Short-Term In Vitro Exposure of Human Blood to 5G Network Frequencies: Do Sex and Frequency Additionally Affect Erythrocyte Morphometry?
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics and Welfare Approval Statement
2.2. Blood Collection
2.3. Exposure of Blood Samples to 5G Radiofrequency Electromagnetic Radiation Under Laboratory Conditions
2.4. Analysis of Complete Blood Count Indicators
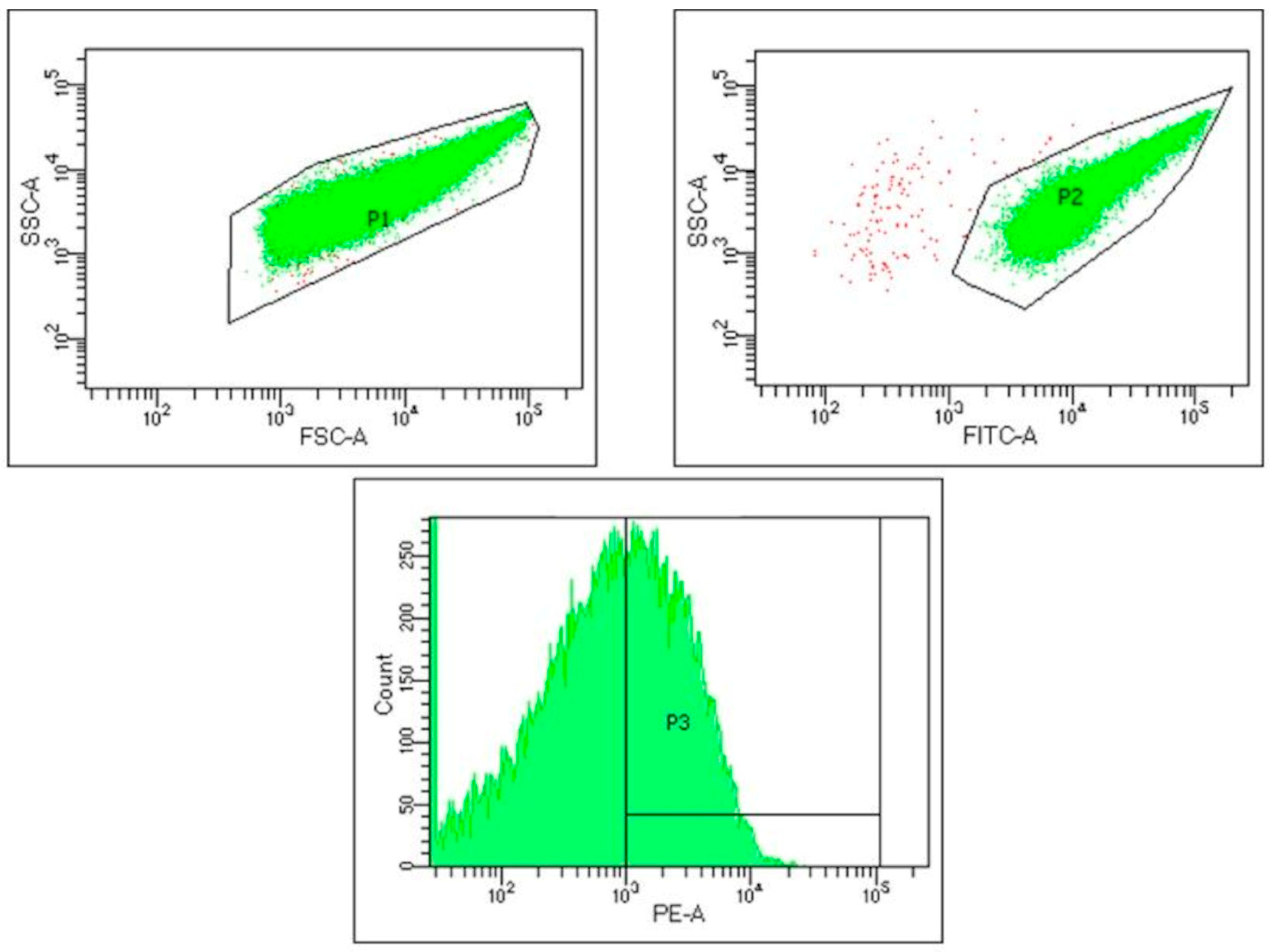
2.5. Analysis of Platelet Activation
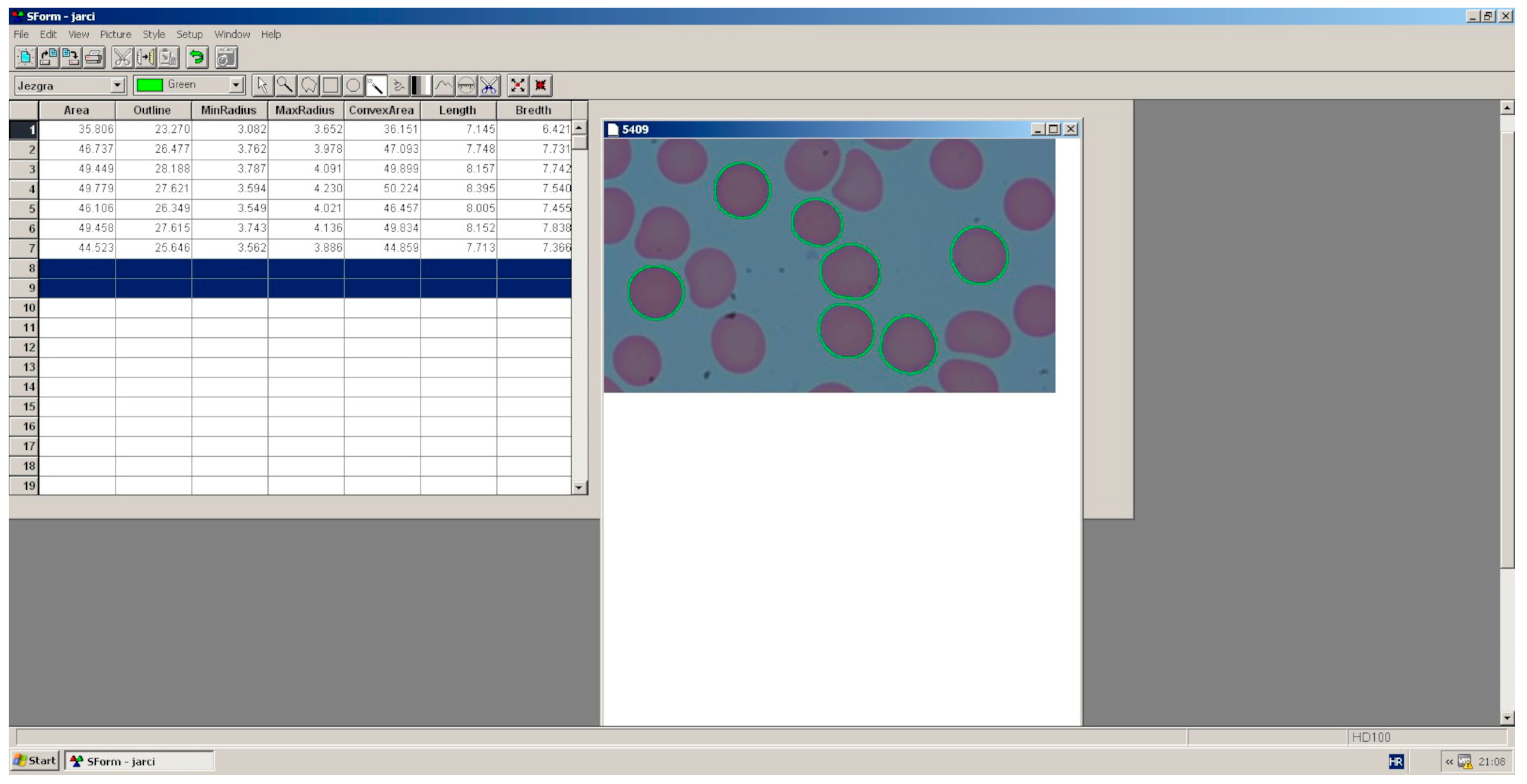
2.6. Morphometric Analysis of Erythrocytes
2.7. Statistical Data Processing
3. Results
3.1. Complete Blood Count Indicators and Platelet Activation Depending on the Frequency of 5G Radiofrequency Electromagnetic Radiation and Sex
3.2. Morphometric Indicators of the Size and Shape of Erythrocytes Depending on the Frequency of 5G Radiofrequency Electromagnetic Radiation and Gender
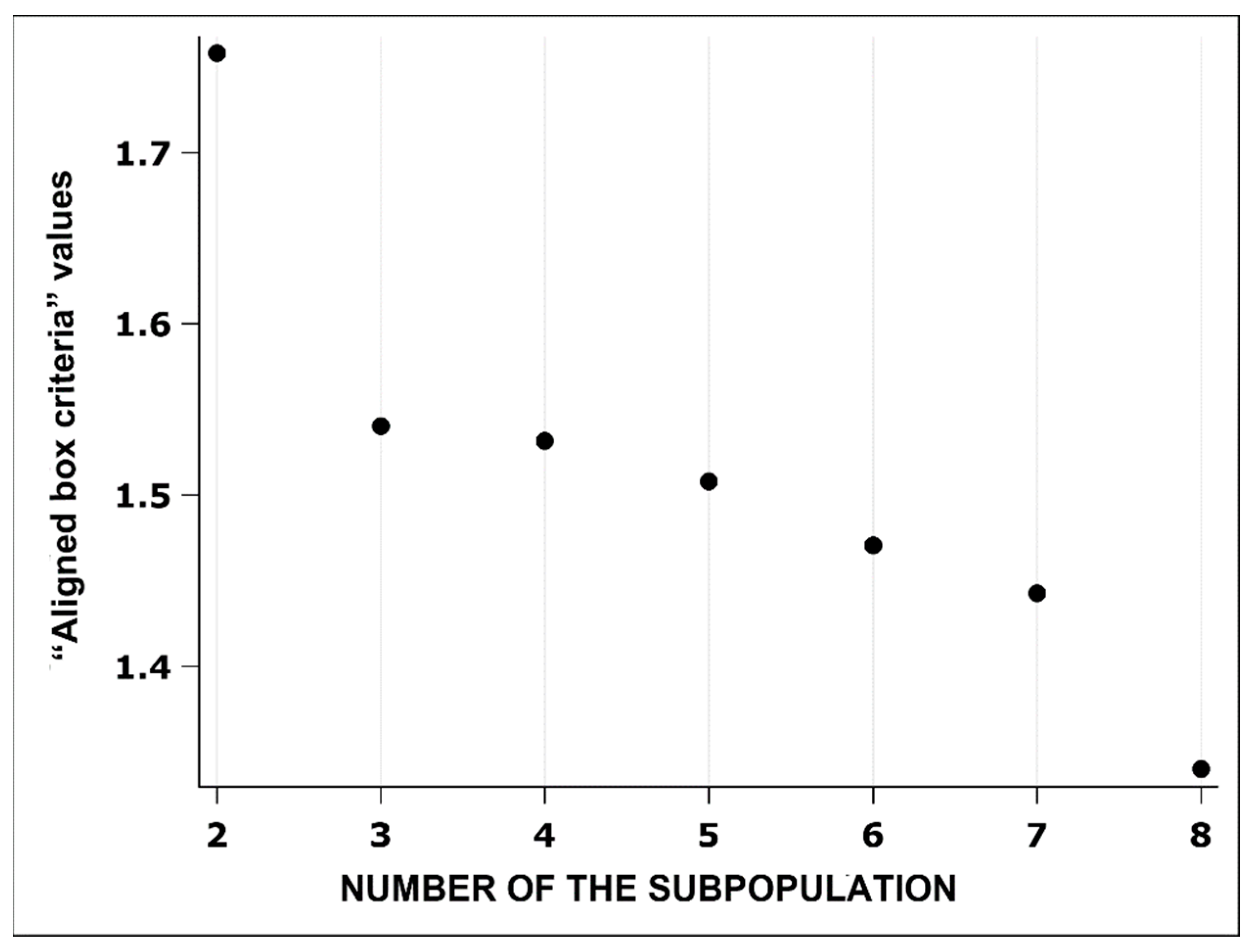
3.3. Distribution of Subpopulations of Erythrocytes in Groups Categorised According to the Morphometric Indicators
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Saliev, T.; Begimbetova, D.; Masoud, A.R.; Matkarimov, B. Biological effects of non-ionizing electromagnetic fields: Two sides of a coin. Prog. Biophys. Mol. Biol. 2019, 141, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Hardell, L.; Carlberg, M. Health risks from radiofrequency radiation, including 5G, should be assessed by experts with no conflicts of interest. Oncol. Lett. 2020, 20, 15. [Google Scholar] [CrossRef]
- Karipidis, K.; Brzozek, C.R.; Bhatt, S.; Loughran, S.; Wood, A. What evidence exists on the impact of anthropogenic radiofrequency electromagnetic fields on animals and plants in the environment? A systematic map protocol. Environ. Evid. 2021, 10, 39. [Google Scholar] [CrossRef]
- Simkó, M.; Mattsson, M.-O. 5G Wireless communication and health effects—A pragmatic review based on available studies regarding 6 to 100 GHz. Int. J. Environ. Res. Public Health 2019, 16, 3406. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.P.; Pham, V.H.; Baulin, V.; Croft, R.J.; Crawford, R.J.; Ivanova, E.P. The effect of a high-frequency electromagnetic field in the microwave range on red blood cells. Sci. Rep. 2017, 7, 10798. [Google Scholar] [CrossRef] [PubMed]
- Jbireal, J.M.; Azab, A.E.; Elsayed, A.S.I. Disturbance in hematological parameters induced by exposure to electromagnetic fields. Hematol. Transfus. Int. J. 2018, 6, 242–251. [Google Scholar] [CrossRef]
- Henschenmacher, B.; Bitsch, A.; De Las Heras Gala, T.; Forman, H.J.; Fragoulis, A.; Ghezzi, P.; Kellner, R.; Koch, W.; Kuhne, J.; Sachno, D.; et al. The effect of radiofrequency electromagnetic fields (RF-EMF) on biomarkers of oxidative stress in vivo and in vitro: A protocol for a systematic review. Environ. Int. 2022, 158, 106932. [Google Scholar] [CrossRef] [PubMed]
- Wust, P.; Kortüm, B.; Strauss, U.; Nadobny, J.; Zschaeck, S.; Beck, M.; Stein, U.; Ghadjar, P. Non-thermal effects of radiofrequency electromagnetic fields. Sci. Rep. 2020, 10, 13488. [Google Scholar] [CrossRef]
- Elder, C.A.; Smith, J.S.; Almosawi, M.; Mills, E.; Janis, B.R.; Kopechek, J.A.; Wolkers, W.F.; Menze, M.A. Cryopreserved red blood cells maintain allosteric control of oxygen binding when utilizing trehalose as a cryoprotectant. Cryobiology 2024, 114, 104793. [Google Scholar] [CrossRef]
- López-Martín, E.; Sueiro-Benavides, R.; Leiro-Vidal, J.M.; Rodríguez-González, J.A.; Ares-Pena, F.J. Redox cell signalling triggered by black carbon and/or radiofrequency electromagnetic fields: Influence on cell death. Sci. Total Environ. 2024, 953, 176023. [Google Scholar] [CrossRef] [PubMed]
- Panagopoulos, D.J.; Karabarbounis, A.; Yakymenko, I.; Chrousos, G.P. Human-made electromagnetic fields: Ion forced-oscillation and voltage-gated ion channel dysfunction, oxidative stress and DNA damage (Review). Int. J. Oncol. 2021, 59, 92. [Google Scholar] [CrossRef]
- Koohestanidehaghi, Y.; Khalili, M.A.; Fesahat, F.; Seify, M.; Mangoli, E.; Kalantar, S.M.; Nottola, S.A.; Macchiarelli, G.; Palmerini, M.G. Detrimental effects of radiofrequency electromagnetic waves emitted by mobile phones on morphokinetics, oxidative stress, and apoptosis in mouse preimplantation embryos. Environ. Pollut. 2023, 336, 122411. [Google Scholar] [CrossRef] [PubMed]
- Massaccesi, L.; Galliera, E.; Corsi Romanelli, M.M. Erythrocytes as markers of oxidative stress related pathologies. Mech. Ageing Dev. 2020, 191, 111333. [Google Scholar] [CrossRef] [PubMed]
