ADAMTS4 Reduction Contributes to Extracellular Matrix Deposition and Impaired Myogenesis in the Skeletal Muscle of Cigarette Smoke-Exposed Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Model
2.2. Cell Culture
2.3. Histology Analysis
2.4. Immunochemistry
2.5. Preparation of CSE
2.6. Small Interfering RNA Transfection
2.7. Polymerase Chain Reaction (PCR)
2.8. Immunoblotting
2.9. Immunofluorescence
2.10. Statistical Analysis
3. Results
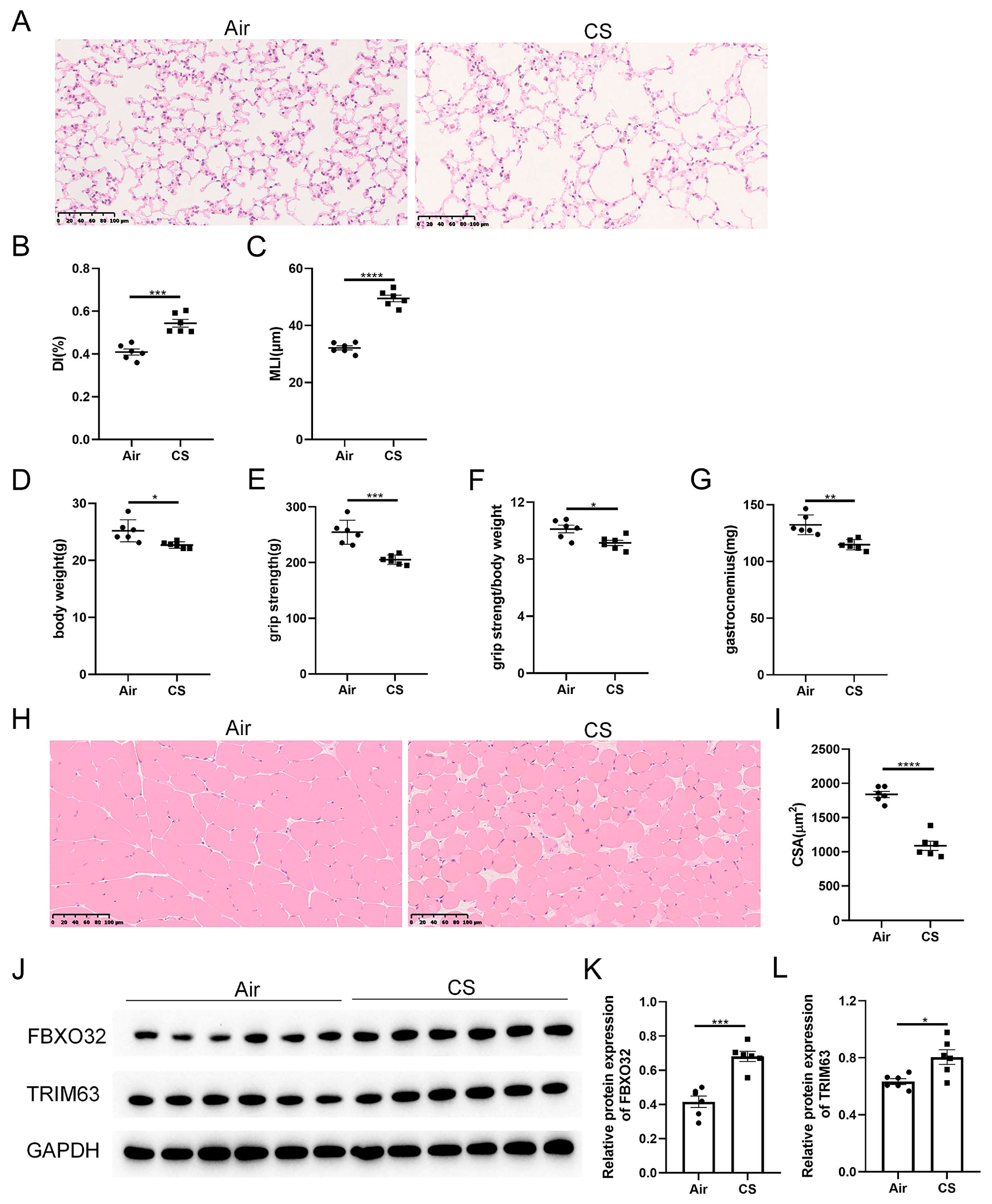
3.1. Chronic CS Exposure Induced the Development of Sarcopenia in C57BL/6J Mice
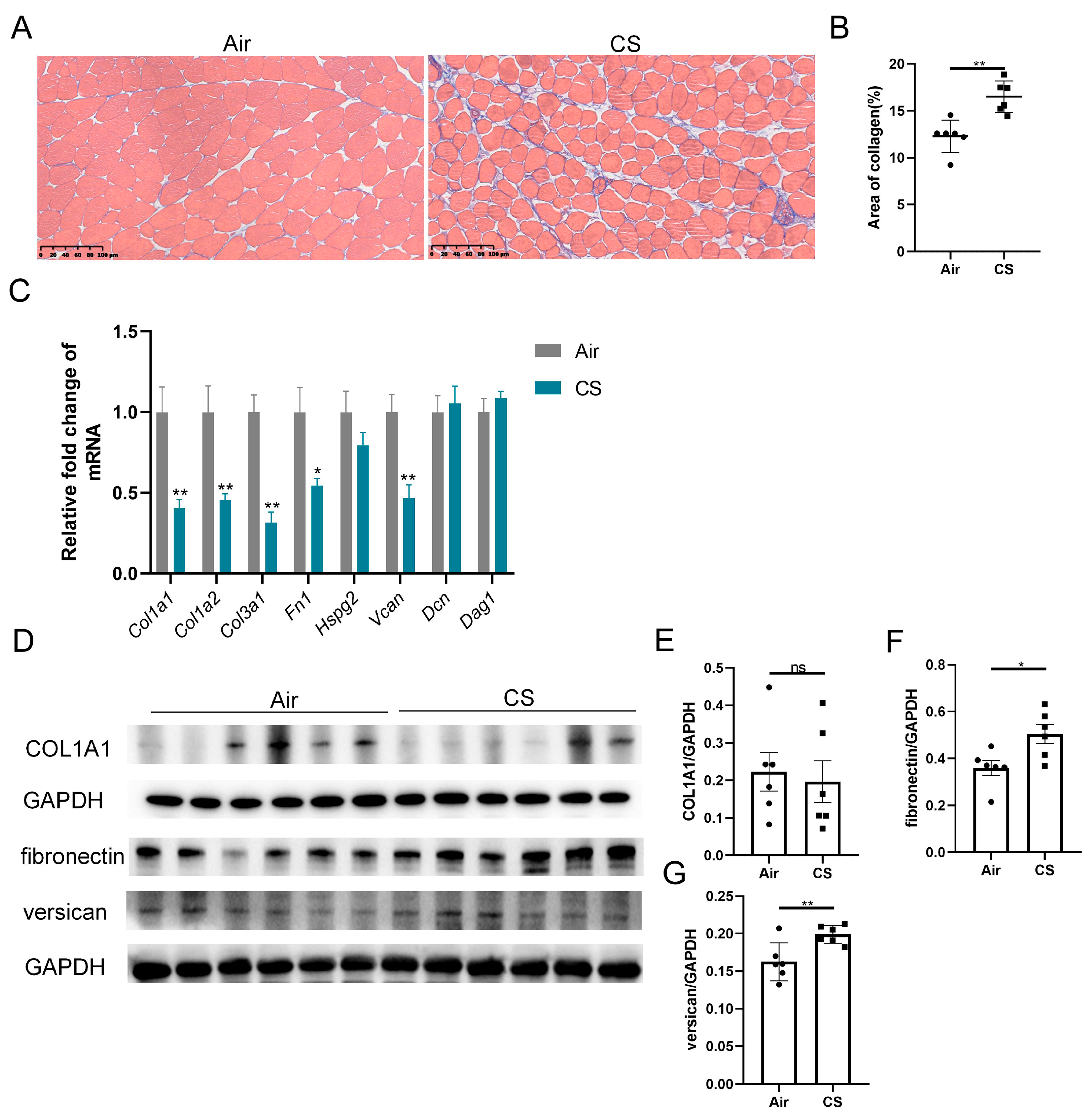
3.2. Chronic CS Exposure Induced Skeletal Muscle Extracellular Matrix Deposition in C57BL/6J Mice
3.3. The Expression of ADAMTS4 Was Decreased in the Gastrocnemius Muscle of CS-Exposed Mice
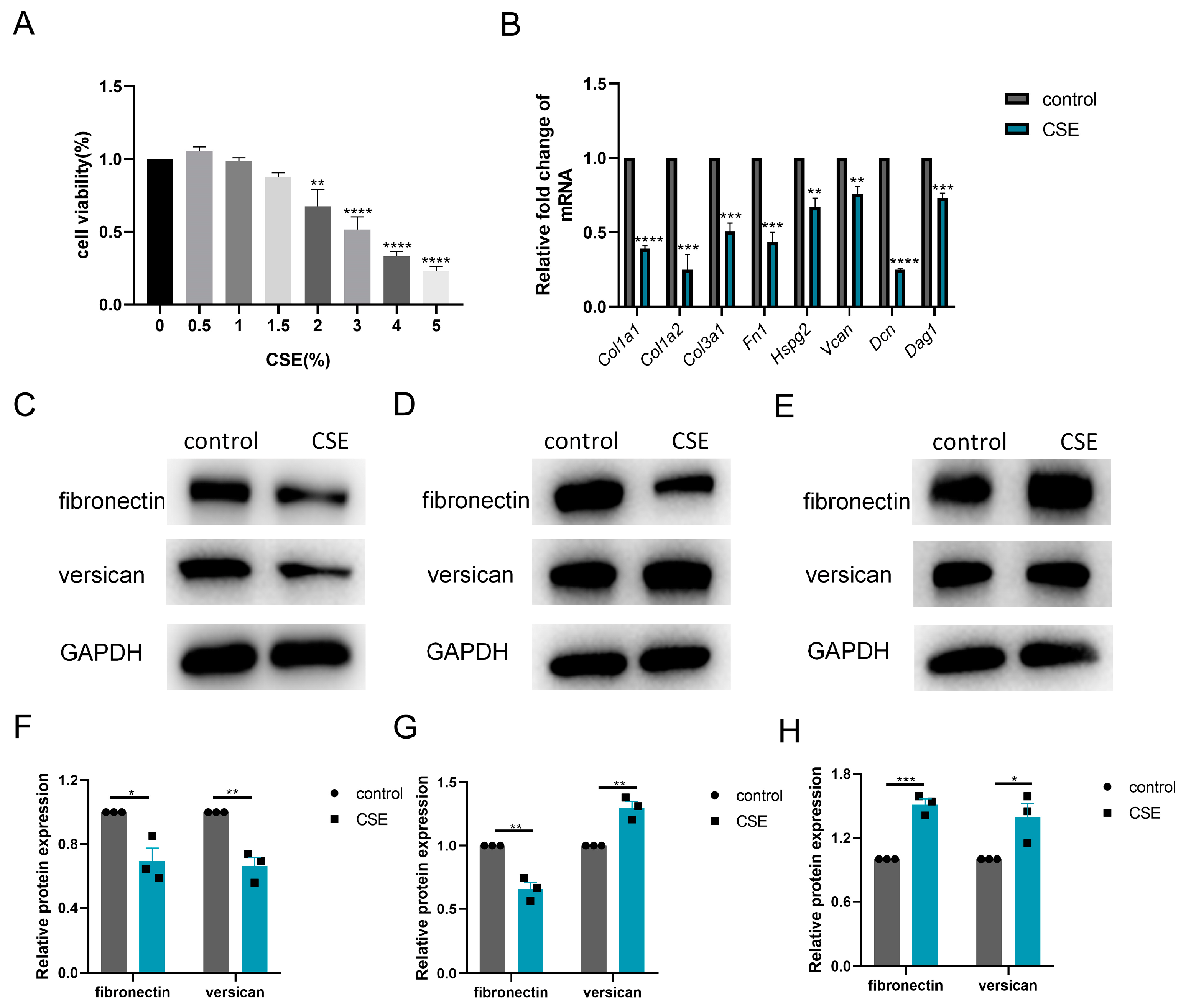
3.4. CSE Induced ADAMTS4 Reduction and Extracellular Matrix Deposition in C2C12 Cells
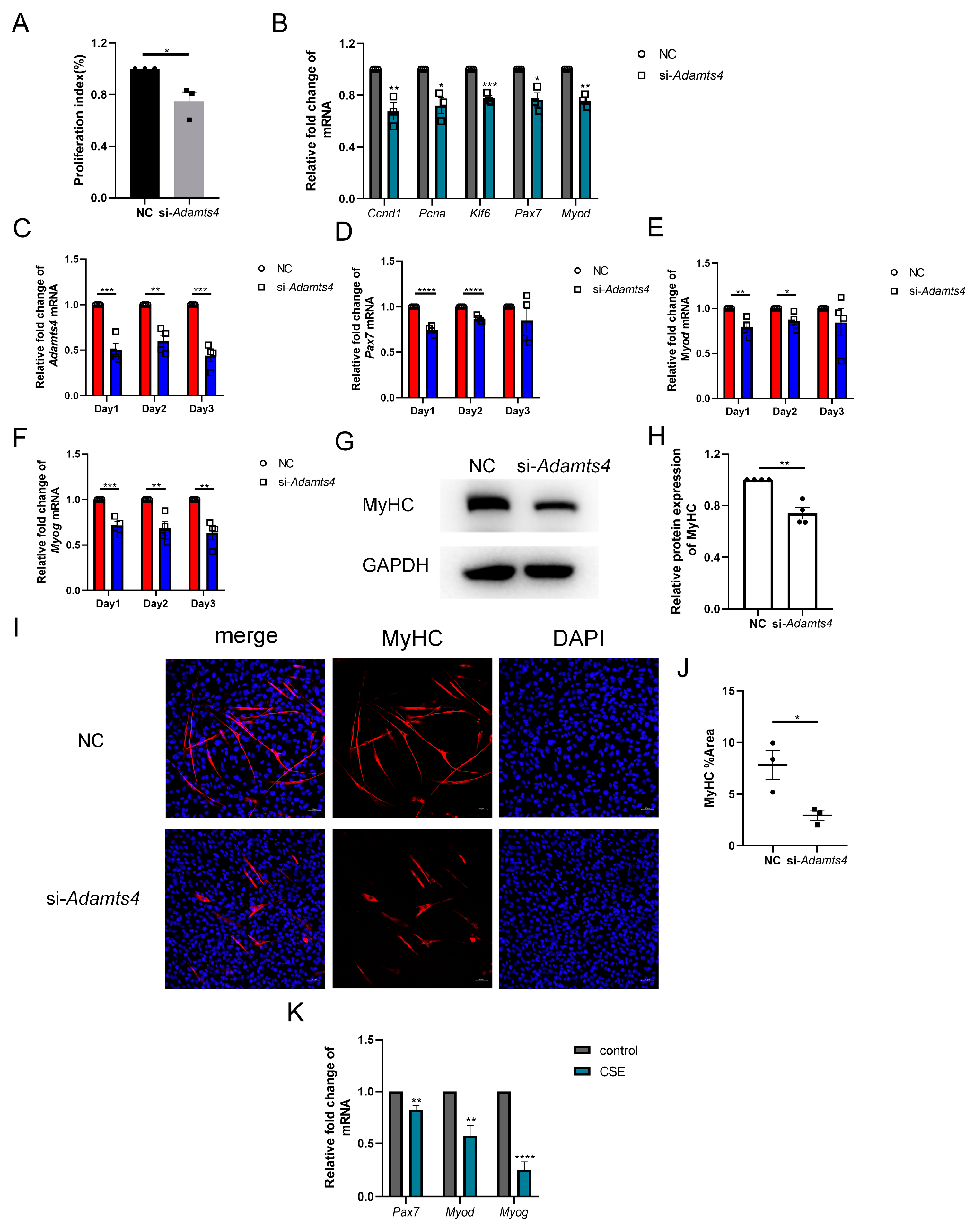
3.5. Knockdown of ADAMTS4 Interfered with Fibronectin and Versican Secretion in C2C12 Cells
3.6. ADAMTS4 Participated in the Activation, Proliferation and Differentiation of C2C12 Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Christenson, S.A.; Smith, B.M.; Bafadhel, M.; Putcha, N. Chronic obstructive pulmonary disease. Lancet 2022, 399, 2227–2242. [Google Scholar] [CrossRef]
- Fabbri, L.M.; Celli, B.R.; Agustí, A.; Criner, G.J.; Dransfield, M.T.; Divo, M.; Krishnan, J.K.; Lahousse, L.; Montes de Oca, M.; Salvi, S.S.; et al. COPD and multimorbidity: Recognising and addressing a syndemic occurrence. Lancet Respir. Med. 2023, 11, 1020–1034. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Sepúlveda-Loyola, W.; Osadnik, C.; Phu, S.; Morita, A.A.; Duque, G.; Probst, V.S. Diagnosis, prevalence, and clinical impact of sarcopenia in COPD: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2020, 11, 1164–1176. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Wu, Y.; Yang, X.; Zhu, J.; Feng, M.; Deng, R.; Yang, C.; Sun, C. Association between muscular atrophy and mortality risk in patients with COPD: A systematic review. Ther. Adv. Respir. Dis. 2024, 18, 17534666241304626. [Google Scholar] [CrossRef]
