A Narrative Review on Cognitive Impairment in Type 2 Diabetes: Global Trends and Diagnostic Approaches
Abstract
1. Introduction
2. Screening Methods for Diabetic Cognitive Impairment Recommended by ADA Guidelines
2.1. MMSE
2.2. MoCA
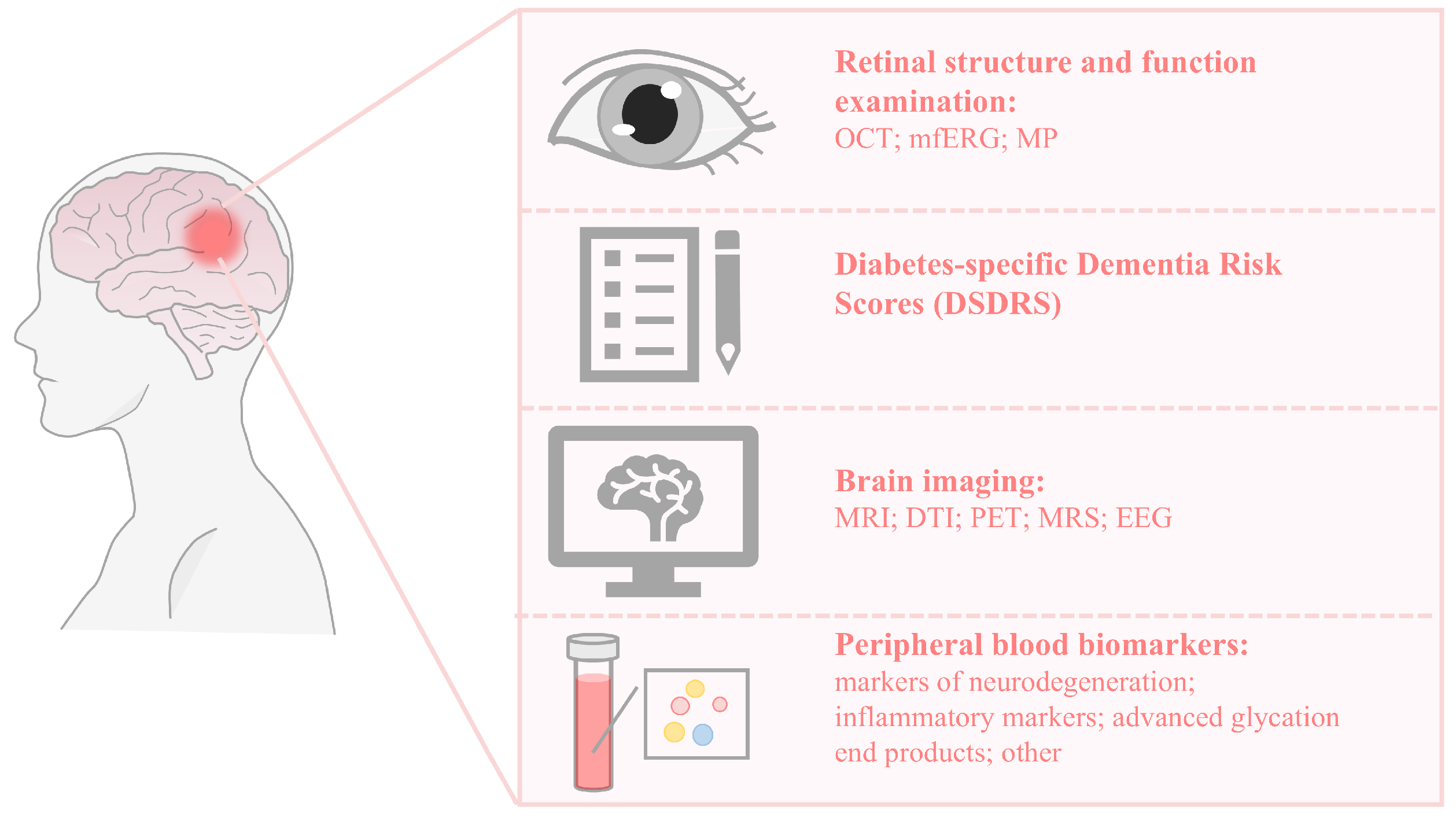
3. Retinal Structure and Function Examination
4. Diabetes-Specific Dementia Risk Score
5. Brain Imaging
5.1. Brain Structural Changes
5.2. Alterations in Cerebral Metabolism
5.3. Brain Function Changes
6. Peripheral Blood Biomarkers
6.1. Biomarkers Related to Neurodegeneration
6.2. Markers of Inflammation
6.3. Advanced Glycation End Products
6.4. Other Markers
7. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Car, J.; GBD 2021 Diabetes Collaborators. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: A systematic analysis for the Global Burden of Disease Study 2021. Lancet 2023, 402, 203–234. [Google Scholar] [CrossRef]
- Koekkoek, P.S.; Kappelle, L.J.; van den Berg, E.; Rutten, G.E.H.M.; Biessels, G.J. Cognitive function in patients with diabetes mellitus: Guidance for daily care. Lancet Neurol. 2015, 14, 329–340. [Google Scholar] [CrossRef]
- Sadanand, S.; Balachandar, R.; Bharath, S. Memory and executive functions in persons with type 2 diabetes: A meta-analysis. Diabetes/Metab. Res. Rev. 2016, 32, 132–142. [Google Scholar] [CrossRef]
- Wei, B.; He, J. Mediation effect of cognitive impairment for the relationship of type 2 diabetes mellitus with mortality among elderly individuals. Front. Endocrinol. 2024, 15, 1392326. [Google Scholar] [CrossRef]
- Xue, M.; Xu, W.; Ou, Y.-N.; Cao, X.-P.; Tan, M.-S.; Tan, L.; Yu, J.-T. Diabetes mellitus and risks of cognitive impairment and dementia: A systematic review and meta-analysis of 144 prospective studies. Ageing Res. Rev. 2019, 55, 100944. [Google Scholar] [CrossRef] [PubMed]
- Dove, A.; Shang, Y.; Xu, W.; Grande, G.; Laukka, E.J.; Fratiglioni, L.; Marseglia, A. The impact of diabetes on cognitive impairment and its progression to dementia. Alzheimer’s Dement. 2021, 17, 1769–1778. [Google Scholar] [CrossRef] [PubMed]
- Kodl, C.T.; Seaquist, E.R. Cognitive Dysfunction and Diabetes Mellitus. Endocr. Rev. 2008, 29, 494–511. [Google Scholar] [CrossRef] [PubMed]
- Crane, P.K.; Walker, R.; Hubbard, R.A.; Li, G.; Nathan, D.M.; Zheng, H.; Haneuse, S.; Craft, S.; Montine, T.J.; Kahn, S.E.; et al. Glucose Levels and Risk of Dementia. N. Engl. J. Med. 2013, 369, 540–548. [Google Scholar] [CrossRef] [PubMed]
- Sommerfield, A.J.; Deary, I.J.; McAulay, V.; Frier, B.M. Short-term, delayed, and working memory are impaired during hypoglycemia in individuals with type 1 diabetes. Diabetes Care 2003, 26, 390–396. [Google Scholar] [CrossRef]
- American Diabetes Association 12. Older Adults: Standards of Medical Care in Diabetes-2020. Diabetes Care 2020, 43, S152–S162. [Google Scholar] [CrossRef] [PubMed]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Tombaugh, T.N.; McIntyre, N.J. The Mini-Mental State Examination: A Comprehensive Review. J. Am. Geriatr. Soc. 1992, 40, 922–935. [Google Scholar] [CrossRef] [PubMed]
- Ciesielska, N.; Sokołowski, R.; Mazur, E.; Podhorecka, M.; Polak-Szabela, A.; Kędziora-Kornatowska, K. Is the Montreal Cognitive Assessment (MoCA) test better suited than the Mini-Mental State Examination (MMSE) in mild cognitive impairment (MCI) detection among people aged over 60? Meta-analysis. Psychiatr. Pol. 2016, 50, 1039–1052. [Google Scholar] [CrossRef] [PubMed]
- Pinto, T.C.C.; Machado, L.; Bulgacov, T.M.; Rodrigues-Júnior, A.L.; Costa, M.L.G.; Ximenes, R.C.C.; Sougey, E.B. Is the Montreal Cognitive Assessment (MoCA) screening superior to the Mini-Mental State Examination (MMSE) in the detection of mild cognitive impairment (MCI) and Alzheimer’s Disease (AD) in the elderly? Int. Psychogeriatr. 2019, 31, 491–504. [Google Scholar] [CrossRef]