- Narayanan, S.N.; Jetti, R.; Kesari, K.K.; Kumar, R.S.; Nayak, S.B.; Bhat, P.G. Radiofrequency electromagnetic radiation-induced behavioral changes and their possible basis. Environ. Sci. Pollut. Res. 2019, 26, 30693–30710. [Google Scholar] [CrossRef] [PubMed]
- Pooam, M.; Jourdan, N.; Aguida, B.; Dahon, C.; Baouz, S.; Terry, C.; Raad, H.; Ahmad, M. Exposure to 1.8 GHz radiofrequency field modulates ROS in human HEK293 cells as a function of signal amplitude. Commun. Integr. Biol. 2022, 15, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Adebayo, E.A.; Adeeyo, A.O.; Ogundiran, M.A.; Olabisi, O. Bio-physical effects of radiofrequency electromagnetic radiation (RF-EMR) on blood parameters, spermatozoa, liver, kidney, and heart of albino rats. J. King Saud Univ. Sci. 2019, 31, 813–821. [Google Scholar] [CrossRef]
- Alchalabi, A.S.H.; Aklilu, E.; Aziz, A.R.; Malek, F.; Ronald, S.H.; Khan, M.A. Exposure to 1800 MHz GSM-like radiofrequency electromagnetic field reduces follicular development and overall fertility of female rats. South Asian J. Exp. Biol. 2015, 5, 127–136. [Google Scholar] [CrossRef]
- Aghajanloo, B.; Hadady, H.; Ejeian, F.; Inglis, D.W.; Hughes, M.P.; Fadaei Tehrani, A.; Nasr-Esfahani, M.H. Biomechanics of circulating cellular and subcellular bioparticles: Beyond separation. Cell Commun. Signal. 2024, 22, 331. [Google Scholar] [CrossRef]
- Simmonds, M.J.; Meiselman, H.J.; Marshall-Gradisnik, S.M.; Pyne, M.; Kakanis, M.; Keane, J.; Brenu, E.; Christy, R.; Baskurt, O.K. Assessment of oxidant susceptibility of red blood cells in various species based on cell deformability. Biorheology 2011, 48, 293–304. [Google Scholar] [CrossRef] [PubMed]
- Mohanty, J.G.; Nagababu, E.; Rifkind, J.M. Red blood cell oxidative stress impairs oxygen delivery and induces red blood cell aging. Front. Physiol. 2014, 28, 5–84. [Google Scholar] [CrossRef]
- Langari, A.; Strijkova, V.; Komsa-Penkova, R.; Danailova, A.; Krumova, S.; Taneva, S.G.; Giosheva, I.; Gartchev, E.; Kercheva, K.; Savov, A.; et al. Morphometric and nanomechanical features of erythrocytes characteristic of early pregnancy loss. Int. J. Mol. Sci. 2022, 23, 4512. [Google Scholar] [CrossRef] [PubMed]
- Kiko, T.; Nakagawa, K.; Satoh, A.; Tsuduki, T.; Furukawa, K.; Arai, H.; Miyazawa, T. Amyloid-β levels in human red blood cells. PLoS ONE 2012, 7, e49620. [Google Scholar] [CrossRef]
- Barbour, R.; Kling, K.; Anderson, J.P.; Banducci, K.; Cole, T.; Diep, L.; Fox, M.; Goldstein, J.M.; Soriano, F.; Seubert, P.; et al. Red blood cells are the major source of alpha-synuclein in blood. Neurodegener. Dis. 2008, 5, 55–59. [Google Scholar] [CrossRef]
- Melzak, K.A.; Spouge, J.L.; Boecker, C.; Kirschhöfer, F.; Brenner-Weiss, G.; Bieback, K. Hemolysis Pathways During Storage of Erythrocytes and Inter-Donor Variability in Erythrocyte Morphology. Transfus. Med. Hemother. 2021, 48, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Lan, J.; Liu, J.; Zhao, Z.; Xue, R.; Zhang, N.; Zhang, P.; Zhao, P.; Zheng, F.; Sun, X. The peripheral blood of Aβ binding RBC as a biomarker for diagnosis of Alzheimer’s disease. Age Ageing 2015, 44, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Melzak, K.A.; Moreno-Flores, S.; Bieback, K. Spicule movement on RBCs during echinocyte formation and possible segregation in the RBC membrane. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183338. [Google Scholar] [CrossRef]
- Strijkova-Kenderova, V.; Todinova, S.; Andreeva, T.; Bogdanova, D.; Langari, A.; Danailova, A.; Krumova, S.; Zlatareva, E.; Kalaydzhiev, N.; Milanov, I.; et al. Morphometry and stiffness of red blood cells—Signatures of neurodegenerative diseases and aging. Int. J. Mol. Sci. 2022, 23, 227. [Google Scholar] [CrossRef] [PubMed]
- Bor-Kucukatay, M.; Wenby, R.B.; Meiselman, H.J.; Baskurt, O.K. Effects of nitric oxide on red blood cell deformability. Am. J. Physiol. Heart Circ. Physiol. 2003, 284, H1577–H1584. [Google Scholar] [CrossRef]
- Pavan, A.R.; Terroni, B.; Dos Santos, J.L. Endothelial dysfunction in Sickle Cell Disease: Strategies for the treatment. Nitric Oxide 2024, 149, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Grau, M.; Cremer, J.M.; Schmeichel, S.; Kunkel, M.; Bloch, W. Comparisons of blood parameters, red blood cell deformability and circulating nitric oxide between males and females considering hormonal contraception: A longitudinal gender study. Front. Physiol. 2018, 9, 1835. [Google Scholar] [CrossRef]