- Gao, Q.; Hu, K.; Yan, C.; Zhao, B.; Mei, F.; Chen, F.; Zhao, L.; Shang, Y.; Ma, Y.; Ma, B. Associated Factors of Sarcopenia in Community-Dwelling Older Adults: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 4291. [Google Scholar] [CrossRef] [PubMed]
- Taivassalo, T.; Hepple, R.T. Integrating Mechanisms of Exacerbated Atrophy and Other Adverse Skeletal Muscle Impact in COPD. Front. Physiol. 2022, 13, 861617. [Google Scholar] [CrossRef] [PubMed]
- Henrot, P.; Dupin, I.; Schilfarth, P.; Esteves, P.; Blervaque, L.; Zysman, M.; Gouzi, F.; Hayot, M.; Pomiès, P.; Berger, P. Main Pathogenic Mechanisms and Recent Advances in COPD Peripheral Skeletal Muscle Wasting. Int. J. Mol. Sci. 2023, 24, 6454. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, Y.; Zhang, H. Extracellular matrix: An important regulator of cell functions and skeletal muscle development. Cell Biosci. 2021, 11, 65. [Google Scholar] [CrossRef]
- Kjaer, M. Role of extracellular matrix in adaptation of tendon and skeletal muscle to mechanical loading. Physiol. Rev. 2004, 84, 649–698. [Google Scholar] [CrossRef] [PubMed]
- Gosker, H.R.; Kubat, B.; Schaart, G.; van der Vusse, G.J.; Wouters, E.F.; Schols, A.M. Myopathological features in skeletal muscle of patients with chronic obstructive pulmonary disease. Eur. Respir. J. 2003, 22, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Kritikaki, E.; Terzis, G.; Soundararajan, M.; Vogiatzis, I.; Simoes, D.C.M. Expression of intramuscular extracellular matrix proteins in vastus lateralis muscle fibres between atrophic and non-atrophic COPD. ERJ Open Res. 2024, 10, 00857-2023. [Google Scholar] [CrossRef]
- Willis-Owen, S.A.G.; Thompson, A.; Kemp, P.R.; Polkey, M.I.; Cookson, W.; Moffatt, M.F.; Natanek, S.A. COPD is accompanied by co-ordinated transcriptional perturbation in the quadriceps affecting the mitochondria and extracellular matrix. Sci. Rep. 2018, 8, 12165. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, K.; Shaikh, S.; Chun, H.J.; Ali, S.; Lim, J.H.; Ahmad, S.S.; Lee, E.J.; Choi, I. Extracellular matrix: The critical contributor to skeletal muscle regeneration-a comprehensive review. Inflamm. Regen. 2023, 43, 58. [Google Scholar] [CrossRef]
