis. Semin. Respir. Med. 2008, 5, 246–254. [Google Scholar] [CrossRef]
- Reboux, G.; Piarroux, R.; Mauny, F.; Madroszyk, A.; Millon, L.; Bardonnet, K.; Dalphin, J.C. Role of molds in farmer’s lung disease in Eastern France. Am. J. Respir. Crit. Care Med. 2001, 163, 1534–1539. [Google Scholar] [CrossRef]
- Mahieu, L.M.; De Dooy, J.J.; Van Laer, F.A.; Jansens, H.; Ieven, M.M. A Prospective Study on Factors Influencing Aspergillus Spore Load in the Air during Renovation Works in a Neonatal Intensive Care Unit. J. Hosp. Infect. 2000, 45, 191–197. [Google Scholar] [CrossRef]
- Cao, C.; Jiang, W.; Wang, B.; Fang, J.; Lang, J.; Tian, G.; Jiang, J.; Zhu, T.F. Inhalable Microorganisms in Beijing’s PM2.5 and PM10 Pollutants during a Severe Smog Event. Environ. Sci. Technol. 2014, 48, 1499–1507. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.H.; Jang, S.; Park, W.M.; Park, J.B. Concentrations and Identification of Culturable Airborne Fungi in Underground Stations of the Seoul Metro. Environ. Sci. Pollut. Res. Int. 2016, 23, 20680–20686. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.-Y.; Fong, J.J.; Park, M.S.; Chang, L.; Lim, Y.W. Identifying Airborne Fungi in Seoul, Korea Using Metagenomics. J. Microbiol. 2014, 52, 465–472. [Google Scholar] [CrossRef]
- Grishkan, I.; Schlesinger, P.; Mamane, Y. Influence of Dust Storms on Concentration and Content of Fungi in the Atmosphere of Haifa, Israel. Aerobiologia 2012, 28, 557–564. [Google Scholar] [CrossRef]
- Oberle, M.; Reichmuth, M.; Laffer, R.; Ottiger, C.; Fankhauser, H.; Bregenzer, T. Non-Seasonal Variation of Airborne Aspergillus Spore Concentration in a Hospital Building. Int. J. Environ. Res. Public Health 2015, 12, 13730–13738. [Google Scholar] [CrossRef] [PubMed]
- Hallen-Adams, H.E.; Suhr, M.J. Fungi in the Healthy Human Gastrointestinal Tract. Virulence 2017, 8, 352–358. [Google Scholar] [CrossRef]
- Drell, T.; Lillsaar, T.; Tummeleht, L.; Simm, J.; Aaspõllu, A.; Väin, E.; Saarma, I.; Salumets, A.; Donders, G.G.G.; Metsis, M. Characterization of the Vaginal Micro- and Mycobiome in Asymptomatic Reproductive-Age Estonian Women. PLoS ONE 2013, 8, e54379. [Google Scholar] [CrossRef] [PubMed]
- Findley, K.; Oh, J.; Yang, J.; Conlan, S.; Deming, C.; Meyer, J.A.; Schoenfeld, D.; Nomicos, E.; Park, M.; NIH Intramural Sequencing Center Comparative Sequencing Program; et al. Topographic Diversity of Fungal and Bacterial Communities in Human Skin. Nature 2013, 498, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Ghannoum, M.A.; Jurevic, R.J.; Mukherjee, P.K.; Cui, F.; Sikaroodi, M.; Naqvi, A.; Gillevet, P.M. Characterization of the Oral Fungal Microbiome (Mycobiome) in Healthy Individuals. PLoS Pathog. 2010, 6, e1000713. [Google Scholar] [CrossRef] [PubMed]
- Varade, R.S.; Burkemper, N.M. Cutaneous Fungal Infections in the Elderly. Clin. Geriatr. Med. 2013, 29, 461–478. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Li, C.; Wang, C.; Li, J.; Ding, M.; Chen, D.; Lao, M. Epidemiology and Mortality-Associated Factors of Invasive Fungal Disease in Elderly Patients: A 20-Year Retrospective Study from Southern China. Infect. Drug Resist. 2020, 13, 711–723. [Google Scholar] [CrossRef] [PubMed]
- Saxena, K.; Shukla, P.; Shaafie, H.I.; Palliwal, G.; Jain, C. Spectrum of fungal infections in the elderly age group. Int. J. Med. Biomed. Stud. 2020, 4, 99–102. [Google Scholar] [CrossRef]
- Mullins, J.; Seaton, A. Fungal Spores in Lung and Sputum. Clin. Allergy 1978, 8, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Rotstein, C.; Tebbi, C.K.; Brass, C. Viral, Bacterial, and Fungal Infections in Adolescent Oncology. In Adolescnet Oncology; Futura Publishing Company: Mt. Kisco, NY, USA, 1987; pp. 429–506. [Google Scholar]