- Rubanyi, G.M.; Freay, A.D.; Kauser, K.; Sukovich, D.; Burton, G.; Lubahn, D.B.; Couse, J.F.; Curtis, S.W.; Korach, K.S. Vascular estrogen receptors and endothelium-derived nitric oxide production in the mouse aorta. Gender difference and effect of estrogen receptor gene disruption. J. Clin. Investig. 1997, 99, 2429–2437. [Google Scholar] [CrossRef] [PubMed]
- Haynes, M.P.; Sinha, D.; Russell, K.S.; Collinge, M.; Fulton, D.; Morales-Ruiz, M.; Sessa, W.C.; Bender, J.R. Membrane estrogen receptor engagement activates endothelial nitric oxide synthase via the PI3-kinase-Akt pathway in human endothelial cells. Circ. Res. 2000, 87, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Unfer, T.C.; Maurer, L.H.; Kemerich, D.M.; Figueiredo, C.G.; Duarte, M.M.; Gelain, D.P.; Moreira, J.C.F.; Emanuelli, T. Non-genomic, direct modulatory effect of 17β-estradiol, progesterone, and their synthetic derivatives on the activity of human erythrocyte CuZn superoxide dismutase. Free Radic. Res. 2013, 47, 219–232. [Google Scholar] [CrossRef] [PubMed]
- Farber, P.L.; Freitas, T.; Saldanha, C.; Silva-Herdade, A.S. Betaestradiol and ethinylestradiol enhance RBC deformability dependent on their blood concentration. Clin. Hemorheol. Microcirc. 2018, 70, 339–345. [Google Scholar] [CrossRef]
- Poljičak-Milas, N.; Kardum-Skelin, I.; Vuđan, M.; Marenjak, T.S.; Ballarin-Perharić, A.; Milas, Z. Blood cell count analyses and erythrocyte morphometry in New Zealand white rabbits. Vet. Arh. 2009, 79, 561–571. [Google Scholar]
- Žaja, I.Ž.; Vince, S.; Butković, I.; Senaši, K.; Poljičak Milas, N.; Malarić, K.; Lojkić, M.; Folnožić, I.; Milinković Tur, S.; Kreszinger, M.; et al. The distribution of boars spermatozoa in morphometrically distinct subpopulations after in vitro exposure to radiofrequency electromagnetic radiation at 2500 MHz and their motility. Animals 2024, 14, 828. [Google Scholar] [CrossRef] [PubMed]
- Kumari, P.; Manjula, S.D.; Gautham, K. In vitro study of effect of radiation emitted by mobile phone on osmotic fragility and other blood parameters. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 1283–1292. [Google Scholar]
- Christopher, B.; Mary, Y.; Khandaker, M.; Bradley, D.; Chew, M.T.; John, J. Effects of mobile phone radiation on certain hematological parameters. Radiat. Phys. Chem. 2020, 166, 108443. [Google Scholar] [CrossRef]
- Lippi, G.; Danese, E.; Brocco, G.; Gelati, M.; Salvagno, G.L.; Montagnana, M. Acute effects of 30 minutes of exposure to a smartphone call on in vitro platelet function. Blood. Transfus. 2017, 15, 249–253. [Google Scholar] [CrossRef]
- Girasole, M.; Dinarelli, S.; Boumis, G. Structure and function in native and pathological erythrocytes: A quantitative view from the nanoscale. Micron 2012, 43, 1273–1286. [Google Scholar] [CrossRef]
- Nowakowski, R.; Luckham, P.; Winlove, P. Imaging erythrocytes under physiological conditions by atomic force microscopy. Biochim. Biophys. Acta 2001, 1514, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Deuticke, B. Membrane lipids and proteins as a basis of red cell shape and its alterations. In Red Cell Membrane Transport in Health and Disease, 1st ed.; Bernhardt, I., Ellory, J.C., Eds.; Springer: Berlin/Heidelberg, Germany, 2003; pp. 27–60. [Google Scholar]
- Betz, T.; Bakowsky, U.; Müller, M.R.; Lehr, C.M.; Bernhardt, I. Conformational change of membrane proteins leads to shape changes of red blood cells. Bioelectrochemistry 2007, 70, 122–126. [Google Scholar] [CrossRef] [PubMed]
- Lenzi, E.; Dinarelli, S.; Longo, G.; Girasole, M.; Mussi, V. Multivariate analysis of mean Raman spectra of erythrocytes for a fast analysis of the biochemical signature of ageing. Talanta 2021, 221, 121442. [Google Scholar] [CrossRef]
- Rudenko, S.V. Erythrocyte morphological states, phases, transitions, and trajectories. Biochim. Biophys. Acta 2010, 1798, 1767–1778. [Google Scholar] [CrossRef] [PubMed]
- Dasdag, S.; Akdag, M.Z. The link between radiofrequencies emitted from wireless technologies and oxidative stress. J. Chem. Neuroanat. 2016, 75, 85–93. [Google Scholar] [CrossRef]
- Challis, L.J. Mechanisms for interaction between RF fields and biological tissue. Bioelectromagnetics 2005, 7, S98–S106. [Google Scholar] [CrossRef]
- Alghamdi, M.S.; El-Ghazaly, N.A. Effects of exposure to electromagnetic field on some hematological parameters in mice. Open J. Med. Chem. 2012, 2, 30–42. [Google Scholar] [CrossRef][Green Version]