- Roalf, D.R.; Moberg, P.J.; Xie, S.X.; Wolk, D.A.; Moelter, S.T.; Arnold, S.E. Comparative accuracies of two common screening instruments for the classification of Alzheimer’s disease, mild cognitive impairment and healthy aging. Alzheimers Dement. 2013, 9, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool for Mild Cognitive Impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, F.N.; Stokholm, L.; Lois, N.; Yang, D.; Cheung, C.Y.; Biessels, G.J.; Exalto, L.; Simó, R.; Peto, T.; Pouwer, F.; et al. Structural and Metabolic Retinal Changes Associated with Mild Cognitive Impairment in Type 2 Diabetes. Diabetes 2023, 72, 1853–1863. [Google Scholar] [CrossRef] [PubMed]
- Patton, N.; Aslam, T.; MacGillivray, T.; Pattie, A.; Deary, I.J.; Dhillon, B. Retinal vascular image analysis as a potential screening tool for cerebrovascular disease: A rationale based on homology between cerebral and retinal microvasculatures. J. Anat. 2005, 206, 319–348. [Google Scholar] [CrossRef] [PubMed]
- Risau, W. Mechanisms of angiogenesis. Nature 1997, 386, 671–674. [Google Scholar] [CrossRef]
- Chan-Ling, T.; McLeod, D.S.; Hughes, S.; Baxter, L.; Chu, Y.; Hasegawa, T.; Lutty, G.A. Astrocyte-endothelial cell relationships during human retinal vascular development. Invest. Ophthalmol. Vis. Sci. 2004, 45, 2020–2032. [Google Scholar] [CrossRef]
- Dorrell, M.I.; Aguilar, E.; Friedlander, M. Retinal Vascular Development Is Mediated by Endothelial Filopodia, a Preexisting Astrocytic Template and Specific R-Cadherin Adhesion. Investig. Ophthalmol. Vis. Sci. 2002, 43, 3500–3510. [Google Scholar]
- Hughes, S.; Yang, H.; Chan-Ling, T. Vascularization of the Human Fetal Retina: Roles of Vasculogenesis and Angiogenesis. Investig. Ophthalmol. Vis. Sci. 2000, 41, 1217–1228. [Google Scholar]
- Lu, X.; Gong, W.; Wen, Z.; Hu, L.; Peng, Z.; Zha, Y. Correlation Between Diabetic Cognitive Impairment and Diabetic Retinopathy in Patients with T2DM by 1H-MRS. Front. Neurol. 2019, 10, 1068. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Mei, F.; Hu, K.; Feng, L.; Wang, Z.; Gao, Q.; Chen, F.; Zhao, L.; Li, X.; Ma, B. Diabetic retinopathy and cognitive dysfunction: A systematic review and meta-analysis. Acta Diabetol. 2022, 59, 443–459. [Google Scholar] [CrossRef]
- Gupta, P.; Gan, A.T.L.; Man, R.E.K.; Fenwick, E.K.; Sabanayagam, C.; Mitchell, P.; Cheung, C.Y.; Cheung, N.; Wong, T.Y.; Cheng, C.-Y.; et al. Association between diabetic retinopathy and incident cognitive impairment. Br. J. Ophthalmol. 2019, 103, 1605–1609. [Google Scholar] [CrossRef]
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A.; et al. Optical Coherence Tomography. Science 1991, 254, 1178–1181. [Google Scholar] [CrossRef] [PubMed]
- Leung, C.K.; Cheung, C.Y.; Weinreb, R.N.; Qiu, Q.; Liu, S.; Li, H.; Xu, G.; Fan, N.; Huang, L.; Pang, C.-P.; et al. Retinal Nerve Fiber Layer Imaging with Spectral-Domain Optical Coherence Tomography: A Variability and Diagnostic Performance Study. Ophthalmology 2009, 116, 1257–1263.e2. [Google Scholar] [CrossRef]
- Yang, Q.; Reisman, C.A.; Wang, Z.; Fukuma, Y.; Hangai, M.; Yoshimura, N.; Tomidokoro, A.; Araie, M.; Raza, A.S.; Hood, D.C.; et al. Automated layer segmentation of macular OCT images using dual-scale gradient information. Opt. Express 2010, 18, 21293–21307. [Google Scholar] [CrossRef]
- Santos, C.Y.; Johnson, L.N.; Sinoff, S.E.; Festa, E.K.; Heindel, W.C.; Snyder, P.J. Change in retinal structural anatomy during the preclinical stage of Alzheimer’s disease. Alzheimers Dement. 2018, 10, 196–209. [Google Scholar] [CrossRef] [PubMed]
- Chan, V.T.T.; Sun, Z.; Tang, S.; Chen, L.J.; Wong, A.; Tham, C.C.; Wong, T.Y.; Chen, C.; Ikram, M.K.; Whitson, H.E.; et al. Spectral Domain-Optical Coherence Tomography Measurements in Alzheimer’s Disease: A Systematic Review and Meta-analysis. Ophthalmology 2019, 126, 497–510. [Google Scholar] [CrossRef]