- Sullivan, D.J.; Moran, G.P.; Coleman, D.C. Fungal Infections of Humans. In Fungi; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 251–273. ISBN 978-1-119-37431-2. [Google Scholar]
- Barrios, N.; Tebbi, C.K.; Rotstein, C.; Siddiqui, S.; Humbert, J.R. Brainstem Invasion by Aspergillus fumigatus in a Child with Leukemia. N. Y. State J. Med. 1988, 88, 656–658. [Google Scholar]
- Garber, G. An Overview of Fungal Infections. Drugs 2001, 61 (Suppl. S1), 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kainz, K.; Bauer, M.A.; Madeo, F.; Carmona-Gutierrez, D. Fungal infections in humans: The silent crisis. Microbial Cell 2020, 7, 143. [Google Scholar] [CrossRef] [PubMed]
- Aykut, B.; Pushalkar, S.; Chen, R.; Li, Q.; Abengozar, R.; Kim, J.I.; Shadaloey, S.A.; Wu, D.; Preiss, P.; Verma, N.; et al. The fungal mycobiome promotes pancreatic oncogenesis via activation of MBL. Nature 2019, 574, 264–267. [Google Scholar] [CrossRef]
- Pathakumari, B.; Liang, G.; Liu, W. Immune Defence to Invasive Fungal Infections: A Comprehensive Review. Biomed. Pharmacother. 2020, 130, 110550. [Google Scholar] [CrossRef] [PubMed]
- Ostry, V.; Malir, F.; Toman, J.; Grosse, Y. Mycotoxins as Human Carcinogens-the IARC Monographs Classification. Mycotoxin Res. 2017, 33, 65–73. [Google Scholar] [CrossRef] [PubMed]
- De Ruyck, K.; De Boevre, M.; Huybrechts, I.; De Saeger, S. Dietary Mycotoxins, Co-Exposure, and Carcinogenesis in Humans: Short Review. Mutat. Res./Rev. Mutat. Res. 2015, 766, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Claeys, L.; Romano, C.; De Ruyck, K.; Wilson, H.; Fervers, B.; Korenjak, M.; Zavadil, J.; Gunter, M.J.; De Saeger, S.; De Boevre, M.; et al. Mycotoxin Exposure and Human Cancer Risk: A Systematic Review of Epidemiological Studies. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1449–1464. [Google Scholar] [CrossRef]
- Arsenijevic, T.; Nicolle, R.; Bouchart, C.; D’Haene, N.; Demetter, P.; Puleo, F.; Van Laethem, J.-L. Pancreatic Cancer Meets Human Microbiota: Close Encounters of the Third Kind. Cancers 2021, 13, 1231. [Google Scholar] [CrossRef]
- Bellotti, R.; Speth, C.; Adolph, T.E.; Lass-Flörl, C.; Effenberger, M.; Öfner, D.; Maglione, M. Micro- and Mycobiota Dysbiosis in Pancreatic Ductal Adenocarcinoma Development. Cancers 2021, 13, 3431. [Google Scholar] [CrossRef]
- Stasiewicz, M.; Kwaśniewski, M.; Karpiński, T.M. Microbial Associations with Pancreatic Cancer: A New Frontier in Biomarkers. Cancers 2021, 13, 3784. [Google Scholar] [CrossRef]
- Wang, H.; Capula, M.; Krom, B.P.; Yee, D.; Giovannetti, E.; Deng, D. Of Fungi and Men: Role of Fungi in Pancreatic Cancer Carcinogenesis. Ann. Transl. Med. 2020, 8, 1257. [Google Scholar] [CrossRef] [PubMed]
- Elaskandrany, M.; Patel, R.; Patel, M.; Miller, G.; Saxena, D.; Saxena, A. Fungi, host immune response, and tumorigenesis. Am. J. Physiol.-Gastrointest. Liver Physiol. 2021, 321, G213–G222. [Google Scholar] [CrossRef] [PubMed]
- Dohlman, A.B.; Klug, J.; Mesko, M.; Gao, I.H.; Lipkin, S.M.; Shen, X.; Iliev, I.D. A Pan-Cancer Mycobiome Analysis Reveals Fungal Involvement in Gastrointestinal and Lung Tumors. Cell 2022, 185, 3807–3822.e12. [Google Scholar] [CrossRef] [PubMed]
- Narunsky-Haziza, L.; Sepich-Poore, G.D.; Livyatan, I.; Asraf, O.; Martino, C.; Nejman, D.; Gavert, N.; Stajich, J.E.; Amit, G.; González, A.; et al. Pan-Cancer Analyses Reveal Cancer-Type-Specific Fungal Ecologies and Bacteriome Interactions. Cell 2022, 185, 3789–3806.e17. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K. Can Candida albicans Induce Oral Cancer Development and Progression? Sch. J. Dent. Sci. 2023, 9, 211–218. [Google Scholar] [CrossRef]