- Hu, C.; Zuo, H.; Li, Y. Effects of radiofrequency electromagnetic radiation on neurotransmitters in the brain. Front. Public Health 2021, 17, 691880. [Google Scholar] [CrossRef]
- Karipidis, K.; Mate, R.; Urban, D.; Tinker, R.; Wood, A. 5G mobile networks and health—A state of the science review of the research into low-level RF fields above 6 GHz. J. Expo. Sci. Environ. Epidemiol. 2021, 31, 585–605. [Google Scholar] [CrossRef] [PubMed]
- Raval, J.S.; Waters, J.H.; Seltsam, A.; Scharberg, E.A.; Richter, E.; Kameneva, M.V.; Yazer, M.H. Menopausal status affects the susceptibility of stored RBCs to mechanical stress. Vox Sang. 2011, 100, 418–421. [Google Scholar] [CrossRef] [PubMed]
- Jordan, A.; Chen, D.; Yi, Q.L.; Kanias, T.; Gladwin, M.T.; Acker, J.P. Assessing the influence of component processing and donor characteristics on quality of red cell concentrates using quality control data. Vox Sang. 2016, 111, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Kanias, T.; Sinchar, D.; Osei-Hwedieh, D.; Baust, J.J.; Jordan, A.; Zimring, J.C.; Waterman, H.R.; De Wolski, K.S.; Acker, J.P.; Gladwin, M.T. Testosterone-dependent sex differences in red blood cell hemolysis in storage, stress, and disease. Transfusion 2016, 56, 2571–2583. [Google Scholar] [CrossRef] [PubMed]
- Žura Žaja, I.; Vince, S.; Poljičak Milas, N.; Lobpreis, I.R.A.; Špoljarić, B.; Shek Vugrovečki, A.; Milinković Tur, S.; Šimpraga, M.; Pajurin, L.; Mikuš, T.; et al. A new method of assessing sheep red blood cell types from their morphology. Animals 2019, 9, 1130. [Google Scholar] [CrossRef] [PubMed]


| 5G Radiofrequency Electromagnetic Radiation (5G RF-EMR) Frequencies | |||||||
|---|---|---|---|---|---|---|---|
| 700 MHz | 2500 MHz | 3500 MHz | |||||
| Sample Size | 30 | 30 | 30 | 30 | 30 | 30 | |
| Group | Gender | Control | Experimental | Control | Experimental | Control | Experimental |
| Neutrophils (%) | M | 56.25 (52.25–60.17) | 56.91 (52.92–60.82) | 55.70 # (51.70–59.63) | 55.91 # (51.91–59.84) | 55.81 (51.81–59.43) | 55.50 (51.50–59.43) |
| F | 58.37 (54.38–62.25) | 58.21 (54.22–62.10) | 61.73 # (57.77–65.73) | 62.37 # (58.42–66.16) | 59.90 (55.92–63.75) | 59.75 (55.77–63.60) | |
| M+F | 57.31 (54.49–60.09) | 57.56 (54.75–60.33) | 58.75 (55.93–61.51) | 59.18 (56.37–61.93) | 57.87 (55.05–60.63) | 57.64 (54.82–60.41) | |
| Basophils (%) | M | 0.49 (0.39–0.61) | 0.48 (0.38–0.60) | 0.50 ## (0.40–0.63) | 0.55 ## (0.45–0.68) | 0.55 # (0.45–0.68) | 0.55 # (0.45–0.68) |
| F | 0.35 (0.26–0.45) | 0.36 (0.28–0.47) | 0.30 ## (0.22–0.40) | 0.34 ## (0.26–0.45) | 0.39 # (0.31–0.51) | 0.37 # (0.29–0.48) | |
| M+F | 0.41 (0.34–0.49) | 0.42 (0.35–0.50) | 0.39 (0.32–0.47) | 0.43 (0.36–0.52) | 0.47 (0.40–0.55) | 0.45 (0.38–0.54) | |
| Eosinophils (%) | M | 3.25 # (2.37–4.46) | 3.25 # (2.36–4.45) | 2.99 # (2.15–4.16) | 3.03 # (2.18–4.20) | 3.20 # (2.32–4.39) | 3.09 # (2.23–4.26) |
| F | 1.84 # (1.20–2.81) | 1.89 # (1.25–2.87) | 1.53 # (0.96–2.44) | 1.66 # (1.06–2.59) | 1.71 # (1.10–2.65) | 1.61 # (1.02–2.53) | |
| M+F | 2.45 (1.88–3.19) | 2.48 (1.91–3.22) | 2.15 (1.61–2.85) | 2.24 (1.70–2.96) | 2.34 (1.79–3.07) | 2.23 (1.69–2.94) | |
| Lymphocytes (%) | M | 33.95 (30.11–38.02) | 33.75 (29.92–37.92) | 34.31 # (30.45–38.38) | 34.17 (30.32–38.24) | 33.88 (30.04–37.95) | 34.18 (30.33–38.25) |
| F | 33.79 (29.96–37.86) | 33.96 (30.12–38.03) | 28.83 # (25.19–32.76) | 30.71 (26.99–34.70) | 32.77 (28.97–36.81) | 32.81 (29.00–36.85) | |
| M+F | 33.87 (31.13–36.73) | 33.86 (31.12–36.71) | 31.50 (28.81–34.32) | 32.42 (29.71–35.26) | 33.32 (30.59–36.17) | 33.49 (30.76–36.34) | |
| Monocytes (%) | M | 6.07 (5.29–6.95) | 5.79 (5.03–6.66) | 6.47 # (5.67–7.38) | 6.33 ## (5.53–7.23) | 6.59 # (5.78–7.50) | 6.65 # (5.84–7.57) |
| F | 5.63 (4.89–6.49) | 5.59 (4.84–6.44) | 5.13 # (4.42–5.96) | 4.89 ## (4.20–5.70) | 5.19 # (4.48–6.02) | 5.49 # (4.75–6.33) | |
| M+F | 5.85 (5.30–6.45) | 5.69 (5.15–6.29) | 5.77 (5.22–6.37) | 5.57 (5.03–6.16) | 5.85 (5.30–6.46) | 6.04 (5.48–6.66) | |
| 5G Radiofrequency Electromagnetic Radiation (5G RF-EMR) Frequencies | |||||||
|---|---|---|---|---|---|---|---|
| 700 MHz | 2500 MHz | 3500 MHz | |||||
| Number of Humans | 30 | 30 | 30 | 30 | 30 | 30 | |
| Group | Gender | Control | Experimental | Control | Experimental | Control | Experimental |
| Total leukocytes (109/L) | M | 6.82 (6.20–7.50) | 6.85 (6.23–7.53) | 6.45 (5.83–7.13) | 6.31 (5.70–7.00) | 6.28 (5.66–6.96) | 6.22 (5.60–6.90) |