- Schüler, S.C.; Kirkpatrick, J.M.; Schmidt, M.; Santinha, D.; Koch, P.; Di Sanzo, S.; Cirri, E.; Hemberg, M.; Ori, A.; von Maltzahn, J. Extensive remodeling of the extracellular matrix during aging contributes to age-dependent impairments of muscle stem cell functionality. Cell Rep. 2021, 35, 109223. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-J.; Lin, I.H.; Lee, C.-W.; Chen, Y.-F. Aged Skeletal Muscle Retains the Ability to Remodel Extracellular Matrix for Degradation of Collagen Deposition after Muscle Injury. Int. J. Mol. Sci. 2021, 22, 2123. [Google Scholar] [CrossRef] [PubMed]
- Brack, A.S.; Conboy, M.J.; Roy, S.; Lee, M.; Kuo, C.J.; Keller, C.; Rando, T.A. Increased Wnt signaling during aging alters muscle stem cell fate and increases fibrosis. Science 2007, 317, 807–810. [Google Scholar] [CrossRef] [PubMed]
- Stearns-Reider, K.M.; D’Amore, A.; Beezhold, K.; Rothrauff, B.; Cavalli, L.; Wagner, W.R.; Vorp, D.A.; Tsamis, A.; Shinde, S.; Zhang, C.; et al. Aging of the skeletal muscle extracellular matrix drives a stem cell fibrogenic conversion. Aging Cell 2017, 16, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Kumar, L.; Bisen, M.; Khan, A.; Kumar, P.; Patel, S.K.S. Role of Matrix Metalloproteinases in Musculoskeletal Diseases. Biomedicines 2022, 10, 2477. [Google Scholar] [CrossRef] [PubMed]
- Mead, T.J.; Apte, S.S. ADAMTS proteins in human disorders. Matrix Biol. 2018, 71–72, 225–239. [Google Scholar] [CrossRef]
- Du, H.; Shih, C.H.; Wosczyna, M.N.; Mueller, A.A.; Cho, J.; Aggarwal, A.; Rando, T.A.; Feldman, B.J. Macrophage-released ADAMTS1 promotes muscle stem cell activation. Nat. Commun. 2017, 8, 669. [Google Scholar] [CrossRef] [PubMed]
- Stupka, N.; Kintakas, C.; White, J.D.; Fraser, F.W.; Hanciu, M.; Aramaki-Hattori, N.; Martin, S.; Coles, C.; Collier, F.; Ward, A.C.; et al. Versican processing by a disintegrin-like and metalloproteinase domain with thrombospondin-1 repeats proteinases-5 and -15 facilitates myoblast fusion. J. Biol. Chem. 2013, 288, 1907–1917. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Le, Y.; Rao, Y.; Zhou, L.; Hu, Y.; Guo, S.; Sun, Y. RANKL Mediates Muscle Atrophy and Dysfunction in a Cigarette Smoke-induced Model of Chronic Obstructive Pulmonary Disease. Am. J. Respir. Cell Mol. Biol. 2021, 64, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, T.; Ma, Q.; Zhou, L.; Le, Y.; Rao, Y.; Jin, L.; Pei, Y.; Cheng, Y.; Huang, C.; et al. IL-17A Promotes Epithelial ADAM9 Expression in Cigarette Smoke-Related COPD. Int. J. Chronic Obs. Pulmon Dis. 2022, 17, 2589–2602. [Google Scholar] [CrossRef] [PubMed]