- den Haan, J.; Verbraak, F.D.; Visser, P.J.; Bouwman, F.H. Retinal thickness in Alzheimer’s disease: A systematic review and meta-analysis. Alzheimers Dement. 2017, 6, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, D.; Votruba, M. Can the retina be used to diagnose and plot the progression of Alzheimer’s disease? Acta Ophthalmol. 2017, 95, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Ko, F.; Muthy, Z.A.; Gallacher, J.; Sudlow, C.; Rees, G.; Yang, Q.; Keane, P.A.; Petzold, A.; Khaw, P.T.; Reisman, C.; et al. Association of Retinal Nerve Fiber Layer Thinning with Current and Future Cognitive Decline: A Study Using Optical Coherence Tomography. JAMA Neurol. 2018, 75, 1198–1205. [Google Scholar] [CrossRef]
- Simó, R.; Hernández, C. Novel approaches for treating diabetic retinopathy based on recent pathogenic evidence. Prog. Retin. Eye Res. 2015, 48, 160–180. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, M.B.; Bach, M.; Kondo, M.; Li, S.; Walker, S.; Holopigian, K.; Viswanathan, S.; Robson, A.G. ISCEV standard for clinical multifocal electroretinography (mfERG) (2021 update). Doc. Ophthalmol. 2021, 142, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Ciudin, A.; Hernández, C.; Simó-Servat, O.; Simó, R. The usefulness of the retina for identifying people with type 2 diabetes with prodromal stages of dementia. Neurosci. Biobehav. Rev. 2024, 159, 105592. [Google Scholar] [CrossRef] [PubMed]
- Acton, J.H.; Greenstein, V.C. Fundus-driven perimetry (microperimetry) compared to conventional static automated perimetry: Similarities, differences and clinical applications. Can. J. Ophthalmol. 2013, 48, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Ayton, L.N.; Guymer, R.H.; Luu, C.D. Comparison between multifocal electroretinography and microperimetry in age-related macular degeneration. Invest. Ophthalmol. Vis. Sci. 2014, 55, 6431–6439. [Google Scholar] [CrossRef] [PubMed]
- Ortiz-Zúñiga, Á.M.; Simó-Servat, O.; Rojano-Toimil, A.; Sebastian, J.V.; Castellano-Tejedor, C.; Hernández, C.; Simó, R.; Ciudin, A. The Gaze Fixation Assessed by Microperimetry: A Useful Tool for the Monitoring of the Cognitive Function in Patients with Type 2 Diabetes. J. Pers. Med. 2021, 11, 698. [Google Scholar] [CrossRef]
- Ciudin, A.; Simó-Servat, O.; Hernández, C.; Arcos, G.; Diego, S.; Sanabria, Á.; Sotolongo, Ó.; Hernández, I.; Boada, M.; Simó, R. Retinal Microperimetry: A New Tool for Identifying Patients with Type 2 Diabetes at Risk for Developing Alzheimer Disease. Diabetes 2017, 66, 3098–3104. [Google Scholar] [CrossRef]
- Balasubramanian, S.; Uji, A.; Lei, J.; Velaga, S.; Nittala, M.; Sadda, S. Interdevice comparison of retinal sensitivity assessments in a healthy population: The CenterVue MAIA and the Nidek MP-3 microperimeters. Br. J. Ophthalmol. 2018, 102, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Ciudin, A.; Simó, R. New methods for the diagnosis and monitoring of cognitive function in patients with type 2 diabetes. Front. Endocrinol. 2022, 13, 1024794. [Google Scholar] [CrossRef]
- Exalto, L.G.; Biessels, G.J.; Karter, A.J.; Huang, E.S.; Katon, W.J.; Minkoff, J.R.; Whitmer, R.A. Risk score for prediction of 10 year dementia risk in individuals with type 2 diabetes: A cohort study. Lancet Diabetes Endocrinol. 2013, 1, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Verhagen, C.; Janssen, J.; Exalto, L.G.; van den Berg, E.; Johansen, O.E.; Biessels, G.J. Diabetes-specific dementia risk score (DSDRS) predicts cognitive performance in patients with type 2 diabetes at high cardio-renal risk. J. Diabetes Its Complicat. 2020, 34, 107674. [Google Scholar] [CrossRef] [PubMed]
- Ortiz Zuñiga, A.M.; Simó, R.; Rodriguez-Gómez, O.; Hernández, C.; Rodrigo, A.; Jamilis, L.; Campo, L.; Alegret, M.; Boada, M.; Ciudin, A. Clinical Applicability of the Specific Risk Score of Dementia in Type 2 Diabetes in the Identification of Patients with Early Cognitive Impairment: Results of the MOPEAD Study in Spain. J. Clin. Med. 2020, 9, 2726. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhao, Y.; Chen, Z.; Long, J.; Dai, J.; Huang, X.; Lui, S.; Radua, J.; Vieta, E.; Kemp, G.J.; et al. Meta-analysis of cortical thickness abnormalities in medication-free patients with major depressive disorder. Neuropsychopharmacology 2020, 45, 703–712. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, X.; Zhang, J.; Liu, C.; Yuan, Q.; Yin, X.; Wei, L.; Cui, J.; Tao, R.; Wei, P.; et al. Gray matter volume abnormalities in type 2 diabetes mellitus with and without mild cognitive impairment. Neurosci. Lett. 2014, 562, 1–6. [Google Scholar] [CrossRef]