| F | 6.99 (6.37–7.67) | 7.13 (6.51–7.81) | 6.77 (6.15–7.45) | 6.78 (6.17–7.47) | 7.06 (6.44–7.74) | 7.10 (6.48–7.78) | |
| M+F | 6.91 (6.46–7.38) | 6.99 (6.54–7.46) | 6.61 (6.16–7.08) | 6.54 (6.10–7.02) | 6.66 (6.21–7.13) | 6.64 (6.20–7.12) | |
| Neutrophils (109/L) | M | 3.94 (3.43–4.63) | 3.98 (3.47–4.67) | 3.57 (3.07–4.27) | 3.55 (3.04–4.25) | 3.51 (3.00–4.21) | 3.45 # (2.95–4.16) |
| F | 4.11 (3.60–4.80) | 4.18 (3.67–4.86) | 4.16 (3.64–4.84) | 4.22 (3.71–4.90) | 4.31 (3.79–4.99) | 4.33 # (3.81–5.01) | |
| M+F | 4.03 (3.65–4.49) | 4.08 (3.70–4.54) | 3.84 (3.46–4.32) | 3.85 (3.47–4.33) | 3.87 (3.48–4.35) | 3.84 (3.45–4.32) | |
| Basophils (109/L) | M | 0.031 (0.024–0.038) | 0.031 (0.024–0.038) | 0.031 # (0.024–0.039) | 0.035 # (0.029–0.043) | 0.035 (0.028–0.042) | 0.035 (0.028–0.042) |
| F | 0.023 (0.016–0.030) | 0.027 (0.020–0.034) | 0.021 # (0.014–0.028) | 0.023 # (0.016–0.031) | 0.027 (0.020–0.034) | 0.027 (0.021–0.035) | |
| M+F | 0.027 (0.022–0.032) | 0.029 (0.024–0.034) | 0.026 (0.021–0.031) | 0.029 (0.024–0.034) | 0.031 (0.026–0.036) | 0.031 (0.026–0.036) | |
| Eosinophils (109/L) | M | 0.22 (0.16–0.29) | 0.22 (0.16–0.29) | 0.19 (0.14–0.27) | 0.19 (0.14–0.27) | 0.21 (0.15–0.28) | 0.20 (0.15–0.28) |
| F | 0.13 (0.08–0.21) | 0.13 (0.08–0.21) | 0.10 (0.06–0.19) | 0.11 (0.07–0.20) | 0.11 (0.07–0.20) | 0.11 (0.06–0.19) | |
| M+F | 0.17 (0.13–0.22) | 0.17 (0.13–0.23) | 0.14 (0.10–0.20) | 0.15 (0.11–0.21) | 0.15 (0.11–0.21) | 0.15 (0.10–0.20) | |
| Lymphocytes (109/L) | M | 2.22 (1.95–2.49) | 2.22 (1.94–2.49) | 2.19 (1.91–2.46) | 2.15 (1.87–2.42) | 2.10 (1.83–2.38) | 2.10 (1.83–2.37) |
| F | 2.33 (2.06–2.31) | 2.39 (2.12–2.67) | 1.95 * (1.67–2.22) | 2.10 * (1.83–2.37) | 2.24 (1.97–2.52) | 2.25 (1.97–2.52) | |
| M+F | 2.27 (2.08–2.47) | 2.31 (2.11–2.50) | 2.07 (1.87–2.26) | 2.12 (1.93–2.32) | 2.17 (1.98–2.37) | 2.18 (1.98–2.37) | |
| Monocytes (109/L) | M | 0.41 (0.35–0.49) | 0.39 (0.33–0.48) | 0.41 (0.35–0.49) | 0.39 (0.33–0.48) | 0.43 (0.36–0.51) | 0.43 (0.37–0.51) |
| F | 0.39 (0.33–0.48) | 0.39 (0.33–0.48) | 0.34 (0.28–0.43) | 0.33 (0.27–0.42) | 0.37 (0.31–0.45) | 0.39 (0.33–0.48) | |
| M+F | 0.40 (0.35–0.46) | 0.39 (0.35–0.45) | 0.37 (0.33–0.43) | 0.36 (0.31–0.42) | 0.39 (0.35–0.45) | 0.41 (0.36–0.47) | |
| 5G Radiofrequency Electromagnetic Radiation (5G RF-EMR) Frequencies | |||||||
|---|---|---|---|---|---|---|---|
| 700 MHz | 2500 MHz | 3500 MHz | |||||
| Number of Humans | 30 | 30 | 30 | 30 | 30 | 30 | |
| Group | SEX | Control | Experimental | Control | Experimental | Control | Experimental |
| Total erythrocytes (1012/L) | M | 5.05 ## (4.88–5.22) | 5.06 ## (4.89–5.23) | 5.13 ## (4.96–5.30) | 5.11 ## (4.93–5.28) | 5.01 ## (4.84–5.18) | 5.04 ## (4.87–5.21) |
| F | 4.54 ## (4.37–4.71) | 4.55 ## (4.38–4.73) | 4.48 ## (4.31–4.65) | 4.50 ## (4.33–4.67) | 4.48 ## (4.31–4.65) | 4.49 ## (4.32–4.67) | |
| M+F | 4.80 (4.67–4.92) | 4.81 (4.68–4.93) | 4.80 (4.68–4.92) | 4.80 (4.68–4.92) | 4.75 (4.63–4.87) | 4.77 (4.65–4.89) | |
| Reticulocytes (109/L) | M | 57.13 (48.03–67.96) | 57.20 (48.10–68.03) | 58.50 (48.40–70.70) | 59.77 (50.01–71.43) | 57.14 (47.75–68.38) | 59.21 (49.79–70.42) |
| F | 48.47 (39.50–59.47) | 47.80 (38.85–58.82) | 53.58 (43.57–65.90) | 54.25 (44.22–66.55) | 43.47 (34.60–54.60) | 46.47 (37.54–57.52) | |
| M+F | 52.62 (46.02–60.18) | 52.29 (45.68–59.86) | 55.99 (48.66–64.42) | 56.94 (49.72–65.21) | 49.84 (43.10–57.62) | 52.45 (45.72–60.18) | |
| Reticulocytes (%) | M | 11.23 (9.43–13.31) | 11.22 (9.43–13.31) | 11.32 (9.32–13.68) | 11.65 (9.70–13.94) | 11.40 (9.53–13.58) | 11.73 (9.84–13.93) |
| F | 10.60 (8.86–12.64) | 10.41 (8.64–12.44) | 11.75 (9.72–14.14) | 11.87 (9.82–14.27) | 9.59 (7.93–11.55) | 10.23 (8.52–12.25) | |
| M+F | 10.91 (9.63–12.34) | 10.81 (9.54–12.23) | 11.53 (10.07–13.17) | 11.76 (10.31–13.38) | 10.46 (9.18–11.89) | 10.96 (9.65–12.41) | |
| Haematocrit (L/L) | M | 0.46 ## (0.45–0.48) | 0.46 ## (0.45–0.48) | 0.47 ## (0.45–0.49) | 0.47 ## (0.45–0.48) | 0.46 ## (0.44–0.48) | 0.47 ## (0.45–0.48) |
| F | 0.42 ## (0.40–0.44) | 0.42 ## (0.41–0.44) | 0.41 ## (0.40–0.43) | 0.42 ## (0.40–0.43) | 0.42 ## (0.40–0.43) | 0.42 ## (0.40–0.43) | |
| M+F | 0.44 (0.43–0.45) | 0.44 (0.43–0.45) | 0.44 (0.43–0.45) | 0.44 (0.43–0.45) | 0.44 (0.43–0.45) | 0.44 (0.43–0.45) | |
| Haemoglobin (g/L) | M | 152 ## (147–157) | 151 ## (146–156) | 153 ## (148–158) | 153 ## (148–158) | 151 ## (146–156) | 151 ## (146–156) |