- Vistnes, M.; Erusappan, P.M.; Sasi, A.; Nordén, E.S.; Bergo, K.K.; Romaine, A.; Lunde, I.G.; Zhang, L.; Olsen, M.B.; Øgaard, J.; et al. Inhibition of the extracellular enzyme A disintegrin and metalloprotease with thrombospondin motif 4 prevents cardiac fibrosis and dysfunction. Cardiovasc. Res. 2023, 119, 1915–1927. [Google Scholar] [CrossRef] [PubMed]
- Ren, P.; Hughes, M.; Krishnamoorthy, S.; Zou, S.; Zhang, L.; Wu, D.; Zhang, C.; Curci, J.A.; Coselli, J.S.; Milewicz, D.M.; et al. Critical Role of ADAMTS-4 in the Development of Sporadic Aortic Aneurysm and Dissection in Mice. Sci. Rep. 2017, 7, 12351. [Google Scholar] [CrossRef] [PubMed]
- Thériault, M.E.; Paré, M.; Lemire, B.B.; Maltais, F.; Debigaré, R. Regenerative defect in vastus lateralis muscle of patients with chronic obstructive pulmonary disease. Respir. Res. 2014, 15, 35. [Google Scholar] [CrossRef]
- Pomiès, P.; Rodriguez, J.; Blaquière, M.; Sedraoui, S.; Gouzi, F.; Carnac, G.; Laoudj-Chenivesse, D.; Mercier, J.; Préfaut, C.; Hayot, M. Reduced myotube diameter, atrophic signalling and elevated oxidative stress in cultured satellite cells from COPD patients. J. Cell Mol. Med. 2015, 19, 175–186. [Google Scholar] [CrossRef]
- Balnis, J.; Drake, L.A.; Singer, D.V.; Vincent, C.E.; Korponay, T.C.; D’Armiento, J.; Lee, C.G.; Elias, J.A.; Singer, H.A.; Jaitovich, A. Deaccelerated Myogenesis and Autophagy in Genetically Induced Pulmonary Emphysema. Am. J. Respir. Cell Mol. Biol. 2022, 66, 623–637. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Li, C.; Chen, X.; Deng, Z.; Xie, T.; Huo, Z.; Wei, X.; Huang, Y.; Zeng, X.; Luo, Y.; et al. HDAC9 inhibition reduces skeletal muscle atrophy and enhances regeneration in mice with cigarette smoke-induced COPD. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 167023. [Google Scholar] [CrossRef] [PubMed]
- Li, C.W.; Yu, K.; Shyh-Chang, N.; Jiang, Z.; Liu, T.; Ma, S.; Luo, L.; Guang, L.; Liang, K.; Ma, W.; et al. Pathogenesis of sarcopenia and the relationship with fat mass: Descriptive review. J. Cachexia Sarcopenia Muscle 2022, 13, 781–794. [Google Scholar] [CrossRef]