- Greve, D.N.; Van der Haegen, L.; Cai, Q.; Stufflebeam, S.; Sabuncu, M.R.; Fischl, B.; Bysbaert, M. A Surface-based Analysis of Language Lateralization and Cortical Asymmetry. J. Cogn. Neurosci. 2013, 25, 1477–1492. [Google Scholar] [CrossRef]
- Li, C.; Zuo, Z.; Liu, D.; Jiang, R.; Li, Y.; Li, H.; Yin, X.; Lai, Y.; Wang, J.; Xiong, K. Type 2 Diabetes Mellitus May Exacerbate Gray Matter Atrophy in Patients with Early-Onset Mild Cognitive Impairment. Front. Neurosci. 2020, 14, 856. [Google Scholar] [CrossRef]
- Sanjari Moghaddam, H.; Ghazi Sherbaf, F.; Aarabi, M.H. Brain microstructural abnormalities in type 2 diabetes mellitus: A systematic review of diffusion tensor imaging studies. Front. Neuroendocrinol. 2019, 55, 100782. [Google Scholar] [CrossRef]
- Wardlaw, J.M.; Smith, E.E.; Biessels, G.J.; Cordonnier, C.; Fazekas, F.; Frayne, R.; Lindley, R.I.; O’Brien, J.T.; Barkhof, F.; Benavente, O.R.; et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol. 2013, 12, 822–838. [Google Scholar] [CrossRef]
- Brundel, M.; Kappelle, L.J.; Biessels, G.J. Brain imaging in type 2 diabetes. Eur. Neuropsychopharmacol. 2014, 24, 1967–1981. [Google Scholar] [CrossRef] [PubMed]
- Biessels, G.J.; Nobili, F.; Teunissen, C.E.; Simó, R.; Scheltens, P. Understanding multifactorial brain changes in type 2 diabetes: A biomarker perspective. Lancet Neurol. 2020, 19, 699–710. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.-L.; Rothman, D.L. What have novel imaging techniques revealed about metabolism in the aging brain? Future Neurol. 2014, 9, 341–354. [Google Scholar] [CrossRef]
- Mistur, R.; Mosconi, L.; Santi, S.D.; Guzman, M.; Li, Y.; Tsui, W.; de Leon, M.J. Current Challenges for the Early Detection of Alzheimer’s Disease: Brain Imaging and CSF Studies. J. Clin. Neurol. 2009, 5, 153–166. [Google Scholar] [CrossRef]
- Rooijackers, H.M.M.; Wiegers, E.C.; Tack, C.J.; van der Graaf, M.; de Galan, B.E. Brain glucose metabolism during hypoglycemia in type 1 diabetes: Insights from functional and metabolic neuroimaging studies. Cell Mol. Life Sci. 2015, 73, 705–722. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.L. A review of chemical issues in 1H NMR spectroscopy: N-acetyl-L-aspartate, creatine and choline. NMR Biomed. 1991, 4, 47–52. [Google Scholar] [CrossRef]
- Zhao, X.; Han, Q.; Gang, X.; Wang, G. Altered brain metabolites in patients with diabetes mellitus and related complications—Evidence from 1H MRS study. Biosci. Rep. 2018, 38, BSR20180660. [Google Scholar] [CrossRef]
- Wang, K.; Liang, M.; Wang, L.; Tian, L.; Zhang, X.; Li, K.; Jiang, T. Altered functional connectivity in early Alzheimer’s disease: A resting-state fMRI study. Hum. Brain Mapp. 2006, 28, 967–978. [Google Scholar] [CrossRef]
- Fekete, T.; Wilf, M.; Rubin, D.; Edelman, S.; Malach, R.; Mujica-Parodi, L.R. Combining Classification with fMRI-Derived Complex Network Measures for Potential Neurodiagnostics. PLoS ONE 2013, 8, e62867. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Chen, X.; Shi, F.; Li, G.; Kim, M.; Giannakopoulos, P.; Haller, S.; Shen, D. Topographical Information-Based High-Order Functional Connectivity and Its Application in Abnormality Detection for Mild Cognitive Impairment. J. Alzheimers Dis. 2016, 54, 1095–1112. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, H.; Zhang, L.; Shen, C.; Lee, S.-W.; Shen, D. Extraction of dynamic functional connectivity from brain grey matter and white matter for MCI classification. Hum. Brain Mapp. 2017, 38, 5019. [Google Scholar] [CrossRef] [PubMed]
- Friston, K.J.; Frith, C.D.; Liddle, P.F.; Frackowiak, R.S. Functional connectivity: The principal-component analysis of large (PET) data sets. J. Cereb. Blood Flow. Metab. 1993, 13, 5–14. [Google Scholar] [CrossRef]