| F | 136 ## (131–141) | 136 ## (131–141) | 134 ## (129–139) | 134 ## (129–139) | 134 ## (129–139) | 135 ## (130–140) | |
| M+F | 144 (140–147) | 144 (140–147) | 143 (140–147) | 144 (140–147) | 142 * (139–146) | 143 * (139–146) | |
| 5G Radiofrequency Electromagnetic Radiation (5G RF-EMR) Frequencies | |||||||
|---|---|---|---|---|---|---|---|
| 700 MHz | 2500 MHz | 3500 MHz | |||||
| Number of Humans | 30 | 30 | 30 | 30 | 30 | 30 | |
| Group | Gender | Control | Experimental | Control | Experimental | Control | Experimental |
| MCV (fL) | M | 91.72 (89.22–94.09) | 91.81 (89.30–94.18) | 91.61 * (89.10–93.98) | 89.69 * (87.18–92.06) | 92.23 (89.73–94.61) | 92.24 (89.74–94.61) |
| F | 92.80 (90.30–95.18) | 92.79 (90.28–95.16) | 92.74 (90.24–95.12) | 92.82 (90.32–95.20) | 93.35 (90.85–95.73) | 93.34 (90.84–95.72) | |
| M+F | 92.26 (90.51–93.95) | 92.30 (90.54–94.00) | 92.18 (90.42–93.88) | 91.28 (89.52–92.97) | 92.80 (91.04–94.49) | 92.80 (91.04–94.49) | |
| MCH (pg) | M | 30.02 (2932–30.70) | 29.95 (29.25–30.63) | 29.81 (29.12–30.49) | 29.97 (29.27–30.65) | 30.02 (29.32–30.70) | 29.97 (29.27–30.65) |
| F | 29.91 (29.21–30.59) | 29.87 (29.17–30.55) | 29.95 (29.25–30.63) | 29.88 (29.18–30.56) | 29.99 (29.29–30.67) | 30.00 (29.30–30.68) | |
| M+F | 29.96 (29.47–30.45) | 29.91 (29.42–30.39) | 29.88 (29.39–30.37) | 29.92 (29.43–30.41) | 30.00 (29.51–30.49) | 29.98 (29.49–30.47) | |
| MCHC (g/L) | M | 327 # (324–330) | 326 # (323–329) | 325 (322–328) | 327 # (324–330) | 326 # (323–329) | 325 (322–328) |
| F | 322 # (319–325) | 322 # (319–325) | 323 (320–326) | 322 # (319–325) | 321 # (318–324) | 321 (319–324) | |
| M+F | 325 (323–327) | 324 (322–326) | 324 (322–326) | 325 (322–327) | 324 (321–326) | 323 (321–325) | |
| RDW (%) | M | 12.98 (12.69–13.28) | 12.97 (12.68–13.26) | 13.03 (12.74–13.33) | 13.02 (12.73–13.32) | 13.07 (12.78–13.36) | 13.06 (12.77–13.36) |
| F | 13.21 (12.92–13.51) | 13.18 (12.89–13.48) | 13.22 (12.93–13.52) | 13.25 (12.95–13.54) | 13.19 (12.90–13.49) | 13.21 (12.92–13.51) | |
| M+F | 13.10 (12.89–13.31) | 13.07 (12.89–13.28) | 13.13 (12.92–13.34) | 13.13 (12.93–13.34) | 13.13 (12.92–13.34) | 13.14 (12.93–13.35) | |
| 5G Radiofrequency Electromagnetic Radiation (5G RF-EMR) Frequencies | |||||||
|---|---|---|---|---|---|---|---|
| 700 MHz | 2500 MHz | 3500 MHz | |||||
| Number of Humans | 30 | 30 | 30 | 30 | 30 | 30 | |
| Group | Gender | Control | Experimental | Control | Experimental | Control | Experimental |
| Platelets (109/L) | M | 257 (226–288) | 263 (232–294) | 259 (228–290) | 259 (227–290) | 246 ## (215–277) | 250 # (219–281) |
| F | 298 (267–329) | 304 (272–335) | 296 (265–327) | 294 (263–326) | 308 ## (277–339) | 304 # (273–335) | |
| M+F | 277 (255–299) | 283 (261–305) | 277 (255–291) | 277 (255–298) | 277 (255–299) | 277 (255–299) | |
| MPV (fL) | M | 8.55 (8.00–9.23) | 8.52 (7.97–9.19) | 8.75 (8.26–9.35) | 8.75 (8.26–9.35) | 8.84 (8.34–9.44) | 8.89 (8.40–9.49) |
| F | 8.32 (7.75–9.03) | 8.23 (7.66–8.94) | 8.73 (8.23–9.32) | 8.63 (8.14–9.23) | 8.69 (8.19–9.28) * | 8.52 (8.02–9.11) * | |
| M+F | 8.43 (8.03–8.91) | 8.37 (7.96–8.84) | 8.74 (8.38–9.15) | 8.69 (8.33–9.10) | 8.76 (8.40–9.17) | 8.70 (8.34–9.11) | |
| Platelet activation (%) | M | 0.002 (0.001–0.003) | 0.002 (0.001–0.003) | 0.002 (0.001–0.003) | 0.002 (0.001–0.003) | 0.002 (0.001–0.003) | 0.002 (0.001–0.003) |
| F | 0.001 (0.001–0.002) | 0.002 (0.001–0.003) | 0.002 (0.001–0.003) | 0.001 (0.001–0.002) | 0.002 (0.001–0.003) | 0.001 (0.001–0.002) | |
| M+F | 0.002 (0.001–0.002) | 0.002 (0.001–0.002) | 0.002 (0.001–0.002) | 0.002 (0.001–0.002) | 0.002 (0.001–0.002) | 0.002 (0.001–0.002) | |
| 5G Radiofrequency Electromagnetic Radiation (5G RF-EMR) Frequencies | ||||||||
|---|---|---|---|---|---|---|---|---|
| 700 MHz | 2500 MHz | 3500 MHz | ||||||
| Number of Humans | 30 | 30 | 30 | 30 | 30 | 30 | ||
| Group | Gender | Control | Experimental | Control | Experimental | Control | Experimental | |
| Erythrocyte size indicators | Area (μm2) | M | 44.51 ###* (44.26–44.77) | 45.16 ###* (44.91–45.41) | 45.11 (44.85–45.38) | 45.45 (45.19–45.71) | 45.28 ### (45.04–45.53) | 45.68 ### (45.43–45.93) |
| F | 45.53 ###*** (45.27–45.79) | 46.52 ###*** (46.26–46.78) | 45.59 (45.33–45.84) | 45.79 (45.53–46.04) | 47.96 ### (47.70–48.20) | 48.03 ### (47.77–48.28) | ||
| M+F | 45.02 *** (44.84–45.20) | 45.84*** (45.66–46.02) | 45.35 (45.17–45.53) | 45.62 (45.43–45.80) | 46.62 (46.44–46.79) | 46.86 (46.68–47.03) | ||