- David, H.; Solomon, A.P. Molecular Association of Candida albicans and Vulvovaginal Candidiasis: Focusing on a Solution. Front. Cell Infect. Microbiol. 2023, 13, 1245808. [Google Scholar] [CrossRef]
- Li, L.; Huang, X.; Chen, H. Unveiling the Hidden Players: Exploring the Role of Gut Mycobiome in Cancer Development and Treatment Dynamics. Gut Microbes 2024, 16, 2328868. [Google Scholar] [CrossRef]
- Sutcliffe, S.; De Marzo, A.M.; Sfanos, K.S.; Laurence, M. MSMB variation and prostate cancer risk: Clues towards a possible fungal etiology. Prostate 2014, 74, 569–578. [Google Scholar] [CrossRef]
- Domingues-Ferreira, M.; Grumach, A.S.; Duarte, A.J.D.S.; De Moraes-Vasconcelos, D. Esophageal Cancer Associated with Chronic Mucocutaneous Candidiasis. Could Chronic Candidiasis Lead to Esophageal Cancer? Med. Mycol. 2009, 47, 201–205. [Google Scholar] [CrossRef] [PubMed]
- Scott, B.B.; Jenkins, D. Gastro-Oesophageal Candidiasis. Gut 1982, 23, 137–139. [Google Scholar] [CrossRef]
- Delam, H.; Izanloo, S.; Bazrafshan, M.-R.; Eidi, A. Risk Factors for Cervical Cancer: An Epidemiological Review. J. Health Sci. Surveill. Syst. 2020, 8, 105–109. [Google Scholar] [CrossRef]
- Zwolinska-Wcisło, M.; Budak, A.; Bogdał, J.; Trojanowska, D.; Stachura, J. Fungal Colonization of Gastric Mucosa and Its Clinical Relevance. Med. Sci. Monit. 2001, 7, 982–988. [Google Scholar]
- Velez-Haro, J.M.; Pérez-Rodríguez, F.; Velázquez-Márquez, S.; Ramírez Medina, H.; Velázquez-Márquez, N. Mycology in Oncology: Exploring the Role of the Mycobiome in Human Cancer, Etiology, Progression, Epidemiology, Mycoses, and Mycotoxins. In Pathogens Associated with the Development of Cancer in Humans: OMICs, Immunological, and Pathophysiological Studies; Springer: Cham, Switzerland, 2024; pp. 303–348. [Google Scholar]
- Gamal, A.; Elshaer, M.; Alabdely, M.; Kadry, A.; McCormick, T.S.; Ghannoum, M. The mycobiome: Cancer pathogenesis, diagnosis, and therapy. Cancers 2022, 14, 2875. [Google Scholar] [CrossRef]
- Heng, W.; Wang, W.; Dai, T.; Jiang, P.; Lu, Y.; Li, R.; Zhang, M.; Xie, R.; Zhou, Y.; Zhao, M.; et al. Oral bacteriome and mycobiome across stages of oral carcinogenesis. Microbiol. Spectr. 2022, 10, e02737-22. [Google Scholar] [CrossRef] [PubMed]
- Wainwright, M. Do fungi play a role in the aetiology of cancer? Rev. Res. Med. Microbiol. 2002, 13, 37–42. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, Y.; Zhou, Y.; Feng, Y.; Sun, T.; Xu, J. Tumor-related fungi and crosstalk with gut fungi in the tumor microenvironment. Cancer Biol. Med. 2024, 21, 977. [Google Scholar] [CrossRef]
- Tebbi, C.K.; Sahakian, E.; Patel, S. Leukemogenesis of Mycovirus-Containing Aspergillus flavus. In Proceedings of the 2nd International Conference on Cancer Research and Oncology, Imperial Conferences, London, UK, 16 October 2023. [Google Scholar]
- Tebbi, C.K.; Kotta-Loizou, I.; Coutts, R.H. Mycovirus containing Aspergillus flavus and acute lymphoblastic leukemia: Carcinogenesis beyond mycotoxin production. In The Genus Aspergillus-Pathogenicity, Mycotoxin Production and Industrial Applications; IntechOpen: London, UK, 2021. [Google Scholar]
- Denning, D.W.; O’Driscoll, B.R.; Hogaboam, C.M.; Bowyer, P.; Niven, R.M. The Link between Fungi and Severe Asthma: A Summary of the Evidence. Eur. Respir. J. 2006, 27, 615–626. [Google Scholar] [CrossRef] [PubMed]
- Packe, G.E.; Ayres, J.G. Asthma Outbreak during a Thunderstorm. Lancet 1985, 2, 199–204. [Google Scholar] [CrossRef]
- Agarwal, R.; Gupta, D. Severe Asthma and Fungi: Current Evidence. Med. Mycol. 2011, 49 (Suppl. S1), S150–S157. [Google Scholar] [CrossRef] [PubMed]