- Farup, J.; Just, J.; de Paoli, F.; Lin, L.; Jensen, J.B.; Billeskov, T.; Roman, I.S.; Cömert, C.; Møller, A.B.; Madaro, L.; et al. Human skeletal muscle CD90(+) fibro-adipogenic progenitors are associated with muscle degeneration in type 2 diabetic patients. Cell Metab. 2021, 33, 2201–2214.e11. [Google Scholar] [CrossRef] [PubMed]
- Lukjanenko, L.; Jung, M.J.; Hegde, N.; Perruisseau-Carrier, C.; Migliavacca, E.; Rozo, M.; Karaz, S.; Jacot, G.; Schmidt, M.; Li, L.; et al. Loss of fibronectin from the aged stem cell niche affects the regenerative capacity of skeletal muscle in mice. Nat. Med. 2016, 22, 897–905. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Zhang, M.; Li, J.; Zhou, L.; Tamm, M.; Roth, M. Airway Smooth Muscle Cell Mitochondria Damage and Mitophagy in COPD via ERK1/2 MAPK. Int. J. Mol. Sci. 2022, 23, 13987. [Google Scholar] [CrossRef] [PubMed]
- Chijiiwa, M.; Mochizuki, S.; Kimura, T.; Abe, H.; Tanaka, Y.; Fujii, Y.; Shimizu, H.; Enomoto, H.; Toyama, Y.; Okada, Y. CCN1 (Cyr61) Is Overexpressed in Human Osteoarthritic Cartilage and Inhibits ADAMTS-4 (Aggrecanase 1) Activity. Arthritis Rheumatol. 2015, 67, 1557–1567. [Google Scholar] [CrossRef] [PubMed]
- de Groot, R.; Folgado, P.B.; Yamamoto, K.; Martin, D.R.; Koch, C.D.; Debruin, D.; Blagg, S.; Minns, A.F.; Bhutada, S.; Ahnström, J.; et al. Cleavage of Cartilage Oligomeric Matrix Protein (COMP) by ADAMTS4 generates a neoepitope associated with osteoarthritis and other forms of degenerative joint disease. Matrix Biol. 2025, 135, 106–124. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Chen, M.; Li, Y.; Wong, F.H.; Thiam, C.W.; Hossain, M.Z.; Poh, K.K.; Hirohata, S.; Ogawa, H.; Angeli, V.; et al. Loss of ADAMTS4 reduces high fat diet-induced atherosclerosis and enhances plaque stability in ApoE(-/-) mice. Sci. Rep. 2016, 6, 31130. [Google Scholar] [CrossRef]
- Mangarova, D.B.; Kaufmann, J.O.; Brangsch, J.; Kader, A.; Möckel, J.; Heyl, J.L.; Verlemann, C.; Adams, L.C.; Ludwig, A.; Reimann, C.; et al. ADAMTS4-Specific MR Peptide Probe for the Assessment of Atherosclerotic Plaque Burden in a Mouse Model. Investig. Radiol. 2025. [Google Scholar] [CrossRef] [PubMed]