- Fox, M.D.; Raichle, M.E. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat. Rev. Neurosci. 2007, 8, 700–711. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Fang, Y.; Tan, X.; Kang, S.; Yue, X.; Rao, Y.; Huang, H.; Liu, M.; Qiu, S.; Yap, P.-T. Detecting type 2 diabetes mellitus cognitive impairment using whole-brain functional connectivity. Sci. Rep. 2023, 13, 3940. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Jiao, Y.; Chen, Y.-C.; Wang, K.; Gao, B.; Wen, S.; Ju, S.; Teng, G.-J. Altered spontaneous brain activity in type 2 diabetes: A resting-state functional MRI study. Diabetes 2014, 63, 749–760. [Google Scholar] [CrossRef]
- Xia, W.; Wang, S.; Sun, Z.; Bai, F.; Zhou, Y.; Yang, Y.; Wang, P.; Huang, Y.; Yuan, Y. Altered baseline brain activity in type 2 diabetes: A resting-state fMRI study. Psychoneuroendocrinology 2013, 38, 2493–2501. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, Y.; Tan, X.; Qin, C.; Chen, Y.; Liang, Y.; Qiu, S.; An, J. Resting-state neural activity and cerebral blood flow alterations in type 2 diabetes mellitus: Insights from hippocampal subfields. Brain Behav. 2024, 14, e3600. [Google Scholar] [CrossRef] [PubMed]
- Wee, C.-Y.; Yang, S.; Yap, P.-T.; Shen, D. Sparse Temporally Dynamic Resting-State Functional Connectivity Networks for Early MCI Identification. Brain Imaging Behav. 2016, 10, 342–356. [Google Scholar] [CrossRef]
- Hutchison, R.M.; Womelsdorf, T.; Allen, E.A.; Bandettini, P.A.; Calhoun, V.D.; Corbetta, M.; Penna, S.D.; Duyn, J.H.; Glover, G.H.; Gonzalez-Castillo, J.; et al. Dynamic functional connectivity: Promise, issues, and interpretations. Neuroimage 2013, 80, 360–378. [Google Scholar] [CrossRef]
- Leonardi, N.; Richiardi, J.; Gschwind, M.; Simioni, S.; Annoni, J.-M.; Schluep, M.; Vuilleumier, P.; Van De Ville, D. Principal components of functional connectivity: A new approach to study dynamic brain connectivity during rest. NeuroImage 2013, 83, 937–950. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Glover, G.H. Time-frequency dynamics of resting-state brain connectivity measured with fMRI. Neuroimage 2010, 50, 81–98. [Google Scholar] [CrossRef]
- Allen, E.A.; Damaraju, E.; Plis, S.M.; Erhardt, E.B.; Eichele, T.; Calhoun, V.D. Tracking Whole-Brain Connectivity Dynamics in the Resting State. Cereb. Cortex 2014, 24, 663–676. [Google Scholar] [CrossRef]
- Lyu, W.; Wu, Y.; Huang, H.; Chen, Y.; Tan, X.; Liang, Y.; Ma, X.; Feng, Y.; Wu, J.; Kang, S.; et al. Aberrant dynamic functional network connectivity in type 2 diabetes mellitus individuals. Cogn. Neurodyn 2023, 17, 1525–1539. [Google Scholar] [CrossRef]
- Öksüz, Ö.; Günver, M.G.; Arıkan, M.K. Quantitative Electroencephalography Findings in Patients with Diabetes Mellitus. Clin. EEG Neurosci. 2022, 53, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.; Wu, Z.; Xia, R.; Li, X.; Liu, J.; Dai, Y.; Wang, D.; Chen, S. Phase Lag Index of Resting-State EEG for Identification of Mild Cognitive Impairment Patients with Type 2 Diabetes. Brain Sci. 2022, 12, 1399. [Google Scholar] [CrossRef]
- Braak, H.; Braak, E. Frequency of Stages of Alzheimer-Related Lesions in Different Age Categories. Neurobiol. Aging 1997, 18, 351–357. [Google Scholar] [CrossRef]
- Tumminia, A.; Vinciguerra, F.; Parisi, M.; Frittitta, L. Type 2 Diabetes Mellitus and Alzheimer’s Disease: Role of Insulin Signalling and Therapeutic Implications. Int. J. Mol. Sci. 2018, 19, 3306. [Google Scholar] [CrossRef]
- Moran, C.; Beare, R.; Phan, T.G.; Bruce, D.G.; Callisaya, M.L.; Srikanth, V. Type 2 diabetes mellitus and biomarkers of neurodegeneration. Neurology 2015, 85, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.-P.; Yang, S.-L.; Zhao, S.; Zheng, C.-H.; Li, H.-H.; Zhang, Y.; Huang, R.-X.; Li, M.-Z.; Gao, Y.; Zhang, S.-J.; et al. Biomarkers for Early Diagnostic of Mild Cognitive Impairment in Type-2 Diabetes Patients: A Multicentre, Retrospective, Nested Case–Control Study. eBioMedicine 2016, 5, 105–113. [Google Scholar] [CrossRef]