- Mendell, M.J.; Mirer, A.G.; Cheung, K.; Tong, M.; Douwes, J. Respiratory and Allergic Health Effects of Dampness, Mold, and Dampness-Related Agents: A Review of the Epidemiologic Evidence. Environ. Health Perspect. 2011, 119, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, R.A.; Bearman, N.; Thornton, C.R.; Husk, K.; Osborne, N.J. Indoor fungal diversity and asthma: A meta-analysis and systematic review of risk factors. J. Allergy Clin. Immunol. 2015, 135, 110–122. [Google Scholar] [CrossRef]
- Pasqualotto, A.C.; Powell, G.; Niven, R.; Denning, D.W. The effects of antifungal therapy on severe asthma with fungal sensitization and allergic bronchopulmonary aspergillosis. Respirology 2009, 14, 1121–1127. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Hemminki, K.; Försti, A.; Sundquist, J.; Sundquist, K.; Ji, J. Cancer Risk and Mortality in Asthma Patients: A Swedish National Cohort Study. Acta Oncol. 2015, 54, 1120–1127. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Bian, J.; Chen, Z.; Fishe, J.N.; Zhang, D.; Braithwaite, D.; George, T.J.; Shenkman, E.A.; Licht, J.D. Cancer incidence after asthma diagnosis: Evidence from a large clinical research network in the United States. Cancer Med. 2023, 12, 11871–11877. [Google Scholar] [CrossRef] [PubMed]
- Beckstead, J.; Mehrotra, K.; Wilson, K.; Fingleton, B. Asthma is associated with a lower incidence of metastatic colorectal cancer in a US patient cohort. Front. Oncol. 2023, 13, 1253660. [Google Scholar] [CrossRef] [PubMed]
- Kantor, E.D.; Hsu, M.; Du, M.; Signorello, L.B. Allergies and Asthma in Relation to Cancer Risk. Cancer Epidemiol. Biomark. Prev. 2019, 28, 1395–1403. [Google Scholar] [CrossRef]
- Vesterinen, E.; Pukkala, E.; Timonen, T.; Aromaa, A. Cancer Incidence among 78,000 Asthmatic Patients. Int. J. Epidemiol. 1993, 22, 976–982. [Google Scholar] [CrossRef] [PubMed]
- Atmaj, E.; Schuiling-Veninga, C.C.; van Tuinen, E.L.; Bos, J.H.; de Vries, T.W. The relationship between childhood leukaemia and childhood asthma: A pharmacoepidemiological study from the Netherlands. Pediatr. Blood Cancer 2023, 70, e30231. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.-H.; Yang, Q.-M. Association of Asthma with the Risk of Acute Leukemia and Non-Hodgkin Lymphoma. Mol. Clin. Oncol. 2015, 3, 859–864. [Google Scholar] [CrossRef][Green Version]
- Linabery, A.M.; Jurek, A.M.; Duval, S.; Ross, J.A. The Association between Atopy and Childhood/Adolescent Leukemia: A Meta-Analysis. Am. J. Epidemiol. 2010, 171, 749–764. [Google Scholar] [CrossRef]
- Schüz, J.; Morgan, G.; Böhler, E.; Kaatsch, P.; Michaelis, J. Atopic Disease and Childhood Acute Lymphoblastic Leukemia. Int. J. Cancer 2003, 105, 255–260. [Google Scholar] [CrossRef] [PubMed]
- Wen, W.; Shu, X.O.; Linet, M.S.; Neglia, J.P.; Potter, J.D.; Trigg, M.E.; Robison, L.L. Allergic Disorders and the Risk of Childhood Acute Lymphoblastic Leukemia (United States). Cancer Causes Control 2000, 11, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, P.; Kaplan, G.A. Asthma and cancer. Am. J. Epidemiol. 1987, 125, 539–540. [Google Scholar] [CrossRef]
- Ji, J.; Shu, X.; Li, X.; Sundquist, K.; Sundquist, J.; Hemminki, K. Cancer risk in hospitalised asthma patients. Br. J. Cancer 2009, 100, 829–833. [Google Scholar] [CrossRef] [PubMed]
- Söderberg, K.C.; Hagmar, L.; Schwartzbaum, J.; Feychting, M. Allergic Conditions and Risk of Hematological Malignancies in Adults: A Cohort Study. BMC Public Health 2004, 4, 51. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Maza, O.; Moreno, A.D.; Cozen, W. Epidemiological Evidence: IgE, Allergies, and Hematopoietic Malignancies. In Cancer and IgE: Introducing the Concept of AllergoOncology; Penichet, M.L., Jensen-Jarolim, E., Eds.; Humana Press: Totowa, NJ, USA, 2010; pp. 79–136. ISBN 978-1-60761-451-7. [Google Scholar]