| Outline (μm) | M | 26.67 ###*** (26.56–26.78) | 27.16 ###*** (27.05–27.27) | 26.68 ## (26.57–26.80) | 26.87 ### (26.75–26.98) | 27.03 ### (26.92–27.14) | 27.08 ### (26.97–27.19) | |
| F | 27.07 ###*** (26.96–27.19) | 27.55 ###*** (27.43–27.66) | 27.02 ## (26.91–27.13) | 27.28 ### (27.16–27.39) | 28.05 ###*** (27.94–28.16) | 28.52 ###*** (28.41–28.63) | ||
| M+F | 26.87 *** (26.79–26.95) | 27.35 *** (27.27–27.43) | 26.85 ** (26.77–26.93) | 27.07 ** (26.99–27.15) | 27.54 *** (27.46–27.62) | 27.80 *** (27.72–27.88) | ||
| Minimum radius (μm) | M | 3.43 ###** (3.42–3.44) | 3.46 ###** (3.45–3.47) | 3.47 (3.46–3.49) | 3.48 (3.46–3.49) | 3.47 ### (3.46–3.48) | 3.48 ### (3.47–3.49) | |
| F | 3.48 ###*** (3.47–3.49) | 3.52 ###*** (3.51–3.53) | 3.48 (3.47–3.49) | 3.47 (3.46–3.48) | 3.56 ### (3.55–3.57) | 3.58 ### (3.56–3.59) | ||
| M+F | 3.45 *** (3.45–3.46) | 3.49 *** (3.48–3.50) | 3.48 (3.47–3.48) | 3.47 (3.47–3.48) | 3.52 (3.51–3.52) | 3.53 (3.52–3.54) | ||
| Maximum radius (μm) | M | 4.04 ##* (4.03–4.05) | 4.07 ###* (4.06–4.08) | 4.05 # (4.03–4.06) | 4.07 # (4.06–4.09) | 4.07 ### (4.06–4.09) | 4.09 ### (4.08–4.11) | |
| F | 4.08 ##*** (4.06–409) | 4.13 ###*** (4.11–4.14) | 4.09 # (4.07–4.10) | 4.11 # (4.10–4.12) | 4.20 ### (4.19–421) | 4.19 ### (4.18–4.20) | ||
| M+F | 4.06 *** (4.05–4.07) | 4.10 *** (4.09–4.11) | 4.07 ** (4.06–4.08) | 4.09 ** (4.08–4.10) | 4.14 (4.13–4.15) | 4.14 (4.13–4.15) | ||
| Convex area (μm2) | M | 44.99 ###* (44.73–45.25) | 45.68 ###* (45.42–45.94) | 45.57 (45.30–45.83) | 45.91 (45.65–46.18) | 45.78 ### (45.53–46.03) | 46.18 ### (45.93–46.44) | |
| F | 46.02 ###*** (45.76–46.28) | 47.05 ###*** (46.78–47.31) | 46.07 (45.81–46.33) | 45.30 (46.04–46.52) | 48.50 ### (48.25–48.76) | 48.64 ### (48.38–48.90) | ||
| M+F | 45.50 *** (45.32–45.69) | 46.36 *** (46.18–46.55) | 45.82 (45.63–46.00) | 46.11 (45.92–46.29) | 47.14 (46.96–47.32) | 47.41 (47.23–47.59) | ||
| Length (µm) | M | 7.93 ##* (7.90–7.95) | 7.99 ###* (7.97–8.02) | 7.96 ## (7.93–7.99) | 8.00 # (7.97–8.02) | 8.00 ### (7.99–8.03) | 8.05 ### (8.03–8.08) | |
| F | 8.01 ##*** (7.98–8.03) | 8.10 ###*** (8.07–8.13) | 8.04 ## (8.01–8.06) | 8.07 # (8.04–8.09) | 8.25 ### (8.23–8.28) | 8.23 ### (8.20–8.26) | ||
| M+F | 7.97 *** (7.95–7.99) | 8.05 *** (8.03–8.06) | 7.99 (7.98–8.02) | 8.03 (8.01–8.05) | 8.13 (8.11–8.15) | 8.14 (8.12–8.16) | ||
| Width (µm) | M | 7.18 ###** (7.15–7.20) | 7.24 ###** (7.22–7.25) | 7.25 (7.22–7.27) | 7.29 (7.24–7.29) | 7.25 ### (7.22–7.27) | 7.26 ### (7.24–7.28) | |
| F | 7.27 ###*** (7.25–7.30) | 7.37 ###*** (7.34–7.39) | 7.25 (7.23–7.27) | 7.26 (7.24–7.29) | 7.44 ### (7.42–7.47) | 7.48 ### (7.46–7.51) | ||
| M+F | 7.22 *** (7.21–7.24) | 7.30 *** (7.29–7.32) | 7.25 (7.23–7.26) | 7.26 (7.25–7.28) | 7.34 (7.33–7.36) | 7.37 (7.36–7.39) | ||
| 5G Radiofrequency Electromagnetic Radiation (5G RF-EMR) Frequencies | ||||||||
|---|---|---|---|---|---|---|---|---|
| 700 MHz | 2500 MHz | 3500 MHz | ||||||
| Number of Humans | 30 | 30 | 30 | 30 | 30 | 30 | ||
| Group | Gender | Control | Experimental | Control | Experimental | Control | Experimental | |
| Morphometric shape indicators of erythrocytes | Contour index | M | 4.00 *** (3.99–4.01) | 4.05 *** (4.04–4.06) | 3.98 ## (3.97–3.99) | 3.99 ### (3.98–4.00) | 4.02 ### (4.01–4.03) | 4.01 ### (4.00–4.02) |
| F | 4.02 * (4.01–4.03) | 4.04 * (4.03–4.05) | 4.01 ##** (4.00–4.02) | 4.04 ###** (4.03–4.05) | 4.06 ###*** (4.05–4.06) | 4.12 ###*** (4.11–4.13) | ||
| M+F | 4.01*** (4.00–4.02) | 4.04 *** (4.04–4.05) | 3.99 *** (3.96–4.00) | 4.01 *** (4.01–4.02) | 4.04 *** (4.03–4.04) | 4.07 *** (4.06–407) | ||
| Solidity | M | 0.9895 *** (0.9893–0.9897) | 0.9887 ###*** (0.9885–0.9889) | 0.9901 (0.9899–0.9903) | 0.9899 ### (0.9897–0.9901) | 0.9893 ## (0.9891–0.9895) | 0.9893 ### (0.9891–0.9895) | |
| F | 0.9894 * (0.9892–0.9896) | 0.9889 ###* (0.9887–0.9891) | 0.9896 ** (0.9894–0.9898) | 0.9890 ###** (0.9888–0.9892) | 0.9887 ##*** (0.9885–0.9889) | 0.9875 ###*** (0.9873–0.9877) | ||
| M+F | 0.9894 *** (0.9893–0.9896) | 0.9888 *** (0.9887–0.9889) | 0.9899 ** (0.9897–0.9900) | 0.9895 ** (0.9893–0.9896) | 0.9890 *** (0.9889–0.9891) | 0.9884 *** (0.9883–0.9885) | ||
| Roundness | M | 0.868 (0.866–0.871) | 0.867 (0.864–0.869) | 0.875 # (0.872–0.878) | 0.871 ## (0.869–0.874) | 0.868 (0.865–0.870) | 0.867 (0.864–0.869) | |
| F | 0.871 (0.869–0.874) | 0.870 (0.867–0.872) | 0.868 # (0.866–0.871) | 0.863 ## (0.861–0.866) | 0.864 (0.862–0.867) | 0.870 (0.867–0.872) | ||