- Maffei, A.; Lembo, G.; Carnevale, D. PI3Kinases in Diabetes Mellitus and Its Related Complications. Int. J. Mol. Sci. 2018, 19, 4098. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, N.; Yan, F.; Jin, H.; Zhou, S.; Shi, J.; Jin, F. Diabetes mellitus and Alzheimer’s disease: GSK-3β as a potential link. Behav. Brain Res. 2018, 339, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhao, L. Calycosin ameliorates diabetes-induced cognitive impairments in rats by reducing oxidative stress via the PI3K/Akt/GSK-3β signaling pathway. Biochem. Biophys. Res. Commun. 2016, 473, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.-P.; Gan, G.-S.; Liu, Y.-M.; Xiao, J.-S.; Liu, H.-X.; Mei, B.; Zhang, J.-J. Adiponectin Attenuates Streptozotocin-Induced Tau Hyperphosphorylation and Cognitive Deficits by Rescuing PI3K/Akt/GSK-3β Pathway. Neurochem. Res. 2018, 43, 316–323. [Google Scholar] [CrossRef] [PubMed]
- Strachan, M.W.; Deary, I.J.; Ewing, F.M.; Frier, B.M. Is type II diabetes associated with an increased risk of cognitive dysfunction? A critical review of published studies. Diabetes Care 1997, 20, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Marioni, R.E.; Strachan, M.W.J.; Reynolds, R.M.; Lowe, G.D.O.; Mitchell, R.J.; Fowkes, F.G.R.; Frier, B.M.; Lee, A.J.; Butcher, I.; Rumley, A.; et al. Association Between Raised Inflammatory Markers and Cognitive Decline in Elderly People with Type 2 Diabetes. Diabetes 2010, 59, 710–713. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Zhang, Q.; Zhang, X.; Song, Y.; Zheng, J.; An, Y.; Lu, Y. Correlation between inflammatory biomarkers, cognitive function and glycemic and lipid profiles in patients with type 2 diabetes mellitus: A systematic review and meta-analysis. Clin. Biochem. 2023, 121–122, 110683. [Google Scholar] [CrossRef]
- Groeneveld, O.N.; van den Berg, E.; Johansen, O.E.; Schnaidt, S.; Hermansson, K.; Zinman, B.; Espeland, M.A.; Biessels, G.J. Oxidative stress and endothelial dysfunction are associated with reduced cognition in type 2 diabetes. Diab Vasc. Dis. Res. 2019, 16, 577–581. [Google Scholar] [CrossRef]
- Gorska-Ciebiada, M.; Saryusz-Wolska, M.; Borkowska, A.; Ciebiada, M.; Loba, J. C-Reactive Protein, Advanced Glycation End Products, and Their Receptor in Type 2 Diabetic, Elderly Patients with Mild Cognitive Impairment. Front. Aging Neurosci. 2015, 7, 209. [Google Scholar] [CrossRef] [PubMed]
- Anita, N.Z.; Zebarth, J.; Chan, B.; Wu, C.-Y.; Syed, T.; Shahrul, D.; Nguyen, M.M.; Pakosh, M.; Herrmann, N.; Lanctôt, K.L.; et al. Inflammatory markers in type 2 diabetes with vs. without cognitive impairment; a systematic review and meta-analysis. Brain Behav. Immun. 2022, 100, 55–69. [Google Scholar] [CrossRef]
- Wang, P.; Huang, R.; Lu, S.; Xia, W.; Cai, R.; Sun, H.; Wang, S. RAGE and AGEs in Mild Cognitive Impairment of Diabetic Patients: A Cross-Sectional Study. PLoS ONE 2016, 11, e0145521. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Wang, Z.-Y.; Ma, L.-N.; Zhang, T.-T.; Cao, Y.; Li, H. MicroRNAs in Alzheimer’s Disease: Function and Potential Applications as Diagnostic Biomarkers. Front. Mol. Neurosci. 2020, 13, 160. [Google Scholar] [CrossRef]
- Zhao, Y.; Jaber, V.; Alexandrov, P.N.; Vergallo, A.; Lista, S.; Hampel, H.; Lukiw, W.J. microRNA-Based Biomarkers in Alzheimer’s Disease (AD). Front. Neurosci. 2020, 14, 585432. [Google Scholar] [CrossRef] [PubMed]
- Salama, I.I.; Sami, S.M.; Abdellatif, G.A.; Mohsen, A.; Rasmy, H.; Kamel, S.A.; Ibrahim, M.H.; Mostafa, M.; Fouad, W.A.; Raslan, H.M. Plasma microRNAs biomarkers in mild cognitive impairment among patients with type 2 diabetes mellitus. PLoS ONE 2020, 15, e0236453. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, Z.; Chen, D.; Zhang, Z.; Lin, X.; Shen, Z.; Lin, Q.; Fan, K.; Wang, Q.; Zhang, W.; et al. SIRT1 and miR-34a-5p Expression in PBMCs as Potential Biomarkers for Patients with Type 2 Diabetes with Cognitive Impairments. J. Clin. Endocrinol. Metab. 2024, 109, 815–826. [Google Scholar] [CrossRef] [PubMed]