- Chang, J.S.; Tsai, Y.W.; Tsai, C.R.; Wiemels, J.L. Allergy and risk of childhood acute lymphoblastic leukemia: A population-based and record-based study. Am. J. Epidemiol. 2012, 176, 970–978. [Google Scholar] [CrossRef]
- Turner, M.C.; Chen, Y.; Krewski, D.; Ghadirian, P.; Thun, M.J.; Calle, E.E. Cancer mortality among US men and women with asthma and hay fever. Am. J. Epidemiol. 2005, 162, 212–221. [Google Scholar] [CrossRef]
- Bellia, V.; Pedone, C.; Catalano, F.; Zito, A.; Davià, E.; Palange, S.; Forastiere, F.; Incalzi, R.A. Asthma in the elderly: Mortality rate and associated risk factors for mortality. Chest 2007, 132, 1175–1182. [Google Scholar] [CrossRef]
- Merrill, R.M.; Isakson, R.T.; Beck, R.E. The Association between Allergies and Cancer: What Is Currently Known? Ann. Allergy Asthma Immunol. 2007, 99, 102–116; quiz 117–119, 150. [Google Scholar] [CrossRef] [PubMed]
- Rittmeyer, D.; Lorentz, A. Relationship between Allergy and Cancer: An Overview. Int. Arch. Allergy Immunol. 2012, 159, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Karim, A.F.; Westenberg, L.E.H.; Eurelings, L.E.M.; Otten, R.; Gerth van Wijk, R. The association between allergic diseases and cancer: A systematic review of the literature. Neth. J. Med. 2019, 77, 42–66. [Google Scholar]
- Söderberg, K.C.; Jonsson, F.; Winqvist, O.; Hagmar, L.; Feychting, M. Autoimmune Diseases, Asthma and Risk of Haematological Malignancies: A Nationwide Case-Control Study in Sweden. Eur. J. Cancer 2006, 42, 3028–3033. [Google Scholar] [CrossRef]
- Miedema, K.G.; Tissing, W.J.; Te Poele, E.M.; Kamps, W.A.; Alizadeh, B.Z.; Kerkhof, M.; de Jongste, J.C.; Smit, H.A.; de Pagter, A.P.; Bierings, M.; et al. Polymorphisms in the TLR6 gene associated with the inverse association between childhood acute lymphoblastic leukemia and atopic disease. Leukemia 2011, 26, 1203–1210. [Google Scholar] [CrossRef] [PubMed]
- Ajrouche, R.; Chandab, G.; Petit, A.; Strullu, M.; Nelken, B.; Plat, G.; Michel, G.; Domenech, C.; Clavel, J.; Bonaventure, A. Allergies, genetic polymorphisms of Th2 interleukins, and childhood acute lymphoblastic leukemia: The ESTELLE study. Pediatr. Blood Cancer 2022, 69, e29402. [Google Scholar] [CrossRef] [PubMed]
- Musolino, C.; Allegra, A.; Minciullo, P.L.; Gangemi, S. Allergy and risk of hematologic malignancies: Associations and mechanisms. Leuk. Res. 2014, 38, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Dikaliotia, S.K.; Changb, E.T.; Dessyprisa, N.; Papadopouloua, C.; Skenderisd, N.; Pourtsidise, A.; Moschovi, M.; Polychronopoulou, S.; Athanasiadou-Piperopoulou, F.; Sidi, V.; et al. Allergy-associated symptoms in relation to childhood non-Hodgkin’s as contrasted to Hodgkin’s lymphomas: A case–control study in Greece and meta-analysis. Eur. J. Cancer 2012, 48, 1860–1866. [Google Scholar] [CrossRef] [PubMed]
- Dahl, S.; Schmidt, L.S.; Vestergaard, T.; Schüz, J.; Schmiegelow, K. Allergy and the risk of childhood leukemia: A meta-analysis. Leukemia 2009, 23, 2300–2304. [Google Scholar] [CrossRef][Green Version]
- Lariou, M.S.; Dikalioti, S.K.; Dessypris, N.; Baka, M.; Polychronopoulou, S.; Athanasiadou-Piperopoulou, F.; Kalmanti, M.; Fragandrea, I.; Moschovi, M.; Germenis, A.E.; et al. Allergy and risk of acute lymphoblastic leukemia among children: A nationwide case control study in Greece. Cancer Epidemiol. 2013, 37, 146–151. [Google Scholar] [CrossRef]
- Melbye, M.; Smedby, K.E.; Lehtinen, T.; Rostgaard, K.; Glimelius, B.; Munksgaard, L.; Schöllkopf, C.; Sundström, C.; Chang, E.T.; Koskela, P.; et al. Atopy and risk of non-Hodgkin lymphoma. J. Natl. Cancer Inst. 2007, 99, 158–166. [Google Scholar] [CrossRef]