| M+F | 0.870 (0.868–0.871) | 0.868 (0.866–0.870) | 0.872 ** (0.870–0.873) | 0.867 ** (0.865–0.869) | 0.866 (0.864–0.868) | 0.868 (0.867–0.870) | ||
| Ellipticity | M | 1.11 (1.10–1.11) | 1.10 (1.10–1.11) | 1.10 ## (1.10–1.10) | 1.10 ## (1.10–1.11) | 1.11 (1.11–1.12) | 1.11 ## (1.11–1.12) | |
| F | 1.10 (1.10–1.11) | 1.10 (1.10–1.10) | 1.11 ## (1.10–1.11) | 1.11 ## (1.11–1.12) | 1.11 ** (1.11–1.12) | 1.10 ##** (1.10–1.11) | ||
| M+F | 1.11 (1.10–1.11) | 1.11 (1.10–1.11) | 1.11 (1.11–1.11) | 1.11 (1.11–1.11) | 1.11 (1.11–1.11) | 1.11 (1.11–1.11) | ||
| Elongation | M | 0.049 (0.048–0.051) | 0.049 (0.047–0.050) | 0.046 ## (0.045–0.048) | 0.048 ## (0.046–0.049) | 0.049 (0.048–0.050) | 0.051 ## (0.050–0.053) | |
| F | 0.048 (0.046–0.049) | 0.047 (0.045–0.048) | 0.051 ## (0.050–0.053) | 0.052 ## (0.050–0.053) | 0.051 * (0.050–0.052) | 0.047 ##* (0.045–0.048) | ||
| M+F | 0.048 (0.047–0.049) | 0.048 (0.047–0.0549 | 0.049 (0.048–0.050) | 0.050 (0.049–0.051) | 0.050 (0.049–0.051) | 0.049 (0.048–0.050) | ||
| Form factor | M | 0.788 ##*** (0.785–0.791) | 0.773 ###*** (0.770–0.776) | 0.80 ## (0.79–0.80) | 0.793 ### (0.790–0.796) | 0.782 ### (0.780–0.785) | 0.784 ### (0.782–0.787) | |
| F | 0.783 ##** (0.780–0.786) | 0.775 ###** (0.772–0.778) | 0.788 ##*** (0.785–0.790) | 0.778 ###*** (0.775–0.781) | 0.768 ###*** (0.765–0.771) | 0.750 ###*** (0.747–0.752) | ||
| M+F | 0.785 *** (0.783–0.787) | 0.774 *** (0.772–0.776) | 0.793 *** (0.791–0.794) | 0.785 *** (0.783–0.787) | 0.775 *** (0.773–0.777) | 0.767 *** (0.765–0.769) | ||
| Morphometric Size and Shape Indicators of Erythrocytes | Erythrocyte Size | Erythrocyte Form | |
|---|---|---|---|
| Factor 1 | Factor 2 | Factor 3 | |
| Outline (µm) | * 0.97 | ||
| Convexity (µm2) | 0.94 | ||
| Area (µm2) | 0.2 | ||
| Length (µm) | 0.88 | ||
| Width (µm) | 0.80 | ||
| Roundness | 0.91 | ||
| Form factor | 0.70 | ||
| Contour index | −0.74 | ||
| Elongation | * −0.95 | ||
| Ellipticity | −0.94 | ||
| Solidity | * 0.77 | ||
| Characteristic root (λ) andexplained variance (%) | 6.57 (50.6) | 3.71 (28.6) | 2.32 (17.8) |
| Cumulative variance (%) | 50.6 | 79.2 | 97.0 |
| Erythrocyte Subpopulations | Erythrocyte Size | ||||||
|---|---|---|---|---|---|---|---|
| N (%) | Outline (µm) | Convex Area (µm2) | Area (µm2) | Length (µm) | Width (µm) | ||
| ES 1 | 17,592 (67.5) | 25.9 ± 1.71 | 43.0 ± 4.10 | 42.6 ± 4.04 | 7.76 ± 0.46 | 7.03 ± 0.42 | |
| ES 2 | 8476 (32.5) | 29.9 ± 2.53 | 53.4 ± 3.63 | 52.7 ± 3.55 | 8.66 ± 0.41 | 7.83 ± 0.37 | |
| Erythrocyte form | |||||||
| Roundness | Ellipticity | Elongation | Solidity | Form factor | Contour index | ||
| ES 1 | 17,592 (675) | 0.871 ± 0.059 | 1.106 ± 0.078 | 0.049 ± 0.033 | 0.989 ± 0.003 | 0.795 ± 0.049 | 3.98 ± 0.148 |
| ES 2 | 8476 (32.5) | 0.863 ± 0.059 | 1.107 ± 0.074 | 0.049 ± 0.032 | 0.987 ± 0.006 | 0.749 ± 0.085 | 4.12 ± 0.305 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Žura, N.; Vince, S.; Perić, P.; Vilić, M.; Malarić, K.; Rimac, V.; Golubić Ćepulić, B.; Vajdić, M.; Jurak, I.; Milinković Tur, S.; et al. Short-Term In Vitro Exposure of Human Blood to 5G Network Frequencies: Do Sex and Frequency Additionally Affect Erythrocyte Morphometry? Biomedicines 2025, 13, 478. https://doi.org/10.3390/biomedicines13020478
Žura N, Vince S, Perić P, Vilić M, Malarić K, Rimac V, Golubić Ćepulić B, Vajdić M, Jurak I, Milinković Tur S, et al. Short-Term In Vitro Exposure of Human Blood to 5G Network Frequencies: Do Sex and Frequency Additionally Affect Erythrocyte Morphometry? Biomedicines. 2025; 13(2):478. https://doi.org/10.3390/biomedicines13020478
Chicago/Turabian StyleŽura, Nikolino, Silvijo Vince, Porin Perić, Marinko Vilić, Krešimir Malarić, Vladimira Rimac, Branka Golubić Ćepulić, Marina Vajdić, Ivan Jurak, Suzana Milinković Tur, and et al. 2025. "Short-Term In Vitro Exposure of Human Blood to 5G Network Frequencies: Do Sex and Frequency Additionally Affect Erythrocyte Morphometry?" Biomedicines 13, no. 2: 478. https://doi.org/10.3390/biomedicines13020478
APA StyleŽura, N., Vince, S., Perić, P., Vilić, M., Malarić, K., Rimac, V., Golubić Ćepulić, B., Vajdić, M., Jurak, I., Milinković Tur, S., Poljičak Milas, N., Samardžija, M., Nemir, J., Telebuh, M., & Žura Žaja, I. (2025). Short-Term In Vitro Exposure of Human Blood to 5G Network Frequencies: Do Sex and Frequency Additionally Affect Erythrocyte Morphometry? Biomedicines, 13(2), 478. https://doi.org/10.3390/biomedicines13020478