- Li, M.D.; Lu, J.W.; Zhang, F.; Lei, W.J.; Pan, F.; Lin, Y.K.; Ling, L.J.; Myatt, L.; Wang, W.S.; Sun, K. ADAMTS4 is a crucial proteolytic enzyme for versican cleavage in the amnion at parturition. Commun. Biol. 2024, 7, 301. [Google Scholar] [CrossRef] [PubMed]
- Bentzinger, C.F.; Wang, Y.X.; von Maltzahn, J.; Soleimani, V.D.; Yin, H.; Rudnicki, M.A. Fibronectin regulates Wnt7a signaling and satellite cell expansion. Cell Stem Cell 2013, 12, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Vaz, R.; Martins, G.G.; Thorsteinsdóttir, S.; Rodrigues, G. Fibronectin promotes migration, alignment and fusion in an in vitro myoblast cell model. Cell Tissue Res. 2012, 348, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Podleski, T.R.; Greenberg, I.; Schlessinger, J.; Yamada, K.M. Fibronectin delays the fusion of L6 myoblasts. Exp. Cell Res. 1979, 122, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Li, Y.; Li, Y.; Yin, Q.; Li, H.; Li, J.; Zhou, B.; Meng, J.; Lian, H.; Wu, M.; et al. Versican Promotes Cardiomyocyte Proliferation and Cardiac Repair. Circulation 2023, 149, 1004–1015. [Google Scholar] [CrossRef]
- McRae, N.; Forgan, L.; McNeill, B.; Addinsall, A.; McCulloch, D.; Van der Poel, C.; Stupka, N. Glucocorticoids Improve Myogenic Differentiation In Vitro by Suppressing the Synthesis of Versican, a Transitional Matrix Protein Overexpressed in Dystrophic Skeletal Muscles. Int. J. Mol. Sci. 2017, 18, 2629. [Google Scholar] [CrossRef] [PubMed]
- Velleman, S.G.; Sporer, K.R.; Ernst, C.W.; Reed, K.M.; Strasburg, G.M. Versican, matrix Gla protein, and death-associated protein expression affect muscle satellite cell proliferation and differentiation. Poult. Sci. 2012, 91, 1964–1973. [Google Scholar] [CrossRef]
- Zhang, X.H.; Qi, Y.X.; Gao, X.; Li, J.Y.; Xu, S.Z. Expression of ADAMTS4 and ADAMTS5 in longissimus dorsi muscle related to meat tenderness in Nanyang cattle. Genet. Mol. Res. 2013, 12, 4639–4647. [Google Scholar] [CrossRef]
- Nadeau, G.; Ouimet-Grennan, E.; Aaron, M.; Drouin, S.; Bertout, L.; Shalmiev, A.; Beaulieu, P.; St-Onge, P.; Veilleux, L.N.; Rauch, F.; et al. Identification of genetic variants associated with skeletal muscle function deficit in childhood acute lymphoblastic leukemia survivors. Pharmgenomics Pers. Med. 2019, 12, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Taye, N.; Singh, M.; Baldock, C.; Hubmacher, D. Secreted ADAMTS-like 2 promotes myoblast differentiation by potentiating WNT signaling. Matrix Biol. 2023, 120, 24–42. [Google Scholar] [CrossRef] [PubMed]