- Vajdic, C.M.; Falster, M.O.; De Sanjose, S.; Martínez-Maza, O.; Becker, N.; Bracci, P.M.; Melbye, M.; Smedby, K.E.; Engels, E.A.; Turner, J.; et al. Atopic disease and risk of non–Hodgkin lymphoma: An interlymph pooled analysis. Cancer Res. 2009, 69, 6482–6489. [Google Scholar] [CrossRef]
- Sudan, M.; Arah, O.A.; Olsen, J.; Kheifets, L. Reported associations between asthma and acute lymphoblastic leukemia: Insights from a hybrid simulation study. Eur. J. Epidemiol. 2016, 31, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.W.; Xu, S.M.; An, Q.; Wang, P. A review of risk factors for childhood leukemia. Eur. Rev. Med. Pharmacol. Sci. 2016, 20, 3760–3764. [Google Scholar] [PubMed]
- Blair, A.; Malker, H.; Cantor, K.P.; Burmeister, L.; Wiklund, K. Cancer among Farmers. A Review. Scand. J. Work Environ. Health 1985, 11, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Pearce, N.; Reif, J.S. Epidemiologic Studies of Cancer in Agricultural Workers. Am. J. Ind. Med. 1990, 18, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Morton, W.; Marjanovic, D. Leukemia Incidence by Occupation in the Portland-Vancouver Metropolitan Area. Am. J. Ind. Med. 1984, 6, 185–205. [Google Scholar] [CrossRef] [PubMed]
- Pearce, N.E.; Sheppard, R.A.; Howard, J.K.; Fraser, J.; Lilley, B.M. Leukemia among New Zealand Agricultural Workers. A Cancer Registry-Based Study. Am. J. Epidemiol. 1986, 124, 402–409. [Google Scholar] [CrossRef] [PubMed]
- Loomis, D.P.; Savitz, D.A. Occupation and Leukemia Mortality among Men in 16 States: 1985–1987. Am. J. Ind. Med. 1991, 19, 509–521. [Google Scholar] [CrossRef]
- Semenciw, R.M.; Morrison, H.I.; Morison, D.; Mao, Y. Leukemia Mortality and Farming in the Prairie Provinces of Canada. Can. J. Public Health 1994, 85, 208–211. [Google Scholar] [PubMed]
- Keller-Byrne, J.E.; Khuder, S.A.; Schaub, E.A. Meta-Analysis of Leukemia and Farming. Environ. Res. 1995, 71, 1–10. [Google Scholar] [CrossRef]
- Blair, A.; Zheng, T.; Linos, A.; Stewart, P.A.; Zhang, Y.W.; Cantor, K.P. Occupation and Leukemia: A Population-Based Case-Control Study in Iowa and Minnesota. Am. J. Ind. Med. 2001, 40, 3–14. [Google Scholar] [CrossRef]
- Kelleher, C.; Newell, J.; MacDonagh-White, C.; MacHale, E.; Egan, E.; Connolly, E.; Gough, H.; Delaney, B.; Shryane, E. Incidence and Occupational Pattern of Leukaemias, Lymphomas, and Testicular Tumours in Western Ireland over an 11 Year Period. J. Epidemiol. Community Health 1998, 52, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Tsai, R.J.; Luckhaupt, S.E.; Schumacher, P.; Cress, R.D.; Deapen, D.M.; Calvert, G.M. Acute Myeloid Leukemia Risk by Industry and Occupation. Leuk. Lymphoma 2014, 55, 2584–2591. [Google Scholar] [CrossRef]
- Hansen, E.S.; Hasle, H.; Lander, F. A Cohort Study on Cancer Incidence among Danish Gardeners. Am. J. Ind. Med. 1992, 21, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Viel, J.F.; Richardson, S.T. Lymphoma, Multiple Myeloma and Leukaemia among French Farmers in Relation to Pesticide Exposure. Soc. Sci. Med. 1993, 37, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Keller, J.E.; Howe, H.L. Case-Control Studies of Cancer in Illinois Farmers Using Data from the Illinois State Cancer Registry and the U.S. Census of Agriculture. Eur. J. Cancer 1994, 30A, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Järvisalo, J.; Tola, S.; Korkala, M.L.; Järvinen, E. A Cancer Register-Based Case Study of Occupations of Patients with Acute Myeloid Leukemia. Cancer 1984, 54, 785–790. [Google Scholar] [CrossRef]