- Krabbe, K.S.; Nielsen, A.R.; Krogh-Madsen, R.; Plomgaard, P.; Rasmussen, P.; Erikstrup, C.; Fischer, C.P.; Lindegaard, B.; Petersen, A.M.W.; Taudorf, S.; et al. Brain-derived neurotrophic factor (BDNF) and type 2 diabetes. Diabetologia 2007, 50, 431–438. [Google Scholar] [CrossRef]
- Zhen, Y.F.; Zhang, J.; Liu, X.Y.; Fang, H.; Tian, L.B.; Zhou, D.H.; Kosten, T.R.; Zhang, X.Y. Low BDNF is associated with cognitive deficits in patients with type 2 diabetes. Psychopharmacology 2013, 227, 93–100. [Google Scholar] [CrossRef] [PubMed]
| Advantage | Deficiency | Application Situation | |
|---|---|---|---|
| MMSE | Fast administration; wide international translation and use; high acceptance among professionals and non-professionals | The recognition sensitivity of patients with MCI was low; influenced by age, education, race and other factors | Moderate-to-severe cognitive impairment |
| MoCA | More effective than MMSE in identifying MCI | Scores are influenced by education and age | MCI; dementia |
| Content | Main Indicators | Application | Limitations | |
|---|---|---|---|---|
| Optical coherence tomography (OCT) | Measure the thickness of the retinal nerve fiber layer (RNFL) and other retinal layers | RNFL thickness; retinal layer thickness | Used for early detection of retinal diseases, RNFL thinning is associated with cognitive decline and may serve as a screening tool for cognitive decline | Lack of universally accepted cutoffs; potential variability; focus on structural changes |
| Multifocal electroretinogram (mfERG) | Record the electrical activity of multiple retinal areas to assess retinal function | Retinal electrophysiological responses; local retinal function | Used to detect retinal neurodegenerative changes in diabetic retinopathy, which may be associated with the occurrence of diabetes-related cognitive impairment | Complex; requires specialized personnel; time-consuming |
| Microperimetry (MP) | Assess retinal sensitivity (photoreception) and fixation | Retinal sensitivity; ocular fixation | Can be used to assess retinal function, with decreased retinal sensitivity potentially associated with cognitive decline, showing potential as a screening tool for cognitive impairment | Device availability is limited in many healthcare settings; no studies on psychological/mood impact on the test; advanced DR may limit tool effectiveness |
| Content of Assessment | Detection Method | Advantages | Limitations | |
|---|---|---|---|---|
| Brain structural imaging | Changes in gray matter (GM) and white matter (WM) | VBM; SBM; DTI | Non-invasive; quantitative, automated analysis | Highly affected by motion artifacts, unable to provide functional information |
| Brain metabolic imaging | Changes in glucose metabolism and brain metabolites (such as NAA, Cr, and Cho) | PET;1H MRS | Non-invasive; capable of detecting metabolic changes at an early stage | Expensive equipment; time-consuming |
| Brain functional imaging | Functional connectivity (FC); spatiotemporal dynamics of brain networks | fMRI; EEG | Non-invasive; dynamic assessment of brain function | Requires complex calculations |
| Main Biomarkers | Relation to Diabetes | Impact On Cognitive Function | Application Potential | |
|---|---|---|---|---|
| Neurodegenerative biomarkers | Aβ; phosphorylated Tau protein; GSK3; PI3K | Tau and Aβ accumulation, and dysfunction of PI3K and GSK-3β are related to diabetes | Abnormal levels of Tau, Aβ, PI3K, and GSK-3β are associated with cognitive decline | These biomarkers are significantly associated with diabetes-related cognitive impairment, including MCI and Alzheimer’s disease |
| Inflammatory markers | CRP; IL-6; TNF-α | High inflammation levels are related to insulin resistance and glucose metabolism disorders in diabetes | Elevated inflammatory markers (such as CRP, IL-6, TNF-α) are associated with cognitive decline | CRP, IL-6, and TNF-α may serve as biomarkers for diabetes-related cognitive impairment, but research findings are inconsistent |