- Taye, N.; Rodriguez, L.; Iatridis, J.C.; Han, W.M.; Hubmacher, D. Myoblast-derived ADAMTS-like 2 promotes skeletal muscle regeneration after injury. NPJ Regen. Med. 2024, 9, 39. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, H.; Kusumoto, D.; Hashimoto, H.; Yuasa, S. Stem Cell Aging in Skeletal Muscle Regeneration and Disease. Int. J. Mol. Sci. 2020, 21, 1830. [Google Scholar] [CrossRef] [PubMed]
- Menon, M.K.; Houchen, L.; Singh, S.J.; Morgan, M.D.; Bradding, P.; Steiner, M.C. Inflammatory and satellite cells in the quadriceps of patients with COPD and response to resistance training. Chest 2012, 142, 1134–1142. [Google Scholar] [CrossRef] [PubMed]
- Thériault, M.E.; Paré, M.; Maltais, F.; Debigaré, R. Satellite cells senescence in limb muscle of severe patients with COPD. PLoS ONE 2012, 7, e39124. [Google Scholar] [CrossRef] [PubMed]
- Sancho-Muñoz, A.; Guitart, M.; Rodríguez, D.A.; Gea, J.; Martínez-Llorens, J.; Barreiro, E. Deficient muscle regeneration potential in sarcopenic COPD patients: Role of satellite cells. J. Cell. Physiol. 2021, 236, 3083–3098. [Google Scholar] [CrossRef] [PubMed]
- Kneppers, A.E.M.; Langen, R.C.J.; Gosker, H.R.; Verdijk, L.B.; Cebron Lipovec, N.; Leermakers, P.A.; Kelders, M.; de Theije, C.C.; Omersa, D.; Lainscak, M.; et al. Increased Myogenic and Protein Turnover Signaling in Skeletal Muscle of Chronic Obstructive Pulmonary Disease Patients with Sarcopenia. J. Am. Med. Dir. Assoc. 2017, 18, 637.e1–637.e11. [Google Scholar] [CrossRef]
- Plant, P.J.; Brooks, D.; Faughnan, M.; Bayley, T.; Bain, J.; Singer, L.; Correa, J.; Pearce, D.; Binnie, M.; Batt, J. Cellular markers of muscle atrophy in chronic obstructive pulmonary disease. Am. J. Respir. Cell Mol. Biol. 2010, 42, 461–471. [Google Scholar] [CrossRef]



| Target | Forward Sequence (5′-3′) | Reverse Sequence (5′-3′) |
|---|---|---|
| Col1a1 | GCTCCTCTTAGGGGCCACT | CCACGTCTCACCATTGGGG |
| Col1a2 | GGTGAGCCTGGTCAAACGG | ACTGTGTCCTTTCACGCCTTT |
| Col3a1 | ACGTAGATGAATTGGGATGCAG | GGGTTGGGGCAGTCTAGTG |
| Fn1 | GCTCAGCAAATCGTGCAGC | CTAGGTAGGTCCGTTCCCACT |