- Patel, T.Y.; Buttner, M.; Rivas, D.; Cross, C.; Bazylinski, D.A.; Seggev, J. Variation in Airborne Fungal Spore Concentrations among Five Monitoring Locations in a Desert Urban Environment. Environ. Monit. Assess. 2018, 190, 634. [Google Scholar] [CrossRef] [PubMed]
- Halstensen, A.S.; Nordby, K.C.; Wouters, I.M.; Eduard, W. Determinants of Microbial Exposure in Grain Farming. Ann. Occup. Hyg. 2007, 51, 581–592. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.L.; Bhatnagar, J.M. Chapter 4—Fungi in Soil: A Rich Community with Diverse Functions. In Soil Microbiology, Ecology and Biochemistry, 5th ed.; Paul, E.A., Frey, S.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2024; pp. 75–129. ISBN 978-0-12-822941-5. [Google Scholar]
- Rodriguez-Romero, J.; Hedtke, M.; Kastner, C.; Müller, S.; Fischer, R. Fungi, Hidden in Soil or up in the Air: Light Makes a Difference. Annu. Rev. Microbiol. 2010, 64, 585–610. [Google Scholar] [CrossRef] [PubMed]
- Abrego, N.; Crosier, B.; Somervuo, P.; Ivanova, N.; Abrahamyan, A.; Abdi, A.; Hämäläinen, K.; Junninen, K.; Maunula, M.; Purhonen, J.; et al. Fungal Communities Decline with Urbanization-More in Air than in Soil. ISME J. 2020, 14, 2806–2815. [Google Scholar] [CrossRef] [PubMed]
- Berg, M.P.; Kniese, J.P.; Verhoef, H.A. Dynamics and Stratification of Bacteria and Fungi in the Organic Layers of a Scots Pine Forest Soil. Biol. Fertil. Soils 1998, 26, 313–322. [Google Scholar] [CrossRef]
- Fischer, G.; Dott, W. Relevance of Airborne Fungi and Their Secondary Metabolites for Environmental, Occupational and Indoor Hygiene. Arch. Microbiol. 2003, 179, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Booth, B.J.; Ward, M.H.; Turyk, M.E.; Stayner, L.T. Agricultural Crop Density and Risk of Childhood Cancer in the Midwestern United States: An Ecologic Study. Environ. Health 2015, 14, 82. [Google Scholar] [CrossRef] [PubMed]
- Shu, X.O.; Gao, Y.T.; Brinton, L.A.; Linet, M.S.; Tu, J.T.; Zheng, W.; Fraumeni, J.F. A Population-Based Case-Control Study of Childhood Leukemia in Shanghai. Cancer 1988, 62, 635–644. [Google Scholar] [CrossRef]
- Patel, D.M.; Gyldenkærne, S.; Jones, R.R.; Olsen, S.F.; Tikellis, G.; Granström, C.; Dwyer, T.; Stayner, L.T.; Ward, M.H. Residential Proximity to Agriculture and Risk of Childhood Leukemia and Central Nervous System Tumors in the Danish National Birth Cohort. Environ. Int. 2020, 143, 105955. [Google Scholar] [CrossRef]
- Rull, R.P.; Gunier, R.; Von Behren, J.; Hertz, A.; Crouse, V.; Buffler, P.A.; Reynolds, P. Residential Proximity to Agricultural Pesticide Applications and Childhood Acute Lymphoblastic Leukemia. Environ. Res. 2009, 109, 891–899. [Google Scholar] [CrossRef]
- Zorlu, P.; Ergor, G.; Tezic, T.; Duru, F.; Ertem, U. Evaluation of Risk Factors in Children with Acute Lymphoblastic Leukemia. Turk. J. Cancer 2002, 32, 5. [Google Scholar]
- Meinert, R.; Schüz, J.; Kaletsch, U.; Kaatsch, P.; Michaelis, J. Leukemia and Non-Hodgkin’s Lymphoma in Childhood and Exposure to Pesticides: Results of a Register-Based Case-Control Study in Germany. Am. J. Epidemiol. 2000, 151, 639–646; discussion 647–650. [Google Scholar] [CrossRef] [PubMed]
- Cha, E.S.; Hwang, S.; Lee, W.J. Childhood Leukemia Mortality and Farming Exposure in South Korea: A National Population-Based Birth Cohort Study. Cancer Epidemiol. 2014, 38, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Tebbi, C.K.; Sahakian, E. Comment on “Association between Residential Proximity to Viticultural Areas and Childhood Acute Leukemia Risk in Mainland France: GEOCAP Case–Control Study, 2006–2013”. Environ. Health Perspect. 2024, 132, 048003. [Google Scholar] [CrossRef] [PubMed]
| Cancer Type | Fungi Associated with Cancer |