| Advanced glycation end products | AGEs; RAGE | AGEs and RAGE levels are elevated in diabetic patients, especially under poor blood sugar control | High levels of AGEs are associated with cognitive dysfunction | AGEs and RAGE may serve as biomarkers for diabetes-related cognitive impairment, but further research is needed to confirm their potential |
| Other biomarkers | miRNA; BDNF; adipokines; islet amyloid peptide; glycosylated hemoglobin |
| Content | Main Indicators/Biomarkers | Application | |
|---|---|---|---|
| Retinal Structure and Function Examination | OCT | RNFL thickness; retinal layer thickness | Used for the early detection of retinal diseases, RNFL thinning is associated with cognitive decline and may serve as a screening tool for cognitive decline |
| mfERG | Retinal electrophysiological responses; local retinal function | Used to detect retinal neurodegenerative changes in diabetic retinopathy, which may be associated with the occurrence of diabetes-related cognitive impairment | |
| MP | Retinal sensitivity; ocular fixation | Can be used to assess retinal function, with decreased retinal sensitivity potentially associated with cognitive decline, showing potential as a screening tool for cognitive impairment | |
| Diabetes-Specific Dementia Risk Score | DSDRS | Score | Estimates a person’s overall risk of developing dementia within the next decade based on diabetes-related co-morbidities and complications, age, and education level |
| Brain Imaging | Brain Structural Changes | Changes in gray matter (GM) and white matter (WM) | Enabling the early detection of brain structural abnormalities in T2DM patients and serving as a structural basis for screening diabetes-related cognitive impairment |
| Brain metabolic imaging | Changes in glucose metabolism and brain metabolites (such as NAA, Cr, and Cho) | Providing metabolic biomarkers for the early screening of cognitive decline | |
| Brain functional imaging | Functional connectivity (FC); spatiotemporal dynamics of brain networks | Revealing abnormal brain network activities in T2DM patients and offering functional biomarkers for screening cognitive dysfunction | |
| Peripheral Blood Biomarkers | Neurodegenerative biomarkers | Aβ; phosphorylated Tau protein; GSK3; PI3K | These biomarkers are significantly associated with diabetes-related cognitive impairment, including MCI and Alzheimer’s disease |
| Inflammatory markers | CRP; IL-6; TNF-α | CRP, IL-6, and TNF-α may serve as biomarkers for diabetes-related cognitive impairment, but research findings are inconsistent | |
| Advanced glycation end products | AGEs; RAGE | AGEs and RAGE may serve as biomarkers for diabetes-related cognitive impairment, but further research is needed to confirm their potential | |
| Other biomarkers | miRNA; BDNF; adipokines; islet amyloid peptide; glycosylated hemoglobin |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, X.; Zhang, Y.; Xu, J.; Yin, J.; Li, S.; Dong, K.; Shi, X.; Xu, W.; Ma, D.; Chen, X.; et al. A Narrative Review on Cognitive Impairment in Type 2 Diabetes: Global Trends and Diagnostic Approaches. Biomedicines 2025, 13, 473. https://doi.org/10.3390/biomedicines13020473
Liao X, Zhang Y, Xu J, Yin J, Li S, Dong K, Shi X, Xu W, Ma D, Chen X, et al. A Narrative Review on Cognitive Impairment in Type 2 Diabetes: Global Trends and Diagnostic Approaches. Biomedicines. 2025; 13(2):473. https://doi.org/10.3390/biomedicines13020473
Chicago/Turabian StyleLiao, Xiaobin, Yibin Zhang, Jialu Xu, Jiaxin Yin, Shan Li, Kun Dong, Xiaoli Shi, Weijie Xu, Delin Ma, Xi Chen, and et al. 2025. "A Narrative Review on Cognitive Impairment in Type 2 Diabetes: Global Trends and Diagnostic Approaches" Biomedicines 13, no. 2: 473. https://doi.org/10.3390/biomedicines13020473
APA StyleLiao, X., Zhang, Y., Xu, J., Yin, J., Li, S., Dong, K., Shi, X., Xu, W., Ma, D., Chen, X., Yu, X., & Yang, Y. (2025). A Narrative Review on Cognitive Impairment in Type 2 Diabetes: Global Trends and Diagnostic Approaches. Biomedicines, 13(2), 473. https://doi.org/10.3390/biomedicines13020473