| Hspg2 | TGGAGCCCGAATACAGGAAGA | AGATCCGTCCGCATTCCCT |
| V0/V1 Vcan | ACCAAGGAGAAGTTCGAGCA | CTTCCCAGGTAGCCAAATCA |
| Dcn | TCTTGGGCTGGACCATTTGAA | CATCGGTAGGGGCACATAGA |
| Dag1 | CTTGAGGCGTCCATGCACT | GGCAATTAAATCCGTTGGAATGC |
| Mmp2 | CAAGTTCCCCGGCGATGTC | TTCTGGTCAAGGTCACCTGTC |
| Mmp3 | ACATGGAGACTTTGTCCCTTTTG | TTGGCTGAGTGGTAGAGTCCC |
| Adamts1 | AAGGAAGAAGCGATTTGTGTCC | CCACCGAGAACAGGGTTAGA |
| Adamts4 | ATGGCCTCAATCCATCCCAG | AAGCAGGGTTGGAATCTTTGC |
| Adamts5 | GGAGCGAGGCCATTTACAAC | CGTAGACAAGGTAGCCCACTTT |
| Adamts9 | GACTTGTGGGCAAGGTAAGG | TCAGTCTCGGGGATGTAATCTG |
| Adamts15 | GCTCATCTGCCGAGCCAAT | CAGCCAGCCTTGATGCACTT |
| Ccnd1 | GCGTACCCTGACACCAATCTC | CTCCTCTTCGCACTTCTGCTC |
| Pcna | TTTGAGGCACGCCTGATCC | GGAGACGTGAGACGAGTCCAT |
| Klf6 | GTTTCTGCTCGGACTCCTGAT | TTCCTGGAAGATGCTACACATTG |
| Pax7 | CGATTAGCCGAGTGCTCAGA | GGAGGTCGGGTTCTGATTCC |
| Myod | ATAGACTTGACAGGCCCCGA | GTAGGGAAGTGTGCGTGCT |
| Myog | AATGCACTGGAGTTCGGTCC | TTCGTCTGGGAAGGCAACAG |
| Gapdh | AGGTCGGTGTGAACGGATTTG | GGGGTCGTTGATGGCAACA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, D.; Pei, Y.; Liang, L.; Wang, Z.; Gai, X.; Sun, Y. ADAMTS4 Reduction Contributes to Extracellular Matrix Deposition and Impaired Myogenesis in the Skeletal Muscle of Cigarette Smoke-Exposed Mice. Biomedicines 2025, 13, 474. https://doi.org/10.3390/biomedicines13020474
Li D, Pei Y, Liang L, Wang Z, Gai X, Sun Y. ADAMTS4 Reduction Contributes to Extracellular Matrix Deposition and Impaired Myogenesis in the Skeletal Muscle of Cigarette Smoke-Exposed Mice. Biomedicines. 2025; 13(2):474. https://doi.org/10.3390/biomedicines13020474
Chicago/Turabian StyleLi, Danyang, Yuqiang Pei, Long Liang, Zihan Wang, Xiaoyan Gai, and Yongchang Sun. 2025. "ADAMTS4 Reduction Contributes to Extracellular Matrix Deposition and Impaired Myogenesis in the Skeletal Muscle of Cigarette Smoke-Exposed Mice" Biomedicines 13, no. 2: 474. https://doi.org/10.3390/biomedicines13020474
APA StyleLi, D., Pei, Y., Liang, L., Wang, Z., Gai, X., & Sun, Y. (2025). ADAMTS4 Reduction Contributes to Extracellular Matrix Deposition and Impaired Myogenesis in the Skeletal Muscle of Cigarette Smoke-Exposed Mice. Biomedicines, 13(2), 474. https://doi.org/10.3390/biomedicines13020474