|---|---|
| Skin cancer | Candida species: C. albicans, C. cladosporioides, C. glabrata, C. parapsilosis, C. tropicalis; Alternaria species: A. alternata, A. infectoria, M. arundinis, E. oligosperma |
| Lung cancer | A. fumigatus, Cryptococcus sp., Fusarium, H. immitis, Histoplasma capsulatum, P. jiroveci, Pneumocystis sp., Rhizopus, Talaromyces marneffei, Trichosporon |
| Oral cancer | C. albicans |
| Esophageal carcinoma | Aspergillus sp.: A. flavus, A. parasiticus; Candida sp.: C. albicans, C. glabrata, C. tropicalis, C. krusei, C. parapsilosis; Fusarium species.: F. verticillioides, F. proliferatum; Torulosis sp.: T. glabrata, T. tomata |
| Gastric cancer | Aspergillus spp., Blastomyces spp., Candida sp.: C. albicans, Coccidioides spp., Cryptococcus spp., Fusarium spp., Histoplasma spp., Malassezia spp., Mucor spp., Paracoccidioides spp., Penicillium spp., Phialemonium spp., Rhodotorula spp., Saccharomyces cerevisiae, Trichosporon spp. |
| Colorectal cancer | Aspergillus sp.: A. flavus, A. sydowii, A. ochraceoroseus; Candida sp.: C. albicans, C. tropicalis, Cladosporium, Cryptococcus, Debaryomyces fabryi, Histoplasma, Kwoniella mangrovensis, Malassezia globosa, Moniliophthora perniciosa, Paracoccidioides, Phoma, Pneumocystis, Plectosphaerella, Pseudogymnoascus sp., Rhodotorula, Scedosporiosi, Talaromyces islandicus, Trichosporon, Thanatephorus, Zygomycetes |
| Cholangiocarcinoma | Aspergillus sp.: A. flavus, A. parasiticus, Penicillium; Candida sp.: C. albicans, C. glabrata, C. tropicalis, Penicillium |
| Pancreatic ductal adenocarcinoma | Malassezia |
| Breast cancer | Aspergillus, Candida, Coccidioides, Cunninghamella, Geotrichum, Pleistophora, Rhodotorula, Filobasidiella, Mucor, Trichophyton, Epidermophyton, Fonsecaea, Pseudallescheria, Penicillium, Ajellomyces, Alternaria, Rhizomucor, Piedraia, Malassezia |
| Cervical cancer | Candida, Cryptococcus laurentii, Gjaerumia, Pleosporales, Malassezia, Nakaseomyces, Sporidiobolacea, Saccharomyces |
| Ovarian cancer | Pneumocystis, Acremonium, Cladophialophora, Malassezia, Microsporidia Pleistophora, Ajellomyces, Aspergillus sp., Candida sp., Cladosporium, Coccidioides, Cryptococcus, Cunninghamella, Issatchenkia, Nosema, Paracoccidioides, Penicillium, Pleistophora, Rhizomucor, Rhizopus, Rhodotorula, Trichophyton |
| Prostate cancer | Aspergillus sp., Candida sp.: C. neoformans, C. immitis, H. capsulatum, B. dermatitidis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tebbi, C.K.; Sahakian, E.; Shah, B.; Yan, J.; Mediavilla-Varela, M.; Patel, S. Aspergillus flavus with Mycovirus as an Etiologic Factor for Acute Leukemias in Susceptible Individuals: Evidence and Discussion. Biomedicines 2025, 13, 488. https://doi.org/10.3390/biomedicines13020488
Tebbi CK, Sahakian E, Shah B, Yan J, Mediavilla-Varela M, Patel S. Aspergillus flavus with Mycovirus as an Etiologic Factor for Acute Leukemias in Susceptible Individuals: Evidence and Discussion. Biomedicines. 2025; 13(2):488. https://doi.org/10.3390/biomedicines13020488
Chicago/Turabian StyleTebbi, Cameron K., Eva Sahakian, Bijal Shah, Jiyu Yan, Melanie Mediavilla-Varela, and Saumil Patel. 2025. "Aspergillus flavus with Mycovirus as an Etiologic Factor for Acute Leukemias in Susceptible Individuals: Evidence and Discussion" Biomedicines 13, no. 2: 488. https://doi.org/10.3390/biomedicines13020488
APA StyleTebbi, C. K., Sahakian, E., Shah, B., Yan, J., Mediavilla-Varela, M., & Patel, S. (2025). Aspergillus flavus with Mycovirus as an Etiologic Factor for Acute Leukemias in Susceptible Individuals: Evidence and Discussion. Biomedicines, 13(2), 488. https://doi.org/10.3390/biomedicines13020488







